Introduction
Pancreatic carcinoma refers to cancer in the
pancreatic exocrine secretion and accounts for 8–10% of all
gastrointestinal tumors. The mortality rate associated with
pancreatic cancer has been increasing globally. Due to the fact
that pancreatic cancer is asymptomatic, it is difficult to diagnose
this disease during early stages (1).
The 5-year survival rate is only 1–4% and, with the exception of
surgery, there is no clear way of improving the survival of
patients with pancreatic cancer (2).
Pancreatic cancer is not sensitive to traditional chemoradiotherapy
(3). Additionally, when using
chemotherapeutics to treat pancreatic cancer, chemotherapy
resistance and high recurrence rates are two challenges faced in
the clinic (4). If the primary tumor
is not surgically removed at an early stage, even if patients
receive first-line chemotherapy, the median survival rate of
pancreatic cancer is only ~8 months (3).
Long non-coding RNAs (lncRNAs) were originally
regarded as the ‘noise’ of genome transcription and the by-product
of RNA polymerase II transcription. lcnRNAs have a transcript
length of >200 nucleotides and were considered to have no
biological function (5). However,
more recent research indicates that lncRNAs participate in multiple
important regulatory processes, including genome blotting,
chromatin modification, transcriptional activation, transcriptional
interference and intranuclear transportation (6). Additionally, lcnRNAs are associated with
the genesis and development of cancer (6). For example, abnormal lncRNA expression
has been observed in colon, breast and liver cancer (7–9). HOTAIR
and HOTTIP are two recently identified functional lncRNAs (10). An in vitro experiment indicated
that HOTAIR regulates HOXD10 and HOXC11, and that HOTTIP regulates
the transcription of HOXA13 (10).
Certain studies demonstrate that HOTAIR is abnormally expressed in
breast, pancreatic, colon and liver cancer (5,11).
Metabotropic glutamate receptors (mGluRs) belong to
the group of G protein-coupled receptors (GPCRs). Despite
possessing the same structure, with seven transmembrane domains,
mGluRs exhibit amino acid sequences and molecular structures that
differ from those of the majority of GPCRs. mGluR1 was separated
from other mGluRs early on and is widely distributed in the brain
(12). At present, glutamate
receptors are widely studied (13).
The activation of glutamate receptors may activate multiple signal
pathways, two of which are the phosphoinositide 3-kinase (PI3K)/Akt
and the mitogen-activated protein kinase (MAPK) pathways (14). The PI3K and Akt signaling pathways
serve an important role in regulating cell proliferation, and
restraining apoptosis (15). Akt is
the serine/threonine protein kinase that regulates cell
proliferation and balances apoptosis. Increased Akt activity has
been observed in a number of types of human cancer, including colon
cancer, ovarian cancer, thymic carcinoma and gastric carcinoma,
indicating that there is an interaction between Akt and cell
proliferation rates (16). The
present study attempted to assess the function of HOTTIP in
regulating human pancreatic cancer via the mGluR1 pathway.
Materials and methods
Patient samples
The present study was approved by the Research
Ethics Committee of Sun Yat-sen Memorial Hospital (Guangzhou,
China) and written informed consent was obtained from all patients
(age range 56–67 years, mean 66.55±9.12 years; 3 male and 3
female). The pancreatic cancer tissue samples and adjacent
non-tumor tissues of 8 patients were available from the Department
of Hepatobiliary Surgery, Sun Yat-sen Memorial Hospital from June
2015 to September 2015. All the tissue samples were collected and
immediately snap frozen in liquid nitrogen and stored at −80°C.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (PCR)
analyses
Total RNA was isolated from tissues using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacturer's protocol. cDNA was
synthesized with PrimeScript™ 1st Strand cDNA Synthesis Kit (6110A;
Takara Biotechnology Co., Ltd., Dalian, China) at 37°C for 60 min
and oligo (dT) primer (Takara Biotechnology Co., Ltd.) according to
the manufacturer's protocol. ABI Prism 7500 real-time system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used,
along with a SYBR Premix Ex Taq™ II kit (Takara Biotechnology Co.,
Ltd.). The primer sequences were as follows: HOTTIP forward,
5′-CCTAAAGCCACGCTTCTTTG-3′ and reverse, 5′-TGCAGGCTGGAGATCCTACT-3′;
GAPDH forward, 5′-GTCAACGGATTTGGTCTGTATT-3′ and reverse,
5′-AGTCTTCTGGGTGGCAGTGAT-3′. The thermocycling conditions of the
PCR were as follows: 94°C for 20 sec, 94°C for 15 sec, 58°C for 30
sec to anneal and 72°C for 25 sec, repeated for 40 cycles. The mRNA
expression was quantified using the 2−ΔΔCt method
(17).
