Introduction
Gastric cancer (GC) is associated with high
morbidity and mortality, and remains the third leading cause of
cancer mortality globally and most prevalent cancer in East Asia
(1,2).
The majority of patients are diagnosed at an advanced stage, during
which curative surgery is necessary with extended lymphadenectomy.
Endoscopic screening and laparoscopic distal gastrectomy are
effective treatments for advanced GC (3); however, the 5-year overall survival rate
for patients with GC remains poor, with a <20% survival rate
worldwide due to distant and high frequency metastasis in 2017
(4). Therefore, it is of great
importance to investigate the underlying mechanisms of GC distant
metastasis, and to identify effective diagnostic biomarkers of and
treatments for GC.
Tumor cells disseminate from the primary tumor to
other locations in the body, and rely on modifications in signaling
pathways to affect proliferation, migration and invasion. An
improved understanding of the molecular mechanisms underlying
metastasis may prolong survival and maintain quality of life by
relieving tumor-associated symptoms. The tumor microenvironment is
a pivotal factor in tumorigenesis and tumor metastasis for
coordinating the morphological transformation of cancer cells
(5). Studies have highlighted the
important role of the ADAM family, which may be associated with
tumor microenvironment (6–8).
The ADAM proteins are a family of membrane-anchored
glycoproteins mediating cell-matrix interactions (9). They are essential and are singularly
active during the reprogramming of pluripotent stem cells (10). Their protease and adhesion domains are
responsible for cell fusion (11),
adhesion and signaling (9,12). Previously, several studies identified
aberrant expression of ADAMs in human cancer tissues (13,14), which
may be associated with carcinogenesis. For example, the
overexpression of ADAM metallopeptidase domain 12 (ADAM12)
contributed to increased cell proliferation through regulating the
expression of phosphorylated (p)-protein kinase B and p-glycogen
synthase kinase-3β in non-Hodgkin's lymphoma (15). ADAM17 promoted growth, migration and
invasion in gastric carcinoma cells via the transforming growth
factor (TGF)-β/Smad pathway (16).
Furthermore, ADAM17 was demonstrated to be a potential therapeutic
target for GC and serve a key role in GC progression (16).
ADAM29 is a member of the ADAM family, located on
human chromosome 4q34 (17). Previous
studies indicated that ADAM29 was involved in cancer development
and progression (18–21). ADAM29 is frequently mutated in several
tumor types, including breast and lung cancer, in the form of 4
missense mutations, all located in the same residue (p.Q814H),
potentially forming a mini-hotspot (18). A previous study demonstrated that the
mutation of ADAM29 in melanoma affected melanoma cell adherence to
specific extracellular matrix proteins, and in a number of cases
promoted the migratory ability of the cells (19). Studies regarding ADAM29 in breast
cancer have also been conducted: For example, genome-wide studies
indicated ADAM29 was a susceptible locus and a risk factor for
breast cancer (20). In addition, a
previous study detected an increased expression level of ADAM29 in
breast cancer tissues compared with normal tissues (21). ADAM29 overexpression and its mutation
in various domains affected the migration, growth and invasion of
breast cancer cells in vitro (22). The roles of ADAM29 in other malignant
tumor types were also investigated: ADAM29 was demonstrated to
exhibit a high mutation rate in several cancer types, including
colorectal cancer (23,24), esophageal cancer (18) and melanoma (19). Previous data demonstrated that ADAM29
was upregulated in the MKN45 gastric carcinoma cell line with Mucin
1, cell surface associated downregulated clones, which decreased
the rate of cell proliferation (25);
however, the molecular mechanism underlying ADAM29 and its
biological function in GC remains unknown.
In the present study, an investigation into the
expression of ADAM29 in tumor and paracancerous tissues (2 cm
adjacent from the tumor) of patients with GC, and the association
between the expression level of this molecule with the clinical
outcomes of patients, was conducted. Additionally, the biological
functions of ADAM29 in human GC AGS and MGC803 cell lines were
examined following the manipulation of ADAM29 expression in
vitro.
Materials and methods
Human GC specimens
Human gastric tissue samples were collected from 83
patients aged 53 to 87 years with GC (comprised of 31 females, mean
age 68.24±8.92 years; 52 males, mean age 72.34±9.34 years) who had
received curative resection, and immediately stored at −80°C for
further use. Inclusion criteria for patient selection were: i)
Histological or cytological confirmation of GC; ii) Positron
emission tomography-computed tomography revealed no clinically
positive nodes; iii) patients were ≥18 years of age. Exclusion
criteria included: i) Inconsistent results of qPCR of 3 repeated
experiments; ii) or if patients had previously been diagnosed with
severe vascular, cerebrovascular or heart disease. The samples
included gastric tumor tissues (n=83) and normal paracancerous
paired tissues (n=25) an equivalent number of paracancerous tissues
could not be obtained due to limitations of tissue size, and were
collected from patients in Yantai Yuhuangding Hospital from January
2010 to December 2017. The number and sample classifications of
specimens were verified by two pathologists from Yantai Yuhuangding
Hospital (Yantai, China), were confirmed to be free from tumor
deposits. The present study was executed accordingly under the
protocol approved by the Institutional Review Board and Research
Ethical Committee of the Affiliated Yantai Yuhuangding Hospital of
Qingdao University (Yantai, China). Written informed consent was
obtained from all patients. Clinicopathological factors, including,
sex, tumor stage, Tumor-Node-Metastasis staging and lymph node
metastasis (26), were analyzed and
are presented in Table I.
 | Table I.Association between ADAM29 mRNA and
clinical parameters. |
Table I.
