Introduction
Angiogenesis is a vital process in the growth,
development and spread of tumors. It is a multi-step process that
is determined by a net balance between pro- and antiangiogenesis
regulators. At present, anti-angiogenesis is regarded as a target
for cancer therapy (1).
Human endothelial cell protein C receptor (EPCR) is
a type 1 transmembrane glycoprotein that is expressed primarily by
the vascular endothelium of larger blood vessels (2,3). On the
endothelial surface, EPCR binds and presents protein C (PC) to the
thrombin:thrombomodulin (TM) complex to generate activated protein
C (APC). It binds both PC and APC with equally high affinity
(4). APC is critical for the negative
regulation of blood coagulation by inactivating two key cofactors
FVIIIa and FVa, which are responsible for amplification of blood
coagulation reactions, and which promote thrombin generation
(4). APC plays a cytoprotective role
in endothelial tissue, which involves altering gene expression
profiles, anti-apoptotic activity, anti-inflammatory activity and
protection of endothelial barriers (2,5). This
cytoprotective effect of APC requires EPCR and the protease
activated receptor 1 (PAR1). In addition, APC can induce
endothelial cell proliferation and angiogenesis by activating
mitogen-activated protein kinase (MAPK) (6). Recent research showed that EPCR is
expressed in tumor cells, including leukemia U937 cells (7), mesothelioma (8), ovarian cancer (9), lung cancer (10,11) and
breast cancer cells (12,13). In our previous study, we had found
that EPCR is expressed in gastric carcinoma tissue and BGC803,
HGC27, AGS and SGC7901 gastric carcinoma cancer cells, and that it
can promote MGC803 gastric cancer cells proliferation and migration
(14). However the role of EPCR in
tumor angiogenesis is not clear.
In this study, we investigated the correlation
between the expression of EPCR and the microvessel density (MVD) of
tumors in the primary respectable gastric carcinoma, through
quantification of MVD using the specific endothelial cell markers
CD31 and CD34. From this, we observed that the mean MVD value was
higher in EPCR-positive gastric cancer samples compared with that
in negative samples. Additionally, the proliferation, migration and
tubule formation of human umbilical vein endothelial cells
(HUVECs), when cultured with the tumor-conditioned medium of MGC803
cells treated with PAR1 antibody or subject to EPCR knockdown, were
inhibited. These findings indicate a novel role of EPCR in gastric
cancer progression.
Materials and methods
Antibodies
Mouse monoclonal anti-EPCR (ab151403; Abcam,
Cambridge, MA, USA), rabbit polyclonal anti-PAR1 (ab63445; Abcam,
Cambridge, UK), rabbit monoclonal anti-pERK1/2 (4370), anti-pAKT
(Ser473) (4060), anti-pAKT (Thr308) (13038), and anti-AKT (9272)
(all from Cell Signaling Technology, Danvers, MA, USA), rabbit
polyclonal anti-ERK1/2 (c0185; Anbo Biotech Co., Ltd., San
Francisco, CA, USA), mouse monoclonal anti-GAPDH (TA505454;
Zhongshan Biotech Co., Ltd., Beijing, China), rabbit anti-mouse
(ab6728) and goat anti-rabbit (ab6721) (both from Abcam, Cambridge,
UK) were used in the present study.
Cell culture
Human gastric cancer cell line MGC803 and HUVECs,
were purchased from the Shanghai Institute of Biochemistry and Cell
Biology, Chinese Academy of Sciences (Shanghai, China). Cells were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100 IU/ml
penicillin and 100 ng/ml streptomycin in a 37°C, 5% CO2
humidified atmosphere.
siRNA transfection
Stealth™ RNA duplexes against human EPCR
(sense, 5′-GCACUCGGUAUGAACUGCGGGAAUU-3′ and antisense,
5′-AAUUCCCGCAGUUCAUACCGAGUGC-3′) were designed and synthesized as
previously described (14). The
anti-EPCR siRNA (50 nM) was transfected using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Immunohistochemical analysis of CD31
and CD34
Section of a tissue microarray with 61 gastric
carcinoma tissues collected from the Affiliated Hospital of Xuzhou
Medical University (14) were
incubated at 4°C overnight in a moist chamber with mouse monoclonal
anti-CD31 and CD34 antibodies (Maxim-Bio Ltd., Fuzhou, China). The
sections were then incubated at room temperature for 10 min with
biotinylated anti-mouse immunoglobulin after being washed with 0.02
M phosphate buffered saline (PBS) pH 7.4. Then, the sections were
incubated for 10 min with Streptavidin-Biotin Complex (Boster Ltd.,
Wuhan, China) after being washed with PBS. CD31 and CD34 labeling
was visualized by incubating the sections in DAB solution (Boster
Ltd.). MVD was assessed by counting the vessel numbers under three
different fields at high-power magnification fields (×400). An
average was calculated for each case and statistically presented as
the mean ± SD. The isolated immuno-reactive endothelial cells or
groups of endothelial cells separated by the adjacent microvessels
were considered to be quantifiable individual vessels. Visible
lumens or the presence of associated red cells were not
obligatory.
