Introduction
Malignant melanoma (MM) is a type of tumor arising
from melanocytes, which originate from neural crest cells (1). The common primary sites of MM are the
skin and mucosal surfaces (2). MM is
associated with a very high mortality rate and an extremely poor
prognosis (3). Therefore, the
accurate diagnosis of MM is essential for providing appropriate and
timely treatment. Sinonasal MM accounts for <1% of all melanomas
and <5% of all sinonasal tract neoplasms (4). Patients with sinonasal MM often display
non-specific symptoms, including nasal obstruction or epistaxis,
causing clinical misdiagnosis (1).
Based on melanin pigmentation, MM can be histopathologically
categorized into melanotic and amelanotic subtypes (1,5). It is
difficult to diagnose amelanotic MM in undifferentiated tumor cells
as they exhibit negative expression of all melanocytic markers
(6). The present study reports a case
of undifferentiated sinonasal MM, which mimics a poorly
differentiated carcinoma with aberrant expression of epithelial
markers. The ultrastructural characteristics of the tumor cells and
the tumor phenotypes, including the proportion of cancer stem cells
and vaculogenic mimicry (VM), were determined.
Case report
Written informed consent was acquired from the
patient discussed in the present report, and the following study
was ethically approved by the Ethics Committee of the Anhui
Provincial Hospital (Anhui, China). A 41-year-old male patient was
admitted to Anhui Provincial Hospital (Hefei, China) in July 2016.
The patient reported a month of congestion of the right nasal
passage with no rhinorrhea or bleeding until the 4 days prior to
presentation, in which progressive epistaxis was experienced.
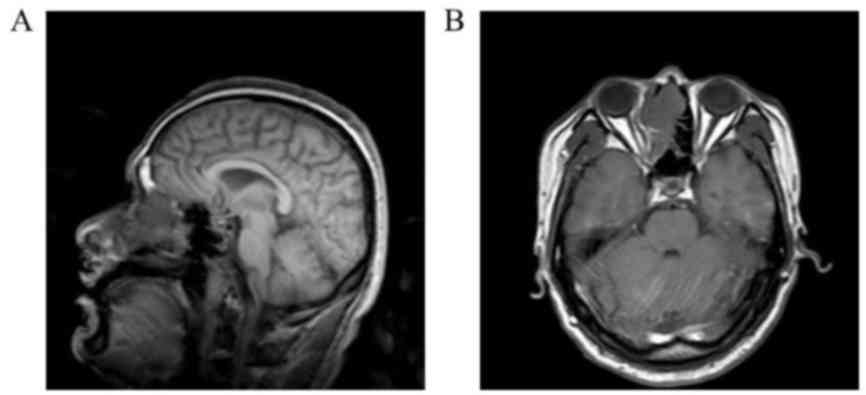
Magnetic resonance imaging (MRI) revealed a lesion in the right
nasal cavity and ethmoid sinus (Fig. 1A
and B). Nasal endoscopic examination indicated a red mass
occupying the right side of the nasal cavity and the middle meatus.
The mass had invaded the middle turbinate, nasal septum and sieve
plate, and bone destruction had occurred. Complete excision of the
primary lesion with at least 1.5 cm of normal tissue was performed,
and a sentinel lymph node biopsy achieved a negative result. The
otolaryngologist identified the surface of the mass to be rough,
brittle and to bleed easily when palpated. The patient exhibited
stage II disease at diagnosis, and radiotherapy was indicated.
The specimen was examined by two pathologists. The
excised tissue was fixed in 10% neutral-buffered formalin for 10 h
at room temperature, then tissue samples were dehydrated in an
ethanol/xylene series, embedded using fresh paraffin wax and
maintained at 55–60°C. Tissue samples were serially sectioned (4-µm
thick); 4 underwent hematoxylin and eosin (H&E) staining and 23
underwent immunohistochemical staining. Immunohistochemistry was
performed using the ChemMate Envision kit including secondary
antibody (cat. no., DAKOK500711; Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA), according to the manufacturer's protocol
(7). Sections were deparaffinized in
xylene and dehydrated with descending alcohol series. Endogenous
peroxidase was blocked with 0.1% hydrogen peroxide-methanol for 30
min at room temperature. Sections were washed once with PBS, and
then microwaved for 15 min in 0.05 mol Tris buffer (pH 9.0) for
antigen retrieval, followed by washing three times with PBS, and
then incubated with primary antibodies at 4°C overnight. Following
washing three times with PBS, they were incubated with horseradish
peroxidase-conjugated dextran polymer reagent (dilution, 1:2,000;
cat. no., sc-2004; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The sections were incubated with the following 23
primary antibodies: Human melanoma black-45 (mouse monoclonal; cat.