Association between ADAM29 mRNA and
clinical parameters.
| Category | N | Median | IQR | P-value |
|---|
| Tissue sample |
|
|
|
|
|
Normal | 25 | <0.001 |
<0.001–3.095 | 0.047a |
|
Tumor | 83 | 0.635 |
<0.001–30.737 |
|
| Sex |
|
|
|
|
|
Male | 52 | 0.693 |
<0.001–26.377 |
|
|
Female | 31 | 0.572 |
<0.001–15.562 | 0.672 |
| Tumor grade
(27) |
|
|
|
|
| 1 | 16 | 0.359 |
<0.001–0.531 |
|
| 2 | 29 | 0.725 |
<0.001–13.127 | 0.345 |
| 3 | 38 | 4.397 | 0.353–22.772 | 0.054 |
| TNM staging |
|
|
|
|
| I | 19 | <0.001 |
<0.001–0.317 |
|
| II | 27 | 0.816 |
<0.001–15.185 | 0.572 |
| III and
IV | 37 | 5.447 |
<0.001–32.146 | 0.038a |
| Location |
|
|
|
|
|
Cardia | 27 | 0.158 |
<0.001–17.172 |
|
|
Non-cardia | 56 | 0.698 |
<0.001–25.398 | 0.296 |
Cell lines and culture conditions
Human GC MGC803 (low ADAM29 expression) and AGS
(high ADAM29 expression) cell lines were purchased from American
Type Culture Collection (Manassas, VA, USA). These cells were
incubated with RPMI-1640 supplemented with 1X
penicillin/streptomycin and 10% fetal calf serum (Gibco, Thermo
Fisher Scientific. Inc., Waltham, MA, USA), in an incubator at 37°C
with an atmosphere containing 5% CO2 and 95%
humidity.
Plasmids and transfection
The Flag-ADAM29 plasmid and ADAM29-small interfering
(si)RNA were purchased from Shanghai GeneChem Co., Ltd. (Shanghai,
China). MGC803 cells were either transfected with 1 µg Flag-ADAM29
or the control vector pCMV (GeneChem, Shanghai, China) respectively
using the X-tremeGENE HP DNA Transfection Reagent (Roche
Diagnostics GmbH, Mannheim, Germany). AGS were transiently
transfected with 1 µg ADAM29-siRNA and corresponding control vector
GV112 (GeneChem) using the aforementioned transfection reagent. The
si-ADAM29 sequence was: Forward
5′-CCGGGCACTCTGACTGATGGTTCTACTCGAGTAGAACCATCAGTCAGAGTGCTTTTTG-3′,
and reverse
5′-AATTCAAAAAGCACTCTGACTGATGGTTCTACTCGAGTAGAACCATCAGTCAGAGTGC-3′.
ADAM29 expression was verified using western blotting (described
subsequently) following transfection for 48 h.
Immunohistochemical (IHC)
staining
All GC sections were dewaxed and rehydrated using
routine methods (27), and incubated
with 5% bovine serum albumin (GeneChem) blocking solution for 30
min at room temperature. Slides were probed with the ADAM29
antibody (1:200; Abnova, Taipei, Taiwan) or with PBS for the
negative control. Following extensive washing three times, sections
were incubated for 30 min with a peroxidase-conjugated goat
anti-mouse IgG (1:200; TA130004, OriGene Technologies, Beijing,
China) at room temperature. Following washing three times to remove
any unbound secondary antibodies, the section color was developed
with 3,3-diaminobenzidine chromogen (Cell Signaling Technology,
Inc., Danvers, MA, USA). A light microscope with ×10 and ×20
magnification (BX43; Olympus Corporation, Tokyo, Japan) was used
for observation and capturing images.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Following the manufacturer's protocol, total RNA
isolated from the GC samples with the ABgene Total RNA Isolation
Reagent (Advanced Biotechnologies Ltd., Epsom, Surrey, UK). cDNA
was generated from 1 µg of each RNA sample using
GoScript™ Reverse Transcription system kit (Promega
Corporation, Madison, WI, USA). ADAM29 mRNA level was quantified by
RT-qPCR using a QuantiNova SYBR Green PCR kit (Qiagen GmbH, Hilden,
Germany). Data was analyzed using the 2−ΔΔCt method
(28). The qPCR primers were as
follows: ADAM29 forward, 5′-CAGAGGCATGACACCTCCAG-3′; and reverse,
5′-TGGACAAATGGCTGGTCCTC-3′; β-actin forward,
5′-CTGGACTTCGAGCAAGAGATG-3′; and reverse,
5′-GAGTTGAAGGTAGTTTCGTGGA-3′. qPCR was programmed as followed: 95°C
for 15 min, then 60 cycles of 95°C for 20 sec, 55°C for 30 sec and
72°C for 20 sec.
Western blot analysis
To investigate the expression level of ADAM29 in the
MGC803 and AGS cells, confluent cells were centrifuged at 2,400 × g
for 10 min at 4°C and lysed with lysis buffer (Beyotime Institute
of Biotechnology, Shanghai, China). The protein concentration was
measured with a bicinchoninic acid protein assay kit (Beijing
ComWin Biotech Co., Ltd., Beijing, China). A total of 5 µg 20 µl
proteins were separated with 10% SDS-PAGE and blotted on to a
nitrocellulose membrane. Following protein transfer, the membrane
was treated with 5% skimmed milk to block non-specific proteins for
1 h at room temperature. Specific proteins were separately probed
with the aforementioned anti-ADAM29 primary antibody (1:1,000;
Abnova, Taipei, Taiwan) and anti-GAPDH antibody (1:1,000; cat no.
sc-32233; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C, and then followed by incubation with
aforementioned peroxidase-conjugated secondary antibody (1:200) for
1 h at room temperature. Protein bands were visualized using the
Tanon High-sig ECL (Tanon Science and Technology Co., Ltd.,
Shanghai, China) and analyzed with Vilber Fusion Fx5 Spectra
(Vilber Lourmat, Marne La Vallée, France).
