Introduction
Bladder cancer (BC) is the ninth most common
malignancy worldwide and the most common cancer involving the
urinary system. BC comprises approximately 2% of all cancers, with
about 330,000 new BC cases each year globally (1,2). The
highest incidence rates are observed in highly developed countries,
where BC is the fourth and 14th most common cancer in men and
women, respectively (2). Despite
ongoing effort to improve therapeutic options, there is still no
curative therapy available for metastatic disease. The pre-clinical
development of new treatments requires an animal model that
accurately simulates the disease in people. Rodent animal models
with experimentally induced bladder cancer have been shown to be
valuable, but their translational relevance has been limited
(3–5).
Therefore, well characterized spontaneous bladder cancers in
companion animals could represent a useful tool to advance the
development of improved diagnostics and therapeutics. Indeed dogs
develop spontaneous BC, more specifically urothelial carcinoma
(transitional cell carcinoma), with striking clinical,
histopathological and molecular similarities compared to humans
(6). Furthermore, companion animals
and their owners share the environment, including exposure to
carcinogenic components. In addition, other advantages of using
spontaneous urinary bladder cancer in pet dogs as an animal model
for human UC are: i) reduced use of research animals, ii) the
shorter lifespan of dogs, relative to people, makes time-efficient
clinical studies possible and iii) dogs show a similar response to
therapy used to treat people (7–11). Yet one
limitation to the use of the dog in preclinical studies of BC has
been access to robust clinical and pathologic datasets. For this
reason, the present study, which is one of the largest yet
undertaken comparative studies, aims to evaluate the dog as animal
model for human urothelial carcinoma by comparing the epidemiology
and histology of n=260 canine urinary bladder and urethral
urothelial carcinoma to the data available in literature regarding
their human counterpart.
Materials and methods
The present study was reviewed and approved by the
University of Nottingham's ethical review committee. Informed
written consent was obtained from the dog owners upon sample
submission. All patient data has been anonymized. The present
retrospective study includes archived formalin-fixed
paraffin-embedded (FFPE) tissues from 260 dogs with primary
urethral (n=61) or urinary bladder (n=199) carcinomas. All samples
were taken from pet dogs living in the United Kingdom (UK) and were
originally submitted for diagnostic purposes. Two hundred
fifty-nine cases were provided by a private diagnostic pathology
laboratory (Bridge Pathology Ltd., Bristol, UK) which received the
tissue samples between October 2008 and April 2015. One additional
case of canine urinary bladder carcinoma which was submitted to the
University Nottingham Veterinary Pathology Service in May 2016 was
also included. Non-invasive urothelial lesions, including
papillomas, were excluded from the study due to the low number of
cases in the present dog population. Tissue of canine in
situ UC was not available. From the originally retrieved n=265
cases of UC, two cases were excluded due to poor tissue section
quality, another two cases due to lack of convincing neoplastic
features, and one case due to lack of information about dog breed
and age. The following case information was available: Age at the
time of first tumor diagnosis, sex, neutering status, and dog
breed. All canine cases (n=93,862) submitted to the same laboratory
within the same time period were used as control population. These
control dogs were diagnosed with a wide range of neoplastic and
non-neoplastic diseases in various organs and tissues, excluding
carcinoma of the lower urinary tract. Haematoxylin and eosin
stained tissue sections of all 260 cases were available which were
digitalized (scanner 3DHISTECH Pannoramic 250 Flash III) and
assessed using the software 3DHISTECH Case Viewer by a
board-certified veterinary pathologist (SdB), with support of a
certified human uropathologist (BR) and a second veterinary
pathologist (LGR). All cases were histologically assessed for tumor
stage and histological subtype, based on the World Health
Organization (WHO) tumor classification system 2016 (12). Subgross tumor growth patterns were
evaluated histologically at a low magnification (1 and 2×).
Epidemiological and pathological canine data was compared with data
available from scientific human literature which was available on
PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) in April 2017.
Cancer incidence was calculated in percentage (%) for each dog
breed (n=60 different breeds). 100% cancer incidence was defined as
the total number of dogs of the same breed in the control
population. Breeds with incidence rates above average (>0.823%)
and with a minimum of n=5 cancer cases were defined as risk breeds.
Three different breeds (Scottish terrier, Shetland sheepdog and
West Highland White terrier) fulfilled these criteria and were
grouped together as ‘risk breeds’. ‘Non-risk dog breeds’ were
defined as all dog breeds, including cross breeds, excluding
Scottish terriers, Shetland Sheepdogs, and West Highland White
terriers. Odds ratios were calculated in order to quantify the risk
of dog breed, sex, neutering status with the development of cancer,
and in order to quantify the risk of non-papillary tumor growth
with muscle-invasive tumor growth. An independent t-test was
performed to compare the mean age of risk breeds vs. non-risk
breeds, of dogs with muscle-invasive vs. non muscle-invasive tumor
growth, and of dogs with papillary vs. non-papillary tumor growth,
respectively. Chi-square test was used to test for associations
between tumor incidence and dog breed, sex, and neutering status,
and to analyze the association of tumor invasive growth with tumor
growth pattern, dog breed, and sex. Binary logistic regression
analysis was performed in order to identify and weigh risk factors,
including dog breed, sex, and neutering. P<0.05 was considered
to indicate a statistically significant difference. Statistical
analyses were performed using SPSS v.22.0 (IBM Corp., Armonk, NY,
USA).
Results
A total of 260 dogs with spontaneous urinary bladder
(199/260, 77%) and primary urethral (61/260, 23%) urothelial
carcinoma (UC) were included in this study. Tables I and II summarize the main canine epidemiological
findings and compare them with the corresponding human data
available in the literature.
 | Table I.Risk factors for urothelial carcinoma
in the studied dog population. |
Table I.
Risk factors for urothelial carcinoma
in the studied dog population.
| Variables | Absolute nos. | Prevalence (%) | Univariate OR | 95% CI | P-value | Multivariate
OR | 95% CI | P-value |
|---|
| Female sex |
|
| 3.51 | 2.57–4.79 | <0.001 | 2.92 | 2.17–3.91 | <0.001 |
| With
UC | 170 | 77 |
|
|
|
|
|
|
| Without
UC | 31,243 | 48 |
|
|
|
|
|
|
| Neutering |
|
| 4.57 | 1.87–11.12 | <0.001 | 3.75 | 1.85–7.60 | <0.001 |
| With
UC | 166 | 75 |
|
|
|
|
|
|
| Without
UC | 37,054 | 88a |
|
|
|
|
|
|
| Breed |
|
|
|
|
|
|
|
|
|
Scottish terrier |
|
| 15.11 | 8.99–25.41 | <0.001 | 19.51 | 11.48–33.16 | <0.001 |
| With
UC | 16 | 7 |
|
|
|
|
|
|
| Without
UC | 338 | 0.5 |
|
|
|
|
|
|
|
Shetland sheepdog |
|
| 6.82 | 3.01–15.49 | <0.001 | 12.33 | 5.35–28.40 | <0.001 |
| With
UC | 6 | 3 |
|
|
|
|
|
|
| Without
UC | 268 | 0.4 |
|
|
|
|
|
|
| West
Highland |
|
| 2.79 | 1.80–4.35 | <0.001 | 3.30 | 2.03–5.36 | <0.001 |
| white
terrier |
|
|
|
|
|
|
|
|
| With
UC | 22 | 10 |
|
|
|
|
|
|
| Without
UC | 2,504 | 4 |
|
|
|
|
|
|
 | Table II.Comparison of epidemiological factors
of canine and human urothelial carcinoma. |
Table II.