no., MAB-0098), Melan-A (mouse monoclonal; cat. no., MAB-0275),
smooth muscle actin (SMA; mouse monoclonal; cat. no., Kit-0006),
marker of proliferation Ki67 (mouse monoclonal; cat. no.,
Kit-0005), synaptophysin (Syn; rabbit monoclonal; cat. no.,
Kit-0022), cluster of differentiation 31 (CD31; mouse monoclonal;
cat. no., MAB-0031), friend leukemia integration 1 transcription
factor (Fli-1; mouse monoclonal; cat. no., MAB-0649), cluster of
differentiation 21 (CD21; rabbit monoclonal; cat. no., RMA-0647),
factor VIII (rabbit polyclonal; cat no., RAB-0070; all from Fuzhou
Maixin Biotechnology Development Co., Ltd., Fuzhou, China). The
following antibodies were purchased from ZSGB-BIO (Beijing, China):
pan-cytokeratin (mouse monoclonal; cat. no., ZM-0069), vimentin
(mouse monoclonal; cat. no., ZM-0260), cytokeratin 5 (rabbit
monoclonal; cat. no., ZA-0518), CD34 (mouse monoclonal; cat. no.,
ZM-0046), epithelial membrane antigen (EMA; mouse monoclonal; cat.
no., ZM-0095), S100 (rabbit polyclonal; cat. no., ZA-0225), cluster
of differentiation 45 (CD45; mouse monoclonal; cat. no., ZM-0183),
p40 (mouse monoclonal; cat. no., ZM-0472), sex determining region
Y-box 10 (SOX10; rabbit monoclonal; cat. no., ZA-0624). Primary
antibodies (volume, 100 µl) were provided as ready-to-use products
and did not require further dilution. The cancer stem cells markers
used include the following primary antibodies: Oct4 (rabbit
polyclonal; dilution, 1:200; cat. no., 2750), sex determining
region Y-box 2 (SOX2; rabbit monoclonal; dilution, 1:100; cat. no.,
3579), Nanog (rabbit monoclonal; dilution, 1:800; cat. no., 4903;
all from Cell Signaling Technology, Inc., Danvers, MA, USA),
cluster of differentiation 133 (CD133; rabbit polyclonal; dilution,
1:100; cat. no., sc-30220; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and Nestin (mouse monoclonal; dilution, 1:200; cat. no.,
MAB-0566; Fuzhou Maixin Biotechnology).
