Introduction
Breast cancer ranks second in female cancer
mortality rate, and the incidence rate in developing countries is
on the increase (1). Cell
cycle-dependent kinase inhibitor p57 is a tumor suppressor gene
coding for a multifunctional protein involved in cell proliferation
regulation, apoptosis, cell differentiation, tumor invasion,
metastasis and angiogenesis (2–4). Previous
findings showed that in a variety of human tumor cells, p57 was
downregulated, suggesting that low levels of p57 increased the risk
of malignant tumor development. Thus, p57 downregulation is
important in the pathogenesis of human ovarian cancer, gastric
cancer, colorectal cancer and lymphoma (5,6).
There are reports suggesting that p57 downregulation
is linked to the onset and development of breast cancer (7). In the present study, we investigated the
effects of p57 on two breast cancer cell lines. The results
however did not show any effects of knocked down p57 gene on
breast cancer cell proliferation.
Materials and methods
Materials
Human HMCF-7 and rat SHZ-88 breast cancer cell lines
were purchased from the Shanghai Institute of Cell Research,
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in media containing 10% fetal bovine serum RPMI-1640
(Gibco BRL, Gaithersburg, MD, USA), with 5% CO2 at 37°C
in a humid environment.
Main reagent
Lipofectamine® 2000 and TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA); AMV First Strand
cDNA Synthesis kit (Toyobo Co., Ltd., Osaka, Japan); and anti-p57
antibody (rb-1527) (Lab Vision/neomarkers, San Diego, CA, USA) were
used in the present study.
Small interfering RNA (siRNA) design
synthesis
p57 mRNA sequences in human and rat were obtained
from NCBI, and siRNAs were designed based on mRNA sequences using
siRNA online design software and structure 4.4 (8–10). In
order to enhance the stability of siRNA, the sense chain was
modified by fluorine (chemical was produced by the Shanghai Jima
Company, Shanghai, China).
Experimental method
Transfection
We used Lipofectamine® 2000 for
transfection. Transfection conditions were optimized using the
negative control siRNA with universal fluorescent labeling. Cells
in the logarithmic growth phase were collected and cell density was
adjusted. The cells were inoculated into a 12-well plate containing
RPMI-1640 culture medium with 10% serum without antibiotics.
Lipofectamine® 2000 reagent was diluted in culture
medium without serum and antibiotics. At 24 h later, the previous
culture medium was discarded and replaced with diluted
Lipofectamine® 2000 reagent and siRNA. They were mixed
for 5 min and after 20 min at room temperature they were incubated
for 6 h and then observed under a fluorescent microscope (IX70;
Olympus, Tokyo, Japan).
Cell proliferation was detected by
CCK-8 method
Normal and transfected human MCF-7 and rat SHZ-88
breast cancer cells were inoculated into a 96-well plate
(5×103 cells/well). Cell proliferation was measured at
1, 2, 3 and 4 days after culture. CCK-8 (10 µl) reagent was added
to each well followed by 2 h incubation at 37°C, and absorbance was
measured at 450 nm. The absorbance value represented cell
proliferation.
Immunofluorescence
MCF-7 and SHZ-88 breast cancer cells were inoculated
into 12-well plates with coverslips (1×105/ml, 1 ml per
well). After 48 h incubation, the cells were washed with PBS twice,
and fixed with 4% formaldehyde at room temperature for 10 min.
Then, 0.2% Triton X-100 was added and left at room temperature for
10 min. BSA (2%) was added, followed by 30 min incubation. Anti-p57
antibody (dilution, 1:50) was added and the sample was incubated at
4°C overnight. After incubation, the cells were washed three times
with PBS, 5 min each time, and secondary anti FITC-IgG (dilution,
1:100) was added at room temperature followed by 1 h incubation in
the dark. The samples were then examined under a fluorescent
microscope.
