Introduction
BRAF is one of the most frequently mutated protein
kinases in human cancer (1,2). BRAF protein kinase has been suggested to
be among the most likely protein kinase genes to carry driver
mutations (3). BRAF mutations have
been identified in the majority of malignant melanomas, and the
frequency of BRAF mutations in malignant melanomas is 59% (1). The mutation frequency of BRAF is
relatively low in other types of cancer, including papillary
thyroid carcinoma and colorectal carcinoma. Research by Davies and
his colleagues indicated that the BRAF mutation is a novel
diagnostic and prognostic biomarker in thyroid cancer by analyzing
cytological and histological thyroid samples, which occurs
specifically and with a high prevalence (35.8%) in papillary
thyroid carcinoma, followed by colorectal carcinoma (18%), gliomas
(11%), sarcomas (9%), ovarian carcinomas (4%) and lung cancer (3%)
(1). Mutations in BRAF have been
associated with altered sensitivities to numerous drugs, including
PLX4720, Nutlin-3a, AZ628, bortezomib, embelin, RDEA119, FH535,
CI-1040, CHIR-99021, AP-24534, obatoclax mesylate, PF-562271,
CEP-701, FTI-277, 17-AAG, PD-0325901, SB590885, AZD6244, PD-173074,
ZM-447439, BIBW2992, temsirolimus, metformin, AZD6482 and gefitinib
(http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/;
visited March 10th, 2014.). The most notable mutational hotspot for
BRAF is p.V600E (c.1799T>A), which accounts for ~90% of the
known cancer-associated mutations (4).
The BRAF V600E mutation is strongly associated with
significantly improved treatment response (5–7).
Therefore, it is considered important to screen for this mutation
prior to selecting a therapeutic strategy. However, it has proven
challenging to determine the status of this mutation in clinical
samples (8,9). A major problem has been that the mutant
cells are typically outnumbered by numerous surrounding wild-type
cells at the tissue sampling site (8,9). A number
of methods have been developed to overcome this problem, including
those based on restriction fragment length polymorphism (RFLP)
analysis (10,11), matrix-assisted laser
desorption/ionization time-of-flight mass spectrometry analysis
(12), ligase chain reaction
(13), suspension array (14), amplification refractory mutation
system polymerase chain reaction (ARMS PCR) (15–19),
allele-specific enzymatic amplification (20), mutant-enriched PCR (11,21),
pyrosequencing (22), coamplification
at lower denaturation temperature PCR (COLD-PCR) (23–27), high
resolution melting (28,29), fluorescent amplicon generation
(30), locked nucleic acid/peptide
nucleic acid clamp PCR (31,32), anti-primer quenching-based
quantitative (qPCR) (33) and
SNaPshot analysis (34–36).
Among these techniques, RFLP and ARMS PCR are widely
used (37,38). Restriction enzymes specifically digest
wild-type alleles, leaving the mutant alleles available for
analysis. This approach has been successfully used to detect
mutations in tumor protein 53, Ras and epithelial growth factor
receptor (11,39–41). ARMS
PCR is based on the principle that extension is efficient when the
3′ terminal base of a primer matches its target, but inefficient or
nonexistent when the 3′ terminal base is mismatched. Therefore,
when primers are designed against the mutation of interest,
amplification proceeds only if the mutation is present (38). This strategy has been successfully
used to screen for point mutations (42–44).
However, RFLP analysis involves a number of post-PCR processing
steps, which may increase the risk for contamination of the PCR
product (10,11), and the usefulness of the ARMS method
may be limited by inefficient amplification due to the abundance of
wild-type alleles (19).
The present study describes a novel method that
combines RFLP analysis and ARMS TaqMan qPCR in a one-step reaction
tube, and suggests the use of this technique, termed ‘RFPL-ARMS
TaqMan PCR,’ to screen clinical melanoma samples for the BRAF V600E
mutation.
Materials and methods
Plasmid construction
Recombinants plasmids encoding wild-type and V600E
mutant BRAF were constructed as described by Board et al
(44). Briefly, corresponding outer
and mutant primers were used to yield half fragments with
complimentary ends using wild type tissue DNA as a template (first
half primer sequences: forward, 5′-CCAGGAGTGCCAAGAGAATA-3′, and
reverse, 5′-CCATCGAGATTTCTCTGTAGCTAGACCA-3′; second half primer
sequences, forward, 5′-TGGTCTAGCTACAGAGAAATCTCGATGG-3′, and
reverse, 5′-TTTCAACAGGGTACACAGAACA-3′), with each half fragment
containing a mutant base. PCR was performed in a 50 µl mixture
containing 5 µl 10X PCR Buffer (Takara Bio Inc., Otsu, Japan), 1.25
U Takara TaqTM polymerase (Takara Bio Inc.), 4 µl dNTP mixture
(Takara Bio Inc.), 0.5 µM primers and 5 µl DNA (Sigma-Aldrich,
Merck KGaA, Darmstadt, Germany). The reaction procedure was as
follows: Initial denaturation at 95°C for 5 min, followed by 35
cycles at 95°C for 15 sec, primer annealing at 53°C for 20 sec, and
72°C for 50 sec, final extension took place at 72°C for 5 min. The
two half fragment products were mixed equally as a template for the
second round PCR. The second round of PCR used inner nested primers
(forward, 5′-AGCATCTTCATTCCAATGAAGAGCC-3′, and reverse,
5′-CATCCACAAAATGGATCCAGACAAC-3′. The second round was performed in
a 50 µl mixture containing 5 µl 10X PCR Buffer (Takara Bio Inc.),
1.25 U Takara TaqTM polymerase (Takara Bio Inc.), 4 µl dNTP mixture
(each 2.5 mM; Takara Bio Inc.,), 0.5 µM primers and 5 µl template.