Preparation of tumor-conditioned
medium
Tumor-conditioned medium was prepared as described
in previous studies (15–20). In brief, after MGC803 cells were
treated with 10 µg/ml anti-PAR1 antibody or transfected with EPCR
siRNA, the culture medium was collected and centrifuged, and the
supernatant was collected. The supernatant was combined with fresh
DMEM according to a ratio of fresh DMEM:FBS:Tumor cell culture
medium of 5:1:4, to obtain the tumor-conditioned medium.
Subsequently, the HUVECs were cultured with the prepared
tumor-conditioned medium.
Proliferation analysis
A total of 3×103 HUVECs were plated in
96-well plates in the tumor-conditioned medium, and cultured for
24, 48 and 72 h, respectively. Then, 10 µl WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium] from a CCK-8 kit was added to each well and incubated
for 4 h. The absorbance was measured at 450 nm on a Muti-Detection
Microplate Reader (Thermo 1500; Thermo Fisher Scientific,
Inc.).
Transwell assay
The migration ability of HUVECs was examined using a
Transwell cell culture chamber (Corning Incorporated, Corning, NY,
USA). The lower chamber was filled with the prepared
tumor-conditioned medium (600 µl), and 1×104 cells were
seeded onto the upper chamber. Chambers were incubated for 24 h at
37°C. The cells remaining on the top surface of the membrane were
removed with application of a cotton swab followed by washing with
PBS three times. The cells on the bottom surface of the membrane
were fixed and stained with 0.1% crystal violet. Subsequently, the
number of migrated cells was quantified by counting in 5 fields of
view under a light microscope (×20 objective).
Matrigel-based tube formation
assay
Matrigel (BD Biosciences, San Jose, CA, USA) was
plated into 96-well plates at 50 µl/well and incubated for 30 min
at 37°C. Then, 2×104 HUVECs were re-suspended with
tumor-conditioned medium, seeded onto the Matrigel, and incubated
overnight at 37°C, Each well was analyzed directly under a
microscope, and tubules from 3–5 random fields of each well were
imaged and counted.
Western blot analysis
After treatment with the prepared tumor-conditioned
medium, HUVECs were lysed with RIPA buffer. Cell lysates (30
µg/lane) were subjected to 15% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). After blocking in PBST (10 mmol/l Tris-HCl, pH 7.4, 150
mmol/l NaCl, 0.05% Tween-20) containing 5% nonfat dried milk for 1
h, the membranes were incubated with the mouse monoclonal anti-EPCR
(1:2,000), rabbit polyclonal anti-PAR1 (1:2,000), rabbit monoclonal
anti-pERK1/2 (1:3,000), anti-pAKT (Ser473) (1:3,000), anti-pAKT
(Thr308) (1:3,000), anti-AKT (1:3,000), rabbit polyclonal
anti-ERK1/2 (1:3,000), and mouse monoclonal anti-GAPDH (1:1,000) at
4°C overnight. Secondary antibodies (1:10,000) were incubated at
room temperature for 1 h. Protein bands were detected by the
enhanced chemiluminescence (ECL) reaction (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Cell ELISA
ELISA was performed as described previously
(21). Briefly, 3×104
cells were seeded in a flat-bottomed 96-well microtiter plate and
incubated for 24 h at 37°C in 5% CO2. Cells were washed
3 times with PBS and fixed with 100 µl of 4% paraformaldehyde
solution in 0.01 M PBS for 10 min. Then, cells were incubated with
100 µl of a blocking solution containing 1% (w/v) BSA in 0.01 M PBS
for 1 h. After blocking, 50 µl/well of the primary antibody
(anti-uncleaved PAR1 antibody was designed and prepared by Abgent
Biotechnology Co., Ltd., Suzhou, China, peptide:
NH2-SFLLRNPNDKC-CONH2; peptide control, NH2-DPRSFLLRNPNDKC-CONH2)
or 50 µl/well of the blocking solution was added and incubated for
at least 1 h at 4°C. After washing 5 times with 200 µl/well of the
washing buffer, 50 µl/well of the secondary antibody or 50 µl/well
of the blocking solution was added and incubated for an additional
1 h at 4°C. Then, 100 µl of 3,3′, 5,5′-tetramethylbenzidine (TMB)
substrate solution was added to each well after washing 5 times,
and incubated for 20 min at room temperature. Finally, 25 µl/well
of 2 M sulfuric acid was added to stop the enzyme reaction, and
absorbance was measured at 450 nm using muti-detection microplate
reader (Thermo 1500).