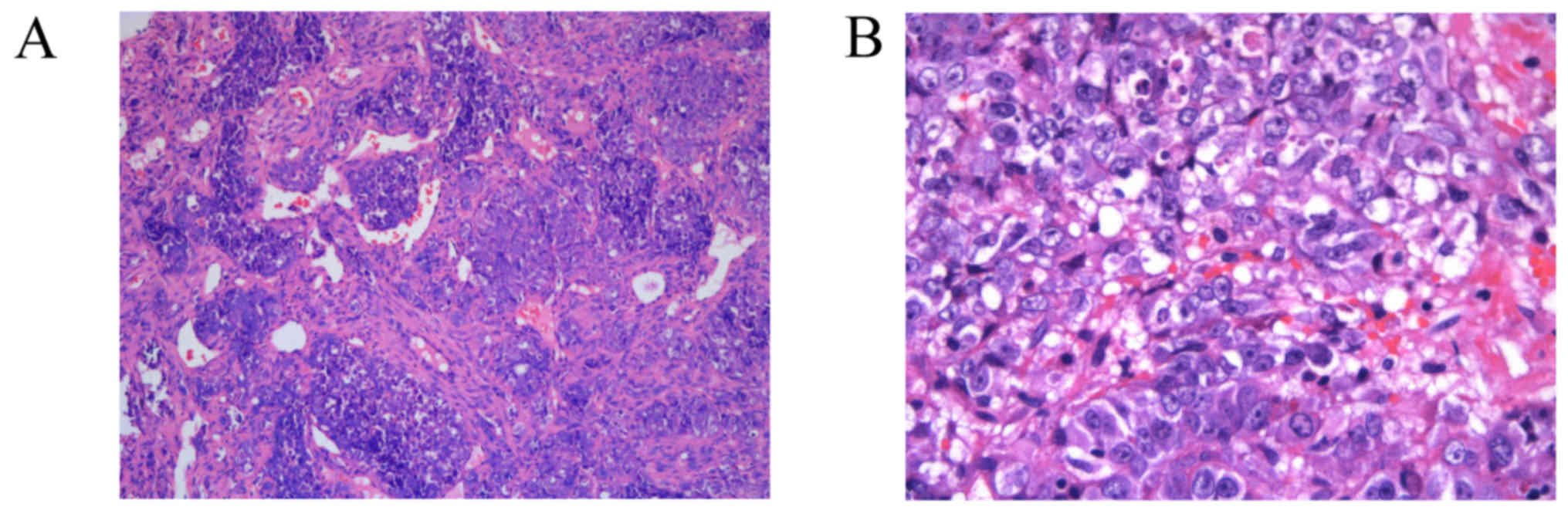
Gross pathological examination resulted in the
description of a grey irregular specimen of 5×5×1 cm3
and moderate hardness. Histopathologically, the mass was composed
of malignant epithelioid cells arranged in nests and sheets
(Fig. 2A). The overall cellularity
was moderate to high with evident atypia. The tumor cells displayed
a hemangiopericytoma-like pattern accompanied by antler-like
branching vessels. The tumor stroma was focally desmoplastic and
necrosis was not evident. Under a light microscope at ×400,
magnification (Leica 2500 microscope; Leica Microsystems, Inc.,
Buffalo Grove, IL, USA), the tumor cells demonstrated relatively
uniform hyperchromatic, rounded nuclei with prominent nucleoli, and
mitotic activity was conspicuous. The cytoplasm of the tumor was
lightly eosinophilic to amphophilic, containing numerous vacuoles
