Introduction
Rectal cancer is one of the most common malignant
tumors of the digestive system in clinical practice, and its
morbidity and mortality rates are among the top 5 in the world,
showing increasing trends year by year (1). The incidence of colorectal cancer is a
result of combined action of multiple factors. Its early symptoms
are not obvious and easily ignored, leading to delayed diagnosis
and treatment, so patients are usually in the advanced stage when
diagnosed, more than half of whom will have distant liver
metastasis (2). At present, rectal
cancer has become one of the killers seriously threatening human
health, and the preferred treatment method for colorectal cancer is
radical surgery (3). Most patients
are unable to receive surgery due to liver metastasis, which is
also the leading cause of death. Patients with colorectal liver
metastasis (CLMs) have a poor prognosis, and if there are no active
and effective treatment measures, the 5-year survival rate is less
than 10% (4). In previous years, with
the application and promotion of multidisciplinary collaborative
diagnosis and treatment modes, some unresectable CLMs can be
resectable via conversion therapy, thus increasing the survival
rate from 10 to 50%, which is of great significance in increasing
the surgical resection rate, extending the survival time and
improving the prognosis of patients with CLMs (5). In the present study, different
conversion therapies were performed for patients with CLMs, so as
to investigate the therapeutic effects and its correlation with the
vascular endothelial growth factor (VEGF) expression, providing a
reference for the treatment of CLMs. It is now reported as
follows.
Materials and methods
General materials
A total of 116 patients with advanced rectal cancer
accompanied by liver metastasis treated in The Second Affiliated
Hospital of Zhengzhou University (Zhengzhou, China) from October
2010 to October 2012 were selected as the objects of study.
Inclusion criteria: i) Patients meeting the diagnostic criteria of
colorectal cancer (6); ii) with liver
metastasis; iii) with the survival time >8 weeks and unilateral
lesions; and iv) who signed the informed consent. Exclusion
criteria: i) Patients with mental diseases; and ii) who refused to
cooperate or had very low compliance. Patients were randomly
divided into control (n=58) and observation group (n=58). There
were no statistically significant differences in general data
between two groups of patients (P>0.05) (Table I).
 | Table I.General data of objects of study. |
Table I.
General data of objects of study.
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Items | Control (n=58) | Observation
(n=58) | t/χ2 | P-value |
|---|
| Sex
(male/female) | 26/32 | 24/34 | 0.035 | 0.851 |
| Age (years) | 40–75 | 40–78 |
|
|
| Average age
(years) | 57.36±7.49 | 57.85±7.58 | 0.350 | 0.727 |
| BMI
(kg/m2) | 22.83±1.54 | 22.56±1.27 | 1.030 | 0.305 |
| Primary tumor site, n
(%) |
| Colon | 34 (58.62) | 32 (55.17) | 0.392 | 0.822 |
| Rectum | 15 (25.86) | 18 (31.03) |
|
|
| Junction between
rectum and sigmoid colon | 9 (15.52) | 8 (13.79) |
|
|
| Hepatic metastasis
size (cm) | 5.68±1.59 | 5.85±1.68 | 0.560 | 0.577 |
Methods
Treatment
Patients in control group were treated with
FOLFOXIRI. On the 1st day, 150–180 mg/m2 irinotecan
(approval no. NMPN H20084572; Qilu Pharmaceutical Hainan Co., Ltd.,
Shandong, China) was intravenously injected for 90 min, and 200
mg/m2 calcium folinate (approval no. NMPN H20000584;
Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) was also
intravenously injected for 2 h; then 400 mg/m2
5-fluorouracil (approval no. NMPN H31020593; Shanghai Xudong Haipu
Pharmaceutical Co., Ltd., Shanghai, China) was intravenously
injected, and then 2,400 mg/m2 5-fluorouracil was pumped
into peripheral vein for 46 h. Patients in observation, based on
the treatment in control group, received intravenous drip of 5
mg/kg bevacizumab (approval no. registration certificate no.
S20120068; Roche Diagnostics, Indianapolis, IN, USA) for ≥1.5 h in
the first, and for ≤1 h in the second time. Two groups of patients
received the above treatment once every two weeks. After treatment,
the curative effects on patients were dynamically evaluated, and
the surgical resection could be scheduled when it was resectable.
This study was approved by the Ethics Committee of The Second
Affiliated Hospital of Zhengzhou University. Signed written
informed consents were obtained from the patients and/or
guardians.