Statistical analysis
All statistical analyses were performed using SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). Measurement
data were representative of experiments repeated at least three
times and presented as mean ± SEM. The results were analyzed among
groups using ANOVA followed by the post-hoc Student-Newman-Keuls
procedure for multiple comparisons. The Student's paired t-test was
used to assess the significance of data comparisons between two
groups. P-values of less than 0.05 were considered statistically
significant (P<0.05). Correlations between qualitative data and
quantitative data were analyzed with the Eta value, with α=0.05 as
the inspection level.
Results
Correlation between EPCR expressions
and MVD in gastric carcinoma
In previous study, we had found EPCR to be highly
expressed in tissue samples of 44/61 (72.13%) cases of gastric
carcinoma (14). In the current
study, the MVD value of these 61 cases of gastric carcinoma was
determined by detecting the microvascular endothelial cells markers
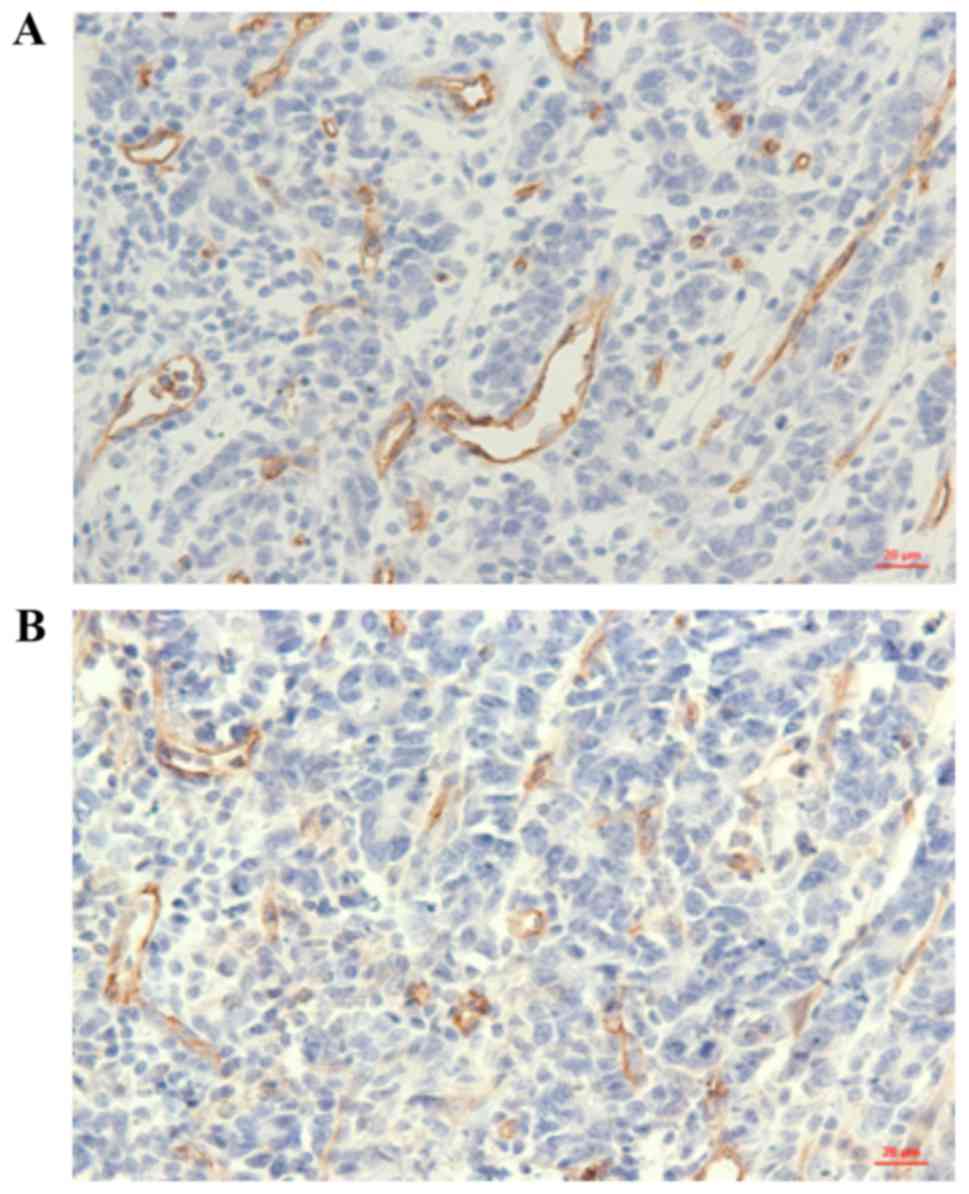
CD31 and CD34 through immunohistochemistry (Fig. 1). Then, the correlation between the
expression of EPCR protein and MVD was analyzed by statistical
analysis. As shown in Table I, we
observed that the mean MVD value was higher in EPCR-positive
gastric cancer samples compared with that in negative samples; and
this association was statistically significant.
 | Table I.Association between EPCR expression
and MVD in gastric carcinoma. |
Table I.