The thermocycling conditions included initial denaturation at 95°C
for 5 min, followed by 40 cycles of 95°C for 15 sec, 50°C for 30
and 72°C for 50 sec, the final extension took place at 72°C for 5
min. Self-priming of the complementary half fragments and the
subsequent amplification created a final product harboring the
mutant base. The products were ligated into the pMD19 plasmid
(Takara Bio Inc., Otsu, Japan), and recombinants containing mutant
alleles were produced and confirmed by sequencing performed by
Sangon Biotech Co., Ltd., (Shanghai, China). The sequencing machine
used was ABl-PRISM 3,730 (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and the analysis software was
DNASTAR 5.0 (DNASTAR, Inc., Madison, WI, USA). Recombinant plasmid
DNA was extracted using a Tiangen Plasmid DNA kit (Tiangen Biotech
Co., Ltd., Beijing, China). As a positive control, the recombinants
were mixed with an equal amount of human genomic DNA
(Sigma-Aldrich, Merck KGaA, Darmstadt, Germany).
Sample collection and DNA
extraction
In total, 53 patients with melanoma treated at the
Department of Dermatology, The Third Affiliated Hospital of Sun
Yat-sen University (Guangzhou, China), were enrolled in the present
study from March 2011 to December 2012. Unrelated patients
diagnosed with melanoma were included. These samples were all
biopsies. The median age of patients was 68 years (age range 39-84
years). A total of 33 cases (62%) were females, and 20 cases (38%)
were males. A total of 10 patients (19%) exhibited Clark level II
disease (penetration of melanoma into the second layer of the skin,
the dermis) (45), and 18 patients
(34%), 24 patients (45%) and 1 patient (2%) exhibited III, IV and V
stage disease, respectively. The study protocol was approved by the
Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen
University, and written informed consent was obtained from all
patients. DNA was extracted from 10% formalin-fixed that was fixed
at room temperature for 24 h, paraffin-embedded melanoma samples
using a QIAamp DNA FFPE Tissue kit (Qiagen GmbH, Hilden, Germany).
Briefly, formalin-fixed, paraffin-embedded blocks containing the
maximum number of tumor-rich areas from the patients were selected
and sliced into 3 5-µm thick sections. Each section was processed
by proteinase K digestion at 56°C for >16 h, and the obtained
lysate was loaded onto a QIAamp column. Following 2 washes, the DNA
was eluted with 100 µl ddH2O. The extracted DNA was kept
at −20°C until it was used for PCR analysis.
RFLP-ARMS TaqMan PCR-based
genotyping
The RFLP-ARMS TaqMan PCR assay described in the
present study was a one-step PCR that used a mutation-enriching
reaction and ARMS primer genotyping process to selectively
eliminate wild-type genes and detect the mutant alleles. For the
BRAF V600E mutation, a restriction enzyme was used to digest the
wild-type genomic DNA, thereby enriching the mutant allele, and
then a pair of RFLP primers: Forward,
5′-AGCATCTTCATTCCAATGAAGAGCC-3′; and reverse,
5′-CATCCACAAAATGGATCCAGACAAC-3′, designed to amplify a 400–500 bp
fragment containing the mutant allele [melting temperature (Tm),
65°C], were used. The ARMS primers were designed to selectively
amplify a mutant allele with a lower Tm (60°C) with the following
respective sequences: Forward, 5′-TAGGTGATTTTGGTCTAGCTACACA-3′
(mismatched base is underlined); and reverse,
5′-CCACAAAATGGATCCAGACAAC-3′. To improve the specificity of the
ARMS primers, an additional mismatch base was introduced at the
penultimate nucleotide of the mutation site (G was replaced by C),
based on the principles described by Newton et al (19). Mutant allele enrichment and genotyping
were performed in a single tube via a 3-phase reaction: i) 65°C for
30 min, allowing the restriction enzyme, TspRI, to cut the
wild-type DNA; ii) enrichment of the mutant allele with the RFLP
primers and thermocycling conditions of 95°C for 10 min, followed
by 5 cycles of 95°C for 15 sec, 65°C for 20 sec and 72°C for 60
sec; and iii) selective amplification of the mutant allele with the
ARMS primers, and 40 cycles of 95°C for 15 sec and 60°C for 35 sec
(fluorescence collection). During the second phase, the ARMS
primers were unable to bind at the higher temperature (65°C), while
in the third phase, the RFLP primers did not function as the
400–500 bp product was incompletely synthesized during the 35 sec
elongation phase.
The PCR reaction mixtures contained 12.5 µl TaqMan
universal PCR MasterMix (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 0.2 µM probe, 0.25 µM primer
(each), 3 µl DNA, 5 IU TspRI (New England Biolabs, Inc., Ipswich,
MA, USA), and ddH2O to 5 µl. qPCR was performed using an
ABI7300 (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). DNA samples were extracted from all clinical
tissue samples using a QIAamp DNA FFPE Tissue kit (Qiagen GmbH,
Hilden, Germany), and tested using our RFLP-ARMS TaqMan PCR
detection system.
Specificity assay
To determine the specificity of the proposed
RFPL-ARMS TaqMan PCR method, reactions were performed with 2–200 ng
of wild-type genomic DNA (Sigma-Aldrich, Saint Louis, Missouri,
USA) per reaction and assessed the inefficiency caused by extension
from wild-type DNA. An internal control assay was used to assess
the total DNA concentration from 2–200 ng in each sample. The
forward primer was designed to begin at c.1798G; it amplified
wild-type and V600E mutant BRAF using the aforementioned reverse
primer and probe. The change in the threshold cycle (ΔCq)
[ΔCq=(mutation Cq)-(control Cq)] was defined for each sample. The
reactions were performed five times for each DNA concentration, and
each reaction was repeated in triplicate to define a cut-off ΔCq
value (46).
Assessing the detection limits of
RFLP-ARMS TaqMan PCR
The wild-type and mutant plasmid DNA samples were
diluted 10-fold. The effective copy number of plasmids was obtained
by comparing the Cq of the diluted samples with that obtained from
Human Random Control DNA Panels (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) with a known concentration of 100 ng/µl. In a
high-quality DNA sample, there was an average of 1 set genomic DNA
per 3 pg.