RT-polymerase chain reaction (PCR)
detection
Total RNA was extracted and the concentration was
measured using a UV spectrophotometer (Hitachi, Ltd., Tokyo, Japan)
and the integrity of RNA was examined by electrophoresis (A260/A280
ratio was 1.8–2). cDNA FastQuant was used for cDNA synthesis. The
reaction conditions were: 95°C for 3 min and 42°C for 15 min, and
then kept on ice. Primer sequences are presented in Table I.
 | Table I.RT-polymerase chain reaction primer
sequences of p57 mRNA of MCF-7 and SHZ-88 cells. |
Table I.
RT-polymerase chain reaction primer
sequences of p57 mRNA of MCF-7 and SHZ-88 cells.
| Gene name | Primer sequence |
|---|
| MCF-7 | 5′-3′
CTGATCTCCGATTTCTTCGC |
|
| 3′-5′
TCTTTGGGCTCTAAATTGG |
| SHZ-88 | 5′-3′
TCTCCTGTCCTGTGTGCCTACC |
|
| 3′-5′
CAGGTCAACTGCCTACACAGAGC |
Western blot analysis
Lysis solution (including protease inhibitor) was
added and the cells were ground (on ice). The sample was
centrifuged at 12,000 × g for 20 min at 4°C and supernatant was
collected. Protein content in the supernatant was quantified and
the sample was electrophoresed (10% SDS-PAGE) and transferred to a
PVDF membrane. The membrane was incubated with skimmed milk (10%)
for 1 h and rabbit monoclonal p57 antibody (dilution, 1:1,000; cat.