Comparison of epidemiological factors
of canine and human urothelial carcinoma.
| Variables | Canine UC (Present
study) | Human UC
(Literature) | Significance |
|---|
| Age | Mean: 10.22 years
(equivalent to 60–70 human years) | Mean: 69–71 years.
[Ref: (36)] | Mean age at UC
diagnosis is comparable between people and dogs |
| Dog
breed/ethnicity | Three breeds are
highly predisposed to develop UC: | White people are
predisposed for | Certain human
ethnic groups and dog breeds |
|
| 1) Scottish terrier
(OR 15.11) | UC (2-fold risk).
Black people are | have a high
predisposition to develop UC. |
|
| 2) Shetland
sheepdog (OR 6.82) | associated with
higher-stage UC and | At risk dog breeds
with UC are significantly |
|
| 3) West Highland
White terrier (OR 2.79) | reduced survival.
[Refs: (37–39)] | younger at the time
of diagnosis compared with non-risk breed dogs with UC |
| Sex | Incidence: Strong
female predominance (OR 3.51). 195/260 (75%) females, 65/260 (25%)
males | Incidence: Strong
male predominance (75%). Ratio male:female=3-4:1 | In contrast to
people, female dogs have an >3 times higher risk to develop UC
compared with males |
|
| Prognosis:
Generally very poor (68% muscle-invasive at first tumor
diagnosis) | Prognosis: Women
have poorer survival rate (56% 5-year survival) compared to men
(68% 5-year survival). [Refs: (1,2,40)] | Female sex is
associated with poor prognosis in human and canine patients |
| Surgical castration
(neutering) | Females: 149/195
(77%) neutered; 3/195 (2%) entire; 43/195 (22%) unknown | Sex hormones and
their receptors serve a crucial role in UC development and
progression. | Surgically neutered
dogs have a four times higher risk to develop UC compared with
entire dogs. Surgical castration in women and female |
|
| Males: 41/65 (63%)
neutered; 4/65 (6%) entire; 20/65 (31%) unknown | Surgical castration
in women increases the risk of developing UC. [Refs: (22,23,41)] | dogs is a risk
factor for UC development. Note: Surgical castration is a routine
procedure in |
|
| Control population:
51,190/93,863 (55%) neutered; 7,632/93,863 (8%) entire;
35,041/93,863 (37%) unknown |
| dogs (55% of our
control dogs are neutered) |
The mean age of dogs diagnosed with UC was older
compared to dogs without UC (10.21 [+/-2.0, range 4–15] years vs.
7.1 [+/-3.6] (mean +/-SD, P<0.001). Dog breeds with only 1 case
of UC were excluded from any further analysis (leaving 66,317
cases; 222 (0.3%) with UC). In univariate analyses [odds ratio (95%
confidence interval), P<0.001], female sex [OR, 3.51
(2.57–4.79)], neutering [OR, 4.57 (1.87–11.12)] and breed (OR 15.11
[8.97–25.41] for Scottish terriers, OR 6.82 [3.01–15.49] for
Shetland sheepdogs, and OR 2.79 [1.80–4.35] for West Highland white
terriers) were identified as significant predictors of UC (Table I). All remained significant
(P<0.001) in multivariate analyses (Table I). In addition, when the three top
risk breed dogs were grouped together, they were found to be
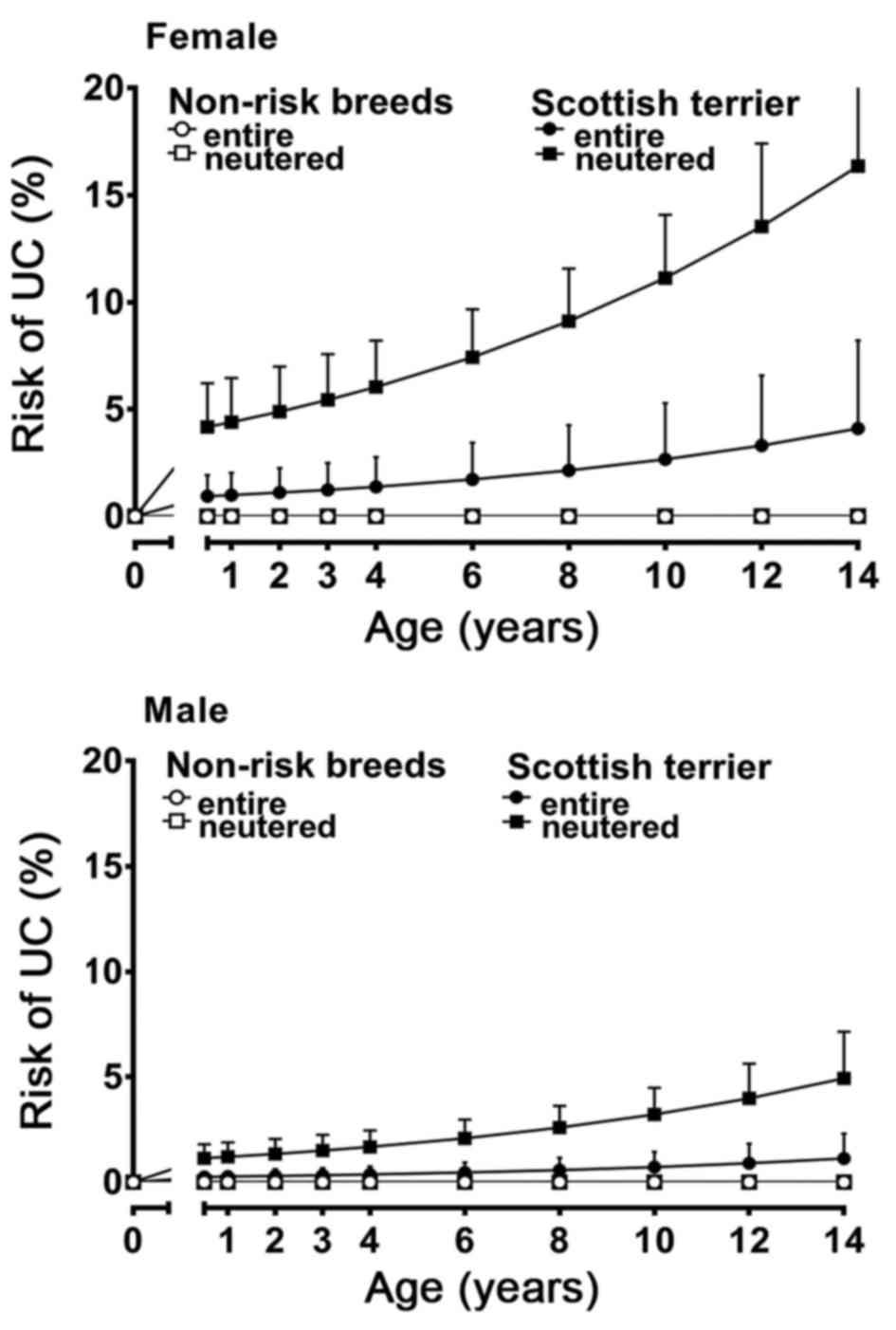
diagnosed with UC at a significantly younger age [mean 112.14
months (9.35 years), SD 19.16] compared to the group containing
‘non-risk dog breeds’ [mean 124.69 months (10.39 years), SD 24.74]
(P<0.001). This effect was most evident for female neutered
Scottish terriers (Fig. 1). ‘Non-risk
dog breeds’ were defined as all dog breeds, including cross breeds,
excluding Scottish terriers, Shetland Sheepdogs, and West Highland
White terriers.