To assess the sensitivity of our assay for the BRAF
V600E mutation, it was compared with the ARMS TaqMan PCR protocol
without a removed enzyme digestion step. The mutant-encoding
plasmid (50,000, 5,000, 500, 50 or 5 copies) was mixed with
wild-type genomic DNA (30 ng/µl), corresponding to 80, 8, 0.8, 0.08
and 0.008% mutation rate. For quantification, a standard curve was
generated by plotting the Cq cycle numbers against the log of each
corresponding DNA copy numbers for the known standards. The linear
correlation coefficients (R2) and slopes were calculated
using ABI7300 software (v.1.3; Applied Biosystems; Thermo Fisher
Scientific, Inc.).
Genotyping by DNA sequencing
All samples were re-analyzed by PCR sequencing,
described as following. Kimura et al (47) previously demonstrated that direct
sequencing failed to yield satisfactory results from samples
containing mixtures of wild-type and mutant DNA. In the present
study, the existing mutation-enriched PCR sequencing method was
improved by restriction enzyme selectively cutting wild type
alleles and leaving the mutant alleles enriched, increasing the
mutation rate. This method was adapted for the detection of the
BRAF V600E mutation. This method involved a first PCR amplification
step, enzymatic digestion (to remove the wild-type DNA), a second
PCR step for mutant enrichment and a final sequencing step. PCR was
performed in a total volume of 25 µl containing 12.5 µl 2X Gold
Fast PCR mix (Tiangen Biotech Co., Ltd., Beijing, China), 0.4 µM
each primer (round 1: forward, 5′-AGCATCTTCATTCCAATGAAGAGCC-3′, and
reverse, 5′-CATCCACAAAATGGATCCAGACAAC-3′; round 2: forward,
5′-CATAATGCTTGCTCTGATAGGA-3′, and reverse,
5′-CCACAAAATGGATCCAGACAAC-3′) and 3 µl DNA (for the first round of
PCR) or 3 µl digested product (for the second round of PCR). The
cycling conditions consisted of 95°C for 10 min, followed by 20