no. ab75974; Abcam, Cambridge, MA, USA) was added and the membrane
was incubated at 4°C overnight. The membrane was washed with TTBS
followed by adding secondary goat anti-rabbit (HRP) IgG antibody
(dilution, 1:2,000; cat. no. ab6721; Abcam) and incubating at room
temperature for 1 h. The membrane was washed with TTBS and
color-substrate solution was added.
Statistical analysis
SPSS 17.0 software (IBM SPSS, Armonk, NY, USA) was
used for statistical analysis. The Kaplan-Meier method was used for
single factor analysis of the relevant data in survival analysis.
Log-rank test was employed for comparisons of significant
difference. The Cox proportional hazard model was used to study the
effects of various clinical and pathological factors on the
recurrence time. P<0.05 was considered to indicate a
statistically significant difference.
Results
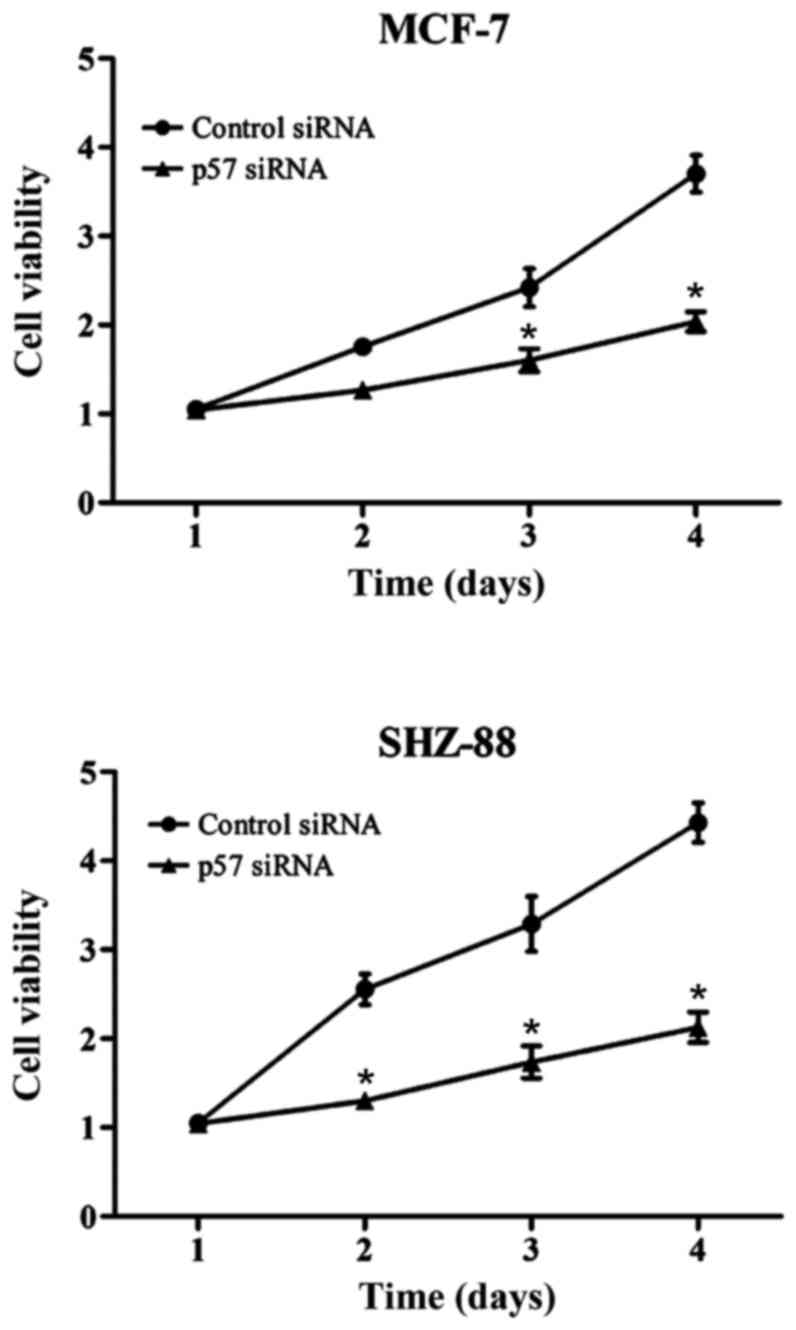
Cell proliferation after p57
interference
Cell proliferation in the experimental group was
significantly reduced. In the MCF-7 cells, absorbance value in the
experimental group and the negative control group were 2.67±0.09
and 3.87±0.12, respectively. In the SHZ-88 cells these values were
2.06±0.07 and 4.37±0.12 in the experimental and negative control