but exhibiting no pigment deposition (Fig. 2B).
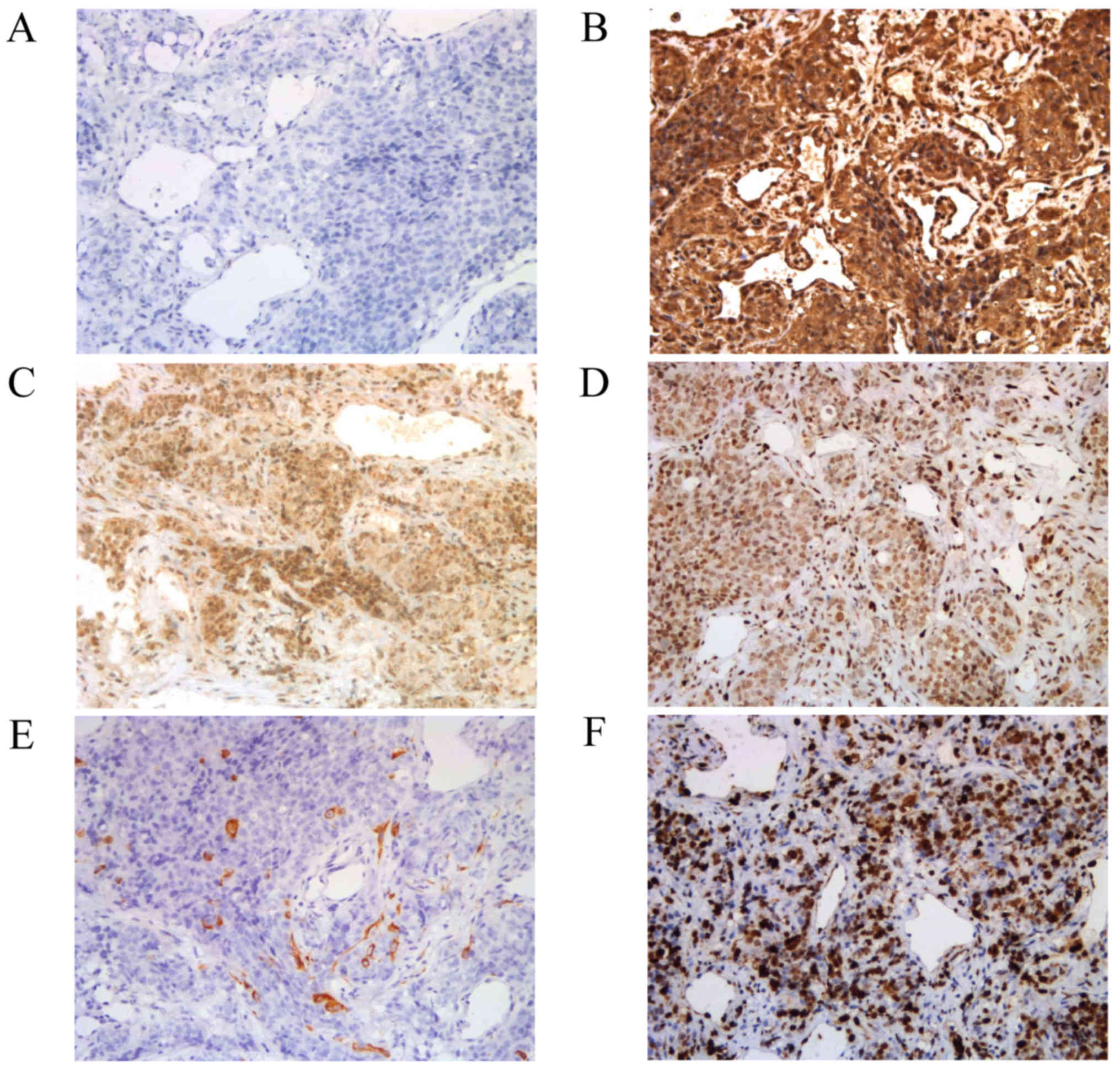
Immunohistochemical analyses demonstrated that the
tumor cells did not express melanocytic markers HMB-45, Melan-A,
S100 or SOX10 (Fig. 3A). However, the
tumor cells did express vimentin (Fig.
3B), EMA (Fig. 3C) and Fli-1
(Fig. 3D). Pan-cytokeratin expression
demonstrated scattered expression among tumor cells (Fig. 3E). The tumor cells did not express
cytokeratin 5, CD31, CD34, Syn, CD45, CD21, SMA, Factor VIII, p63
or p40. A total of 80% of the tumor cells were positive for Ki-67
staining (Fig. 3F). Ultrastructural
analysis by transmission electron microscopy was performed to
determine the malignancy type. The results demonstrated that mature
melanosomes and premelanosomes existed in tumor cells (Fig. 4), supporting the diagnosis of MM.