(round 1) or 35 (round 2) cycles of 95°C for 15 sec, 58°C for 20
sec and 72°C for 30 sec, with a final extension at 72°C for 7 min
and a final hold at 4°C. Following the first round of PCR,
digestion of the wild-type product was performed in a 50 µl volume
containing 10 µl first-round PCR product, 5 µl 10X CutSmart Buffer
(New England Biolabs, Inc., Ipswich, MA, USA) and 5 IU TspRI (New
England Biolabs, Inc.) at 65°C for 30 min. All of the obtained
second-round PCR products were sequenced by Sangon Biotech Co.,
Ltd. (Shanghai, China). The sequencing machine used was ABl-PRISM
3730 (Applied Biosystems; Thermo Fisher Scientific, Inc.) and the
analysis software was DNASTAR (v.5.0; DNASTAR, Inc.).
Statistical analysis
Fisher's exact test was used to compare the
sensitivity difference of the two methods (RFLP-ARMS TaqMan PCR and
PCR sequencing) in the detection of clinical samples with V600E
mutation rates <5%. P<0.05 was considered to indicate a
statistically significant difference. SPSS for Windows was used
(version 23.0; IBM Corp., Armonk, NY, USA).
Results
Specificity
To assess the specificity of the RFPL-ARMS TaqMan
PCR method, RFLP-ARMS TaqMan PCR was performed using wild-type
genomic DNA (2–200 ng/µl). By testing all the wild-type genomic DNA
(2–200 ng) cases, the cut-off ΔCq value was determined to be 3 Cq
below the lowest ΔCq value observed; the final cut-off ΔCt value
was determined to be 14. The ΔCq values were then calculated as the
difference between the mutant and control Cq values. If the
difference was smaller than the cut-off ΔCt value, the sample was
classified as positive (a larger ΔCq reflected the presence of
fewer mutant alleles). If the difference was larger than the
cut-off point, the sample was classified as mutation-negative or
beyond the limits of detection.
Sensitivity
To determine the minimal detection limit of the
RFPL-ARMS TaqMan PCR method, a mimic human genomic DNA panel