In vitro cell growth assay
MGC803 and AGS Cell suspensions were seeded into
96-well plates (3,000 cells/200 µl/well). The growth of the cells
was assessed following a period of incubation (≤5 days) in
quadruplicate (overnight, day 3, day 4 and day 5) in the previously
stated conditions. Following incubation, the medium was removed and
cultured with 10% Cell Counting Kit-8 (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) for 1 h at 37°C. Subsequently,
the absorbance was determined at the wavelength of 450 nm using a
spectrophotometer (BioTek Instruments, Inc., Winooski, VT,
USA).
In vitro cell wound assay
MGC803 and AGS Cells (1×105/600 µl/well)
were added into 12-well plates and cultured overnight at 37°C to
form a confluent monolayer. Subsequently, an artificial wound was
produced in the monolayer with a 200 µl pipette tip and washed
twice with PBS to remove floating cells. The migration of cells was
traced and recorded every 6 h by an inverted microscope at ×10 and
×20 magnification for 24 h. The wound was measured and analyzed
using ImageJ software (version 1.62; National Institute of Health,
Bethesda, MD, USA).
In vitro invasion assay
Transwell chambers (upper inserts) polycarbonate
filter inserts (BD Biosciences, Franklin Lakes, NJ, USA) with 8-µm
pore size were coated with 50 µg Matrigel™/100 µl/insert
(BD Matrigel™ Basement Membrane Matrix; BD Biosciences)
and air-dried overnight at room temperature. Following rehydration,
20,000 cells/200 µl/insert with RPMI-1640 supplemented with 5%
fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.) were
seeded in inserts and 1 ml RPMI-1640 medium with 10% fetal calf
serum (Gibo; Thermo Fisher Scientific, Inc.) in the lower chamber
for 72 h at 37°C. Following incubation, cells that had invaded
through the Matrigel™ and insert membrane and adhered to
the other side of the inserts were fixed with 4% formalin for 20
min and stained with 0.5% crystal violet for 20 min at room
temperature. Subsequently, crystal violet staining was dissolved
with 10% acetic acid and measured at the wavelength of 570 nm
spectrophotometer (BioTek Instruments, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation.
Experimental operations were repeated independently >3 times.
Normally-distributed data were assessed using non-paired
(two-sided) Student's t-test for two groups, whilst the
Mann-Whitney U test was used for non-parametric distribution.
Kaplan-Meier analysis was used to detect the association between
ADAM29 expression and survival time of patients with GC (KM
plotter; http://kmplot.com/analysis/).
Results
ADAM29 expression is associated with
the clinical stages of GC
The expression pattern of ADAM29 was examined in 108
tissue specimens from patients with GC using RT-qPCR, including 83
GC tissues and 25 adjacent normal tissues. The association between
the expression level of ADAM29 and clinicopathological features is
summarized in Table I. In the
analysis of ADAM29 gene expression array data of the human GC
tissue specimens, a significantly increased level of ADAM29
transcripts was observed in GC tumor sections compared with the
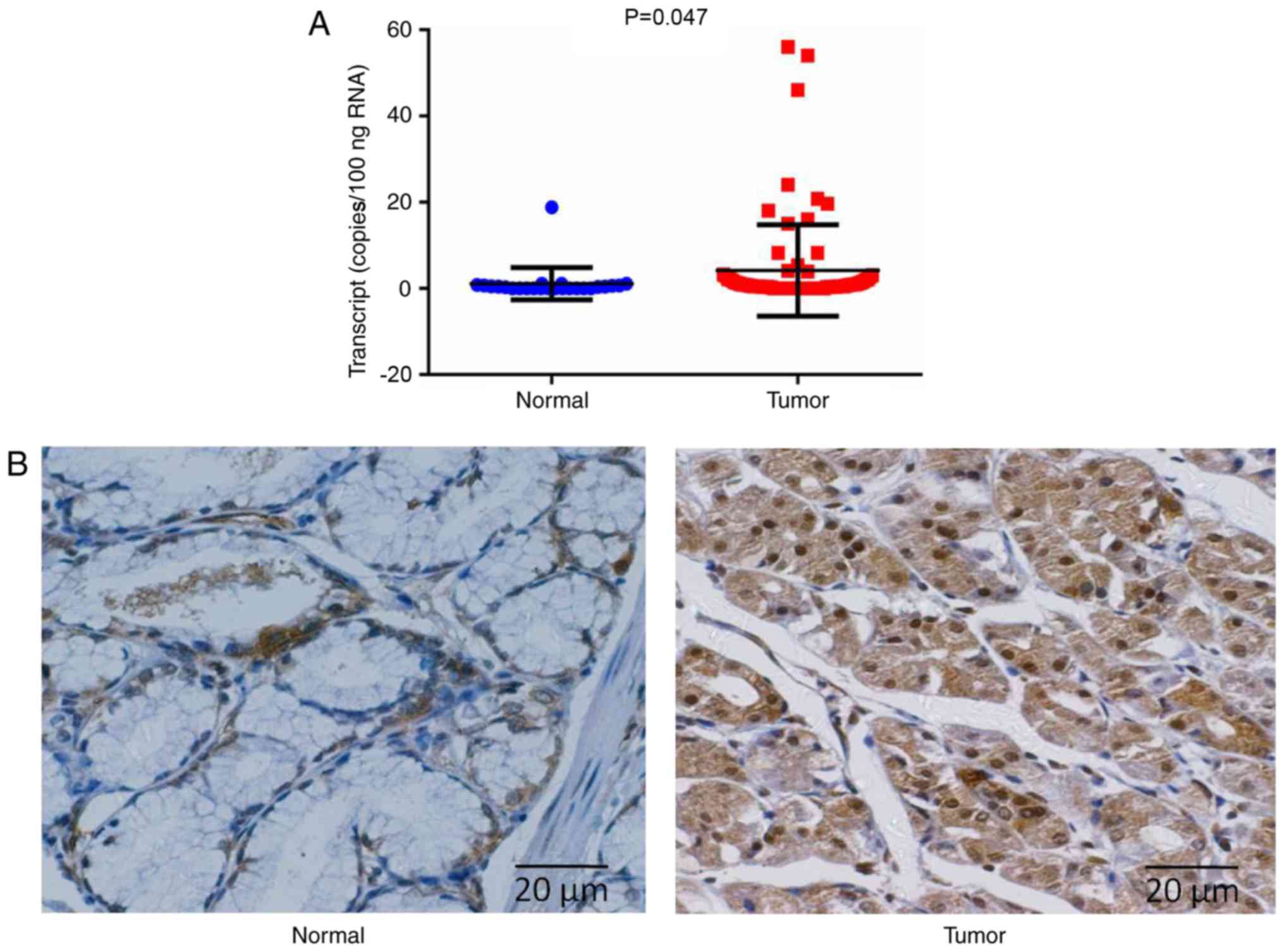
adjacent normal gastric sections (P=0.047; Fig. 1A). A significantly increased level of
ADAM29 transcripts was observed in patients with GC and TNM III or
IV stages compared with patients with TNM I stage (P=0.038).