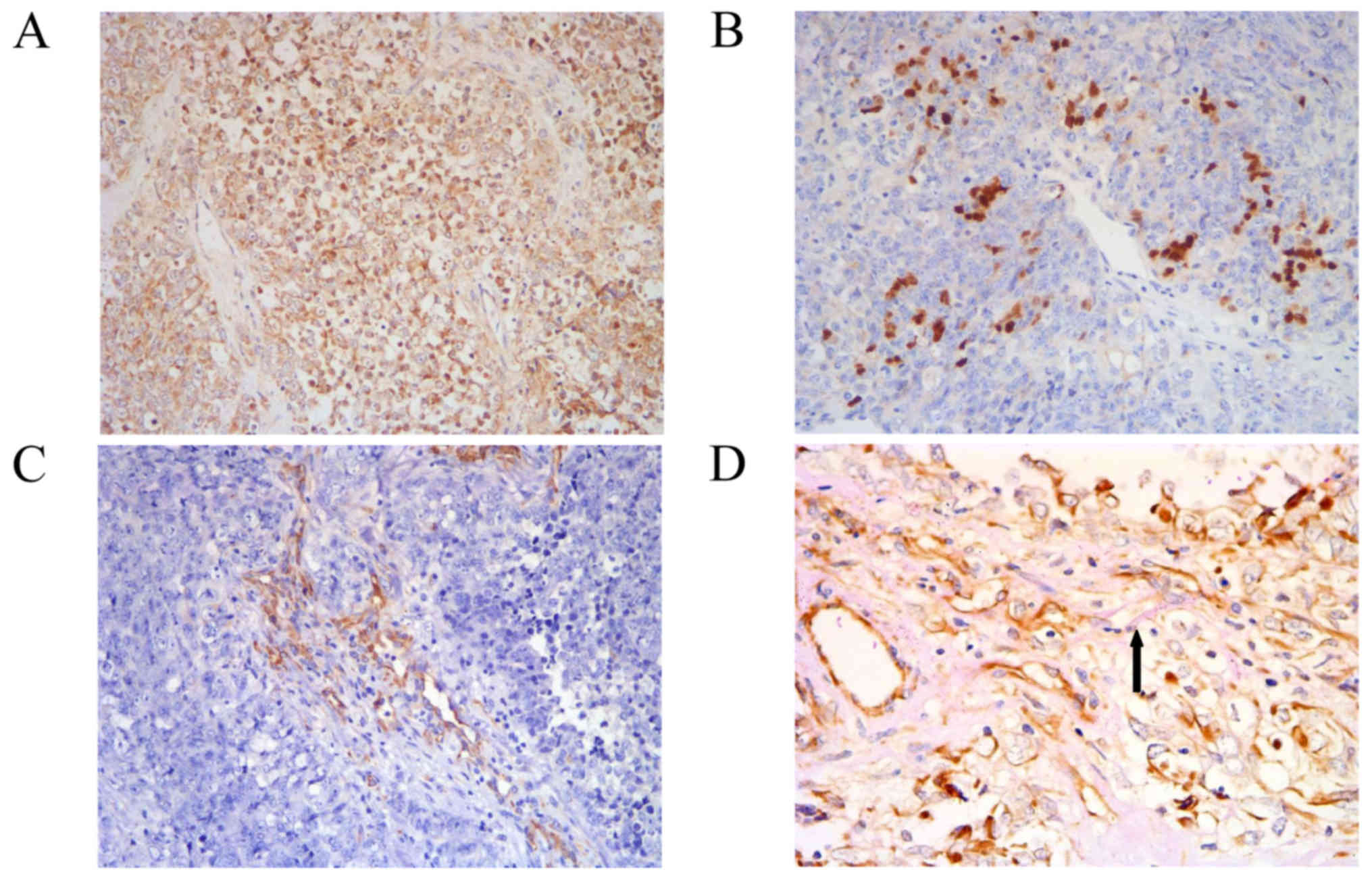
In order to further understand the nature of
undifferentiated MM, cancer stem cell marker expression was also
analyzed, including that of CD133, SOX2, OCT4 and Nanog. The tumor
cells expressed CD133 strongly and diffusely (Fig. 5A). SOX2 was expressed focally around
the vessels (Fig. 5B), while nestin
expression was negative for tumor cells, but positive for vascular
mural cells (Fig. 5C). The tumor
cells exhibited negative expression of OCT4 and Nanog. In order to
investigate VM, CD34 and periodic acid-Schiff (PAS; B24200-250;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) double-staining
was used to distinguish microvessels from VM. Sections were exposed
to sodium periodate for 10 min following immunohistochemical
staining of CD34 at room temperature, then rinsed with distilled
water for 5 min, followed by incubation with 0.1% Periodic
acid-Schiff for 15 min at room temperature. All sections were
counterstained with Mayer's hematoxylin for 2 min at room
temperature, dehydrated with descending alcohol series, and
mounted. VM was characterized as a channel positive for PAS without
exhibiting positive CD34 staining of the endothelium (Fig. 5D).