Association between EPCR expression
and MVD in gastric carcinoma.
| EPCR | n | MVD CD34 (mean ±
SD) | Eta | P-value | MVD CD31(mean ±
SD) | Eta | P-value |
|---|
| + | 44 | 49.523±19.471 | 0.309 | <0.05 | 37.899±20.644 | 0.427 | <0.001 |
| – | 17 | 36.042±17.391 |
|
| 19.421±5.185 |
|
|
Knockdown of EPCR or blockade of PAR1
inhibits HUVECs growth, migration and tubules formation
In our previous study, we found that EPCR knockdown
inhibited the cells growth and migration of MGC803 cells, and that
the role of EPCR may be related to PAR1 (14). To study whether knockdown of EPCR and
blockade of PAR1 in MGC803 cells affects tumor angiogenesis,
tumor-conditioned medium was prepared after MGC803 cells were
transfected with EPCR siRNA or treated with 10 µg/ml anti-PAR1
antibody, and then used to culture HUVECs. Then, the cell
viability, migration, and tubule formation abilities of the HUVECs
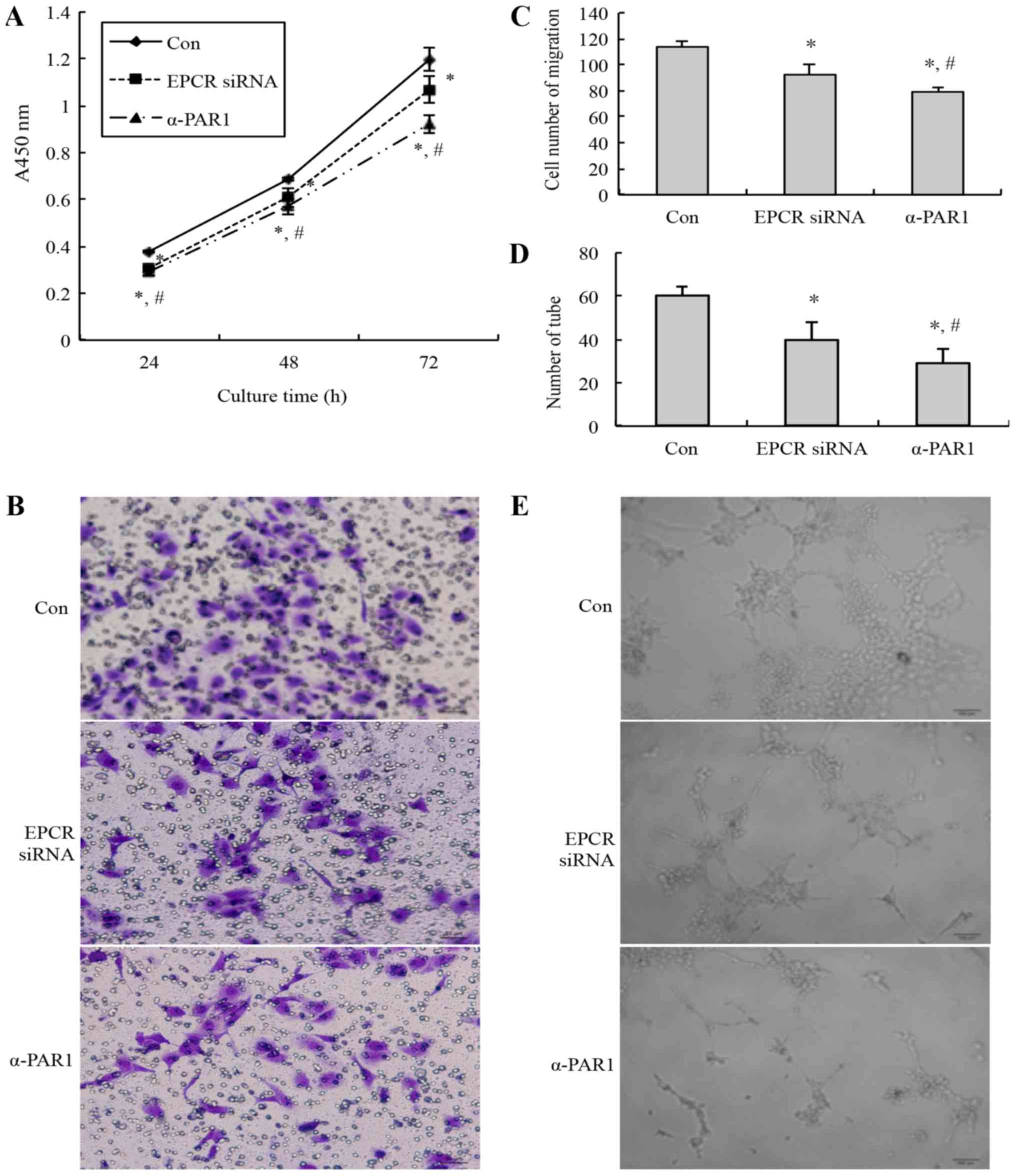
were detected. Compared with the control group, the cell viability
(Fig. 2A), migrated cell number
(Fig. 2B and C), and tubules number
(Fig. 2D and E) of the EPCR
siRNA-treated group and anti-PAR1 antibody treated-group were
decreased significantly. Additionally, compared with the EPCR siRNA
group, the cell viability, migrated cell number, and tubule number
of the anti-PAR1 antibody-treated group was decreased
significantly.