Cell culture and cell proliferation
assay
The human pancreatic cancer SW1990 cell line was
purchased from the Culture Center of Sun Yat-sen University
(Guangzhou, China) and was maintained in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), penicillin (100 IU/ml) and streptomycin (100
µg/ml, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C in a
5% CO2 humidified atmosphere. 500 ng of HOTTIP siRNA and
the negative control siRNA were purchased from Qiagen GmbH (Hilden,
Germany). The siRNA sequences are as follows: HOTTIP,
5′-UGGGAACCCGCUAUUUCACUCUAUU-3′; negative control,
5′-UGACAACUCUUAGGGACCUA-3′. Cells (1×103 cell/well) were
grown on 96-well plates until they reached 70% confluence and were
subsequently transfected using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Following
transfection for 24, 48 and 72 h, cell proliferation was determined
using MTT (20 µl, 50 mg/kg) for 4 h. Dimethyl sulfoxide was added
to every well and the fluorescence intensity was measured using a
fluorescence microplate reader (model no. 680; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 570 nm.
Assessment of apoptosis by flow
cytometry
Following transfection for 48 h, cells were
harvested at 1,000 × g for 10 min at 4°C and washed twice with cold
phosphate buffered saline, then resuspended in 1X Binding buffer
(Invitrogen; Thermo Fisher Scientific, Inc.). A total of 10 µl
Annexin V-fluorescein isothiocyanate (Invitrogen; Thermo Fisher
Scientific, Inc.) and 5 µl propidium iodide (Invitrogen; Thermo
Fisher Scientific, Inc.) were added to each cell. Following
staining for 15 min at room temperature, flow cytometry (Beckman
Coulter, Inc., Brea, CA, USA) was performed at 488 nm in order to
analyze the rate of apoptosis using Flowjo 7.6.1 (FlowJo LLC,
Ashland, OR, USA).
Assessment of caspase-3 and caspase-8
activities
Cultured cells (1×106 cell/well) were
lysed in radioimmunoprecipitation assay (RIPA) buffer (Beyotime
Institute of Biotechnology, Nanjing, China) and protein was
quantified using a bicinchoninic acid (BCA) assay. A total of 10 µg
protein was incubated with caspase-3 (cat. no. C1116; Beyotime
Institute of Biotechnology) and caspase-8 (cat. no. C1152; Beyotime
Institute of Biotechnology) activity kits for 1 h at 37°C. The
fluorescence intensity was measured using a fluorescence microplate
reader (model no. 680; Bio-Rad Laboratories, Inc.) at 450 nm.
Western blot analysis
Cultured cells were lysed in RIPA buffer and protein
was quantified using BCA assay. Protein (50 µg) was loaded and
separated by 10% SDS-PAGE gel and transferred onto polyvinylidene
fluoride (PVDF) membranes. The PVDF membranes were blocked using
Tris-Buffered Saline with 0.1% Tween 20 (TBS-T) buffer containing
5% skimmed milk at 37°C for 1 h and incubated with primary
antibodies against the following: Bax (cat. no. 5023; 1:2,000),
mGluR1 (cat. no. 12551; 1:2,000), PI3K (cat. no. 4249; 1:2,000),
p-Akt (cat. no., 4060; 1:2,000), p-mTOR (cat. no. 5536; 1:2,000)
and GAPDH (cat. no. 2118; 1:5,000; all Cell Signaling Technology,
Inc., Danvers, MA, USA) overnight at 4°C. The membranes were washed
three times with TBS-T for 5 min and incubated with a horseradish
peroxidase-conjugated rabbit IgG or mouse IgG secondary antibody
(cat. no. sc-2004; 1:5,000; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) for 1 h at 37°C. The membrane was visualized using a
Plus-ECL kit (PerkinElmer, Inc., Waltham, MA, USA) and quantified
using ImageJ 3.0 (ImageJ Software; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard error.