groups, respectively. Differences were statistically significant
(P<0.05; Fig. 1).
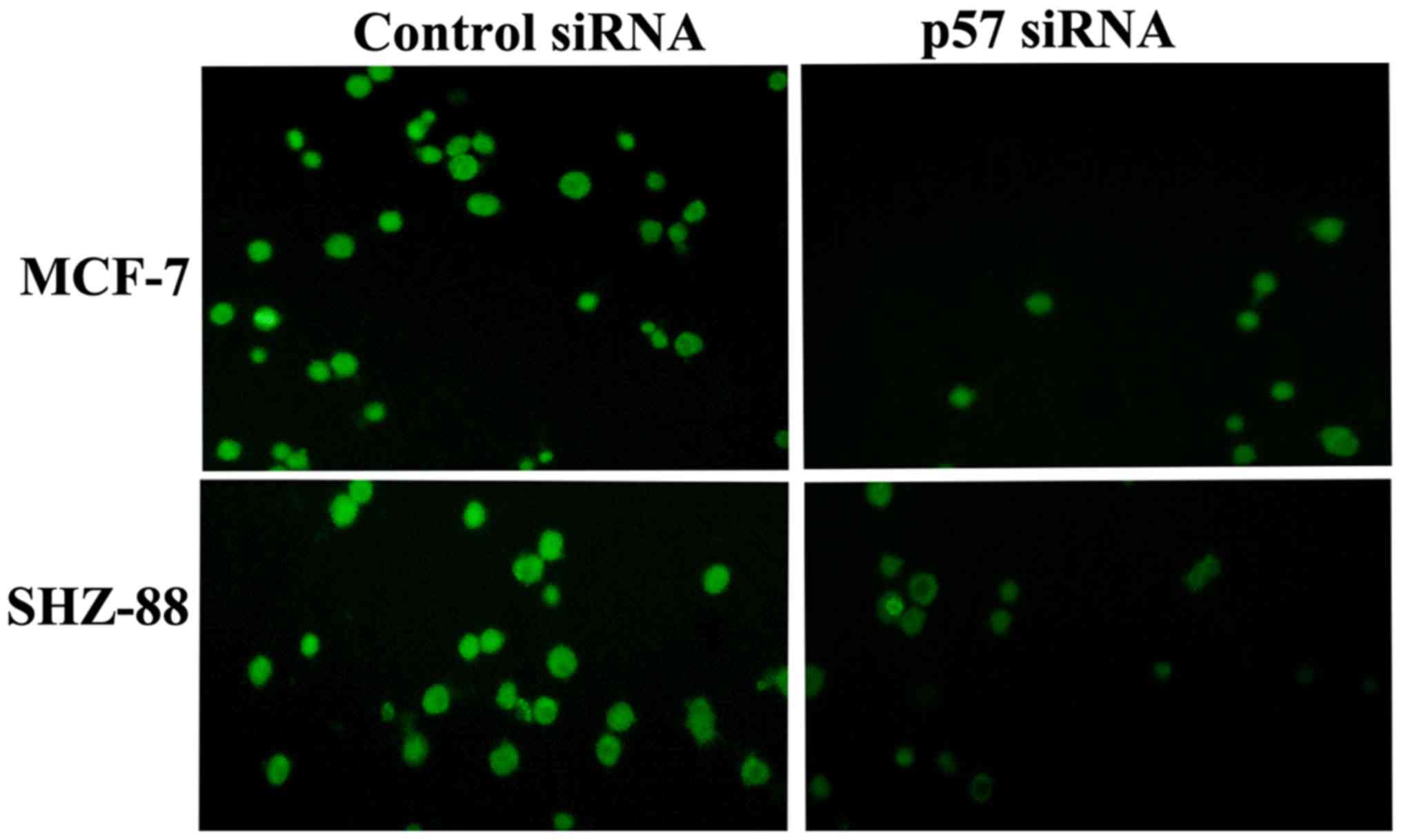
Immunofluorescent assay
siRNA effectively inhibited the p57 expression in
MCF-7 and SHZ-88 cells. Compared with the control group, p57
protein levels were significantly lower in the siRNA-transfected
cells. Immunofluorescent assay showed that p57 was expressed in the
MCF-7 and SHZ-88 cells, 48 h after transfection (Fig. 2).
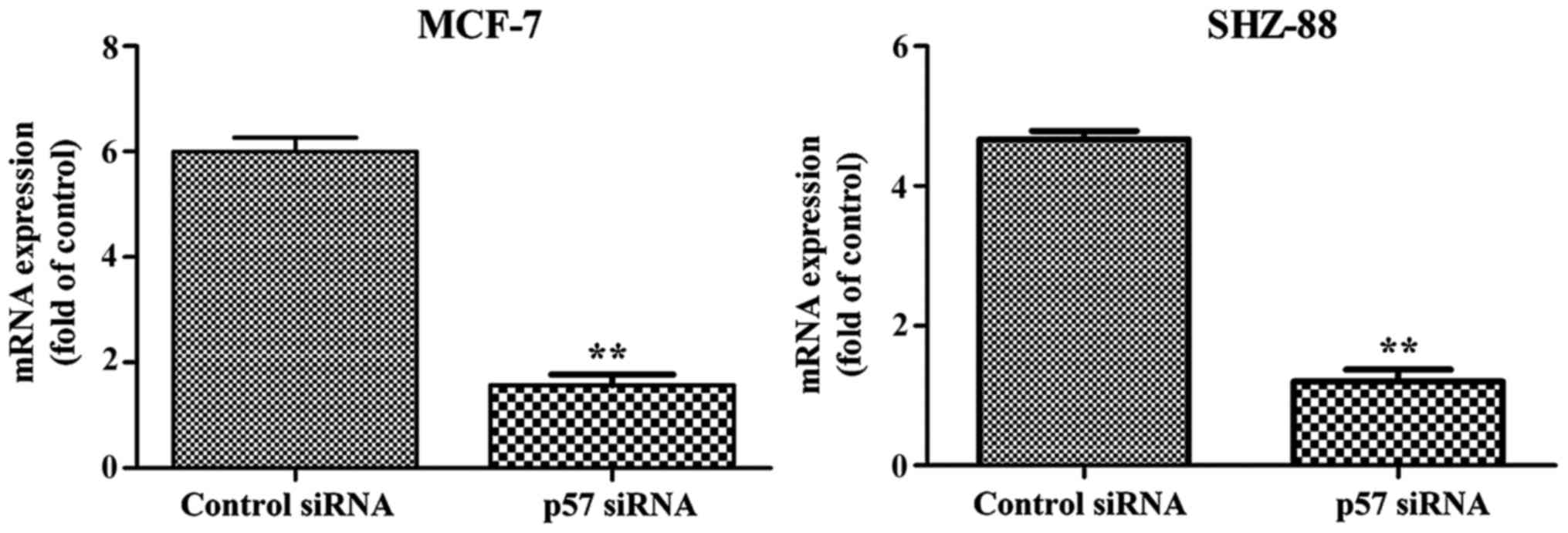
Effect of p57 siRNA on p57
transcription and translation
RT-PCR results showed that 48 h after transfection,
the mRNA level in the transfected group was significantly lower
compared with the control group (Fig.
3). This suggested that the design of our siRNA was successful
and siRNA was able to effectively inhibit the p57 gene
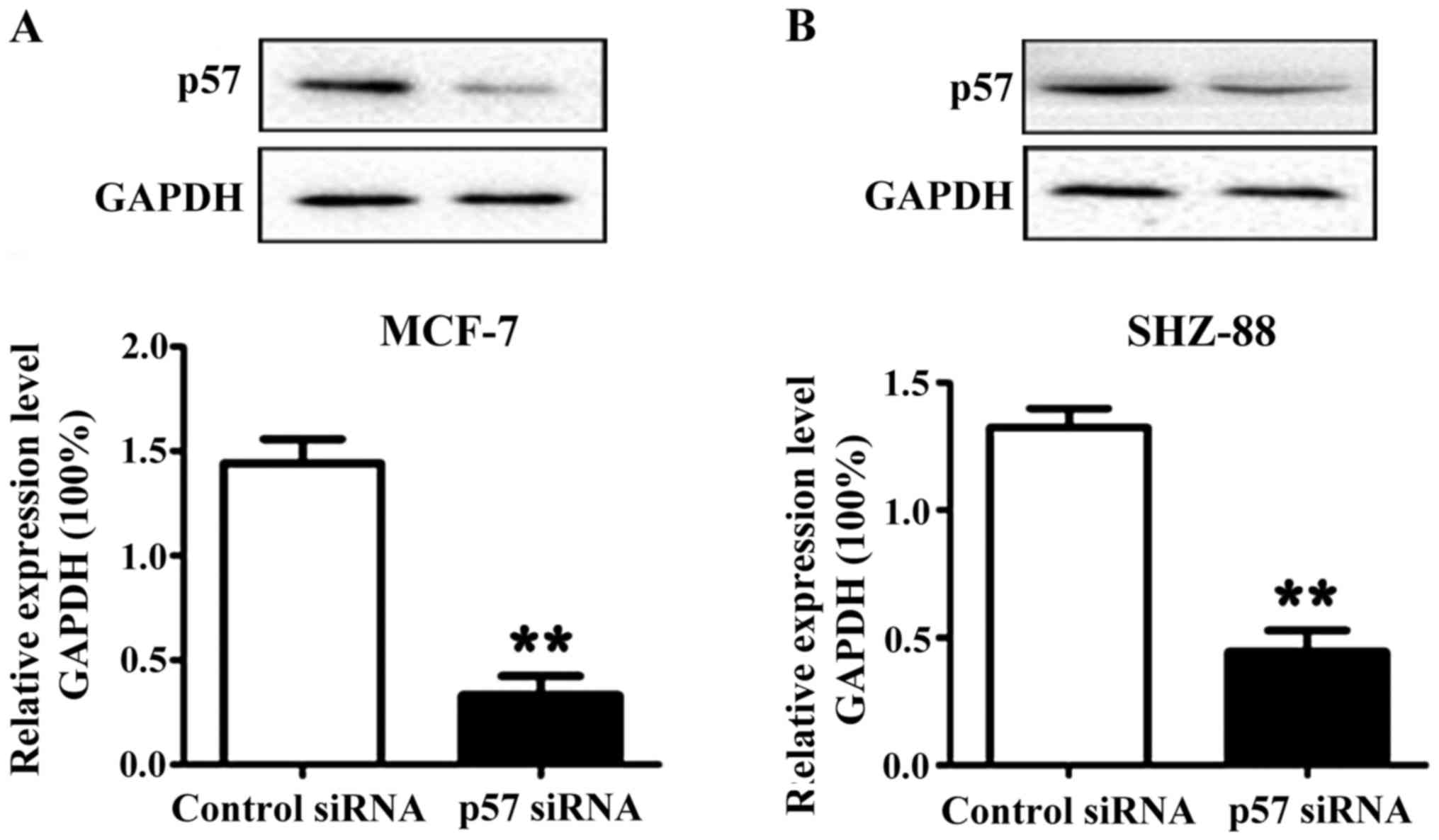
transcription in the MCF-7 and SHZ-88 cells. The results obtained
from western blotting showed that the level of p57 protein was
significantly lower in the transfected cells, which suggested that
the design of our siRNA was successful (Fig. 4).
Discussion
Breast cancer is one of the most common malignant
tumors in women. In China, the incidence of this type of cancer