containing the mutant plasmid and normal wild-type human genomic
DNA was used. The results revealed that the mutation-enriched PCR
sequencing method exhibited increased sensitivity compared with
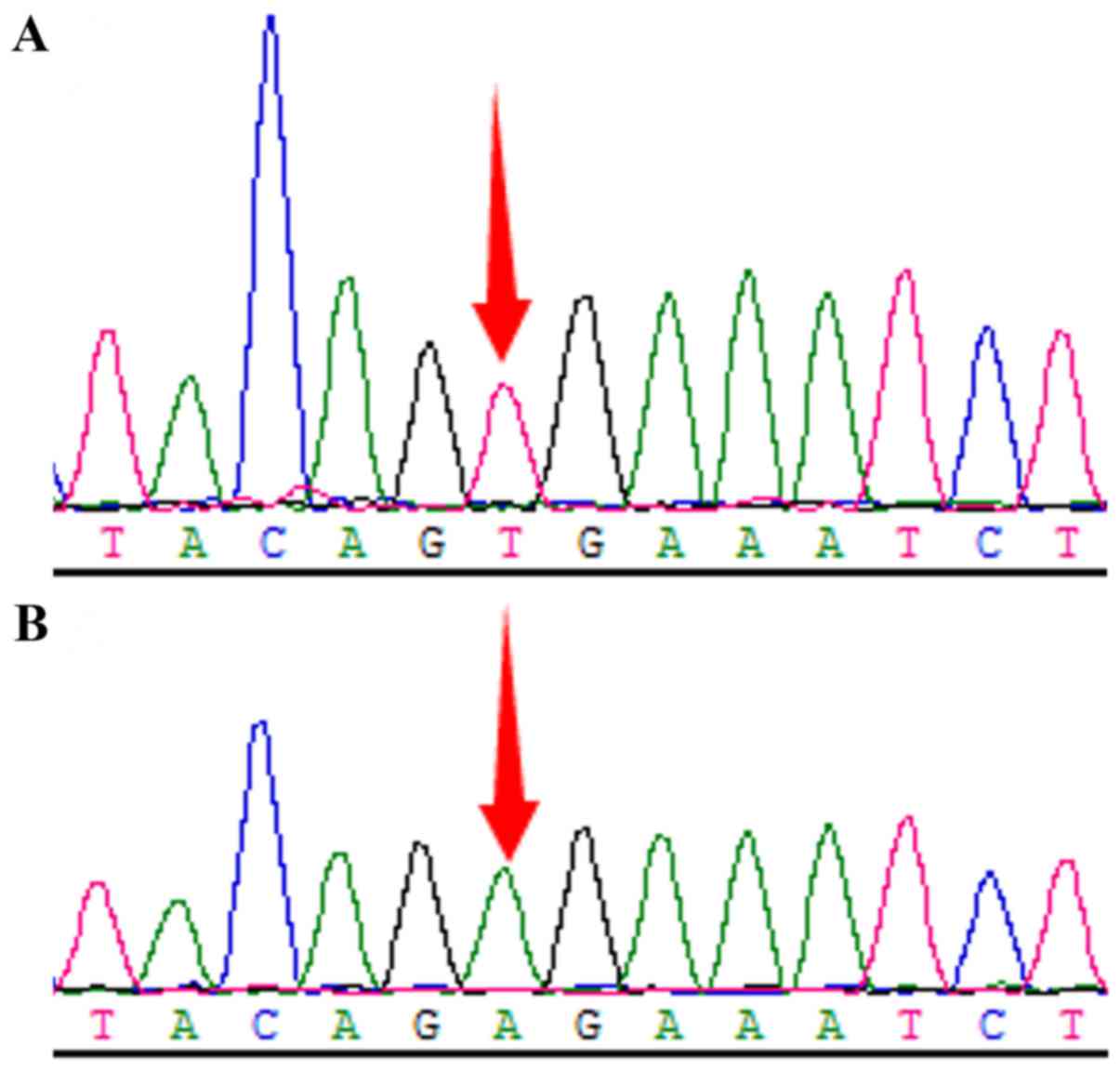
direct PCR sequencing (Fig. 1), and
was able to identify mutations making up ~1% of the total genomic
DNA content. Furthermore, the RFLP-ARMS TaqMan PCR assay was
demonstrated to be a sensitive and practical method to screen for
the BRAF V600E mutation. As indicated in Fig. 2, this RFLP-ARMS TaqMan PCR method
allowed the detection of mutants within mixed samples containing
<0.01% of the V600E mutation (corresponding to <10 copies),
while 0.8% V600E mutations were detected by ARMS TaqMan PCR, but
0.08 and 0.008% gave no amplification signal (Fig. 3).
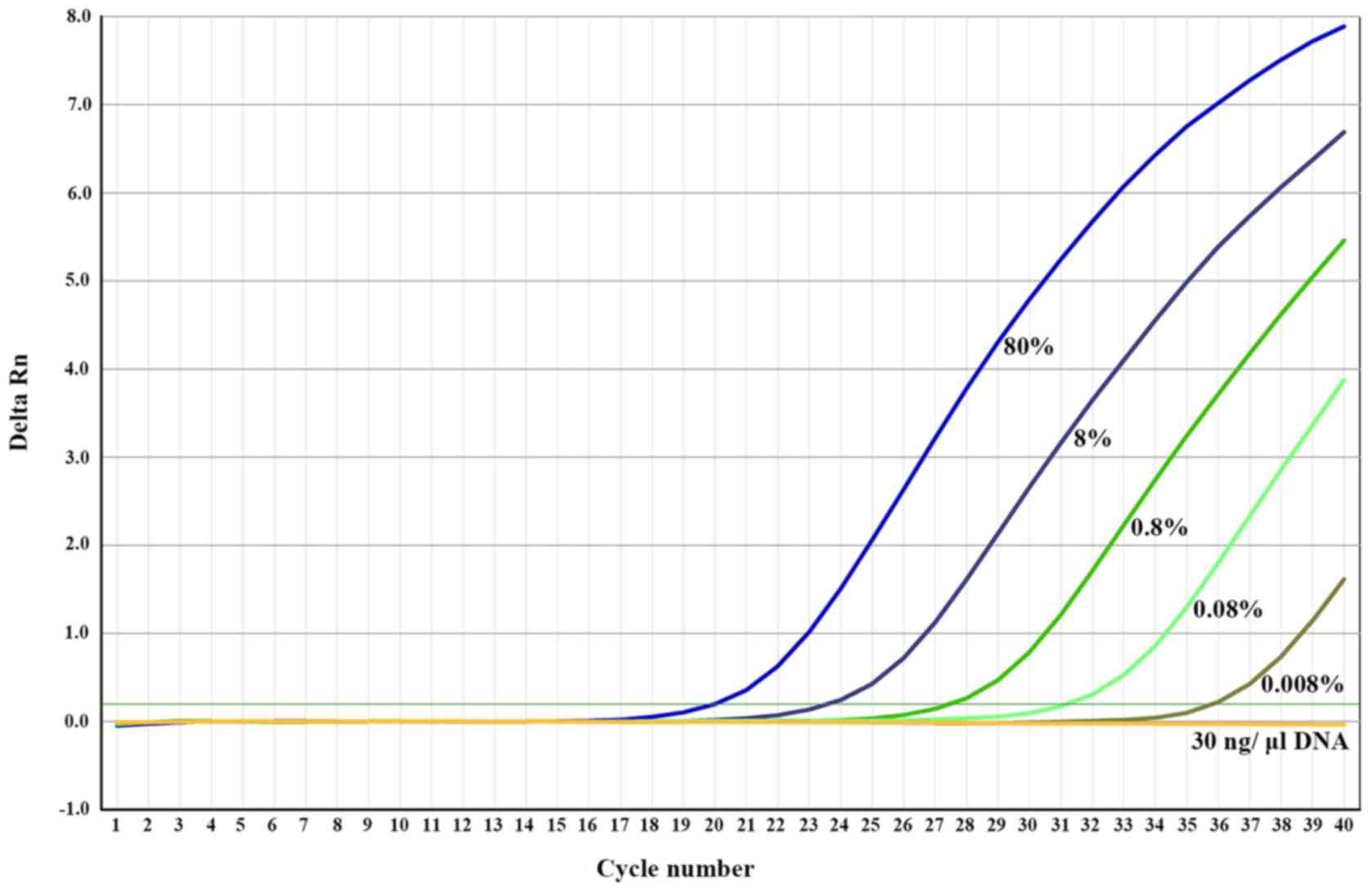 | Figure 2.Sensitivity of the BRAF V600E
mutation detection by RFLP-ARMS quantitative PCR. Mixing the
mutant-encoding plasmid (50,000, 5,000, 500, 50 or 5 copies) with
wild-type genomic DNA (30 ng/µl), corresponding to 80, 8, 0.8, 0.08
and 0.008% mutation rate. RFLP-ARMS, restriction fragment length
polymorphism-amplification refractory mutation system; PCR,
polymerase chain reaction. The horizontal green line represents the
threshold. |
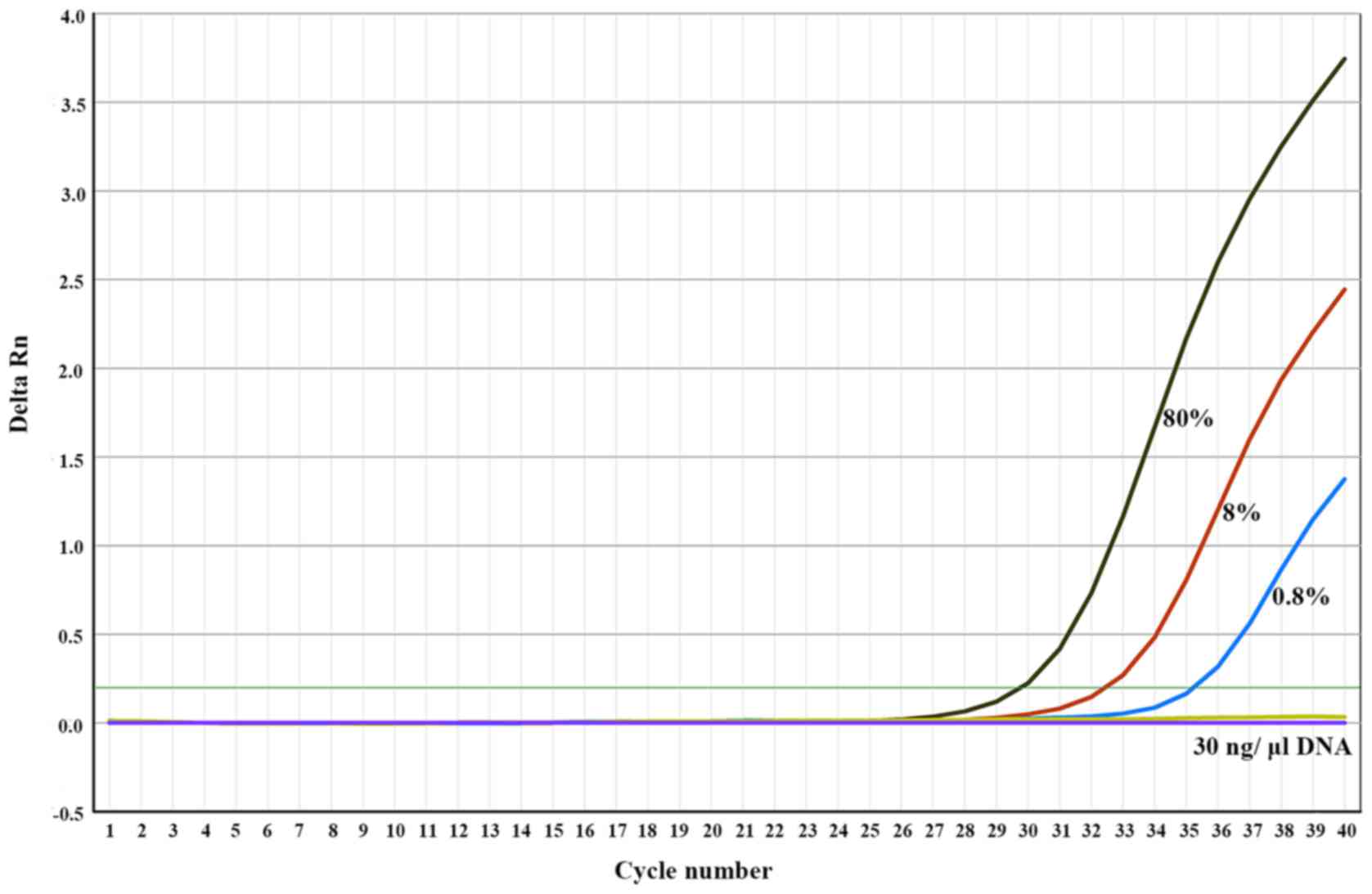 | Figure 3.Sensitivity of the BRAF V600E
mutation detection by ARMS TaqMan PCR. Mixing the mutant-encoding
plasmid (50,000, 5,000, 500, 50 or 5 copies) with wild-type genomic
DNA (30 ng/µl), corresponding to 80, 8, 0.8, 0.08 and 0.008%
mutation rates. AMRS, amplification refractory mutation system;
PCR, polymerase chain reaction. The horizontal green line
represents the threshold. Mutation rates of 0.08 and 0.008% did not
generate an amplified signal. |
Mutation analysis of clinical samples. RFLP-ARMS
TaqMan PCR and PCR sequencing was performed on 53 clinical samples.