Experimental results were assessed using χ2 test,
Student's t-test or one-way analysis of variance followed by
Tukey's post-hoc test as appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
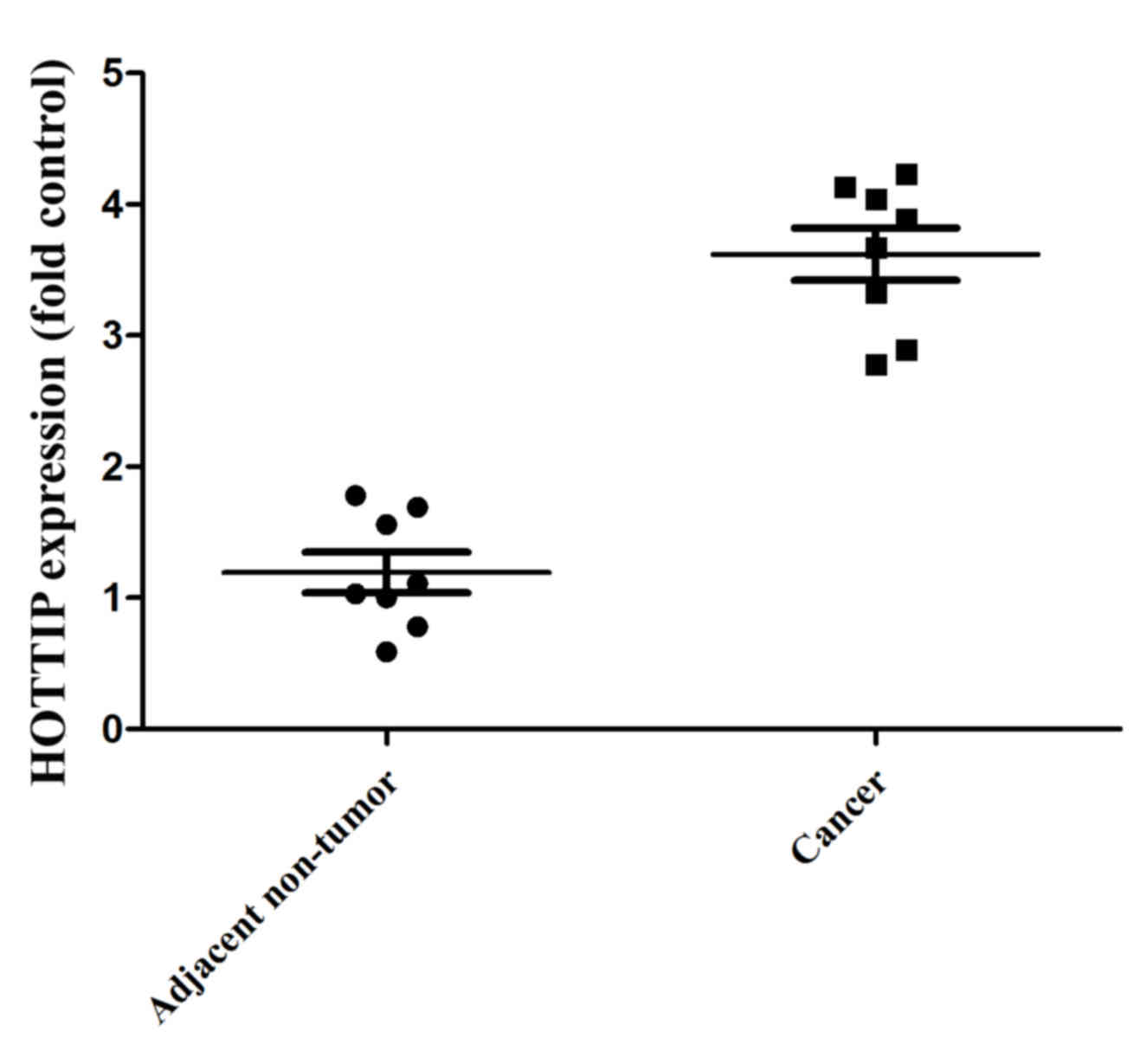
HOTTIP expression in human pancreatic
cancer tissue and para-carcinoma tissue
In the present study, HOTTIP expression in human
pancreatic cancer tissue and para-carcinoma tissue was
investigated. As demonstrated in Fig.
1, HOTTIP expression was higher in the human pancreatic cancer
tissues compared with that in the para-carcinoma tissues.