Discussion
Primary MM of the sinonasal mucosa is a rare
disease, with an equal incidence in males and females. It often
occurs at 60–70 years of age, but rarely prior to 40 years of age
(8). Obstruction or epistaxis are the
most common early symptoms of sinonasal MM (1). Sinonasal MM has a high rate of local
recurrence and distant metastasis (1,3). According
to previous literature, the 5-year survival rate of patients is
44–88.7% (9). One previous study
revealed that the majority of patients with sinonasal MM exhibiting
stage I or II disease demonstrated no evidence of recurrence after
a follow-up period of 13.9 years. However, all patients presenting
with a stage III diagnosis had succumbed to the disease during the
follow-up period (10). The survival
rate was associated with tumor location, tumor spread, tumor
thickness, lymph node and distant metastases (9,10).
Therefore, the early and accurate diagnosis of sinonasal MM is
essential for effective treatment of the disease.
Histopathologically, melanoma is categorized into
melanotic and amelanotic types according to melanin pigmentation
(11). MM tumors are composed of a
variety of cell types: Epithelioid, spindled, clear-cell,
plasmacytoid and mixed-cell (12). In
a number of cases, the melanoma cells may be undifferentiated with
no expression of melanocytic markers, resulting in difficulty
regarding its diagnosis (6,13).
In the present case report, the tumor cells
exhibited negative expression of melanocytic markers, including
HMB-45, Melan-A, S100 and SOX10. However, the tumor cells exhibited
positive expression of epithelial markers, including EMA and
pan-cytokeratin. These characteristics mimic those of
undifferentiated nasopharyngeal carcinomas that occur commonly in
sinonasal regions (14). However,
characteristically, undifferentiated nasopharyngeal carcinomas
exhibit cytokeratin 5, p63 and p40 expression (15), which was not exhibited by the tissue
analyzed in the present study. On the other hand, expression of the
endothelial cell marker, Fli-1, was observed, which raised the
possibility of epithelioid angiosarcoma, characterized by Fli-1,
CD31, CD34 and factor VIII expression (16). However, a previous study demonstrated
that Fli-1 may be strongly expressed in MM, and that Fli-1
expression is associated with tumor cell proliferation rate and
other aggressive behaviors (17). The
immunohistochemical panel of the present study excluded other
possible tumor types of the sinonasal region, including sinonasal
neuroendocrine carcinoma, paraganglioma, olfactory neuroblastoma,
pituitary adenoma, tumors of the Ewing family and follicular
dendritic cell sarcoma. Sinonasal neuroendocrine carcinoma,
paraganglioma, olfactory neuroblastoma, pituitary adenoma and
tumors of the Ewing family which usually express Syn; however this
was not expressed in the present case study. Follicular dendritic
cell sarcoma demonstrated diffuse staining with CD21, while MM was
negative for CD21. The results of transmission electron microscopy
analysis revealed the presence of mature melanosomes and
premelanosomes in the cytoplasm of the tumor cells, which are key
ultrastructures of MM cells (18).
SOX2, CD133 and Nestin have been reported as cancer
stem cell markers in melanoma (19–21). In
the present study, the tumor cells of undifferentiated MM expressed
CD133 and SOX2. Nestin was not expressed in tumor cells but was
expressed in vascular mural cells. It is speculated that cancer
stem cells may transdifferentiate into pericytes and participate in
blood vessel remodeling (22,23). Tumor cell vasculogenic mimicry (VM)
refers to the plasticity of cancer cells forming de novo
vascular networks, and is associated with malignant phenotypes and
a poor prognosis (24,25). Cancer stem cells may contribute to the
formation of VM (26). VM was
originally identified in malignant melanoma, and was subsequently
demonstrated to be an important morphological feature of MM
(27). In the present study, VM is
detectable in undifferentiated MM due to stemness, which may be a
useful tool for MM diagnosis.