Index detection
Fasting venous blood (3–5 ml) was drawn from
patients in two groups, and serum was extracted to detect the VEGF
concentration via enzyme-linked immunosorbent assay (ELISA). The
relevant kits were provided by Reckitt Benckiser LLC (Parsippany,
NJ, USA). According to the instructions of kit, the optical density
value was read at a wavelength of 450 nm using a microplate reader
from Jiangsu Potebio Co., Ltd., (Jiangsu, China), and the
concentration of VEGF was calculated.
The expression of VEGF in tumor tissue samples was
detected using the immunohistochemical method. The rabbit
anti-human VEGF polyclonal antibody (1:800; cat. no. 2479) was
provided by Cell Signaling Technology, Danvers, MA, USA. The
paraffin-embedded tissues were cut into 4 µm-thick sections using a
microtome (Leica, Germany), and baked in an incubator (Shanghai
Medical Equipment Workshop) at 60°C overnight, followed by dewaxing
via xylene. Then sections were placed into 95, 85, 80 and 75%
ethanol for 10 min, respectively, soaked in distilled water for 5
min, added with 50 µl 3% hydrogen peroxide solution and incubated
at 20°C for 10 min. The activity of endogenous peroxidase was
blocked; sections were washed with phosphate-buffered saline for 3
times, and added with 50 µl primary antibody at 4°C overnight.
After that, the goat anti-rabbit secondary polyclonal antibody
(1:1,000; cat. no. 7074; Cell Signaling Technology), was added for
incubation at 20°C for 10 min, followed by color development using
the reagents in DBA kit provided by Beijing Zhongshan Goldenbridge
Biotechnology Co., Ltd. (Beijing, China), and observation under a
microscope (Olympus, Tokyo, Japan). Distilled water was used to
terminate the color development, followed by re-staining via
hematoxylin for 2 min and sealing via neutral gum.
Evaluation criteria
The curative effect was evaluated based on the
therapeutic evaluation criteria of solid tumors: Complete and
partial remission, stable and progressive disease. Objective
response rate (ORR) = (complete remission + partial
remission)/total (7). The conversion
rates of patients in two groups at 8, 12 and 16 weeks after
treatment were compared.
The VEGF concentration in portal
venous blood was detected via ELISA
The expression of VEGF in tumor tissues was detected
via immunohistochemistry, and the brown yellow-stained cells
indicated the positive. Four high-power fields (×400) were randomly
selected in each section, the percentage of positive cells was
calculated, and the percentage point (PP) was scored: i) 0 point,
no positive cells; ii) 1, percentage of positive cells <5%; iii)
2, 5% < percentage of positive cells ≤20%; and iv) 3, percentage
of positive cells >20%. The staining intensity (SI) was also
scored: i) 0 point, no staining; ii) 1, pale yellow; iii) 2, brown
yellow; and iv) 3, dark brown. The immune response score (IRS) was
calculated according to the formula: IRS = PP × SI; IRS >4
points indicated the high expression, while IRS ≤4 points indicated
the low expression (8).
The incidence rates of recent adverse reactions,
including gastrointestinal reaction, bone marrow suppression,
leukopenia and liver dysfunction, were compared; Patients were
followed-up for 5 years, and the survival time and rate of patients
in different groups were recorded.
Statistical analysis
Data were processed using SPSS 19.0 software (SPSS,
Inc., Chicago, IL, USA). Measurement data were presented as mean ±
standard deviation (SD), and t-test was used. Enumeration data were
presented as rate, and Chi-square test was used. Survival analysis
was performed via Kaplan-Meier analysis along with log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparisons of chemotherapeutic
effects between two groups of patients
ORR in observation (79.69%) was significantly higher
than that in control group (50.00%) (P<0.05) (Table II).
 | Table II.Comparisons of chemotherapeutic
effects between two groups of patients n (%). |
Table II.
Comparisons of chemotherapeutic
effects between two groups of patients n (%).
| Groups | No. | Complete
remission | Partial
remission | Stable disease | Progressive
disease |
|---|
| Observation | 58 | 26 (44.83) | 15 (25.86) | 10 (17.24) | 7 (12.07) |
| Control | 58 | 17 (29.31) | 12 (20.69) | 16 (27.59) | 13 (22.41) |
| χ2 |
|
|
|
| 4.359 |
| P-value |
|
|
|
| 0.037 |
Comparisons of conversion rates
between two groups of patients
There was no significant difference in conversion
rate at 8 weeks after treatment between two groups (P>0.05). At
12 and 16 weeks after treatment, the conversion rates in
observation were significantly higher than those in control group
(P<0.05) (Table III).
 | Table III.Comparisons of conversion rates
between two groups of patients n (%). |
Table III.