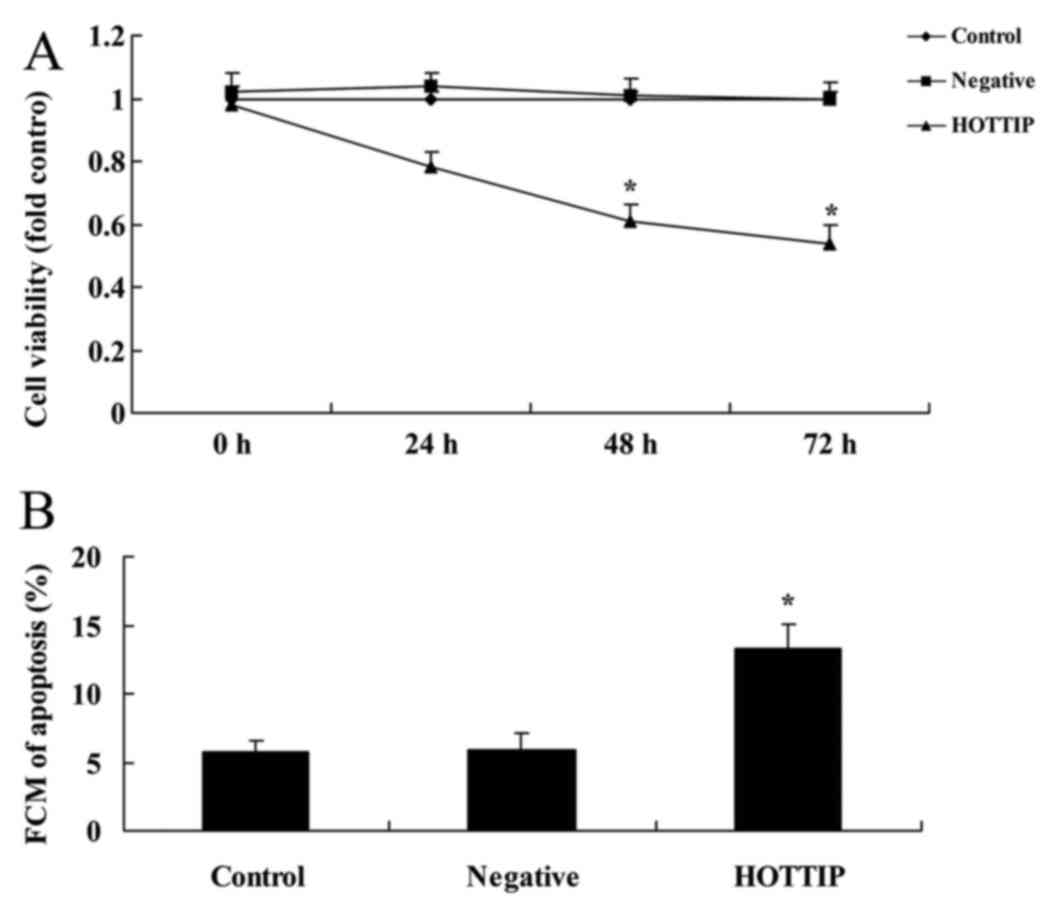
Downregulated expression of HOTTIP
reduces cell growth and induces apoptosis of pancreatic cancer
cells
The effect of HOTTIP on pancreatic cancer cell
growth was analyzed. As demonstrated in Fig. 2, compared with negative group, the
downregulated expression of HOTTIP reduced cell viability at 48 h
and induced apoptosis of pancreatic cancer cells, compared with the
negative group (P=0.0032 and P=0.077).
Downregulated expression of HOTTIP
promotes caspase-3 and caspase-8 activity, and increases Bax
expression in pancreatic cancer cells
The present study also detected the effect of HOTTIP
on pancreatic cancer cell apoptosis, caspase-3 and caspase-8
activities and Bax expression in pancreatic cancer cell. As
demonstrated in Fig. 3, the
downregulated expression of HOTTIP promoted caspase-3 and caspase-8
activities, Bax protein expression, and suppressed mGluR1, PI3K,
p-Akt and p-mTOR protein expression in pancreatic cancer cells.