The present study presents a rare case of
undifferentiated sinonasal MM. Given that an accurate diagnosis of
this type of tumor is challenging, it is demonstrated that the
combination of a panel of conventional immunohistochemical markers,
ultrastructure identification by transmission electron microscopy
and the VM detection may be used to validate the diagnosis of MM,
and to exclude other malignancies of the sinonasal area.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD and LLH contributed to the design of the
research, drafting the manuscript, gave final approval of the
version to be published, and agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. AX, ALZ, XK, MD, WH and ZLG performed
the experiments. WZ, SBS and HL analyzed the data. JC and QS made
substantial contributions to conception and acquisition of data.
LLX made contributions to interpretation of data. HBW contributed
to the design of the research, and revised and finalized the
article. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was ethically approved by the Ethics
Committee of the Anhui Provincial Hospital (Anhui, China). Informed
consent was obtained from the study participant.
Consent for publication
All patients provided consent for the publication of
their data
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MM
|
malignant melanoma
|
|
MRI
|
magnetic resonance imaging
|
|
VM
|
vasculogenic mimicry
|
|
H&E
|
hematoxylin and eosin
|
|
SMA
|
smooth muscle actin
|
|
Fli-1
|
friend leukemia integration 1
transcription factor
|
|
EMA
|
epithelial membrane antigen
|
References
|
1
|
Zhu W, Zou B and Wang S:
Clinicopathological features and prognosis of sinonasal mucosal
malignant melanoma: A retrospective study of 83 cases in a Chinese
population. ORL J Otorhinolaryngol Relat Spec. 78:94–104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Safadi RA, Bader DH, Abdullah NI and
Sughayer MA: Immunohistochemical expression of keratins 6, 7, 8,
14, 16, 18, 19, and MNF-116 pancytokeratin in primary and
metastatic melanoma of the head and neck. Oral Surg Oral Med Oral
Pathol Oral Radiol. 121:510–519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bradley PJ: Primary malignant mucosal
melanoma of the head and neck. Curr Opin Otolaryngol Head Neck
Surg. 14:100–104. 2006.PubMed/NCBI
|
|
4
|
Smith SM, Schmitt AC, Carrau RL and
Iwenofu OH: Primary sinonasal mucosal melanoma with aberrant
diffuse and strong desmin reactivity: A potential diagnostic
pitfall! Head Neck Pathol. 9:165–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thomas NE, Kricker A, Waxweiler WT, Dillon
PM, Busman KJ, From L, Groben PA, Armstrong BK, Anton-Culver H,
Gruber SB, et al: Comparison of clinicopathologic features and
survival of histopathologically amelanotic and pigmented melanomas:
A population-based study. JAMA Dermatol. 150:1306–1314. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agaimy A, Specht K, Stoehr R, Lorey T,
Märkl B, Niedobitek G, Straub M, Hager T, Reis AC, Schilling B, et
al: Metastatic malignant melanoma with complete loss of
differentiation markers (undifferentiated/dedifferentiated
melanoma): Analysis of 14 patients emphasizing phenotypic
plasticity and the value of molecular testing as surrogate
diagnostic marker. Am J Surg Pathol. 40:181–191. 2016.PubMed/NCBI
|
|
7
|
Liu J, Wang Y and Liu Y, Liu Z, Cui Q, Ji
N, Sun S, Wang B, Wang Y, Sun X and Liu Y: Immunohistochemical
profile and prognostic significance in primary central nervous
system lymphoma: Analysis of 89 cases. Oncol Lett. 14:5505–5512.
2017.PubMed/NCBI
|
|
8
|
Montone KT: The diferential diagnosis of
sinonasal/nasopharynheal neuroendocrine/neuroectodermally derived
tumors. Arch Pathol Lab Med. 139:1498–1507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Houette A, Gilain L, Mulliez A, Mom T and
Saroul N: Prognostic value of two tumour staging classifications in
patients with sinonasal mucosal melanoma. Eur Ann Otorhinolaryngol
Head Neck Dis. 133:313–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson LD, Wieneke JA and Miettinen M:
Sinonasal tract and nasopharyngeal melanomas: A clinicopathologic
study of 115 cases with a proposed staging system. Am J Surg
Pathol. 27:594–611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teixeira TF, Gentile LB, da Silva TC,
Mennecier G, Chaible LM, Cogliati B, Roman MA, Gioso MA and Dagli
ML: Cell proliferation and expression of connexins differ in
melanotic and amelanotic canine oral melanomas. Vet Res Commun.