Furthermore, an increased level of ADAM29 was determined in
patients with terminal stage disease when compared with tumor grade
I; however, it was not statistically significant (Table I).
In addition, the expression pattern of ADAM29 at the
protein level also demonstrated similar results to its expression
at the mRNA level in the IHC assay (Fig.
1B). It was determined that ADAM29 was expressed in the
cytoplasm of normal gastric epithelial cells, and in cancerous
cells. The carcinoma specimens demonstrated notably increased
protein expression of ADAM29, whilst the paired paracancerous
tissues consistently indicated weak or undetectable
immunostaining.
Expression of ADAM29 is associated
with the survival of patients with GC
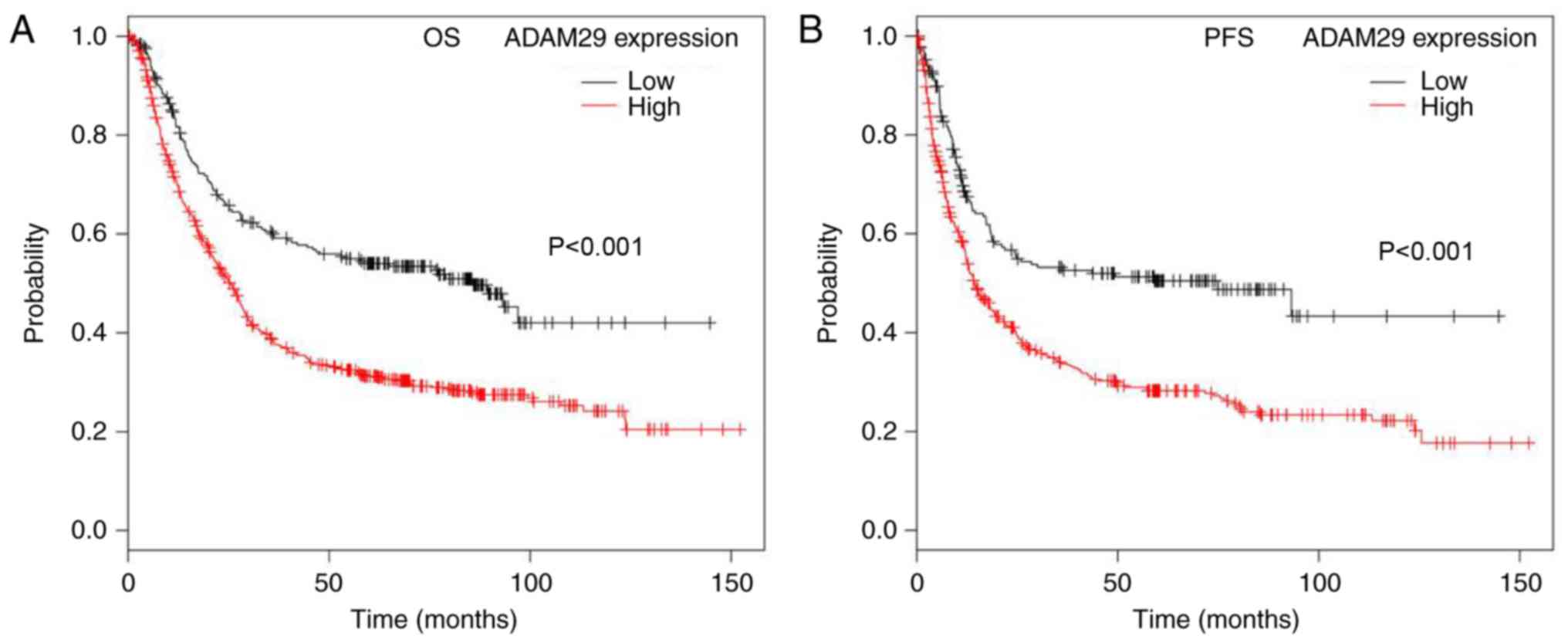
To additionally study the functional aspects of
ADAM29 in GC, the association between ADAM29 expression (Affy ID
221337_s_at) and the survival period of patients with GC was
determined using KMplot. The Kaplan-Meier survival curves
demonstrated that patients with GC and a low ADAM29 transcript
level exhibited a significantly longer overall survival (OS) time
when compared with patients with a high ADAM29 transcript
(P<0.001; Fig. 2A), in a cohort of
882 cases of GC. In addition, decreased ADAM29 expression was also
significantly associated with a reduced progression-free survival
(PFS) of a cohort of 646 samples (P<0.001; Fig. 2B).