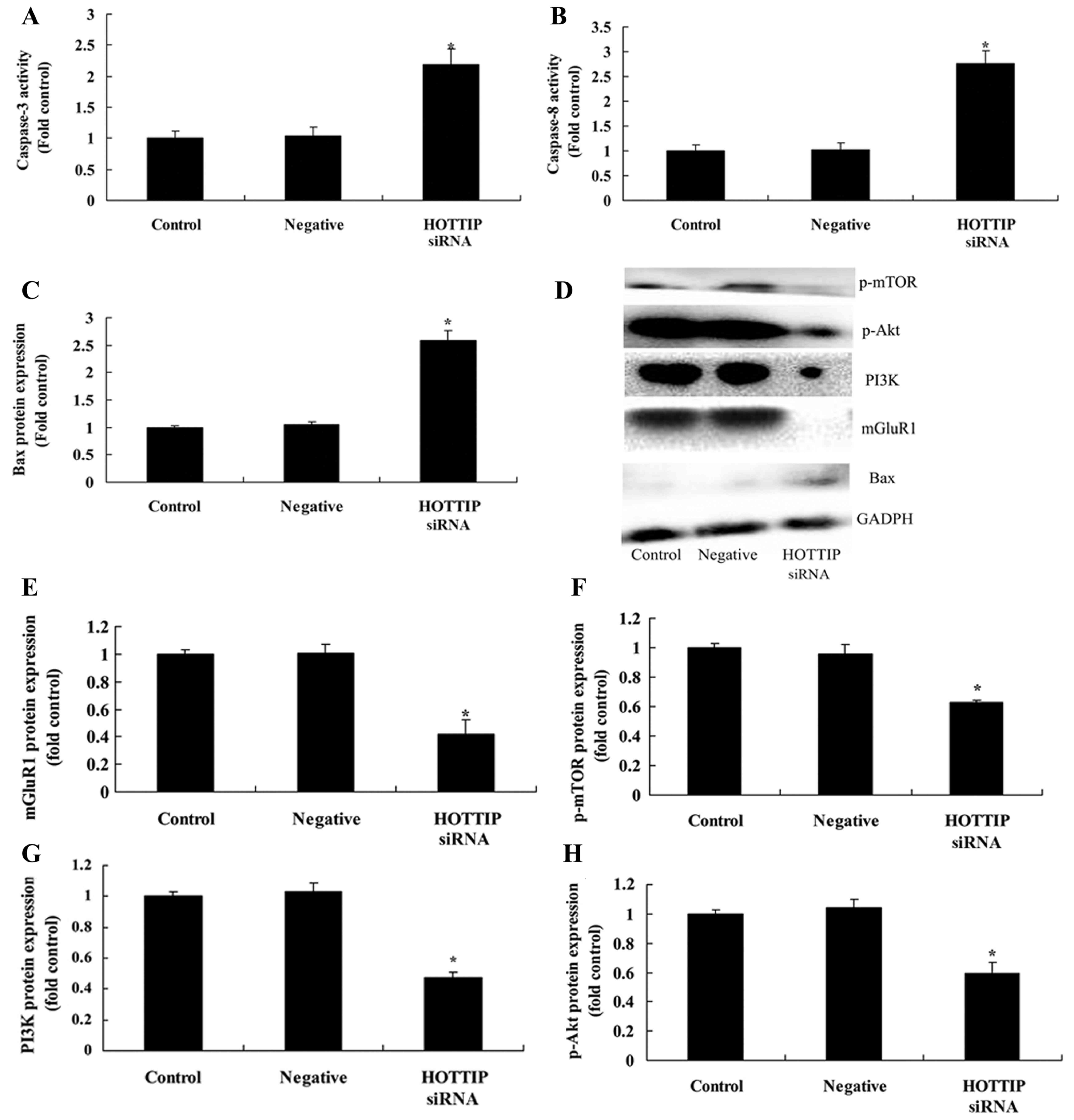 | Figure 3.Downregulated expression of HOTTIP
promotes caspase-3 and caspase-8 activity and Bax expression, and
suppresses mGluR1 and PI3K/Akt/mTOR pathway in pancreatic cancer
cells. Downregulated expression of HOTTIP promoted (A) caspase-3
and (B) caspase-8 activities, and (C) Bax, (D) mGluR1, (E) PI3K,
(F) p-Akt and (G) p-mTOR expression in pancreatic cancer cells, as
determined by statistical analysis and (H) western blot analysis.
Bax; B-cell lymphoma 2-like protein 4; mGluR1, metabotropic
glutamate receptor 1; PI3K, phosphoinositide 3-kinase; p,
phosphorylated; mTOR, mechanistic target of rapamycin; negative,
negative control group. *P<0.05 compared with control group. |
Discussion
Pancreatic cancer is an asymptomatic malignant tumor
with a high invasive capacity and the majority of patients are
diagnosed in middle and advanced stages. The 5-year survival rate
is low and prognosis is poor (18).