All cases were histologically assessed for tumor
stage and histological subtype, based on the World Health
Organization (WHO) tumor classification system 2016 (12,13). Tumor
location, growth pattern and main histological features of the
studied dogs are summarized in Table
III together with the equivalent information extracted from the
human literature.
 | Table III.Comparison of gross and
histopathological features of canine and human urothelial
carcinoma. |
Table III.
Comparison of gross and
histopathological features of canine and human urothelial
carcinoma.
| Variables | Canine UC (Present
study) | Human UC
(Literature) | Significance |
|---|
|
Localization/subsite | 191/260 (74%)
bladder; 61/260 (23%) | Bladder UC is more
common than urethral | UC is more common
in urinary bladder than urethra in |
|
| urethra; 8/260 (3%)
bladder with extension | UC (2% versus
<1% of all malignancies) | both people and
dogs |
|
| into proximal
urethra |
|
|
|
| 14/29 (48.28%)
bladder neck, 9/29 | Bladder: Bladder
neck and trigone | Bladder neck
involvement is associated with poor |
|
| (27.59%) trigone,
3/29 (10.34%) | involvement is
associated with higher tumor | prognosis |
|
| cranial
bladder/dome, 2/29 (6.90%) | grade and worse
prognosis |
|
|
| bifocal (bladder
neck and cranial), 1/29 |
|
|
|
| (3.45%) ventral
bladder, 1/29 (3.45%) dorsal |
|
|
|
| 8/10 (80%) distal
urethra | Urethra: Mostly
proximal location in men and | Urethral UC is more
common in distal portion in |
|
| 2/10 (20%) proximal
urethra | distal in women.
[Refs: (29–31,42–50)] | women and female
dogs |
| Growth pattern | Papillary: 33/66
(50%); T1 (n=22), | Non-papillary UC
are more likely to grow | Non-papillary
growth is associated with higher tumor |
|
| T2a (n=9) | more invasive (74%
muscle-invasive) | grade, muscle
invasion and poor prognosis in both |
|
| Non-papillary
(flat): | compared to
papillary UC | people and
dogs |
|
| 33/66 (50%); T1
(n=2), T2a (n=9), | [Refs: (30,33,51,52)] |
|
|
| T2b (n=6), T3
(n=13); Non-papillary UC |
|
|
|
| are >30 times
more likely to be |
|
|
|
| muscle-invasive
compared to papillary |
|
|
|
| UC (OR 31.00) |
|
|
| Muscle
invasion | Muscle-invasive:
52/76 (68%). | Women suffer from
advanced tumor stages | Tumor grade is
typically higher in canines compared |
|
| − 30/52 (58%)
non-papillary (flat) | compared to men
[Refs: (40,53,54)] | with human UC. High
grade, muscle-invasive UC is |
|
| − 11/52 (21%)
papillary |
| more common in
female than in male in both human |
|
| − 11/52 (21%)
unknown |
| and canine
patients. |
|
|
Non-muscle-invasive: 24/76 (32%). |
|
|
|
| − 22/24 (92%)
papillary |
|
|
|
| − 2/24 (8%)
non-papillary (flat) |
|
|
| Tumor stage at
first | 24/64 (38%) T1 | At the time of
first tumor diagnosis, the | At first tumor
diagnosis, tumor stage is typically |
| diagnosis | 18/64 (28%)
T2a | majority (70–80%)
of UC are non-invasive | higher in canine
compared to human UC |
|
| 8/64 (13%) T2b | or early invasive
[Refs: (1,53,55)] |
|
|
| 14/64 (22%) T3 |
|
|
| Tumor
classification | 234/260 (90%)
UC | 90% UC | UC is the most
common type of bladder cancer in both |
|
| 15/260 (6%) UC with
divergent | 5% SCC and
adenocarcinoma | people and dogs
with comparable frequencies. |
|
|
differentiation | 5% other [Ref:
(55)] | Tumors with
squamous or glandular differentiation |
|
| 5/260 (2%) SCC |
| are the second most
common type of urothelial |
|
| 1/260 (0.4%)
adenocarcinoma |
| tumors in both
people and dogs. Other variants of UC |
|
| 1/260 (0.4%)
plasmacytoid UC |
| are rare in both
species |
|
| 1/260 (0.4%)
neuroendocrine tumor |
|
|
Gross tumor location was available for 29/199 (15%)
bladder and 10/61 (16%) urethral UC cases. The most common location
within the bladder was the neck (14/29, 48%), followed by the
trigone (9/29, 28%), cranial (3/29 10%), ventral (1/29, 4%) and
dorsal (1/29, 4%) bladder. In 2/29 cases (7%), tumor growth was
present in both bladder neck and cranial areas. The location of
urethral UC was more common in the distal (8/10, 80%) than in the
proximal (2/10, 20%) urethra.