Expression level of ADAM29 in GC cell
lines
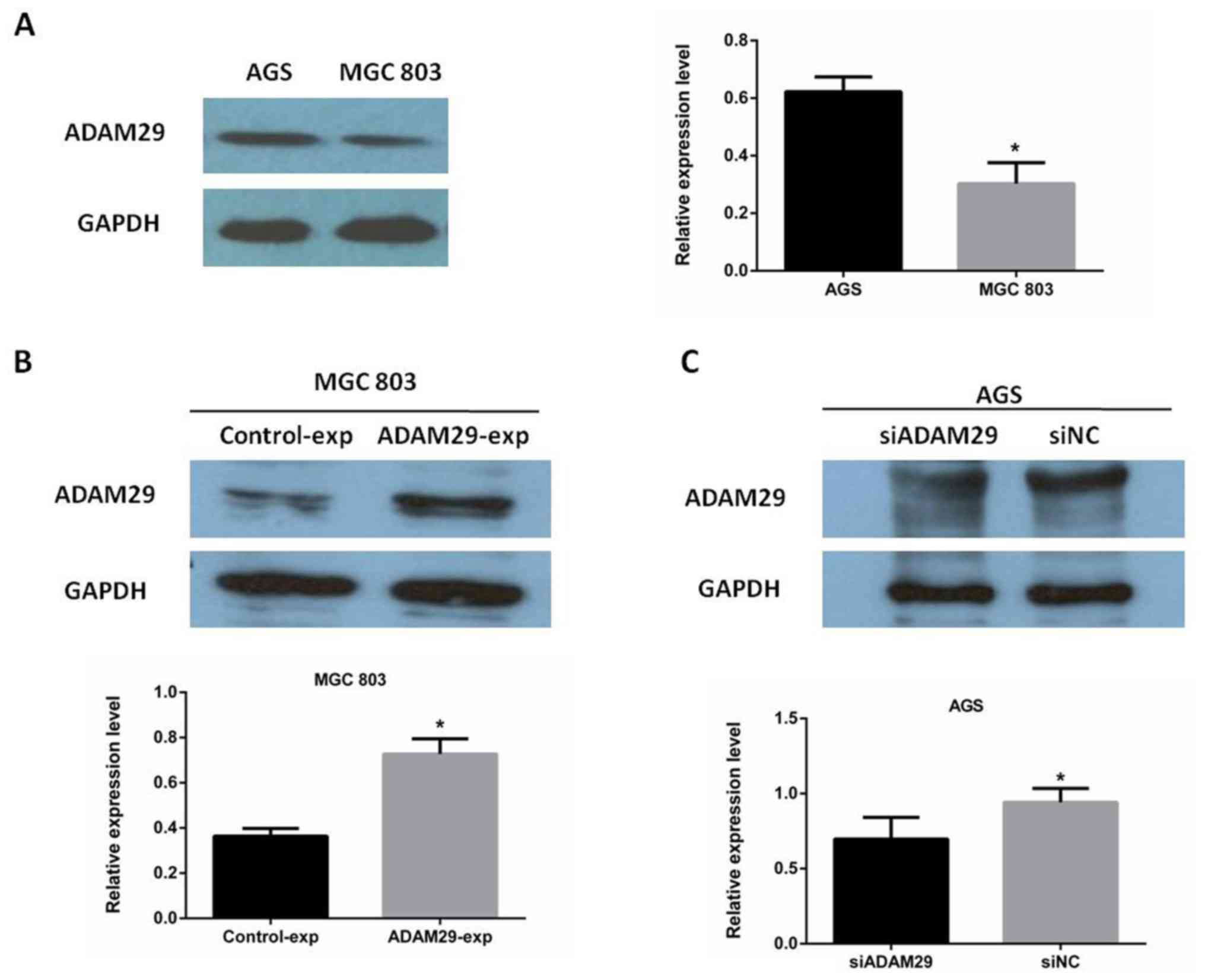
To select suitable cell lines for biological
function of GC cells, the expression of ADAM29 in AGS and MGC803
cells was analyzed using western blotting. It was determined that
ADAM29 was highly expressed in AGS cells and exhibited low
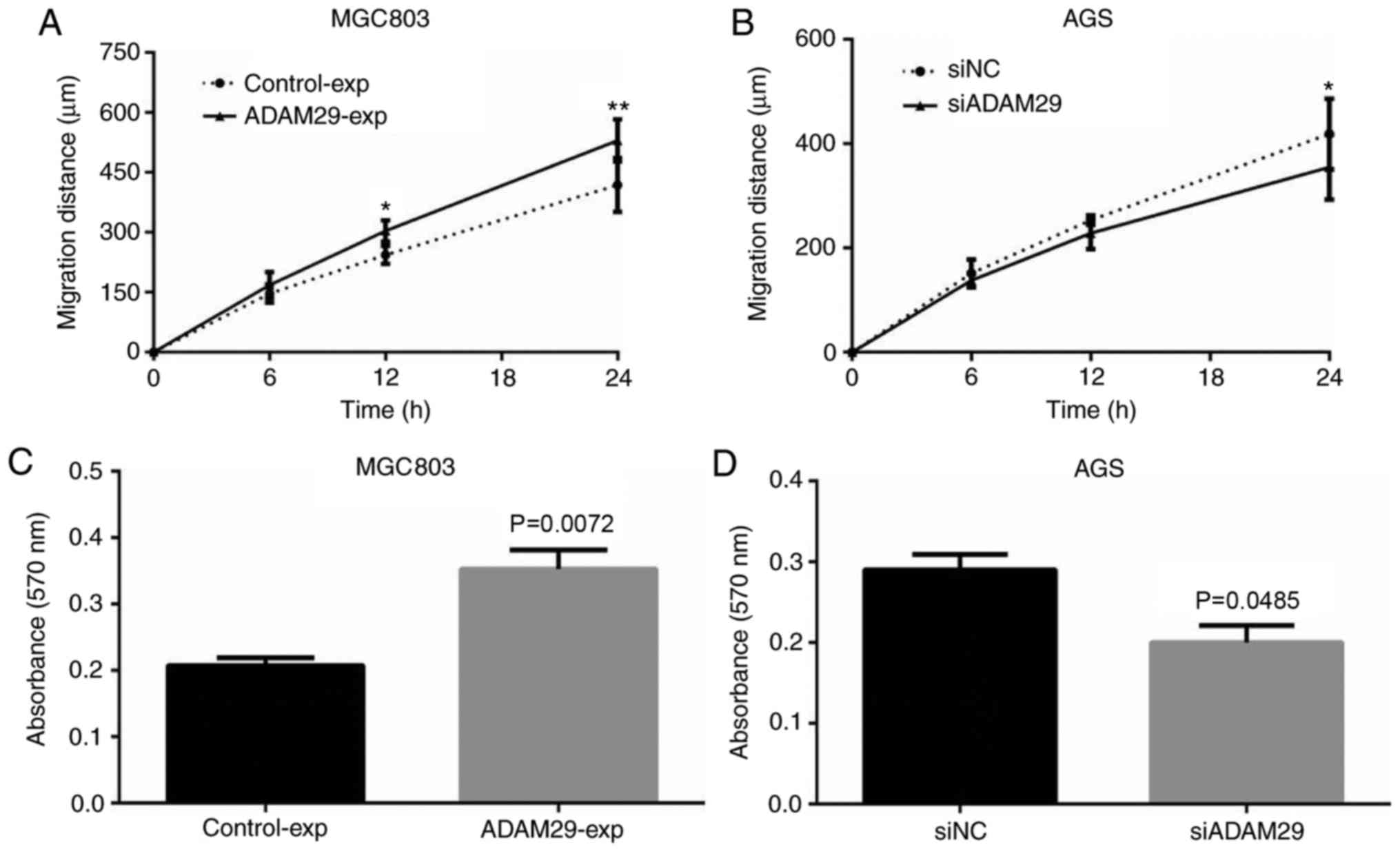
expression in MGC803 cells (Fig. 3A).
Following plasmid and siRNA transfection in MGC803 and AGS cells,
respectively, it was confirmed that the expression level of ADAM29
was considerably increased in the MGC803 cells (Fig. 3B), whilst there was a decreased ADAM29
expression level in the AGS cells (Fig.
3C). Graphical quantification for the overexpressed and
knockdown levels of ADAM29 identified using western blotting is
presented in Fig. 3.
Effect of ADAM29 on GC cells growth in