Pancreatic ductal adenocarcinoma is the most common type of
pancreatic cancer and presents the highest mortality rate (19). Suppression of apoptosis is an
important factor in the genesis and development of pancreatic
cancer. Bcl-2 family proteins dominate in the apoptosis and
regulation of pancreatic cancer cells. The Bcl-2 family includes
apoptosis-inhibiting factors (Bcl-2, Bcl-xL, Bcl-2, Bfl-1, Brag-1,
Mcl-1 and A1) and apoptosis-promoting motors (Bax, Bak, Bcl-xS,
Bad, Bid, Bik and Hrk) (18). The
specific function of them determines whether or not cells undergo
apoptosis. To a certain extent, apoptosis or apoptosis inhibition
is determined by gene regulation (20).
The mechanism of apoptosis remains unclear, but over
time, basic studies on apoptosis have made progress, hypothesizing
that the series of ordered cascade reactions associated with
apoptosis in cells is able to activate a group of proteases known
as caspases (21), which belong to
the cysteine protease family (22).
Caspase promotes apoptosis, reconstructing the cytoskeleton,
terminating DNA repair, destroying DNA and nuclear structure and
reducing formation of the apoptotic body (23). Certain studies in biological chemistry
and genetics have indicated that the caspase protease family has
important functions at every stage of apoptosis (23,24). The
results of the present study demonstrated that the downregulated
expression of HOTTIP reduced cell viability, induced apoptosis,
promoted caspase-3 and caspase-8 activity, and increased Bax
expression in pancreatic cancer cells. Chen et al (25) demonstrated that the higher expression
level of HOTTIP is correlated with positive lymph node metastasis
and poor overall survival rates in different types of human
cancer.
Certain studies have reported that mGluR1 may hinder
apoptosis by activating the PI3K/Akt pathway (26,27). The
PI3K/Akt signaling pathway regulates the crucial cellular
biological process involved in the genesis and development of
cancer, including transcription, translation, metabolism,
angiogenesis, apoptosis, proliferation, and regulation of cell
cycle progression, migration and invasion of cells (26,27). p-Akt
is able to phosphorylate the downstream tyrosine and tryptophan
residues, so as to activate downstream factors, cause the apoptosis
of suppressor cells, promote cellular proliferation, growth,
movement and invasion, and promote tumor angiogenesis (28). In vivo and in vitro
experiments have indicated that following restrained activity of
the PI3K-Akt signaling pathway, pancreatic cancer cells undergo
hindered proliferation, promoted apoptosis and reduced invasive
ability (29). The present study
confirmed that the downregulated expression of HOTTIP suppressed
mGluR1 and mitigated activation of the PI3K/Akt/mTOR pathway in
pancreatic cancer cells.
With regards to lncRNAs, HOTAIR is widely studied.
By reprogramming the chromosome state to control the expression of
numerous genes represented by HOXD10, the functions of HOTAIR may
be elucidated (30). Additionally,
HOTAIR is associated with breast and liver cancer (30). HOTAIR is markedly reduced in lung
cancer tissues, reinforcing the concept that the HOTAIR gene may
have an important function in lung cancer (31). HOXD10 is another gene that is
associated with cancer (30).
Although there is no clear difference in HOXD10 expression in
cancerous tissues and healthy tissues, the gene has been revealed
to be associated with lymphatic metastasis (32). Additionally, HOXD10 expression has
been significantly increased in cancer tissues with lymphatic
metastases (31). An in vitro
experiment indicated that HOXD10 is modulated by the HOTAIR gene
(10).
Taken together, the results of the present study
demonstrated that downregulated expression of HOTTIP suppressed
mGluR1 and mitigated the activation of the PI3K/Akt/mTOR signaling
pathway in pancreatic cancer cells. Therefore, HOTTIP, which
exhibits a significantly higher expression in human pancreatic
cancer tissues compared with that in para-carcinoma tissues, may be
a potential drug for the treatment of pancreatic cancer.
Additionally, the PI3K/Akt/mTOR pathway may provide potential novel
targets for the future treatment of pancreatic cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TC designed the experiment; YY, YL, YW, YX, RW, ZF,
SZ, QZ, YZ, RC performed the experiments. TC and YY analyzed the
data; TC wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Sun Yat-sen Memorial Hospital (Guangzhou,
China). Participants provided written informed consent.