38:29–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerr EH, Hameed O, Lewis JS Jr, Bartolucci
AA, Wang D and Said-AI-Naief N: Head and neck mucosal malignant
melanoma: Clinicopathologic correlation with contemporary review of
prognostic indicators. Int J Surg Pathol. 20:37–46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jalas JR, Vemula S, Bezrookove V, Leboit
PE, Simko JP and Bastian BC: Metastatic melanoma with striking
adenocarcinomatous differentiation illustrating phenotypic
plasticity in melanoma. Am J Surg Pathol. 35:1413–1418. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Franchi A: An update on sinonasal round
cell undifferentiated tumors. Head Neck Pathol. 10:75–84. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh L, Ranjan R, Arava S and Singh MK:
Role of p40 and cytokeratin 5/6 in the differential diagnosis of
sinonasal undifferentiated carcinoma. Ann Diag Pathol. 18:261–265.
2014. View Article : Google Scholar
|
|
16
|
Folpe AL, Chand EM, Goldblum JR and Weiss
SW: Expression of Fli-1, a nuclear transcription factor,
distinguishes vascular neoplasms from potential mimics. Am J Surg
Pathol. 25:1061–1066. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torlakovic EE, Slipicevic A, Flørenes VA,
Chibbar R, DeCoteau JF and Bilalovic N: Fli-1 expression in
malignant melanoma. Histol Histopathol. 23:1309–1314.
2008.PubMed/NCBI
|
|
18
|
Ordóñez NG and Mackay B: Electron
microscopy in tumor diagnosis: Indications for its use in the
immunohistochemical era. Hum Pathol. 29:1403–1411. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Ding N, Li Y, Cheng H, Wang D,
Yang Q, Deng Y, Yang Y, Li Y, Ruan X, et al: Insulin-like growth
factor binding protein 5 (IGFBP5) functions as a tumor suppressor
in human melanoma cells. Oncotarget. 6:20636–20649. 2015.PubMed/NCBI
|
|
20
|
Zimmerer RM, Korn P, Demougin P, Kampmann
A, Kokemüller H, Eckardt AM, Gellrich NC and Tavassol F: Functional
features of cancer stem cells in melanoma cell lines. Cancer Cell
Int. 13:782013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuk SK, Won CH, Lee WJ, Shin WJ, Yoon HJ,
Hong SD, Hong SP and Lee J II: Prognostic significance of nestin in
primary malignant melanoma of the oral cavity. Melanoma Res.
26:457–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klein D, Meissner N, Kleff V, Jastrow H,
Yamaguchi M, Ergün S and Jendrossek V: Nestin (+) tissue-resident
multipotent stem cells contribute to tumor progression by
differentiating into pericytes and smooth muscle cells resulting in
blood vessel remodeling. Front Oncol. 4:1692014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng L, Huang Z, Zhou W, Wu Q, Donnola S,
Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, et al: Glioblastoma
stem cells generate vascular pericytes to support vessel function
and tumor growth. Cell. 153:139–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spiliopoulos K, Peschos D, Batistatou A,
Ntountas I, Agnantis N and Kitsos G: Vasculogenic mimicry: Lesson
from melanocytic tumors. In Vivo. 29:309–317. 2015.PubMed/NCBI
|
|
26
|
Wang SS, Gao XL, Liu X, Gao SY, Fan YL,
Jiang YP, Ma XR, Jiang J, Feng H, Chen QM, et al: CD133+ cancer
stem-like cells promote migration and invasion of salivary adenoid
cystic carcinoma by inducing vasculogenic mimicry formation.
Oncotarget. 7:29051–29062. 2016.PubMed/NCBI
|
|
27
|
Hendrix MJ, Seftor EA, Seftor RE, Chao JT,
Chien DS and Chu YW: Tumor cell vascular mimicry: Novel targeting
opportunity in melanoma. Pharmacol Ther. 159:83–92. 2016.
View Article : Google Scholar : PubMed/NCBI
|