Among them, 21 samples were identified to be positive for the BRAF
V600E mutation by RFLP-ARMS TaqMan PCR, while only 18 positive
samples were identified by PCR sequencing (Table I). The three discordant samples were
then analyzed by Droplet Digital PCR (ddPCR; QX200, Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The results indicate that
the mutation frequency of the three samples was 3.8, 1.2 and 0.6%,
respectively, and the results (3.8, 1.2 and 0.6%) indicate that the
samples were positive for the BRAF V600E mutation, which verified
the sensitivity of RFLP-ARMS TaqMan PCR. The result of Fisher's
exact test revealed a significant difference between the two
methods in detecting low-frequency mutations (<5%). Future
experiments will use a larger sample size, which may result in
increased statistical significance.
 | Table I.Comparison of RFLP-ARMS TaqMan PCR
and PCR sequencing for BRAF V600E mutation detection. |
Table I.
Comparison of RFLP-ARMS TaqMan PCR
and PCR sequencing for BRAF V600E mutation detection.
|
| PCR sequencing |
|
|---|
|
|
|
|
|---|
| RFLP-ARMS TaqMan
PCR | + | − | Total |
|---|
| + | 18 | 3 | 21 |
| − | 0 | 32 | 32 |
| Total | 18 | 35 | 53 |
Discussion
For mutation detection, direct sequencing is a
straightforward and commonly-used method (48–50). The
present study described a novel mutation-enriched PCR sequencing
method and indicated that it was able to detect mutant alleles that
represented >10% of the total genomic DNA content. This strategy
is therefore more sensitive compared with direct PCR sequencing,
which has a lower detection limit of 25–30% (10,51). The
mutation-enriched PCR sequencing method of the present study was
more practical compared with direct PCR sequencing of clinical
samples, as it required fewer steps. It is difficult to obtain
homogeneous tumor samples in the clinical setting, and the
sequencing reaction may fail due to an excess of wild-type
sequences (52). Therefore, it is
critical to develop more sensitive genotyping methods.
Furthermore, the present study described a second
novel method that combines a modified RFLP analysis and ARMS TaqMan
qPCR to screen for the BRAF V600E mutation without any post-PCR
processing. In our previous investigations, we found that when we
introduced an additional mismatch at the 3′-end, there was a marked
decrease (The Cq value increased) in the sensitivity of the ARMS
qPCR method (unpublished). In the present study, the RFLP primers
were introduced into the ARMS TaqMan qPCR to improve the
sensitivity. In contrast to general mutation-enriched PCR, a
restriction enzyme was used to digest the wild-type DNA, enhancing
the proportion of mutant alleles prior to the PCR amplification
step. This protocol has the following advantages: Firstly, the
restriction enzyme digestion step enhances the specificity by
removing the wild-type genomic DNA and enriching the mutant allele;
secondly, the sensitivity was additionally improved by using RFLP
primers with a Tm that was higher (by 5°C) compared with that of
the ARMS primers, which amplified a longer fragment; thirdly, the
digestion and PCR reactions were performed in a single tube,
avoiding the requirement for any post-PCR processing and decreasing
the risk of PCR product contamination; finally, the RFLP and ARMS
PCR steps were independent reactions. Together, these benefits
ensure that the RFLP-ARMS TaqMan PCR assay described in the present
is simple to use and amenable to high-throughput operation.
The results also revealed that the RFLP-ARMS TaqMan
PCR assay was able to detect 0.1% mutant alleles in a background of
~20 copies of total genomic DNA. The sensitivity and selectivity
were significantly higher compared with those achieved by the
existing sequencing-based methods. Using this method, 53 melanoma
samples were successfully screened for the BRAF V600E mutation. The
RFLP-ARMS TaqMan PCR method of the present study identified 21
mutation-positive samples. A total of 18 of these samples were
identified by direct PCR sequencing, indicating the high potential
of the protocol. The mutation frequency of the three discordant
samples was analyzed, which verified that the more sensitive qPCR
method was able to detect mutations in samples containing only a
small proportion of mutant alleles.
In summary, the novel RFLP-ARMS TaqMan PCR protocol
described in the present study offers a means to improve the
sensitivity and specificity of mutation detection, and may be a
promising method for screening mutant alleles in cancer samples
that contain relatively few mutant cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
High-tech R&D Program of China (863 program; grant no.
2011AA02A115).
Availability of data and materials
All datasets generated or analyzed in the present
study are included in this published article.
Authors' contributions
YZ, WL and SCY collected tissue samples and wrote
the article. SQ, TY and JH contributed to study design, the
majority of the experiments, data analysis and article-writing. JZ,
LG, XH and WC participated in the study design, performed the
Droplet Digital PCR experiment, data analysis and interpretation
and revised the manuscript.
Ethics and consent to participate
The study protocol was approved by the Ethics
Committee of The Third Affiliated Hospital of Sun Yat-sen
University, and written informed consent was obtained from all
patients.
Consent for publication
All the study participants have approved the
publication of this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greenman C, Stephens P, Smith R, Dalgliesh
GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C,
et al: Patterns of somatic mutation in human cancer genomes.