Consent for publication
Patients provided written informed consent for
publication.
Authors' information
No additional information.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu X, Wang Q, Zhou X, Fu C, Cheng M, Guo
R, Liu H, Zhang B and Dai M: Celastrol negatively regulates cell
invasion and migration ability of human osteosarcoma via
downregulation of the PI3K/Akt/NF-κB signaling pathway in vitro.
Oncol Lett. 12:3423–3428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cui C and Shi X: miR-187 inhibits tumor
growth and invasion by directly targeting MAPK12 in osteosarcoma.
Exp Ther Med. 14:1045–1050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang H, Tang M, Ou L, Hou M, Feng T, Huang
YE, Jin Y, Zhang H and Zuo G: Biological analysis of cancer
specific microRNAs on function modeling in osteosarcoma. Sci Rep.
7:53822017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su YF, Lin CL, Lee KS, Tsai TH, Wu SC,
Hwang SL, Chen SC and Kwan AL: A modified compression model of
spinal cord injury in rats: Functional assessment and the
expression of nitric oxide synthases. Spinal Cord. 53:432–435.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Xiao X, Shen Y, Chen L, Xu C, Zhao
H, Wu Y, Zhang Q, Zhong J, Tang Z, et al: MicroRNA-32 promotes
calcification in vascular smooth muscle cells: Implications as a
novel marker for coronary artery calcification. PLoS One.
12:e01741382017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vishnubalaji R, Hamam R, Yue S, Al-Obeed
O, Kassem M, Liu FF, Aldahmash A and Alajez NM: MicroRNA-320
suppresses colorectal cancer by targeting SOX4, FOXM1, and FOXQ1.
Oncotarget. 7:35789–35802. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dou L, Lin H, Wang K, Zhu G, Zou X, Chang
E and Zhu Y: Long non-coding RNA CCAT1 modulates neuropathic pain
progression through sponging miR-155. Oncotarget. 8:89949–89957.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu J, Shuang Z, Zhao J, Tang H, Liu P,
Zhang L, Xie X and Xiao X: Linc00152 promotes tumorigenesis by
regulating DNMTs in triple-negative breast cancer. Biomed
Pharmacother. 97:1275–1281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui M, Xiao Z, Wang Y, Zheng M, Song T,
Cai X, Sun B, Ye L and Zhang X: Long noncoding RNA HULC modulates
abnormal lipid metabolism in hepatoma cells through an
miR-9-mediated RXRA signaling pathway. Cancer Res. 75:846–857.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu F, Wu L, Wang A, Xu Y, Luo X, Liu X,
Hua Y, Zhang D, Wu S, Lin T, et al: MicroRNA-138 attenuates
epithelial-to-mesenchymal transition by targeting SOX4 in clear
cell renal cell carcinoma. Am J Transl Res. 9:3611–3622.
2017.PubMed/NCBI
|
|
11
|
Zhang F, Liao L, Ju Y, Song A and Liu Y:
Neurochemical plasticity of nitric oxide synthase isoforms in
neurogenic detrusor overactivity after spinal cord injury.
Neurochem Res. 36:1903–1909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pu Z, Zhang X, Chen Q, Yuan X and Xie H:
Establishment of an expression platform of OATP1B1 388GG and 521CC
genetic polymorphism and the therapeutic effect of tamoxifen in
MCF-7 cells. Oncol Rep. 33:2420–2428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barbie TU, Alexe G, Aref AR, Li S, Zhu Z,
Zhang X, Imamura Y, Thai TC, Huang Y, Bowden M, et al: Targeting an
IKBKE cytokine network impairs triple-negative breast cancer
growth. J Clin Invest. 124:5411–5423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gokal K, Wallis D, Ahmed S, Boiangiu I,
Kancherla K and Munir F: Effects of a self-managed home-based
walking intervention on psychosocial health outcomes for breast
cancer patients receiving chemotherapy: A randomised controlled
trial. Support Care Cancer. 24:1139–1166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalder M, Kyvernitakis I, Albert US,
Baier-Ebert M and Hadji P: Effects of zoledronic acid versus
placebo on bone mineral density and bone texture analysis assessed
by the trabecular bone score in premenopausal women with breast
cancer treatment-induced bone loss: Results of the ProBONE II
substudy. Osteoporos Int. 26:353–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chirico A, D'Aiuto G, Penon A, Mallia L,
de Laurentiis M, Lucidi F, Botti G and Giordano A: Self-efficacy
for coping with cancer enhances the effect of reiki treatments
during the Pre-surgery phase of breast cancer patients. Anticancer
Res. 37:3657–3665. 2017.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren JG, Seth P, Ye H, Guo K, Hanai JI,
Husain Z and Sukhatme VP: Citrate suppresses tumor growth in
multiple models through inhibition of glycolysis, the tricarboxylic
acid cycle and the IGF-1R pathway. Sci Rep. 7:45372017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu H, Dai M, Chen X, Chen X, Qin S and
Dai S: Integrated analysis of the potential roles of miRNAmRNA
networks in triple negative breast cancer. Mol Med Rep.