vitro
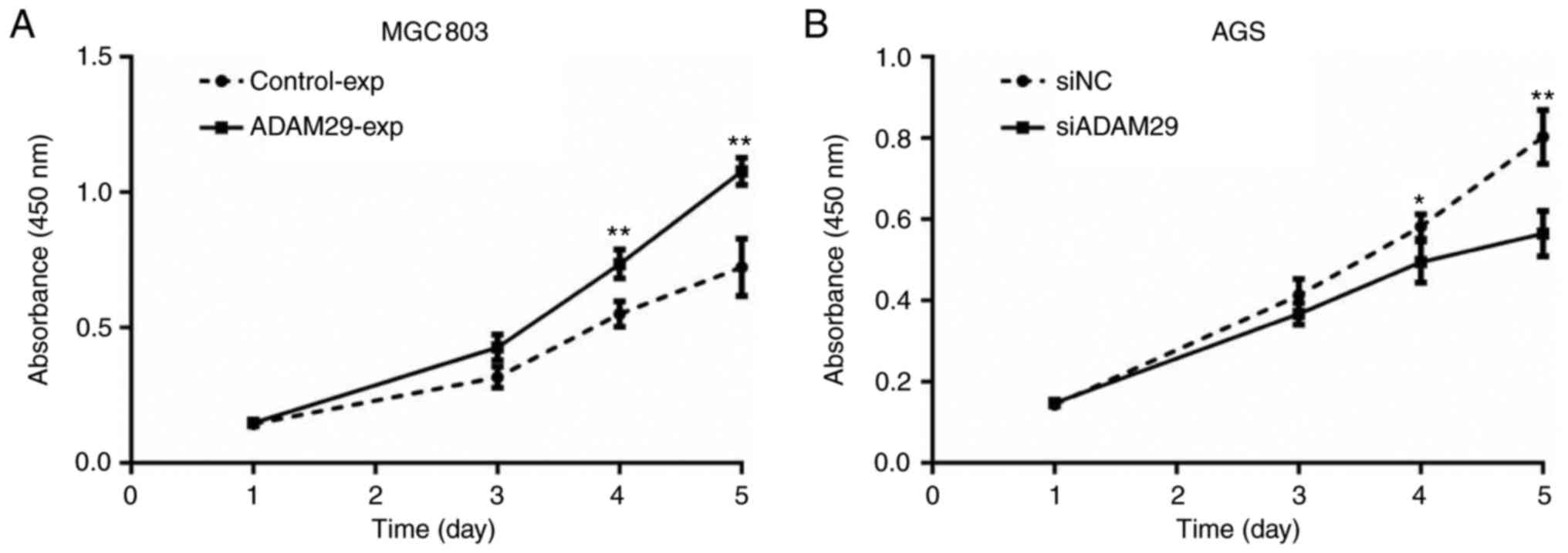
Compared with the control group, increased ADAM29
expression resulted in a significant increase in the growth of
MGC803 cells (Fig. 4A; P<0.01),
whereas the knockdown of ADAM29 caused a significant decrease in
the growth ability of AGS cells (Fig.
4B; P<0.01). These results demonstrated that ADAM29 promoted
GC cell growth.
Effect of ADAM29 on GC cells invasion
and migration
Migration and invasion are important components of
the malignant phenotype of cancer. The effect of ADAM29 on
migration and invasion, respectively, was also assessed through
wound healing and Transwell assays, respectively. Functional
experiments in vitro indicated that the overexpression of
ADAM29 promoted MGC803 cell migration (Fig. 5A; P<0.01) and knockdown of ADAM29
decreased AGS cell migration (Fig.