Comparisons of conversion rates
between two groups of patients n (%).
|
|
| After treatment |
|---|
|
|
|
|
|---|
| Groups | No. | 8 weeks | 12 weeks | 16 weeks |
|---|
| Observation | 58 | 15 (25.86) | 37 (63.79) | 41 (70.69) |
| Control | 58 | 11 (18.97) | 19 (32.76) | 29 (50.00) |
| χ2 |
| 0.446 | 9.977 | 4.359 |
| P-value |
| 0.504 | 0.002 | 0.037 |
Comparisons of adverse reactions
between two groups of patients
The incidence rates of gastrointestinal reaction,
bone marrow suppression, leukopenia and liver dysfunction had no
significant differences between two groups of patients (P>0.05)
(Table IV).
 | Table IV.Comparisons of adverse reactions
between two groups of patients n (%). |
Table IV.
Comparisons of adverse reactions
between two groups of patients n (%).
| Groups | No. | Gastrointestinal
reaction | Bone marrow
suppression | Leucopenia | Liver
dysfunction |
|---|
| Observation | 58 | 2 (3.45) | 1 (1.72) | 1 (1.72) | 1 (1.72) |
| Control | 58 | 4 (6.90) | 3 (5.17) | 3 (5.17) | 4 (6.90) |
| χ2 |
| 0.176 | 0.259 | 0.259 | 0.836 |
| P-value |
| 0.675 | 0.611 | 0.611 | 0.361 |
Comparison of VEGF between two groups
of patients
After treatment, the VEGF concentration in portal
venous blood and positive rate of VEGF expression in cancer tissue
specimens in observation were obviously lower than those in control
group (P<0.05) (Table V).
 | Table V.VEGF concentration in portal venous
blood and VEGF expression in cancer tissue specimens of
patients. |
Table V.
VEGF concentration in portal venous
blood and VEGF expression in cancer tissue specimens of
patients.
| Groups | No. | VEGF concentration
in portal venous blood (µg/l) | Positive rate of
VEGF expression n (%) |
|---|
| Observation | 58 | 185.76±7.75 | 27 (46.55) |
| Control | 58 | 276.83±11.68 | 47 (81.03) |
|
χ2/t |
| 49.479 | 13.474 |
| P-value |
| <0.001 | <0.001 |
Comparisons of survival situations of