16:1139–1146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka H, Hazama S, Iida M, Tsunedomi R,
Takenouchi H, Nakajima M, Tokumitsu Y, Kanekiyo S, Shindo Y,
Tomochika S, et al: miR-125b-1 and miR-378a are predictive
biomarkers for the efficacy of vaccine treatment against colorectal
cancer. Cancer Sci. 108:2229–2238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murphy JP and Pinto DM: Temporal proteomic
analysis of IGF-1R signalling in MCF-7 breast adenocarcinoma cells.
Proteomics. 10:1847–1860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kucab JE and Dunn SE: Role of IGF-1R in
mediating breast cancer invasion and metastasis. Breast Dis.
17:41–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heskamp S, van Laarhoven HW,
Molkenboer-Kuenen JD, Franssen GM, Versleijen-Jonkers YM, Oyen WJ,
van der Graaf WT and Boerman OC: ImmunoSPECT and immunoPET of
IGF-1R expression with the radiolabeled antibody R1507 in a
triple-negative breast cancer model. J Nucl Med. 51:1565–1572.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li ZH, Xiong QY, Xu L, Duan P, Yang QO,
Zhou P and Tu JH: miR-29a regulated ER-positive breast cancer cell
growth and invasion and is involved in the insulin signaling
pathway. Oncotarget. 8:32566–32575. 2017.PubMed/NCBI
|
|
25
|
Chen Z, He A, Wang D, Liu Y and Huang W:
Long noncoding RNA HOTTIP as a novel predictor of lymph node
metastasis and survival in human cancer: A systematic review and
meta-analysis. Oncotarget. 8:14126–14132. 2017.PubMed/NCBI
|
|
26
|
Liu C, Liu Z, Li X, Tang X, He J and Lu S:
MicroRNA-1297 contributes to tumor growth of human breast cancer by
targeting PTEN/PI3K/AKT signaling. Oncol Rep. 38:2435–2443. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iskender B, Izgi K, Sakalar C and Canatan
H: Priming hMSCs with a putative anti-cancer compound,
myrtucommulone-a: A way to harness hMSC cytokine expression via
modulating PI3K/Akt pathway? Tumour Biol. 37:1967–1981. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin Y, Deng W, Pang J, Kemper T, Hu J, Yin
J, Zhang J and Lu M: The microRNA-99 family modulates hepatitis B
virus replication by promoting IGF-1R/PI3K/Akt/mTOR/ULK1
signaling-induced autophagy. Cell Microbiol. 19:2017.doi:
10.1111/cmi.12709. View Article : Google Scholar
|
|
29
|
Ma Y, Fu S, Lu L and Wang X: Role of
androgen receptor on cyclic mechanical stretch-regulated
proliferation of C2C12 myoblasts and its upstream signals:
IGF-1-mediated PI3K/Akt and MAPKs pathways. Mol Cell Endocrinol.
450:83–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farr CJ, Easty DJ, Ragoussis J, Collignon
J, Lovell-Badge R and Goodfellow PN: Characterization and mapping
of the human SOX4 gene. Mamm Genome. 4:577–584. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Huo X, Yang XR, He J, Cheng L,
Wang N, Deng X, Jin H, Wang N, Wang C, et al: STAT3-mediated
upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer
metastasis by regulating SOX4. Mol Cancer. 16:1362017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang T, Huan L, Zhang S, Zhou H, Gu L,
Chen X and Zhang L: MicroRNA-212 functions as a tumor-suppressor in
human non-small cell lung cancer by targeting SOX4. Oncol Rep.
38:2243–2250. 2017. View Article : Google Scholar : PubMed/NCBI
|