Knockdown of EPCR inhibits activation
of PAR1 in MGC803 cells
To study whether the EPCR expression in MGC803 cells
affect tumor angiogenesis through activating PAR1, following EPCR
knockdown, anti-uncleaved PAR1 antibody was used to detect the
uncleaved PAR1 on the cell membrane of MGC803 cells by Cell ELISA.
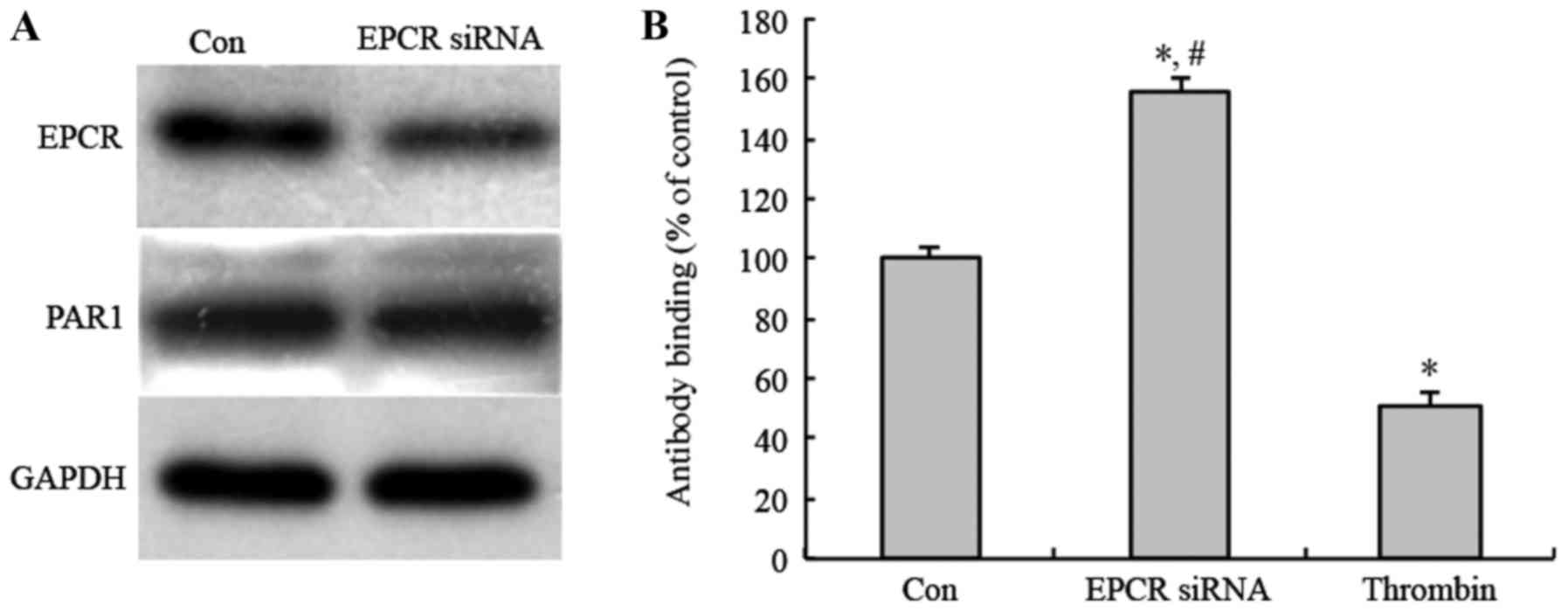
The results showed that PAR1 protein expression level did not
changed after EPCR knockdown (Fig.
3A). However, anti-uncleaved PAR1 antibody-binding rate was
increased after EPCR knockdown, compared with a control group and
positive control group treated with thrombin, as a known activator
of PAR1. Additionally, the anti-uncleaved PAR1 antibody-binding
rate of the thrombin-treated group was decreased compared with the
control (Fig. 3B). These results
indicate that the knockdown of EPCR inhibited PAR1 activation in
MGC803 cells.