The subgross tumor growth pattern could be assessed
in 66/199 (33%) bladder UC cases, which were provided as full
transmural tissue sections. Two different types of subgross growth
appearance were recognized, papillary and non-papillary (also
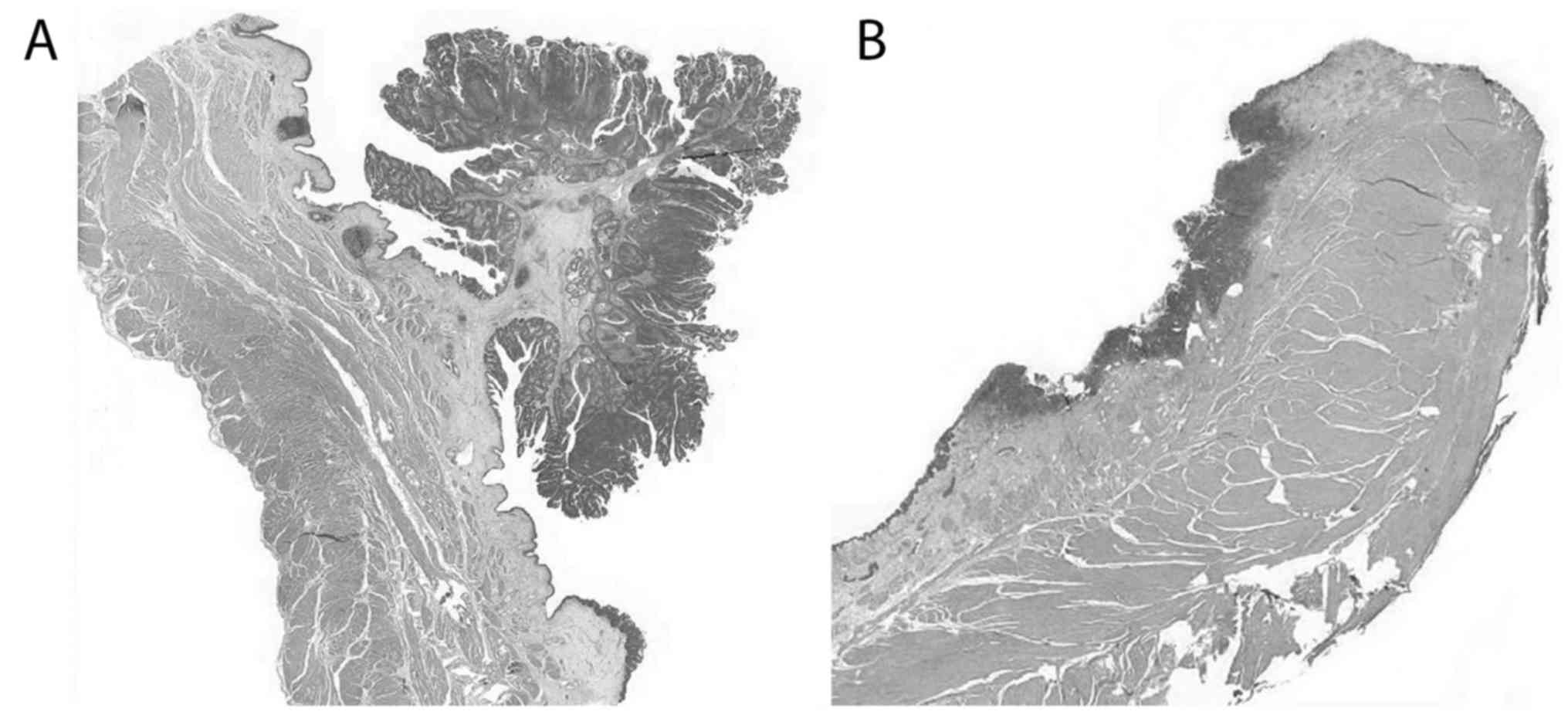
referred to as flat). Papillary UC (Fig.
2A) were equally as common (33/66, 50%) as flat (Fig. 2B) (33/66, 50%) tumors, with identical
female: Male ratio of 1: 1.57 in both growth patterns (21/33, 67%
females, 12/33, 33% males). Irrespective of growth pattern, all UC
were invading beyhond the basement membrane, compatible with
infiltrating UC based on the WHO 2016 tumor classification.
When applying the tumor, node and metastasis (TNM)
staging system provided by the 2016 WHO classification system
(12), tumor characterization was
limited to the assessment of the primary tumor. Information about
the involvement of lymph nodes and presence of distant metastatic
spread was not available, which prevented the performance of an
overall tumor staging. The extent of tumor infiltration into the
bladder wall could be assessed in 64/199 (32%) bladder UC cases
which were available as transmural tissue sections. Thirty-eight %
(24/64) of cases were limited to the lamina propria mucosae (T1),
28% (18/64) invaded the superficial muscularis propria (T2a), and
13% (8/64) the deep muscularis propria (T2b). A substantial number
(14/64, 22%) of UC cases invaded perivesicular tissue (T3).
Muscle-invasive growth could be assessed in 76/199 (38%) bladder UC
cases. More than two thirds (52/76, 68%) were muscle-invasive
tumors, which is compatible with high tumor stage. The tumor growth
pattern of muscle-invasive UC could be assessed in 41/52 (79%)
cases, and it was more commonly flat (30/41, 73%) than papillary
(11/41, 27%). This was in contrasts to the non-muscle-invasive UC,
the vast majority of which (22/24, 92%) presented with a papillary
growth, with only rare flat tumor growth (2/24, 8%). Non-papillary,
flat tumor growth was statistically significantly strongly
associated with tumor muscle invasion (OR 31.00, 95% CI
66.24–153.95).