ranks first. According to GLOBOCAN 2008 statistics, new cases of
breast cancer accounted for 23% of all tumors in 2011, and
accounted for 14% of all cancer deaths (11–14). Tumor
formation is associated with the out-of-control cell cycle
regulation (15). The cell cycle
control mechanism includes cyclins, cyclin-dependent protein
kinases (CDKs) and cyclin-dependent kinase inhibitor (CKI). Any
disturbance in the mechanisms that control these factors can
potentially lead to excessive cell proliferation, reduction of
apoptosis and tumor formation. p57 is a CKI that belongs to the
Cip/Kip family, which includes p21, p27 and p57. p57 can regulate
cell cycle progression and is involved in the regulation of
transcription, apoptosis, differentiation, development, and
migration. p57 is a tumor suppressor that can regulate cyclins,
CDKs and cyclin-CDK complexes in the G1-S transition, modulating
DNA replication (16). Therefore,
absence of p57 may result in an unusual increase in the number of
cells in S phase (16). It has been
reported that P57 protein is downregulated in colon cancer
(17), gastric cancer (18) and human brain glioma (19,20).
In the present study, we blocked the expression of
p57 in human and rat breast cancer cell lines (MCF-7 and SHZ-88) by
transfecting cells using Lipofectamine® 2000 and
specific p57 siRNAs. The results showed that p57 siRNA successfully
knocked down the expression of p57. We demonstrated that the
construction of an appropriate p57 siRNA was directly linked to p57
silencing efficiency. Thus, the knockdown of p57 cells may be
useful in the field of tumor therapy.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bodai BI and Tuso P: Breast cancer
survivorship: A comprehensive review of long-term medical issues
and lifestyle recommendations. Perm J. 19:48–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Furutachi S, Matsumoto A, Nakayama KI and
Gotoh Y: p57 controls adult neural stem cell quiescence and
modulates the pace of lifelong neurogenesis. EMBO J. 32:970–981.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo H, Li Y, Tian T, Han L, Ruan Z, Liang
X, Wang W and Nan K: The role of cytoplasmic p57 in invasion of
hepatocellular carcinoma. BMC Gastroenterol. 15:1042015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsumoto M, Furihata M, Ohtsuki Y,
Sasaguri S and Ogoshi S: Immunohistochemical characterization of
p57KIP2 expression in human esophageal squamous cell carcinoma.
