Introduction
Osteosarcoma is the most common primary malignant
bone tumor, particularly among children and adolescents (1). The estimated incidence rate is 5 per
million per year. With the introduction of adjuvant and
neo-adjuvant chemotherapy into multimodal therapies, the 5-year
survival rate for patients with localized osteosarcoma was improved
to 60–70%. However, the 5-year survival rate for patients with
metastatic disease or relapse is only 20%, and the current
treatment strategies have a limited efficacy (2). Therefore, it is essential to identify
more efficient prognostic factors to develop innovative and
promising therapeutics, so as to further improve the prognosis of
patients with osteosarcoma.
It has long been established that the human
epidermal growth factor (EGF) receptor (HER) family is frequently
overexpressed in the majority of human carcinomas (3). Comprised of EGFR, HER-2, HER-3 and
HER-4, the family makes homodimers or heterodimers to form EGFRs.
Aberrant receptor activation is involved in the improvement of the
proliferation and survival of cancer cells (4).
The overexpression of EGFR in 50–70% of lung, colon
and breast carcinoma cases contributes to cell proliferation, cell
cycle progression and survival (5).
High levels of EGFR are also associated with bone metastasis
(6) and a poor prognosis in human
cancer cases (7). Aberrant expression
of EGFR has been reported in osteosarcoma. However, EGFR
immunohistochemistry studies on osteosarcoma have revealed
discrepancies ranging from no prognostic value to a good clinical
outcome (8–10). HER-2 has been known to be
overexpressed in 20–25% of all ovarian and breast cancer cases, in
35–45% of all pancreatic adenocarcinomas and in up to 90% of
colorectal carcinomas (11).
Overexpression of HER-2 may serve a role in high-grade osteosarcoma
(12). However, favorable,
unfavorable and no independent prognostic significances for HER-2
expression in osteosarcoma have been detected (13–15). The
prognostic value of HER-2 remains controversial, and the efficacy
of HER-2-targeting therapy in the osteosarcoma patient population
has not been established (16).
Increased expression of HER-3 protein has been reported in 50–70%
of human breast cancer cases, and it appears to be associated with
tumor size, metastasis and recurrence (17). Whereas the majority of studies
observed a negative expression pattern for HER-3 across
osteosarcoma cell lines and tumor specimens (18,19), one
previous study showed that HER-4 was involved in the tumorigenicity
of osteosarcoma cells as a protective factor against various
extracellular apoptotic stimuli (20). The associations of HER-3 and HER-4
expression with the survival rate of osteosarcoma patients thus
require further investigation.
To the best of our knowledge, thus far, there have
been no comprehensive studies on the expression of all four members
of the HER family and their associations with the prognosis of
patients with osteosarcoma. Therefore, the aim of the present study
was to investigate the expression levels of the complete members of
the HER family, as well as their co-expression in osteosarcoma
specimens from 60 patients. In addition, any associations of the
expression of the HER family members with clinicopathological
parameters, progression-free survival (PFS) and overall survival
(OS) time in patients with osteosarcoma were evaluated.
Patients and methods
Patients
A total of 72 patients with primary osteosarcoma who
underwent surgical resection at the Department of Orthopedics, The
First Affiliated Hospital of Fujian Medical University (Fuzhou,
Fujian, China) between January 1, 2008, and December 31, 2013, were
selected retrospectively. Of these, 12 cases with distant
metastases at diagnosis or those with defective clinical data were
excluded. The present study was approved by the Institutional
Review Board of The First Affiliated Hospital of Fujian Medical
University, and the protocols conformed to the ethical guidelines
of the Declaration of Helsinki. All participants involved in this
study provided written informed consent. The patients' medical
records, including sex, age at diagnosis, primary tumor size and
location, histological subtype, Enneking stage (21) and distant metastasis status were
reviewed. All patients received the standardized preoperative
neoadjuvant chemotherapy and postoperative chemotherapy for six
courses of treatment. Chemotherapy treatment was performed with
doxorubicin (60–80 mg/m2, intravenous drip for 3 days),
cisplatin (100 mg/m2, intravenous drip over one day) and
ifosfamide (8–12 mg/m2, intravenous drip for 5 days)
twice prior to the operation and 4 times following the operation.
Ifosfamide was replaced with methotrexate (8–12 mg/m2,
intravenous drip over one day) if the tumor necrosis rate was
<90%. The 60 formalin-fixed and paraffin-embedded surgical tumor
samples were obtained from the archives of the Department of
Pathology (The First Affiliated Hospital of Fujian Medical
University) for immunohistochemical staining.
The study cohort consisted of 39 men and 21 women
with a mean age of 24 years (range, 4–55 years). A total of 25
patients presented with a primary tumor size of <5 cm and 35
patients with a tumor size of ≥5 cm. Stage I–IIA disease was
diagnosed in 16 tumors, and stage IIB in 44 tumors, according to
the Enneking staging system. The location of the tumor was the
distal femur in 22 patients; shaft of femur in 9 patients; proximal
tibia and humerus in 6 patients each; pelvis and distal radius in 4
patients; and proximal femur, scapula and maxilla in 3 patients.