patients in different groups
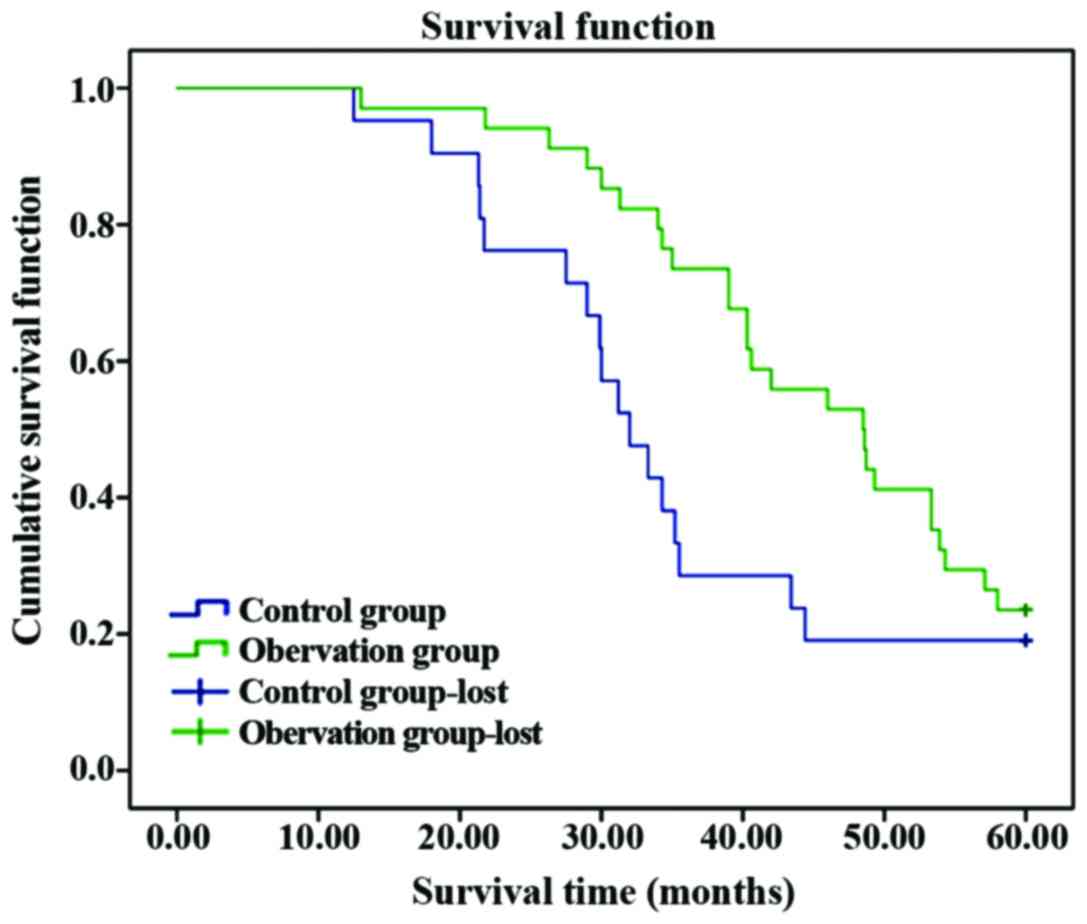
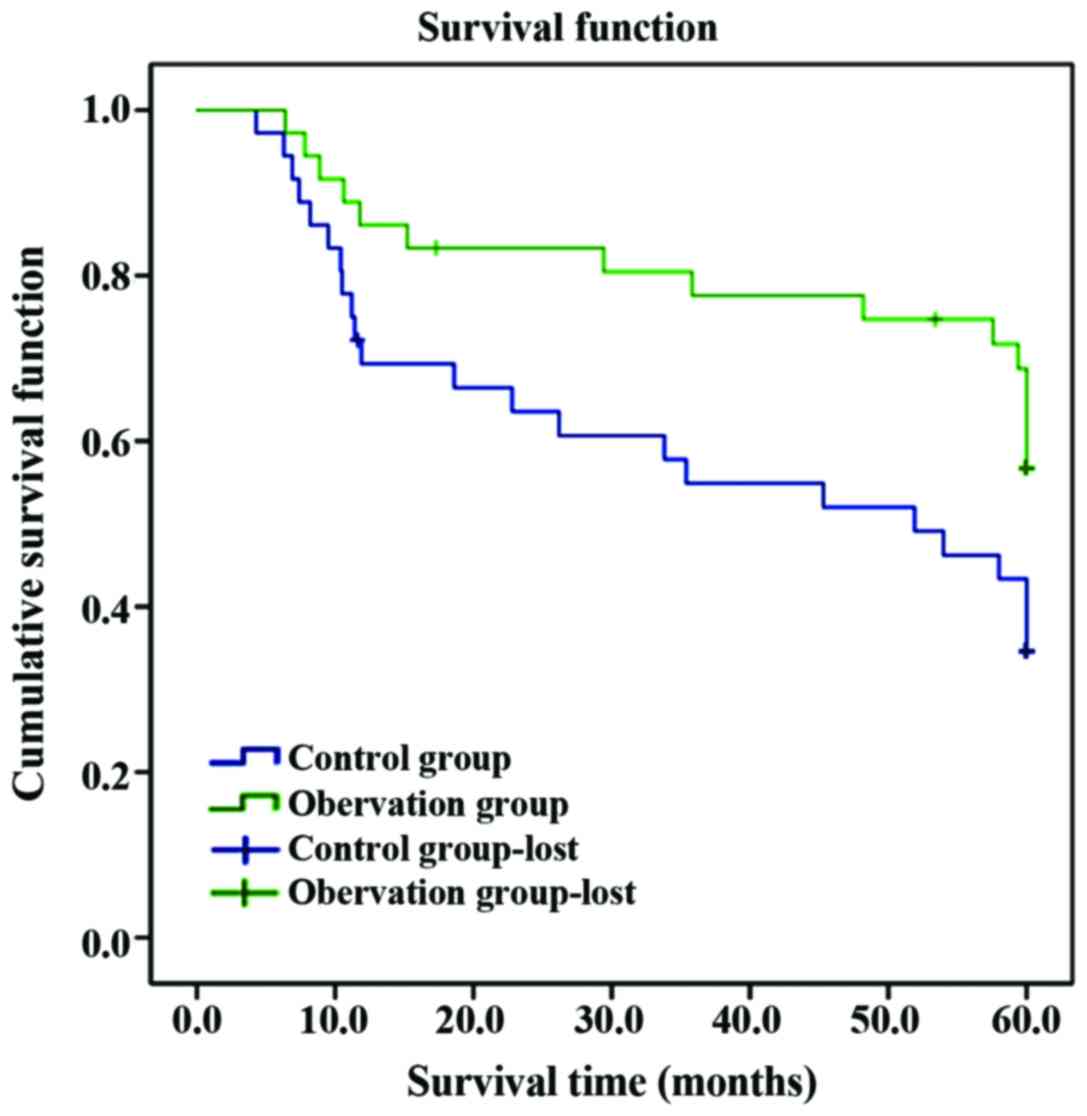
The average survival time in observation was longer
than that in control group, and the postoperative 5-year survival
rate was significantly higher than that in control group
(P<0.05); the 5-year survival rate in high-expression VEGF was
obviously lower than that in low-expression VEGF group, and the
average survival time was obviously shortened compared with that in
low-expression VEGF group (P<0.05) (Table VI and Figs.
1 and 2).
 | Table VI.Comparisons of 5-year follow-up
status of patients in different groups. |
Table VI.
Comparisons of 5-year follow-up
status of patients in different groups.
|
| Groups |
|---|
|
|
|
|---|
| Items | Observation
(n=58) | Control (n=58) | High-expression
VEGF (n=56) | Low-expression VEGF
(n=60) |
|---|
| 5-year survival
rate n (%) | 28 (48.28) | 12 (20.69) | 11 (18.97) | 29 (48.33) |
|
χ2 |
| 8.598 |
| 9.322 |
|
P-value |
| 0.003 |
| 0.002 |
| Average survival
time (month) | 49.83±7.68 | 40.16±6.85 | 38.86±7.52 | 49.93±7.75 |
|
t-test |
| 7.156 |
| 7.807 |
|
P-value |
| <0.001 |
| <0.001 |
Discussion
With the changes in people's living habits and
aggravation of environmental pollution, the incidence rate of
rectal cancer has continued to rise. Colorectal cancer is caused by
various factors, including lifestyle (obesity, smoking, drinking,
drugs and psychosocial factors), diet (high-protein diet, high-fat
diet and trace elements), gastrointestinal diseases (ulcerative
colitis, Helicobacter pylori infection and Crohn's disease) and
genetic factors; in particular, the fat intake was positively
correlated with the incidence of rectal cancer (9,10). The
molecular pathways of occurrence and development of rectal cancer
can be divided into two types; chromosome and microsatellite
instability (MSI). The main pathogenesis of rectal cancer is