Nature. 446:153–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garnett MJ and Marais R: Guilty as
charged; B-RAF is a human oncogene. Cancer Cell. 6:313–319. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wan PT, Garnett MJ, Roe SM, Lee S,
Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ,
Barford D, et al: Mechanism of activation of the RAF-ERK signaling
pathway by oncogenic mutations of B-RAF. Cell. 116:855–867. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartsough EJ, Basile KJ and Aplin AE:
Beneficial effects of RAF inhibitor in mutant BRAF splice
variant-expressing melanoma. Mol Cancer Res. 12:795–802. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dzienis MR and Atkinson VG: Response rate
to vemurafenib in patients with BRAF-positive melanoma brain
metastases: A retrospective review. Melanoma Res. 24:349–353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le K, Blomain ES, Rodeck U and Aplin AE:
Selective RAF inhibitor impairs ERK1/2 phosphorylation and growth
in mutant NRAS, vemurafenib-resistant melanoma cells. Pigment Cell
Melanoma Res. 26:509–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang T, Zhuge J and Zhang WW: Sensitive
detection of BRAF V600E mutation by Amplification Refractory
Mutation System (ARMS)-PCR. Biomark Res. 1:32013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen D, Huang JF, Xia H, Duan GJ, Chuai
ZR, Yang Z, Fu WL and Huang Q: High-sensitivity PCR method for
detecting BRAF V600E mutations in metastatic colorectal cancer
using LNA/DNA chimeras to block wild-type alleles. Anal Bioanal
Chem. 406:2477–2487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Zhao Y, Wang M, Yap WS and Chang
AY: Detection and comparison of epidermal growth factor receptor
mutations in cells and fluid of malignant pleural effusion in
non-small cell lung cancer. Lung Cancer. 60:175–182. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asano H, Toyooka S, Tokumo M, Ichimura K,
Aoe K, Ito S, Tsukuda K, Ouchida M, Aoe M, Katayama H, et al:
Detection of EGFR gene mutation in lung cancer by mutant-enriched
polymerase chain reaction assay. Clin Cancer Res. 12:43–48. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikryannikova LN, Afanas'ev MV, Akopian TA,
Il'ina EN, Kuz'min AV, Larionova EE, Smirnova TG, Chernousova LN
and Govorun VM: Mass-spectrometry based minisequencing method for
the rapid detection of drug resistance in Mycobacterium
tuberculosis. J Microbiol Methods. 70:395–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turner DJ, Zirvi MA, Barany F, Elenitsas R
and Seykora J: Detection of the BRAF V600E mutation in melanocytic
lesions using the ligase detection reaction. J Cutan Pathol.
32:334–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye Y, Wang D, Su C, Rong T and Guo A:
Combined detection of p53, p16, Rb, and EGFR mutations in lung
cancer by suspension microarray. Genet Mol Res. 8:1509–1518. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whitcombe D, Theaker J, Guy SP, Brown T
and Little S: Detection of PCR products using self-probing
amplicons and fluorescence. Nat Biotechnol. 17:804–807. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mattarucchi E, Marsoni M, Binelli G, Passi
A, Lo Curto F, Pasquali F and Porta G: Different real time PCR
approaches for the fine quantification of SNP's alleles in DNA
pools: Assays development, characterization and pre-validation. J
Biochem Mol Biol. 38:555–562. 2005.PubMed/NCBI
|
|
17
|
Wolstencroft EC, Hanlon K, Harries LW,
Standen GR, Sternberg A and Ellard S: Development of a quantitative
real-time polymerase chain reaction assay for the detection of the
JAK2 V617F mutation. J Mol Diagn. 9:42–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thelwell N, Millington S, Solinas A, Booth
J and Brown T: Mode of action and application of Scorpion primers
to mutation detection. Nucleic Acids Res. 28:3752–3761. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Newton CR, Graham A, Heptinstall LE,
Powell SJ, Summers C, Kalsheker N, Smith JC and Markham AF:
Analysis of any point mutation in DNA. The amplification refractory
mutation system (ARMS). Nucleic Acids Res. 17:2503–2516. 1989.
|
|
20
|
Jarry A, Masson D, Cassagnau E, Parois S,
Laboisse C and Denis MG: Real-time allele-specific amplification
for sensitive detection of the BRAF mutation V600E. Mol Cell
Probes. 18:349–352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Es JM, Polak MM, van den Berg FM,
Ramsoekh TB, Craanen ME, Hruban RH and Offerhaus GJ: Molecular
markers for diagnostic cytology of neoplasms in the head region of
the pancreas: Mutation of K-ras and overexpression of the p53
protein product. J Clin Pathol. 48:218–222. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan YH, Liu Y, Eu KW, Ang PW, Li WQ,
Salto-Tellez M, Iacopetta B and Soong R: Detection of BRAF V600E
mutation by pyrosequencing. Pathology. 40:295–298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Wang L, Jänne PA and Makrigiorgos
GM: Coamplification at lower denaturation temperature-PCR increases
mutation-detection selectivity of TaqMan-based real-time PCR. Clin
Chem. 55:748–756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Wang L, Mamon H, Kulke MH, Berbeco R
and Makrigiorgos GM: Replacing PCR with COLD-PCR enriches variant
DNA sequences and redefines the sensitivity of genetic testing. Nat
Med. 14:579–584. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Milbury CA, Correll M, Quackenbush J,
Rubio R and Makrigiorgos GM: COLD-PCR enrichment of rare cancer
mutations prior to targeted amplicon resequencing. Clin Chem.
58:580–589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pritchard CC, Akagi L, Reddy PL, Joseph L
and Tait JF: COLD-PCR enhanced melting curve analysis improves
diagnostic accuracy for KRAS mutations in colorectal carcinoma. BMC
Clin Pathol. 10:62010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zuo Z, Chen SS, Chandra PK, Galbincea JM,
Soape M, Doan S, Barkoh BA, Koeppen H, Medeiros LJ and Luthra R:
Application of COLD-PCR for improved detection of KRAS mutations in
clinical samples. Mod Pathol. 22:1023–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Montgomery J, Wittwer CT, Palais R and
Zhou L: Simultaneous mutation scanning and genotyping by
high-resolution DNA melting analysis. Nat Protoc. 2:59–66. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou L, Wang L, Palais R, Pryor R and
Wittwer CT: High-resolution DNA melting analysis for simultaneous
mutation scanning and genotyping in solution. Clin Chem.
51:1770–1777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amicarelli G, Shehi E, Makrigiorgos GM and
Adlerstein D: FLAG assay as a novel method for real-time signal
generation during PCR: Application to detection and genotyping of
KRAS codon 12 mutations. Nucleic Acids Res. 35:e1312007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Efrati E, Elkin H, Peerless Y, Sabo E,
Ben-Izhak O and Hershkovitz D: LNA-based PCR clamping enrichment
assay for the identification of KRAS mutations. Cancer Biomark.