Knockdown of EPCR or blockade of PAR1
inhibist ERK1/2 and AKT activation in HUVECs
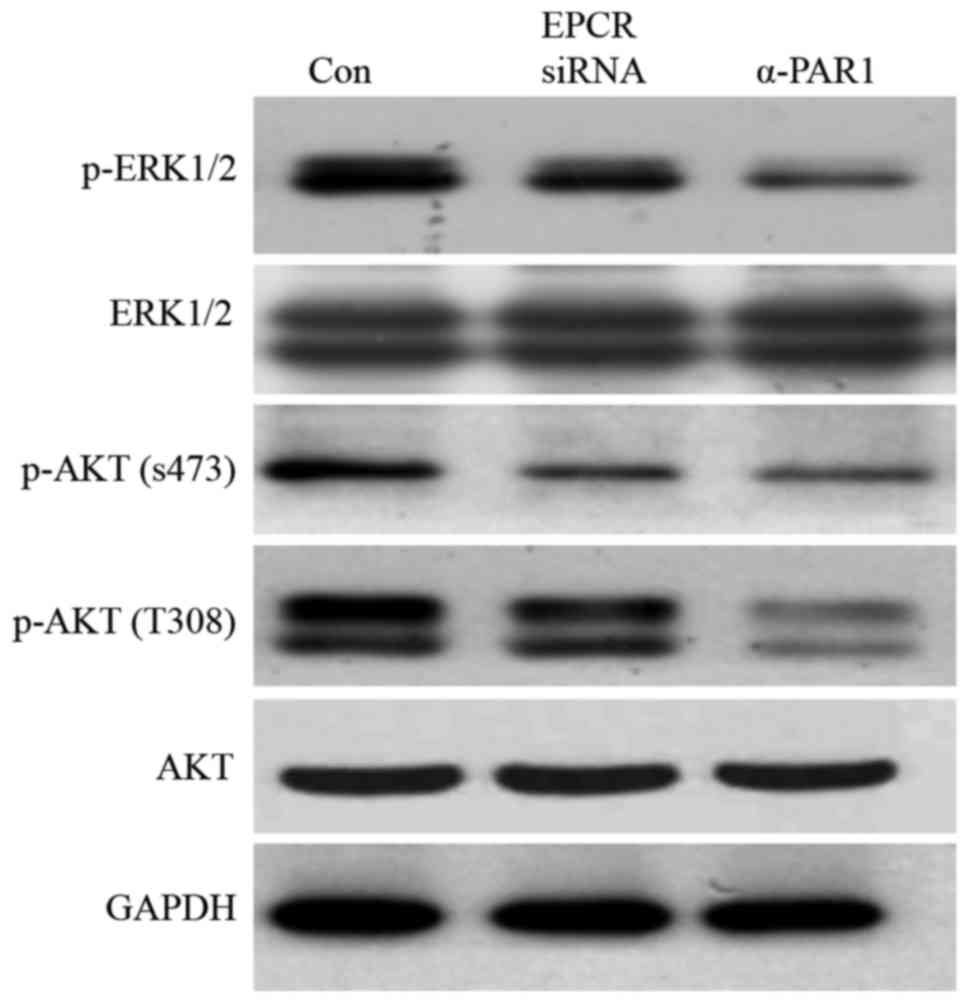
After treatment with tumor-conditioned medium,
compared with the control group, the levels of HUVEC's ERK1/2, AKT
(S473) and AKT (Th308) phosphorylation in HUVECs in the EPCR
siRNA-treated group and anti-PAR1 antibody-treated group were
reduced (Fig. 4). This result
indicates that EPCR and PAR1 may promote HUVECs proliferation and
migration through ERK1/2 and AKT activation in MGC803 gastric
cancer cells.
Discussion
In the present study, we showed that the expression
of EPCR is correlated with MVD in gastric cancer tissues.
Furthermore, when cultured with tumor-conditioned medium of gastric
cancer MGC803 cells treated with EPCR siRNA or blocking antibodies
against PAR1, the proliferation, migration and tubules formation
abilities, and the phosphorylation levels of ERK and AKT in HUVECs
were decreased. PAR1 activation in MGC803 cells was also decreased
after EPCR knockdown.