5B; P<0.05). The invasion assay demonstrated that, compared
with the control groups, the overexpression of ADAM29 in MGC803
cells significantly promoted the invasion ability of GC cells
(Fig. 5C; P=0.0072), whereas
knockdown of ADAM29 resulted in a significant reduction in the
invasion ability of the AGS cells (Fig.
5D; P=0.0485).
Discussion
ADAM29 has been frequently demonstrated to be
associated with the progression of cancer (18–21). In
the present study, it was determined that ADAM29 was upregulated in
GC tissues compared with paracancerous tissues via RT-qPCR and IHC
analyses. Furthermore, the expression of ADAM29 was demonstrated to
be associated with TNM staging of GC.
The important roles of ADAM family in the
progression of various cancer types, including breast and gastric
cancer, have been studied (16,25,28–30).
For example, ADAM10 and ADAM17 may facilitate the migration of
cancer stem cells resulting in a differentiated phenotype that is
more susceptible to treatment (8).
Previously, Micocci et al (29) identified that ADAM9 silencing
inhibited breast cancer cell transmigration via blood and lymphatic
endothelial cells. An additional study demonstrated that ADAM17
promoted the invasion, motility and sprouting of lymphatic
endothelial cells (30). The
anti-lymphangiogenic role of ADAM17 silencing in lymphatic
endothelial cells indicated that ADAM17 may serve as a potential
target in cancer therapy (30). The
role of the ADAM family in GC has been studied previously (16,25).
Previously, a study determined that ADAM17 promoted
epithelial-mesenchymal transition via activating the TGF-β/Smad
pathway in GC cells, which additionally indicated the relevance of
ADAM17 as a potential target in cancer therapy (16). ADAM29 expression also affected the
malignant phenotype of tumor cells (25). For example, ADAM29 overexpression
significantly increased the proliferation, migration and invasion
of breast cancer cells in vitro (22). Furthermore, it has been demonstrated
that the mutation of ADAM29 frequently occurs in cancer (31). It was indicated that ADAM29 may serve
a pivotal role in the process of breast cancer progression and
metastasis (22). An additional study
indicated that somatic mutations of ADAM29 frequently occurred in
melanoma (19); however, the
molecular mechanism underlying ADAM29 in GC remains unclear. To
clarify the function of ADAM29, 7 tumor-derived mutants were
created in melanoma cells, and its effect on growth and adhesion
was examined in a previous study (19). In the present study, the focus was on
the functional aspects of ADAM29 in mediating the malignant
phenotype of GC cells. The present study determined that the
overexpression of ADAM29 is associated with the elevation of
growth, migration and invasion properties of MGC803 in
vitro. Concomitantly, a significant decrease in the cellular
migratory, proliferative and invasive abilities was identified in
AGS cells when ADAM29 was knocked down, when compared with the
control group. In combination, these data indicated the pro-cancer
role of ADAM29 in GC cells; however, whether mutated ADAM29 may
affect these malignant phenotypes, including proliferation,
migration and invasion of GC cells is yet to be investigated. In
future studies, the complete role of the ADAM29 mutation in GC will
be explored.
The effect of ADAM29 on patient survival is not a
negligible factor. Kaplan-Meier survival analyses of ADAM29
transcripts in GC indicated that increased levels of ADAM29
expression was were associated with poor OS and PFS time of
patients with GC, suggesting that ADAM29 promoted disease
progression and relapse of GC. In future studies, clinical outcomes
will be measured to reveal the implications of ADAM29 in patient
survival.
In conclusion, the data of the present study
demonstrated that increased levels of ADAM29 were associated with
poorer clinical outcomes of patients with GC. In vitro cell
function data indicated that ADAM29 served as an oncogene in GC
cells. These data indicated that ADAM29 may serve as a molecular
marker in GC diagnosis and as an indicator for prognosis.
Acknowledgements
Not applicable.
Funding
The work was supported by foundation of Yantai
Yuhuangding Hospital and Yantaishan Hospital (grant no.
YT2017008).
Availability of data and materials
All data generated or analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
HC designed the research; HC and SW performed the
experiments; HC and SW contributed to writing and revision of the
manuscript; all authors read and approved final manuscript.
Ethics approval and consent to
participate
The present study was executed accordingly under the
protocol approved by the Institutional Review Board and Research
Ethical Committee of the Affiliated Yantai Yuhuangding Hospital of
Qingdao University (Yantai, China). Written informed consent was
obtained from all patients.
Consent for publication
All patients provided their written informed consent
for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre L, Bray F, Siegel R, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J,
Xue Y, Suo J, Tao K, He X, et al: Morbidity and mortality of
laparoscopic versus open D2 distal gastrectomy for advanced gastric
cancer: A randomized controlled trial. J Clin Oncol. 34:1350–1357.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Irani S, Baron T, Itoi T and Khashab M:
Endoscopic gastroenterostomy: Techniques and review. Curr Opin
Gastroenterol. 33:320–329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu T, Hu X, Wei P and Shan G: Molecular
background of the regional lymph node metastasis of gastric cancer.
Oncol Lett. 15:3409–3414. 2018.PubMed/NCBI
|
|
6
|
Walkiewicz K, Gętek M, Muc-Wierzgoń M,
Kokot T and Nowakowska-Zajdel E: The importance of ADAM family
proteins in malignant tumors. Postepy Hig Med Dosw (Online).
70:67–73. 2016.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brown RV, Gaerig VC, Simmons T and Brooks
TA: Helping Eve overcome ADAM: G-quadruplexes in the ADAM-15
promoter as new molecular targets for breast cancer therapeutics.