Conventional, not further classifiable infiltrating
UC was the most common type of tumor (234/260, 90%), followed by UC
with divergent differentiation (15/260, 6%) (squamous [8/260, 3%),
glandular (5/260, 2%) or both (2/260, 1%)], and squamous cell
carcinoma (5/260, 2%). One case was identified to be a bladder
adenocarcinoma (not otherwise specified). Two rare urinary tract
tumors, including primary urethral plasmacytoid UC and
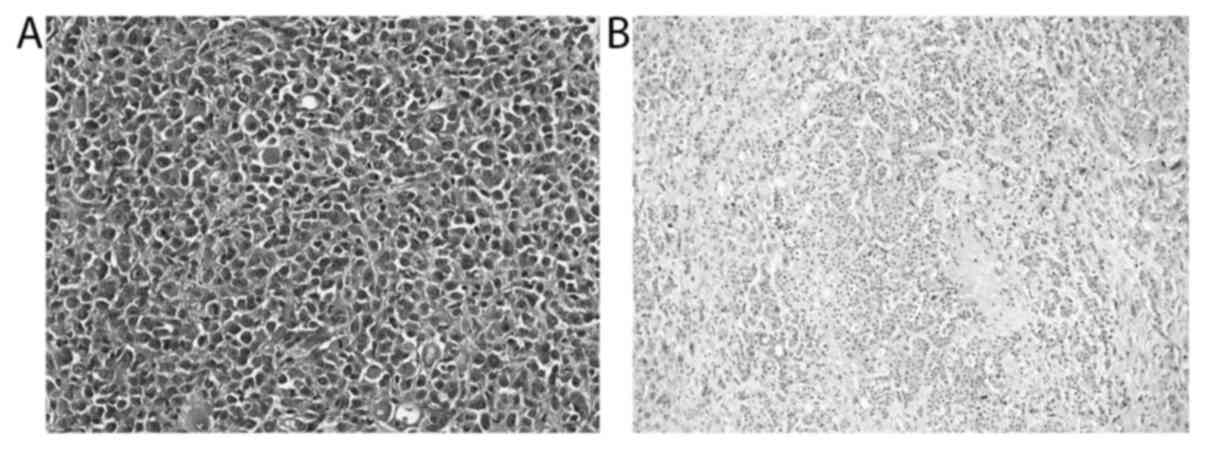
neuroendocrine bladder tumor, have been identified in one dog each
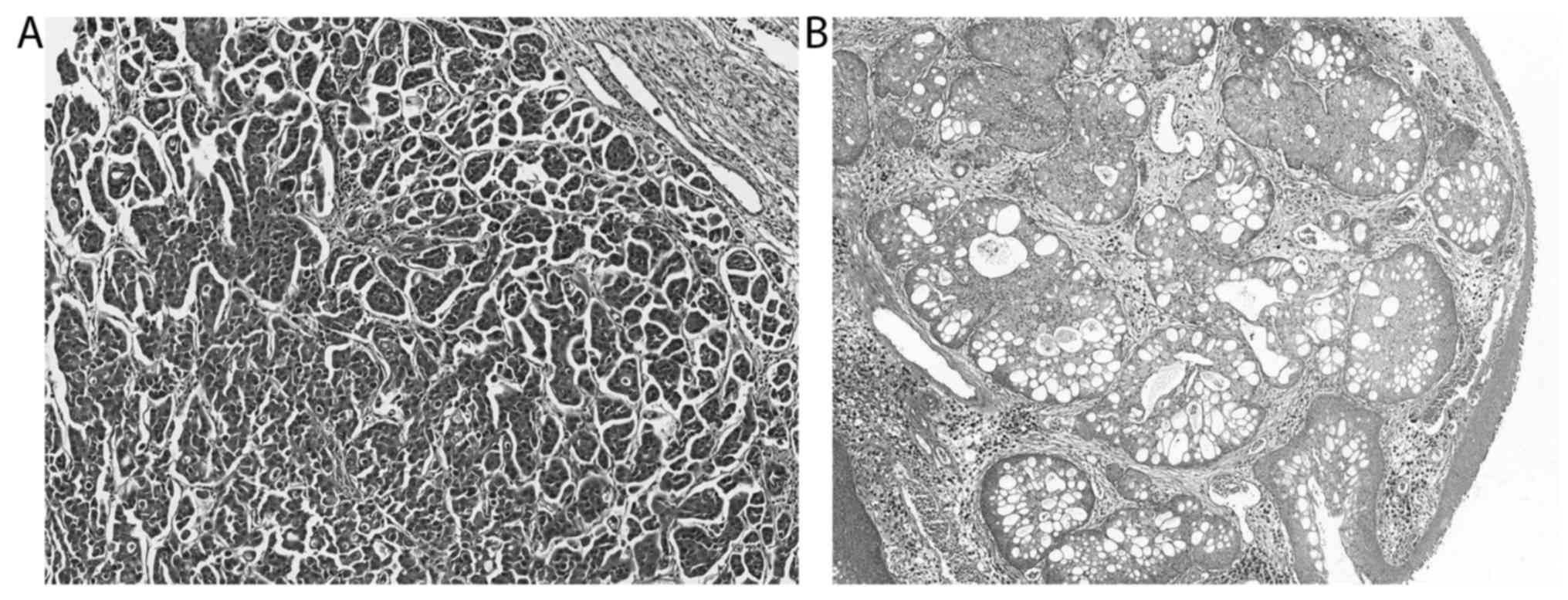
(Fig. 3). Eight of the 234 not
further classifiable infiltrating UC cases presented with certain
characteristic histological features within a low percentage (1 to
20%) of the studied tissue section, including micropapillary and
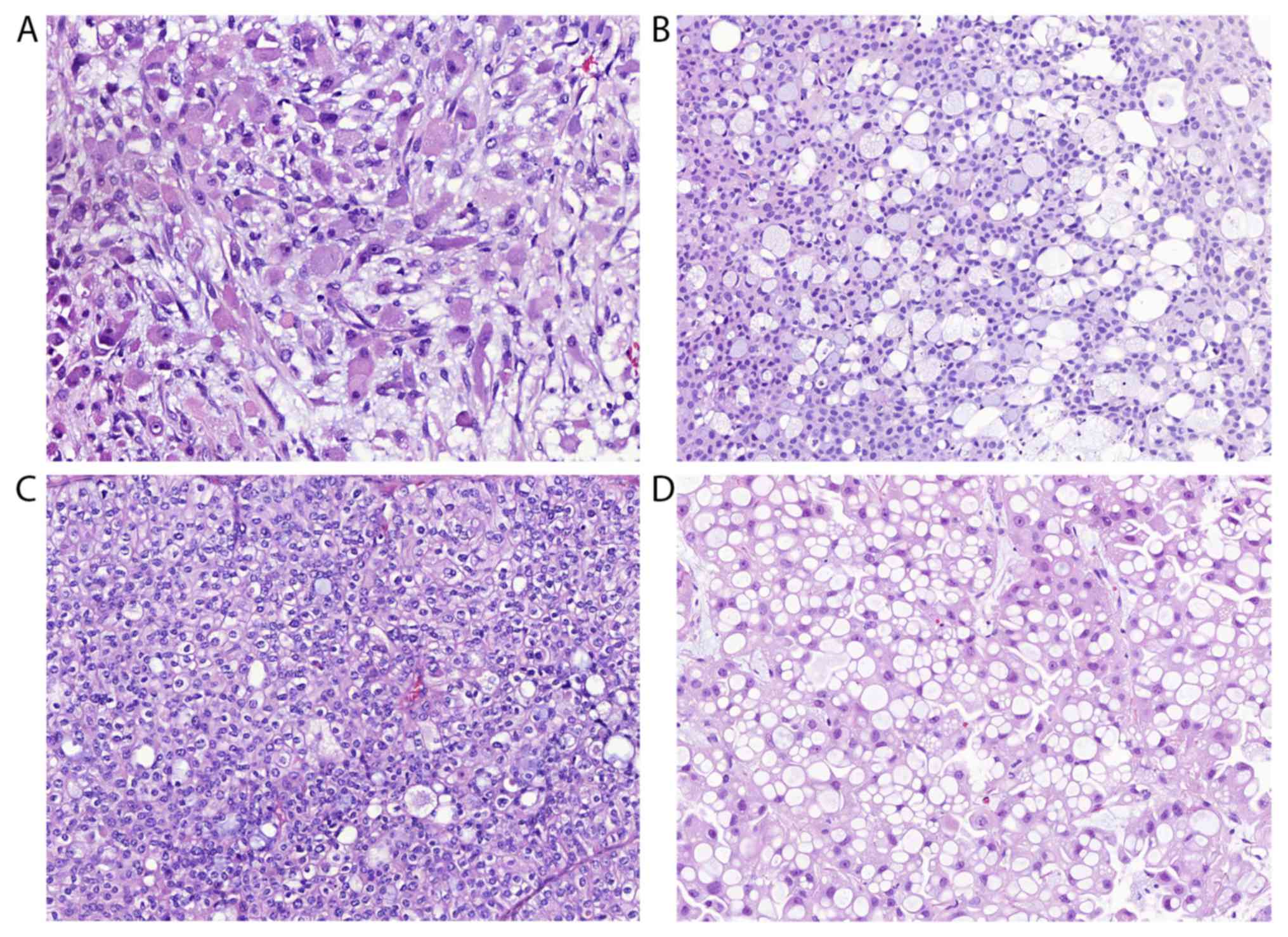
microcystic growth (Fig. 4),
neuroendocrine differentiation as well as the presence of
neoplastic cells with plasmacytoid, rhabdoid, signet-ring cell,
lipid-rich or glycogen-rich appearance (Fig. 5). Since the divergent differentiation
was however only observed in a minor portion of the examined tumor
tissue, these tumors were diagnosed as not further classifiable
infiltrating UC and not as UC with divergent differentiation. The
urethral plasmacytoid UC was found in a 9-year-old male neutered
crossbreed dog with a history of dyschezia and prostate
enlargement. The submitted and histologically examined tissue was
limited to urethra. Prostate tissue was not available and no
further information about the follow-up of this dog was given. The
neuroendocrine bladder tumour was diagnosed in a 13-year-old female
neutered 7 kg crossbreed dog with reported weight loss since a few
months and a history of polyuria and polydipsia, pollakisuria,
haematuria, and increasing lethargy over a period of less than 2
months. The tumor was diagnosed based on cystoscopy and biopsy. Due
to late tumor diagnosis with very advanced disease and poor
prognosis, the dog's owner decided against surgery and chemotherapy
and elected euthanasia 3 weeks after the tumor diagnosis was made.