It has been reported that APC induces HUVEC
proliferation, morphogenetic changes resembling tube-like
structures, and angiogenesis in the mouse cornea; while antibodies
against EPCR inhibit HUVEC proliferation (6). Niessen et al found that
EPCR/APC-PAR1 signaling prevented inflammation-induced vascular
leakage, and pharmacological or genetic blockade of this pathway
leaded to mice sensitivity to LPS-induced lethality in mice
(22). Sundaram et al found
FVIIa could reduce LPS-induced vascular leakage in the lung and
kidney; but the protective effect was attenuated in EPCR-deficient
mice, and blocked by PAR1. In addition they found VEGF-induced
vascular leakage in the skin was highly dependent on EPCR
expression levels (23). Mosnier and
Griffin found that APC could inhibit staurosporine-induced
apoptosis of EAhy926 endothelial cells. APC elicits anti-apoptotic
effects requiring PAR1 and EPCR (24). Hun Lee et al found that
progesterone could attenuate thrombin-induced blood-brain barrier
disruption by blocking the degradation of tight junction proteins
and EPCR in mouse brain endothelial cells bEnd.3 (25). All these studies show that EPCR exerts
vascular barrier-protective effect, but the role of EPCR in tumor
angiogenesis is not clear. Our results revealed that the expression
of EPCR is correlated with MVD in gastric cancer tissue. Knockdown
of EPCR expression in MGC803 cells could decrease the
proliferation, migration and tubule formation of HUVECs in the
presence of the MGC803-conditioned medium, with the medium of
MGC803 cells treated with PAR1 antibody having the same effect.
Furthermore, EPCR knockdown decreased PAR1 activation. These in
vitro events may explain the angiogenic activity of
EPCR-PAR1signaling in gastric tumor cells. Uchiba et al
found that APC activated the MAPK pathway and induced HUVECs
proliferation in vitro. In addition, APC activated
endothelial nitric oxide synthase via PI3K phosphorylation, leading
to protein kinase G activation, suggesting that APC bound to EPCR
may activate the endothelial MAPK pathway through a mechanism
similar to that of VEGF (6). Sen
et al found APC-mediated activation of PAR1 and p44/42 MAPK
in endothelial cell was enhanced by Zinc ions (26). Gramling et al found that APC
enhanced endothelial cell motility and MDA-MB-231 breast cancer
cells migration by activating ERK1/2, Akt and NF-κB, but not the
JNK pathway (12). The present study
showed that the phosphorylation level of ERK1/2 and AKT (S473 and
T308) is decreased in HUVECs cultured with the tumor-conditioned
medium of MGC803 gastric cancer cells treated with PAR1 antibody or
EPCR siRNA. However, further studies are required to investigate
EPCR expressed on the tumor cell is how to regulate ERK1/2 and AKT
pathway of endothelial cell; whether the role of EPCR is dependent
on some pro-angiogenic factors, such as VEGF.
In conclusion, EPCR exhibits a stimulatory effect on
tumor angiogenesis in the human gastric cancer cell line MGC803 by
activating ERK1/2 and AKT, and this effect of EPCR requires PAR1
activation.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81101493), a General
Financial Grant from the China Postdoctoral Science Foundation
(grant no. 2014M561713), the Jiangsu Undergraduate Training Program
for Innovation and Entrepreneurship (grant no. 201610313008Z) and
the Dean Special Foundation of Xuzhou Medical University (grant no.
2012KJZ07).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ designed the study. QW performed the experiments
and drafted the manuscript. YT, TW, HY, XW and HM performed the
tissue collection, cell culture and data analysis. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Medical
Ethics Committee at the Affiliated Hospital of Xuzhou Medical
University (approval no. xyfylw2012002).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EPCR
|
endothelial protein C receptor
|
|
PAR1
|
protease-activated receptor 1
|
|
MVD
|
microvessel density
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
References
|
1
|
Liu CC, Shen Z, Kung HF and Lin MC: Cancer
gene therapy targeting angiogenesis: An updated review. World J
Gastroenterol. 12:6941–6948. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thiyagarajan M, Cheng T and Zlokovic BV:
Endothelial cell protein C receptor: Role beyond endothelium? Circ
Res. 100:155–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crawley JT: Multiple roles of the
endothelial cell protein C receptor. J Thromb Haemost. 5:1813–1816.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohan Rao LV, Esmon CT and Pendurthi UR:
Endothelial cell protein C receptor: A multiliganded and
multifunctional receptor. Blood. 124:1553–1562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riewald M, Petrovan RJ, Donner A and Ruf
W: Activated protein C signals through the thrombin receptor PAR1
in endothelial cells. J Endotoxin Res. 9:317–321. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uchiba M, Okajima K, Oike Y, Ito Y,
Fukudome K, Isobe H and Suda T: Activated protein C induces
endothelial cell proliferation by mitogen-activated protein kinase
activation in vitro and angiogenesis in vivo. Circ Res. 95:34–41.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shua F, Kobayashia H, Fukudomeb K,
Tsuneyoshib N, Kimotob M and Teraoa T: Activated protein C
suppresses tissue factor expression on U937 cells in the
endothelial protein C receptor-dependent manner. FEBS Lett.
477:208–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keshava S, Sahoo S, Tucker TA, Idell S,
Rao LV and Pendurthi UR: Endothelial cell protein C receptor
opposes mesothelioma growth driven by tissue factor. Cancer Res.