chromosomal deletion, and MSI is another important pathogenesis of
colorectal cancer, among which MSI pathway is mainly caused by the
defects and changes in DNA mismatch repair (MMR) system (11). MMR gene is one of important members in
DNA repair system. The incidence of many malignant tumors,
especially colorectal cancer, is closely related to the changes in
MMR gene (12).
Colorectal cancer, especially early rectal cancer,
can be cured only by surgical treatment, but most patients have
been in the late stage when diagnosed, and those with advanced CLMs
cannot receive surgical treatment (13). Conversion chemotherapy refers to a
treatment strategy that the unresectable cancer initially becomes
resectable after chemotherapy, and the surgical indications are
expanded through conversion therapy, thus curing the patients
(14). The results of this study
showed that ORR and conversion rate in observation were
significantly higher than those in control group, and the
postoperative survival rate in observation was also significantly
higher than that in control group (P<0.05). This is because the
conversion therapy and efficacy evaluation of CLMs patients can
make the cancer in some patients become resectable, and timely
surgical treatment is performed before the disappearance of
lesions, thus effectively prolonging the survival time of patients
with CLMs. FOLFOXIRI program includes three drugs: 5-fluorouracil,
irinotecan and leucovorin, among which irinotecan is a kind of
semi-synthetic camptothecin derivative, as well as an effective
drug in the treatment of rectal cancer, and it can also inhibit DNA
replication (15). Leucovorin and
5-fluorouracil are drugs for the treatment of advanced rectal
cancer, and they can be combined with definite effects (16). Bevacizumab is a kind of recombinant
humanized, human-mouse chimeric anti-VEGF monoclonal antibody drug.