Consent for a full post-mortem examination was given and confirmed
the primary bladder tumor with metastases in lung, medial iliac and
sacral lymph nodes.
Finally, non-conventional UC was associated with
muscle-invasive tumor growth (P<0.001), being compatible with
higher tumor stage.
Discussion
The present study confirms the previously observed
striking epidemiological and pathological similarities between
canine and human UC (6,14). The number of comparative human and
canine bladder cancer studies available in the literature is still
very low, with the most relevant studies being done in North
America (15–20). The present study is the largest study
with comparative epidemiology and histology, which includes 260
cases. It demonstrates that similar to the situation in people,
canine UC mainly affects elderly patients. The mean age of dogs
diagnosed with UC was over 10 years, which is equivalent to
approximately 60 to 70 human years (21), compatible with the mean age of people
diagnosed with UC. Both canine and human UC have a strong sex
predilection. While men are at a three-fold higher risk to develop
UC compared to women, the situation is exactly the opposite in
dogs. Similar to the situation in people, the reason for the sex
predisposition is unknown in dogs. Behavioral, anatomic,
physiological and hormonal gender differences have been discussed,
but remain speculative (22,23). It is important to take into account
that most of the dogs in the present study were surgically
castrated which could potentially have contributed to the observed
female predisposition for developing UC. Indeed, neutering appeared
as a risk factor for UC for both female and male dogs in the
present and previous studies (6).
Interestingly, a similar phenomenon can be seen in women with a
history of bilateral oophorectomy, who have a higher risk to
develop UC (22,23). Given that castration causes a change
in the level of sex hormones and their receptors, these proteins
likely play a major role in the carcinogenesis of UC. Previous
studies have shown that reduced androgen receptor and increased
estrogen receptor-beta expression are associated with higher tumor
stage and grade, respectively (24–28).
Another remarkable feature of canine UC is its high
predisposition for certain dog breeds (14,17), which
we could confirm in our study. Dogs of the same breed are
genetically closely related and can be compared to related human
families. The Scottish terrier has been previously shown to be the
breed with the highest risk (up to over 20-fold) to develop UC
(14,17). Present results confirm this
observation, which raises high suspicion of a strong genetic
component of UC in certain dog breeds. Interestingly, the three dog
breeds with highest risk to develop UC were significantly younger
at the time of tumor diagnosis, compared to other dog breeds. The
age difference was just over one year, which would be equivalent to
approximately 7 human years (21).
Pathologically, canine and human UC are very
similar, both grossly and histologically. UC affects most commonly
the urinary bladder in both people and dogs and represents the most
common (over 90%) type of bladder cancer in both species. The
lateral and posterior wall are the most common sites of UC in
people (29–31), whereas dogs in our and previous
studies tend to have UC within the neck and trigone area (6,32). Reasons
for the difference in preferred bladder subsites of UC between men
and dogs are not known, but they may be due to the different
orientation of the bladder within the body, leading to different
intravesical urine flow. The prognostic significance of the
different tumor bladder subsites is reported with some conflicting
results, but involvement of neck or trigone appears to be
associated with higher tumor stage and worse prognosis in people
(29,31). This could potentially explain the
higher UC tumor stages in dogs compared to men.
The vast majority, between 80 and 90%, of human UC
grow papillary (30). Non-papillary
(flat or endophytic) growth is much less common in people. This is
in contrast to the findings of the here studied canine tumors which
presented with an equal number of papillary and flat tumors.
Interestingly, non-papillary growth has been demonstrated to be
associated with higher tumor grade, muscle invasion and poor
prognosis in people (30,33). The studied canine data confirms this
observation.
Histologically, the present study demonstrates that
UC of people and dogs are strikingly similar and directly
comparable. Non-conventional UC are less common in both species and
most frequently comprise UC with divergent, glandular and/or
squamous, differentiation. Other UC subtypes are rare in both
species, but important to recognize given their association with
poor prognosis (34). In the studied
canine population, one case of primary urethral plasmacytoid UC in
a male neutered dog, and one case of neuroendocrine tumor in the
bladder of a female neutered dog were identified. Both tumors were
high grade with evidence of intravascular neoplastic growth in the
plasmacytoid UC and widespread lymph node and distant metastases in
the neuroendocrine tumor, respectively. Neither of these two tumor
types have been described in any non-experimental animal species
and are herein reported for the first time as spontaneous tumors in
an animal species. Other histological features, including
micropapillary and microcystic growth, and rhabdoid, signet-ring
cell, lipid-rich and glycogen-rich appearance of neoplastic cells
were observed in a small proportion of the herein studied canine
cases. These histological tumor features are poorly described in
animals, but are well known features of UC in people (13,34,35). These
results therefore further support the value and suitability of the
dog as animal model for UC in people.