73:3963–3973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ducros E, Mirshahi S, Azzazene D,
Camilleri-Broët S, Mery E, Al Farsi H, Althawadi H, Besbess S,
Chidiac J, Pujade-Lauraine E, et al: Endothelial protein C receptor
expressed by ovarian cancer cells as a possible biomarker of cancer
onset. Int J Oncol. 41:433–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antón I, Molina E, Luis-Ravelo D, Zandueta
C, Valencia K, Ormazabal C, Martínez-Canarias S, Perurena N,
Pajares MJ, Agorreta J, et al: Receptor of activated protein C
promotes metastasis and correlates with clinical outcome in lung
adenocarcinoma. Am J Respir Crit Care Med. 186:96–105. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heng W, Mu CY, Chen C, Huang JA and Wang
ZY: Endothelial cell protein C receptor (EPCR) is expressed by lung
carcinoma and correlated with clinical parameters. Clin Lab.
59:375–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gramling MW, Beaulieu LM and Church FC:
Activated protein C enhances cell motility of endothelial cells and
MDA-MB-231 breast cancer cells by intracellular signal
transduction. Exp Cell Res. 316:314–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perurena N, Zandueta C, Martínez-Canarias
S, Moreno H, Vicent S, Almeida AS, Guruceaga E, Gomis RR,
Santisteban M, Egeblad M, et al: EPCR promotes breast cancer
progression by altering SPOCK1/testican 1-mediated 3D growth. J
Hematol Oncol. 10:232017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Q, Liu Q, Wang T, Yang H, Han Z and
Zhang P: Endothelial cell protein C receptor promotes MGC803
gastric cancer cells proliferation and migration by activating
ERK1/2. Med Oncol. 32:1622015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang HK, Kin BS, Han J, Yoon JK, Lee JR,
Jeong GJ and Shin JY: Therapeutic angiogenesis using tumor
cell-conditioned medium. Biotechnol Prog. 32:456–464. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu LN, Xu BN, Cai J, Yang JB and Lin N:
Tumor-associated fibroblast-conditioned medium promotes tumor cell
proliferation and angiogenesis. Genet Mol Res. 12:5863–5871. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang T and Jiang CL: Tumor conditioned
medium regulates the proliferation, adhesion and migration of human
umbilical vein endothelial cells. Sheng Li Xue Bao. 63:256–260.
2011.(In Chinese). PubMed/NCBI
|
|
18
|
Liu T, Jabbes M, Nedrow-Byers JR, Wu LY,
Bryan JN and Berkman CE: Detection of prostate-specific membrane
antigen on HUVECs in response to breast tumor-conditioned medium.
Int J Oncol. 38:1349–1355. 2011.PubMed/NCBI
|
|
19
|
Koizumi S, Gu C, Amano S, Yamamoto S,
Ihara H, Tokuyama T and Namba H: Migration of mouse-induced
pluripotent stem cells to glioma-conditioned medium is mediated by
tumor-associated specific growth factors. Oncol Lett. 2:283–288.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng Y, Li J and Geng M: The glycan
profile of endothelial cells in the present of tumor-conditioned
medium and potential roles of beta-1,6-GlcNAc branching on HUVEC
conformation. Mol Cell Biochem. 340:143–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Falahat R, Wiranowska M, Gallant ND,
Toomey R, Hill R and Alcantar N: A cell ELISA for the
quantification of MUC1 mucin (CD227) expressed by cancer cells of
epithelial and neuroectodermal origin. Cell Immunol. 298:96–103.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niessen F, Furlan-Freguia C, Fernández JA,
Mosnier LO, Castellino FJ, Weiler H, Rosen H, Griffin JH and Ruf W:
Endogenous EPCR/aPC-PAR1 signaling prevents inflammation-induced
vascular leakage and lethality. Blood. 113:2859–2866. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sundaram J, Keshava S, Gopalakrishnan R,
Esmon CT, Pendurthi UR and Rao LV: Factor VIIa binding to
endothelial cell protein C receptor protects vascular barrier
integrity in vivo. J Thromb Haemost. 12:690–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mosnier LO and Griffin JH: Inhibition of
staurosporine-induced apoptosis of endothelial cells by activated
protein C requires protease-activated receptor-1 and endothelial
cell protein C receptor. Biochem J. 373:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hun Lee J, Won S and Stein DG:
Progesterone attenuates thrombin-induced endothelial barrier
disruption in the brain endothelial cell line bEnd.3: The role of
tight junction proteins and the endothelial protein C receptor.
Brain Res. 1613:73–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sen P, Sahoo S, Pendurthi UR and Rao LV:
Zinc modulates the interaction of protein C and activated protein C
with endothelial cell protein C receptor. J Biol Chem.
285:20410–20420. 2010. View Article : Google Scholar : PubMed/NCBI
|