The combined application of the above drugs based on FOLFOXIRI
program can obtain more definite effects, resulting in a higher
remission rate and more obvious conversion effect (17).
VEGF is a specific angiogenic factor with the
highest and strongest activity, which is a member in the
platelet-derived growth factor family that can stimulate vascular
endothelial cells and promote the division and proliferation,
eventually promoting the neovascularization (18). VEGF is highly expressed in many tumor
tissues and is closely related to the pathological grading of
malignant tumors (19). Venous blood
returns to the liver mainly via the portal vein system and then
enters the venous system. The detection of VEGF concentration in
venous blood of patients with CLMs can effectively evaluate the
condition of disease (20). The
results of this study showed that after treatment, the VEGF
concentration in portal venous blood and the positive rate of VEGF
expression in cancer tissue specimens in observation were
significantly lower than those in control group (P<0.05), and
the incidence rates of gastrointestinal reaction, bone marrow
suppression, leucopenia and liver dysfunction had no significant
differences between two groups of patients (P>0.05). This is
because FOLFOXIRI program combined with bevacizumab can directly
block the activation of VEGF and regulate or inhibit the
vasculature of tumors, thus preventing the neovascularization of
tumor. At the same time, bevacizumab can inhibit tumor
differentiation factors, control the neovascularization from the
source, result in cell hypoxia and apoptosis, and prevent the
process of pseudo-vascular normalization. Neovascularization is an
important basic condition for the growth and migration of tumor
cells. Bevacizumab can effectively lower the pressure in the tumor
stroma and reduce the exudation by decreasing the tumor vascular
bed and changing its permeability, and more effectively release the
5-fluorouracil, irinotecan and leucovorin into tumor cells, thereby
inhibiting the VEGF overexpression, reducing the neovascularization
and enhancing the anticancer effect, without increasing the damage
to normal cells and incidence rate of adverse reactions. FOLFOXIRI
program combined with bevacizumab conversion therapy leaves a large
space for the conversion therapy and increases the resectability.
Patients were followed-up for 5 years, and it was found that the
5-year survival rate in high-expression VEGF was significantly
lower than that in low-expression VEGF group, and the average
survival time was significantly shortened compared with that in
low-expression VEGF group.
In the conversion therapy, it is believed that the
over-conversion should be avoided without pursuing the remission
rate excessively. When the cancer is resectable, surgery should be
performed as soon as possible before the disappearance of
metastasis, and chemotherapy should be withdrawn at this time. If
not, the metastasis may continue to grow and become unresectable
once again, missing the window of surgery. If metastasis
disappears, liver segment resection or hepatic lobectomy is still
needed in the original lesions.
In conclusion, the FOLFOXIRI program combined with
bevacizumab target therapy for CLMs patients can improve the
effective rate of conversion therapy, and its therapeutic effect is
closely related to the expression of VEGF. After conversion
therapy, performing active surgical resection can effectively
improve the survival time of patients, which has a very great
clinical significance.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GH, RS and JY designed the study. YZ, CX and CW
collected the data, JW and TC analysed the data, GH and ZL prepared
the manuscript. ZL performed ELISA. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Second Affiliated Hospital of Zhengzhou University (Zhengzhou,
China). Signed written informed consents were obtained from the
patients and/or guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kahi CJ, Boland CR, Dominitz JA,
Giardiello FM, Johnson DA, Kaltenbach T, Lieberman D, Levin TR,
Robertson DJ and Rex DK: Colonoscopy surveillance after colorectal
cancer resection: Recommendations of the US multi-society task
force on colorectal cancer. Gastrointest Endosc. 83:489–98.e10.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Page AJ, Weiss MJ and Pawlik TM: Surgical
management of noncolorectal cancer liver metastases. Cancer.
120:3111–3121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sotirchos VS, Petrovic LM, Gönen M,
Klimstra DS, Do RK, Petre EN, Garcia AR, Barlas A, Erinjeri JP,
Brown KT, et al: Colorectal cancer liver metastases: Biopsy of the
ablation zone and margins can be used to predict oncologic outcome.