In conclusion, canine and human UC are
epidemiologically and pathologically directly comparable, which
promotes the dog as a valuable animal model. Canine UC has an
extraordinarily high predisposition for certain genetically closely
related dog breeds which suggests a strong genetic component which
needs further investigation. Female surgically neutered dogs are at
highest risk to develop UC which leads to the speculation that sex
hormones are crucial in the carcinogenesis of UC. Given the
typically high tumor grades in dogs, canine UC appears to mimic the
malignant muscle-invasive form of UC in people which is known to
lack a well-established animal model that is so urgently needed to
discover new therapeutic options. Finally, rare urinary tract
tumors, including plasmacytoid UC and neuroendocrine tumor, occur
spontaneously in dogs and are herein described for the first time
in a non-experimental animal species.
Acknowledgements
The authors would like to thank Mrs. Deepthi Chandy
(Bridge Pathology Ltd., Bristol, UK) for her valuable assistance in
producing high quality histological slides and Chris Nolan (City
Hospital, University Nottingham, UK) for digitalizing all of the
slides.
Funding
The present study was supported by PetPlan
Charitable Trust (grant no. 542127), PetSavers and the University
of Nottingham Interdisciplinary Centre for Analytical Science
(UNICAS).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SdB, EW, TS and DG contributed to the acquisition
and analysis of the epidemiological data. SdB, LGR, BDR and TS
performed the histological examinations. SdB, EW and DG performed
the statistical analysis. SdB, SAB, BDR and NPM compared the canine
data with data available from scientific human literature. SdB and
NPM were major contributors in writing the manuscript. All authors
critically reviewed, read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
University of Nottingham's Ethical Review Committee (Nottingham,
UK). Informed written consent was obtained from the dog owners upon
sample submission.
Consent for publication
Informed written consent was obtained from the dog
owners upon sample submission.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC cancer base no. 11 [Internet]. Lyon, France;
International Agency for Research on Cancer; 2013, Available from:.
http://globocan.iarc.fr04
12–2017
|
|
3
|
John BA and Said N: Insights from animal
models of bladder cancer: Recent advances, challenges, and
opportunities. Oncotarget. 8:57766–57781. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobayashi T, Owczarek TB, McKiernan JM and
Abate-Shen C: Modelling bladder cancer in mice: Opportunities and
challenges. Nat Rev Cancer. 15:42–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding J, Xu D, Pan C, Ye M, Kang J, Bai Q
and Qi J: Current animal models of bladder cancer: Awareness of
translatability (Review). Exp Ther Med. 8:691–699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knapp DW, Glickman NW, Denicola DB, Bonney
PL, Lin TL and Glickman LT: Naturally-occurring canine transitional
cell carcinoma of the urinary bladder A relevant model of human
invasive bladder cancer. Urol Oncol. 5:47–59. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choy K and Fidel J: Tolerability and tumor
response of a novel low-dose palliative radiation therapy protocol
in dogs with transitional cell carcinoma of the bladder and
urethra. Vet Radiol Ultrasound. 57:341–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Knapp DW, Ruple-Czerniak A, Ramos-Vara JA,
Naughton JF, Fulkerson CM and Honkisz SI: A nonselective
cyclooxygenase inhibitor enhances the activity of vinblastine in a
naturally-occurring canine model of invasive urothelial carcinoma.
Bladder Cancer. 2:241–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arnold EJ, Childress MO, Fourez LM, Tan
KM, Stewart JC, Bonney PL and Knapp DW: Clinical trial of
vinblastine in dogs with transitional cell carcinoma of the urinary
bladder. J Vet Intern Med. 25:1385–1390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbo AH, Jones DR, Masters AR, Stewart JC,
Fourez L and Knapp DW: Phase I clinical trial and pharmacokinetics
of intravesical mitomycin C in dogs with localized transitional
cell carcinoma of the urinary bladder. J Vet Intern Med.
24:1124–1130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Henry CJ, McCaw DL, Turnquist SE, Tyler
JW, Bravo L, Sheafor S, Straw RC, Dernell WS, Madewell BR,
Jorgensen L, et al: Clinical evaluation of mitoxantrone and
piroxicam in a canine model of human invasive urinary bladder
carcinoma. Clin Cancer Res. 9:906–911. 2003.PubMed/NCBI
|
|
12
|
Moch H, Humphrey PA, Ulbright TM and
Reuter VE: Tumours of the urinary tract. WHO Classification of
Tumours of the Urinary System and Male Genital Organs. 4th edition.
IARC; Lyon. pp; pp. 77–pp134. 2016
|
|
13
|
Humphrey PA, Moch H, Cubilla AL, Ulbright
TM and Reuter VE: The 2016 WHO Classification of Tumours of the
Urinary System and Male Genital Organs-Part B: Prostate and bladder
tumours. Eur Urol. 70:106–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Knapp DW, Ramos-Vara JA, Moore GE, Dhawan
D, Bonney PL and Young KE: Urinary bladder cancer in dogs, a
naturally occurring model for cancer biology and drug development.
ILAR J. 55:100–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Valli VE, Norris A, Jacobs RM, Laing E,
Withrow S, Macy D, Tomlinson J, McCaw D, Ogilvie GK, Pidgeon G, et
al: Pathology of canine bladder and urethral cancer and correlation
with tumour progression and survival. J Comp Pathol. 113:113–130.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patrick DJ, Fitzgerald SD, Sesterhenn IA,
Davis CJ and Kiupel M: Classification of canine urinary bladder
urothelial tumours based on the World Health
Organization/International Society of Urological Pathology
consensus classification. J Comp Pathol. 135:190–199. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Norris AM, Laing EJ, Valli VE, Withrow SJ,
Macy DW, Ogilvie GK, Tomlinson J, McCaw D, Pidgeon G and Jacobs RM:
Canine bladder and urethral tumors: A retrospective study of 115
cases (1980–1985). J Vet Intern Med. 6:145–153. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shapiro SG, Raghunath S, Williams C,
Motsinger-Reif AA, Cullen JM, Liu T, Albertson D, Ruvolo M,
Bergstrom Lucas A, Jin J, et al: Canine urothelial carcinoma:
Genomically aberrant and comparatively relevant. Chromosome Res.
23:311–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dhawan D, Paoloni M, Shukradas S,
Choudhury DR, Craig BA, Ramos-Vara JA, Hahn N, Bonney PL, Khanna C
and Knapp DW: Comparative gene expression analyses identify luminal
and basal subtypes of canine invasive urothelial carcinoma that
mimic patterns in human invasive bladder cancer. PLoS One.