Anticancer Res. 20:1947–1952. 2000.PubMed/NCBI
|
|
5
|
Ma YL, Peng JY, Zhang P, Liu WJ, Huang L
and Qin HL: Immunohistochemical analysis revealed CD34 and Ki67
protein expression as significant prognostic factors in colorectal
cancer. Med Oncol. 27:304–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma Y and Cress WD: Transcriptional
upregulation of p57 (Kip2) by the cyclin-dependent kinase inhibitor
BMS-387032 is E2F dependent and serves as a negative feedback loop
limiting cytotoxicity. Oncogene. 26:3532–3540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Wang Y, Yang Y, Liu J, Song Y, Cao
Y, Chen X, Yang W, Wang F, Gao J, et al: MicroRNA-222 controls
human pancreatic cancer cell line capan-2 proliferation by P57
targeting. J Cancer. 6:1230–1235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banan M and Puri N: The ins and outs of
RNAi in mammalian cells. Curr Pharm Biotechnol. 5:441–450. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu JY, DeRuiter SL and Turner DL: RNA
interference by expression of short-interfering RNAs and hairpin
RNAs in mammalian cells. Proc Natl Acad Sci USA. 99:6047–6052.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyagishi M and Taira K: U6
promoter-driven siRNAs with four uridine 3′ overhangs efficiently
suppress targeted gene expression in mammalian cells. Nat
Biotechnol. 20:497–500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palmer TD, Ashby WJ, Lewis JD and Zijlstra
A: Targeting tumor cell motility to prevent metastasis. Adv Drug
Deliv Rev. 63:568–581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valastyan S and Weinberg RA: Tumor
metastasis: molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holen I, Whitworth J, Nutter F, Evans A,
Brown HK, Lefley DV, Barbaric I, Jones M and Ottewell PD: Loss of
plakoglobin promotes decreased cell-cell contact, increased
invasion, and breast cancer cell dissemination in vivo. Breast
Cancer Res. 14:R862012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Li G, Finch JW, Geromanos SJ and
Gebler JC: P57-T Development of an automated RP/RP 2D nanoLC/MS
method for proteomic analysis. J Biomol Tech. 18:202007.
|
|
16
|
Bek S, Kreppel D, Bscheider M, Lin CC,
Haas T and Poeck H: Activation of RIG-I induces immunogenic cell
death. J Immunother Cancer. 2:P312014. View Article : Google Scholar
|
|
17
|
Tsabouri S, Valari M, Douros K,
Gemou-Engesaeth V, Magiakou MA, Papadavid E, Theodoridou M and
Priftis K: Prognostic factors for asthma at school age in infants
with atopic dermatitis. Clin Transl Allergy. 4:P1122014. View Article : Google Scholar
|
|
18
|
Ullah Z, Kohn MJ, Yagi R, Vassilev LT and
DePamphilis ML: Differentiation of trophoblast stem cells into
giant cells is triggered by p57/Kip2 inhibition of CDK1 activity.
Genes Dev. 22:3024–3036. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Li DQ, Tong L, Stewart P, Chu C
and Pflugfelder SC: Targeted inhibition of p57 and p15 blocks
transforming growth factor β-inhibited proliferation of primary
cultured human limbal epithelial cells. Mol Vis. 12:983–994.
2006.PubMed/NCBI
|
|
20
|
Joaquin M, Gubern A, González-Nuñez D,
Ruiz Josué E, Ferreiro I, de Nadal E, Nebreda AR and Posas F: The
p57 CDKi integrates stress signals into cell-cycle progression to
promote cell survival upon stress. EMBO J. 31:2952–2964. 2012.
View Article : Google Scholar : PubMed/NCBI
|