Radiology. 280:949–959. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fulong W and Pan Z: Operation time of
colorectal liver metastasis and choice of operation after
convertible therapy. Chin J Pract Surg. 33:656–659. 2013.
|
|
6
|
Corley DA, Jensen CD, Marks AR, Zhao WK,
Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger
JE, et al: Adenoma detection rate and risk of colorectal cancer and
death. N Engl J Med. 370:1298–1306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Hao D, Wang L, Wang H, Wang Y, Zhao
Z, Li P, Deng C and Di LJ: Epigenetic targeting drugs potentiate
chemotherapeutic effects in solid tumor therapy. Sci Rep.
7:40352017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jagadish N, Parashar D, Gupta N, Agarwal
S, Sharma A, Fatima R, Suri V, Kumar R, Gupta A, Lohiya NK, et al:
A novel cancer testis antigen target A-kinase anchor protein
(AKAP4) for the early diagnosis and immunotherapy of colon cancer.
OncoImmunology. 5:e10789652016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dunet V, Halkic N, Prior JO, Anaye A,
Meuli RA, Sempoux C, Denys A and Schmidt S: Detection and viability
of colorectal liver metastases after neoadjuvant chemotherapy: A
multiparametric PET/CT-MRI study. Clin Nucl Med. 42:258–263. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Truant S, Séquier C, Leteurtre E,
Boleslawski E, Elamrani M, Huet G, Duhamel A, Hebbar M and Pruvot
FR: Tumour biology of colorectal liver metastasis is a more
important factor in survival than surgical margin clearance in the
era of modern chemotherapy regimens. HPB. 17:176–184. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Venderbosch S, Nagtegaal ID, Maughan TS,
Smith CG, Cheadle JP, Fisher D, Kaplan R, Quirke P, Seymour MT,
Richman SD, et al: Mismatch repair status and BRAF mutation status
in metastatic colorectal cancer patients: A pooled analysis of the
CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res.
20:5322–5330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alexandrescu S, Diaconescu A and Popescu
I.: Surg options synchronous liver metastases colorectal cancer.
Transl. J. Med. Res. 22:10–21. 2017.
|
|
13
|
Wagner M, Ronot M, Doblas S, Giraudeau C,
van Beers B, Belghiti J, Paradis V and Vilgrain V: Assessment of
the residual tumour of colorectal liver metastases after
chemotherapy: Diffusion-weighted MR magnetic resonance imaging in
the peripheral and entire tumour. Eur Radiol. 26:206–215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bregendahl S, Emmertsen KJ, Fassov J,
Krogh K, Zhao J, Gregersen H and Laurberg S: Neorectal
hyposensitivity after neoadjuvant therapy for rectal cancer.
Radiother Oncol. 108:331–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glimelius B, Ristamäki R, Kjaer M,
Pfeiffer P, Skovsgaard T, Tveit KM, Linné T, Frödin JE, Boussard B,
Oulid-Aïssa D, et al: Irinotecan combined with bolus 5-fluorouracil
and folinic acid Nordic schedule as first-line therapy in advanced
colorectal cancer. Ann Oncol. 13:1868–1873. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Cutsem E, Lenz HJ, Köhne CH, Heinemann
V, Tejpar S, Melezínek I, Beier F, Stroh C, Rougier P, van Krieken
JH, et al: Fluorouracil, leucovorin, and irinotecan plus cetuximab
treatment and RAS mutations in colorectal cancer. J Clin Oncol.
33:692–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamnvik OPR, Choueiri TK, Turchin A, McKay
RR, Goyal L, Davis M, Kaymakcalan MD and Williams JS: Clinical risk
factors for the development of hypertension in patients treated
with inhibitors of the VEGF signaling pathway. Cancer. 121:311–319.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ciamporcero E, Miles KM, Adelaiye R,
Ramakrishnan S, Shen L, Ku S, Pizzimenti S, Sennino B, Barrera G
and Pili R: Combination strategy targeting VEGF and HGF/c-met in
human renal cell carcinoma models. Mol Cancer Ther. 14:101–110.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jannuzzi AT, Özhan G, Yanar HT and
Alpertunga B: VEGF gene polymorphisms and susceptibility to
colorectal cancer. Genet Test Mol Biomarkers. 19:133–137. 2015.
View Article : Google Scholar : PubMed/NCBI
|