8:89–94. 2010-2011. View Article : Google Scholar
|
|
32
|
Kobunai T, Watanabe T, Yamamoto Y and
Eshima K: The frequency of KRAS mutation detection in human colon
carcinoma is influenced by the sensitivity of assay methodology: A
comparison between direct sequencing and real-time PCR. Biochem
Biophys Res Commun. 395:158–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Wang F, Mamon H, Kulke MH, Harris L,
Maher E, Wang L and Makrigiorgos GM: Antiprimer quenching-based
real-time PCR and its application to the analysis of clinical
cancer samples. Clin Chem. 52:624–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fariña Sarasqueta A, Moerland E, de Bruyne
H, de Graaf H, Vrancken T, van Lijnschoten G and van den Brule AJ:
SNaPshot and StripAssay as valuable alternatives to direct
sequencing for KRAS mutation detection in colon cancer routine
diagnostics. J Mol Diagn. 13:199–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Magnin S, Viel E, Baraquin A,
Valmary-Degano S, Kantelip B, Pretet JL, Mougin C, Bigand M,
Girardo B, Borg C and Ferrand C: A multiplex SNaPshot assay as a
rapid method for detecting KRAS and BRAF mutations in advanced
colorectal cancers. J Mol Diagn. 13:485–492. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zinsky R, Bölükbas S, Bartsch H, Schirren
J and Fisseler-Eckhoff A: Analysis of KRAS mutations of exon 2
Codons 12 and 13 by SNaPshot analysis in comparison to common DNA
sequencing. Gastroenterol Res Pract. 2010:7893632010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grau O and Griffais R: Diagnosis of
mutations by the PCR double RFLP method (PCR-dRFLP). Nucleic Acids
Res. 22:5773–5774. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Newton CR, Graham A, Heptinstall LE,
Powell SJ, Summers C, Kalshekerl N, Smith JC and Markham AF:
Analysis of any point mutation in DNA. The amplification refractory
mutation system (ARMS). Nucleic Acids Res. 17:2503–2516. 1989.
|
|
39
|
Toyooka S, Tsukuda K, Ouchida M, Tanino M,
Inaki Y, Kobayashi K, Yano M, Soh J, Kobatake T, Shimizu N and
Shimizu K: Detection of codon 61 point mutations of the K-ras gene
in lung and colorectal cancers by enriched PCR. Oncol Rep.
10:1455–1459. 2003.PubMed/NCBI
|
|
40
|
Behn M, Qun S, Pankow W, Havemann K and
Schuermann M: Frequent detection of ras and p53 mutations in brush
cytology samples from lung cancer patients by a restriction
fragment length polymorphism-based ‘enriched PCR’ technique. Clin
Cancer Res. 4:361–371. 1998.PubMed/NCBI
|
|
41
|
Behn M and Schuermann M: Sensitive
detection of p53 gene mutations by a ‘mutant enriched’ PCR-SSCP
technique. Nucleic Acids Res. 26:1356–1358. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scobie GA, Ho ST, Dolan G and Kalsheker
NA: Development of a rapid DNA screening procedure for the Factor V
Leiden mutation. Clin Mol Pathol. 49:M361–M363. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bai RK and Wong LJ: Detection and
quantification of heteroplasmic mutant mitochondrial DNA by
real-time amplification refractory mutation system quantitative PCR
analysis: A single-step approach. Clin Chem. 50:996–1001. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Board RE, Thelwell NJ, Ravetto PF, Little
S, Ranson M, Dive C, Hughes A and Whitcombe D: Multiplexed assays
for detection of mutations in PIK3CA. Clin Chem. 54:757–760. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Marghoob AA, Koenig K, Bittencourt FV,
Kopf AW and Bart RS: Breslow thickness and clark level in melanoma:
Uupport for including level in pathology reports and in American
Joint Committee on Cancer Staging. Cancer. 88:589–595. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kimura H, Kasahara K, Kawaishi M, Kunitoh
H, Tamura T, Holloway B and Nishio K: Detection of epidermal growth
factor receptor mutations in serum as a predictor of the response
to gefitinib in patients with non-small-cell lung cancer. Clin
Cancer Res. 12:3915–3921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kalikaki A, Koutsopoulos A, Hatzidaki D,
Trypaki M, Kontopodis E, Stathopoulos E, Mavroudis D, Georgoulias V
and Voutsina A: Clinical outcome of patients with non-small cell
lung cancer receiving front-line chemotherapy according to EGFR and
K-RAS mutation status. Lung Cancer. 69:110–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Uruga H, Kishi K, Fujii T, Beika Y,
Enomoto T, Takaya H, Miyamoto A, Morokawa N, Kurosaki A and
Yoshimura K: Efficacy of gefitinib for elderly patients with
advanced non-small cell lung cancer harboring epidermal growth
factor receptor gene mutations: A retrospective analysis. Intern
Med. 49:103–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tamura K, Okamoto I, Kashii T, Negoro S,
Hirashima T, Kudoh S, Ichinose Y, Ebi N, Shibata K, Nishimura T, et
al: Multicentre prospective phase II trial of gefitinib for
advanced non-small cell lung cancer with epidermal growth factor
receptor mutations: Results of the West Japan Thoracic Oncology
Group trial (WJTOG0403). Br J Cancer. 98:907–914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Endo K, Konishi A, Sasaki H, Takada M,
Tanaka H, Okumura M, Kawahara M, Sugiura H, Kuwabara Y, Fukai I, et
al: Epidermal growth factor receptor gene mutation in non-small
cell lung cancer using highly sensitive and fast TaqMan PCR assay.
Lung Cancer. 50:375–384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao J, Xie F, Zhong W, Wu W, Qu S, Gao S,
Liu L, Zhao J, Wang M, Zhou J, et al: Restriction
endonuclease-mediated real-time digestion-PCR for somatic mutation
detection. Int J Cancer. 132:2858–2866. 2013. View Article : Google Scholar : PubMed/NCBI
|