10:e01366882015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Decker B, Parker HG, Dhawan D, Kwon EM,
Karlins E, Davis BW, Ramos-Vara JA, Bonney PL, McNiel EA, Knapp DW
and Ostrander EA: Homologous mutation to human BRAF V600E is common
in naturally occurring canine bladder cancer-evidence for a
relevant model system and urine-based diagnostic test. Mol Cancer
Res. 13:993–1002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patronek GJ, Waters DJ and Glickman LT:
Comparative longevity of pet dogs and humans: Implications for
gerontology research. J Gerontol A Biol Sci Med Sci. 52:B171–B178.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dietrich K, Demidenko E, Schned A, Zens
MS, Heaney J and Karagas MR: Parity, early menopause and the
incidence of bladder cancer in women: A case-control study and
meta-analysis. Eur J Cancer. 47:592–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prizment AE, Anderson KE, Harlow BL and
Folsom AR: Reproductive risk factors for incident bladder cancer:
Iowa Women's Health Study. Int J Cancer. 120:1093–1098. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kauffman EC, Robinson BD, Downes M,
Marcinkiewicz K, Vourganti S, Scherr DS, Gudas LJ and Mongan NP:
Estrogen receptor-β expression and pharmacological targeting in
bladder cancer. Oncol Rep. 30:131–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu
I, Izumi K, Chang C, Messing EM, Netto GJ and Yeh S: Expression of
androgen and oestrogen receptors and its prognostic significance in
urothelial neoplasm of the urinary bladder. BJU Int. 109:1716–1726.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tuygun C, Kankaya D, Imamoglu A, Sertcelik
A, Zengin K, Oktay M and Sertcelik N: Sex-specific hormone
receptors in urothelial carcinomas of the human urinary bladder: A
comparative analysis of clinicopathological features and survival
outcomes according to receptor expression. Urol Oncol. 29:43–51.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen SS, Smith CL, Hsieh JT, Yu J, Kim IY,
Jian W, Sonpavde G, Ayala GE, Younes M and Lerner SP: Expression of
estrogen receptors-alpha and -beta in bladder cancer cell lines and
human bladder tumor tissue. Cancer. 106:2610–2616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boorjian S, Ugras S, Mongan NP, Gudas LJ,
You X, Tickoo SK and Scherr DS: Androgen receptor expression is
inversely correlated with pathologic tumor stage in bladder cancer.
Urology. 64:383–388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao GQ and Rashid H: Bladder neck
urothelial carcinoma: A urinary bladder subsite carcinoma with
distinct clinicopathology. Int J Surg Pathol. 23:517–523. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sciarra A, de Matteis A, Mariotti G, Voria
G, Lucera R and Di Silverio F: Histopathological aspects of
transitional cell carcinoma of the bladder: Analysis of 20 years
experience. Int J Urol. 11:467–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stephenson WT, Holmes FF, Noble MJ and
Gerald KB: Analysis of bladder carcinoma by subsite. Cystoscopic
location may have prognostic value. Cancer. 66:1630–1635. 1990.
|
|
32
|
Fulkerson CM and Knapp DW: Management of
transitional cell carcinoma of the urinary bladder in dogs: A
review. Vet J. 205:217–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo A, Liu A and Teng X: The pathology of
urinary bladder lesions with an inverted growth pattern. Chin J
Cancer Res. 28:107–121. 2016.PubMed/NCBI
|
|
34
|
Amin M: Histological variants of
urothelial carcinoma: Diagnostic, therapeutic and prognostic
implications. Mod Pathol. 2222. (Suppl 2): S96–S118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhai QJ, Black J, Ayala AG and Ro JY:
Histologic variants of infiltrating urothelial carcinoma. Arch
Pathol Lab Med. 131:1244–1256. 2007.PubMed/NCBI
|
|
36
|
Madeb R and Messing EM: Gender, racial and
age differences in bladder cancer incidence and mortality. Urol
Oncol. 22:86–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yee DS, Ishill NM, Lowrance WT, Herr HW
and Elkin EB: Ethnic differences in bladder cancer survival.
Urology. 78:544–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee CT, Dunn RL, Williams C and Underwood
W III: Racial disparity in bladder cancer: Trends in tumor
presentation at diagnosis. J Urol. 176:927–933. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hankey BF and Myers MH: Black/white
differences in bladder cancer patient survival. J Chronic Dis.
40:65–73. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tracey E, Watt H, Currow D, Young J and
Armstrong B: Investigation of poorer bladder cancer survival in
women in NSW, Australia: A data linkage study. BJU Int.
113:437–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McGrath M, Michaud DS and de Vivo I:
Hormonal and reproductive factors and the risk of bladder cancer in
women. Am J Epidemiol. 163:236–244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Annan AC, Stevens KA and Osunkoya AO:
Urothelial carcinoma involving the ureteral orifice: A
clinicopathologic analysis of 93 cases. Hum Pathol. 65:101–106.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sui W, RoyChoudhury A, Wenske S, Decastro
GJ, McKiernan JM and Anderson CB: Outcomes and prognostic factors
of primary urethral cancer. Urology. 100:180–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou M, Netto GJ and Epstein JI:
Uropathology: Elsevier Saunders 1st Edition. 2012, View Article : Google Scholar
|
|
45
|
Dayyani F, Hoffman K, Eifel P, Guo C,
Vikram R, Pagliaro LC and Pettaway C: Management of advanced
primary urethral carcinomas. BJU Int. 114:25–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gakis G, Witjes JA, Compérat E, Cowan NC,
de Santis M, Lebret T, Ribal MJ and Sherif AM; European Association
of Urology: EAU guidelines on primary urethral carcinoma. Eur Urol.
64:823–830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Svatek RS, Clinton TN, Wilson CA, Kamat
AM, Grossman HB, Dinney CP and Shah JB: Intravesical tumor
involvement of the trigone is associated with nodal metastasis in
patients undergoing radical cystectomy. Urology. 84:1147–1151.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kobayashi S, Fujii Y, Koga F, Yokoyama M,
Ishioka J, Matsuoka Y, Numao N, Saito K, Masuda H and Kihara K:
Impact of bladder neck involvement on progression in patients with
primary non-muscle invasive bladder cancer: A prospective
validation study. Urol Oncol. 32:38.e29–e36. 2014. View Article : Google Scholar
|
|
49
|
Reis LO, Ferreira F, Almeida M and
Ferreira U: Urethral carcinoma: Critical view on contemporary
consecutive series. Med Oncol. 28:1405–1410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Swartz MA, Porter MP, Lin DW and Weiss NS:
Incidence of primary urethral carcinoma in the United States.
Urology. 68:1164–1168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Terada T: Inverted variant of urothelial
carcinoma of the urinary bladder: A report of three cases and a
proposal for a new clinicopathologic entity. Int J Clin Ex Pathol.
6:766–770. 2013.
|
|
52
|
Kern WH: The grade and pathologic stage of
bladder cancer. Cancer. 53:1185–1189. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Comperat E, Sylvester RJ, Kaasinen E, Böhle A,
Palou Redorta J, et al: EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder: Update 2013. Eur Urol.
64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mungan NA, Aben KK, Schoenberg MP, Visser
O, Coebergh JW, Witjes JA and Kiemeney LA: Gender differences in
stage-adjusted bladder cancer survival. Urology. 55:876–880. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Amin MB and Tickoo SK: Genitourinary
diagnostic pathology. IInd Edition. Elsevier; 2016
|