Molecules. 18:15019–15034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pham D, Kim J, Kim S, Shin D, Uong N, Hyun
H, Yoon MS, Kang SJ, Ryu YJ, Cho JS, et al: Effects of ADAM10 and
ADAM17 inhibitors on natural killer cell expansion and
antibody-dependent cellular cytotoxicity against breast cancer
cells in vitro. Anticancer Res. 37:5507–5513. 2017.PubMed/NCBI
|
|
9
|
Edwards DR, Handsley MM and Pennington CJ:
The ADAM metalloproteinases. Mol Aspects Med. 29:258–289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin H, Diaz A, Blouin L, Lebbink R, Patena
W, Tanbun P, LeProust EM, McManus MT, Song JS and Ramalho-Santos M:
Systematic identification of barriers to human iPSC generation.
Cell. 158:449–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nyren-Erickson E, Jones J, Srivastava D
and Mallik S: A disintegrin and metalloproteinase-12 (ADAM12):
Function, roles in disease progression, and clinical implications.
Biochim Biophys Acta. 1830:4445–4455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolfsberg T, Straight P, Gerena R, Huovila
A, Primakoff P, Myles D and White JM: ADAM, a widely distributed
and developmentally regulated gene family encoding membrane
proteins with a disintegrin and metalloprotease domain. Dev Biol.
169:378–383. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohtsuka T, Shiomi T, Shimoda M, Kodama T,
Amour A, Murphy G, Ohuchi E, Kobayashi K and Okada Y: ADAM28 is
overexpressed in human non-small cell lung carcinomas and
correlates with cell proliferation and lymph node metastasis. Int J
Cancer. 118:263–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le Pabic H, Bonnier D, Wewer U, Coutand A,
Musso O, Baffet G, Clément B and Théret N: ADAM12 in human liver
cancers: TGF-beta-regulated expression in stellate cells is
associated with matrix remodeling. Hepatology. 37:1056–1066. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin H, Zhong F, Ouyang Y, Wang Q, Ding L
and He S: Upregulation of ADAM12 contributes to accelerated cell
proliferation and cell adhesion-mediated drug resistance (CAM-DR)
in Non-Hodgkin's Lymphoma. Hematology. 22:527–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu M, Zhou H, Zhang C, He J, Wei H, Zhou
M, Lu Y, Sun Y, Ding JW, Zeng J, et al: ADAM17 promotes
epithelial-mesenchymal transition via TGF-β/Smad pathway in gastric
carcinoma cells. Int J Oncol. 49:2520–2528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cerretti D, DuBose R, Black R and Nelson
N: Isolation of two novel metalloproteinase-disintegrin (ADAM)
cDNAs that show testis-specific gene expression. Biochem Biophys
Res Commun. 263:810–815. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in oesophageal squamous cell cancer. Nature. 509:91–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei X, Moncada-Pazos A, Cal S,
Soria-Valles C, Gartner J, Rudloff U and Lin JC; NISC Comparative
Sequencing Program; Rosenberg SA, López-Otín C and Samuels Y:
Analysis of the disintegrin-metalloproteinases family reveals
ADAM29 and ADAM7 are often mutated in melanoma. Hum Mutat.
32:E2148–E2175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang B, Li Y, Li L, Chen M, Zhang C, Zuo
X, Zhou FS, Liang B, Zhu J, Li P, et al: Association study of
susceptibility loci with specific breast cancer subtypes in Chinese
women. Breast Cancer Res Treat. 146:503–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Purrington K, Slager S, Eccles D,
Yannoukakos D, Fasching P, Miron P, Carpenter J, Chang-Claude J,
Martin NG, Montgomery GW, et al: Genome-wide association study
identifies 25 known breast cancer susceptibility loci as risk
factors for triple-negative breast cancer. Carcinogenesis.
35:1012–1019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao M, Jia W, Jiang W, Wang P, DU G,
Cheng S and Song M: ADAM29 expression in human breast cancer and
its effects on breast cancer cells in vitro. Anticancer Res.
36:1251–1258. 2016.PubMed/NCBI
|
|
23
|
Brim H, Abu-Asab M, Nouraie M, Salazar J,
Deleo J, Razjouyan H, Mokarram P, Schaffer AA, Naghibhossaini F and
Ashktorab H: An integrative CGH, MSI and candidate genes
methylation analysis of colorectal tumors. PloS One. 9:e821852014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ashktorab H, Schäffer A, Daremipouran M,
Smoot D, Lee E and Brim H: Distinct genetic alterations in
colorectal cancer. PloS One. 5:e88792010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Costa N, Paulo P, Caffrey T, Hollingsworth
M and Santos-Silva F: Impact of MUC1 mucin downregulation in the
phenotypic characteristics of MKN45 gastric carcinoma cell line.
PloS One. 6:e269702011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SG and Hwang SH: The association
between the duration of fluoropyrimidine-based adjuvant
chemotherapy and survival in stage II or III gastric cancer. World
J Surg Oncol. 14:1022016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu H, Ye L, Mansel RE, Zhang Y and Jiang
WG: Clinical implications of the influence of Ehm2 on the
aggressiveness of breast cancer cells through regulation of matrix
metalloproteinase-9 expression. Mol Cancer Res. 8:1501–1512. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Micocci KC, Moritz MN, Lino RL, Fernandes
LR, Lima AG, Figueiredo CC, Morandi V and Selistre-de-Araujo HS:
ADAM9 silencing inhibits breast tumor cells transmigration through
blood and lymphatic endothelial cells. Biochimie. 128–129:174–182.
2016. View Article : Google Scholar
|
|
30
|
Mężyk-Kopeć R, Wyroba B, Stalińska K,
Próchnicki T, Wiatrowska K, Kilarski WW, Swartz MA and Bereta J:
ADAM17 promotes motility, invasion, and sprouting of lymphatic
endothelial cells. PLoS One. 10:e01326612015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Motycková M, Zák P, Vroblová V, Andrýs C,
Belada D, Malý J and Smolej L: Prognostic markers in chronic
lymphocytic leukemia. Vnitr Lek. 57:847–857. 2011.PubMed/NCBI
|