Histological subtypes were osteoblastic in 41 tumors,
chondroblastic in 8, fibroblastic in 6 and special types including
telangiectatic in 3 and small cell type in 2. Subsequent to
surgical resection, all patients were monitored by X-ray, lung CT
scans and/or bone scanning every 3 months during the first 3 years
and every 6 months thereafter, in order to evaluate the development
of local recurrence and distant metastases. PFS time was defined as
the interval between the date of diagnosis and first tumor
progression or the last follow-up. OS time was defined as the
interval between diagnosis and mortality or the last follow-up. The
mean follow-up time was 44.9 months (range, 13–86 months). Among
the 60 patients, 21 succumbed to osteosarcoma, 27 patients showed
no evidence of disease and 12 remained alive with disease at the
last follow-up.
Immunohistochemistry
Archival osteosarcoma specimens resected following
neoadjuvant chemotherapy were examined for the expression of all
four HER family members by immunohistochemical analysis, and 60
corresponding osteochondroma tissues were used as controls. All
specimens were fixed in 10% formalin for 24–48 h at room
temperature, embedded in paraffin, serially sectioned (4-µm thick),
and stained with hematoxylin (room temperature for 10 min) and
eosin (room temperature for 1 min) for histological observation
under a light microscope (magnification, ×200). The PV9000
immunohistochemical kit (Origene Technologies, Inc., Beijing,
China) was used to perform the two-stage immunohistochemical
method. Subsequent to incubation for 1 h at 60°C, the tissue
sections were deparaffinized, dehydrated and incubated with 3%
hydrogen peroxide for 10 min at room temperature to block
endogenous peroxidase activity. During antigen retrieval process,
the sections were microwaved in citrate buffer (pH 6.0) for 2 min
and then naturally cooled to room temperature. The sections were
then immunostained with anti-EGFR (rabbit monoclonal EP38Y; cat no.
ab52894; 1:50 dilution), anti-HER-2 (mousemonoclonalHRB2/451; cat
no. ab187288; 1:100 dilution), anti-HER-3 (mouse monoclonalRTJ2;
cat no. ab20161; 1:50 dilution) or anti-HER-4 (mouse
monoclonal5G6B4; cat. no. ab204959; 1:50 dilution) (all Abcam,
Cambridge, MA, USA) and incubated overnight at 4°C. Next, the
sections were incubated with Polymer Helper reagent (Origene
Technologies, Inc.) for 20 min at 37°C and rinsed with
phosphate-buffered saline (PBS). The sections were then incubated
with poly peroxidase-anti-mouse/rabbit IgG (part of the PV9000 kit;
ready-to-use dilution) for 20 min at room temperature. Subsequent
to being washed again, the sections were stained with
diaminobenzidine (both Origene Technologies, Inc.) for 3–5 min at
room temperature, counterstained with hematoxylin for 2 min at room
temperature, dehydrated, and mounted. Negative (PBS rather than
primary antibodies) and known positive controls (esophagus
carcinoma tissue for EGFR; breast carcinoma tissue for HER-2; oral
carcinoma tissue for HER-3 and HER-4) were stained in parallel with
each set of sections studied.
Observation indices and result
determination
Two pathologists participated in the staining
assessment of tumor specimens independently. EGFR-positive and
HER-2-positive cells were those with specific brown particles in
the cytoplasm or cytomembrane. Cells with brown-stained cytoplasmic
or nuclear particles were determined as HER-3-positive or
HER-4-positive cells. Expression was scored according to the
intensity of staining of tumor cells from 0 to 3 as follows: 0,
negative; 1, weakly positive; 2, moderately positive; and 3,
strongly positive. The mean percentage from 10 random high-power
fields of staining of positive tumor cells was also scored from 1
to 3 as follows: 1, <25%; 2, 25–75%; and 3, >75%. For
statistical analysis, the final score was calculated by the product
of the density and the percentage of positive staining tumor cells,
including scores 0, 1, 2, 3, 4, 6 and 9. Scores >2 were defined
as high expression, while scores ≤2 were considered as low
expression (22).
Statistical analysis
The associations between HER family expression and
clinicopathological parameters, including sex, age, tumor size,
tumor location, histological subtype, Enneking stage and distant
metastasis, were analyzed using the χ2 test and Fisher's exact
test. The correlation between the expression of HER family members
was investigated using Spearman's rank coefficient, and the
difference between them was assessed by Friedman test, with the
box-plot obtained using GraphPad Prism 5.0 (GraphPad Software Inc.,
San Diego, CA, USA). Survival analysis was performed using the
Kaplan-Meier method, and differences in survival distributions were
compared by the log-rank test. The Cox proportional hazards
regression model was adopted to perform multivariate survival
analysis for the expression of HER family members that was found to
be significant in the univariate analysis (23). All statistical analyses were performed
using SPSS 19.0 software (IBM, Corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Immunohistochemical expression of HER
family members in osteosarcoma
Of the 60 osteosarcoma patients,18 (30%), 13 (22%),
23 (38%) and 19 (32%) cases presented with EGFR, HER-2, HER-3 and
HER-4 high expression, respectively, the expression rates of which
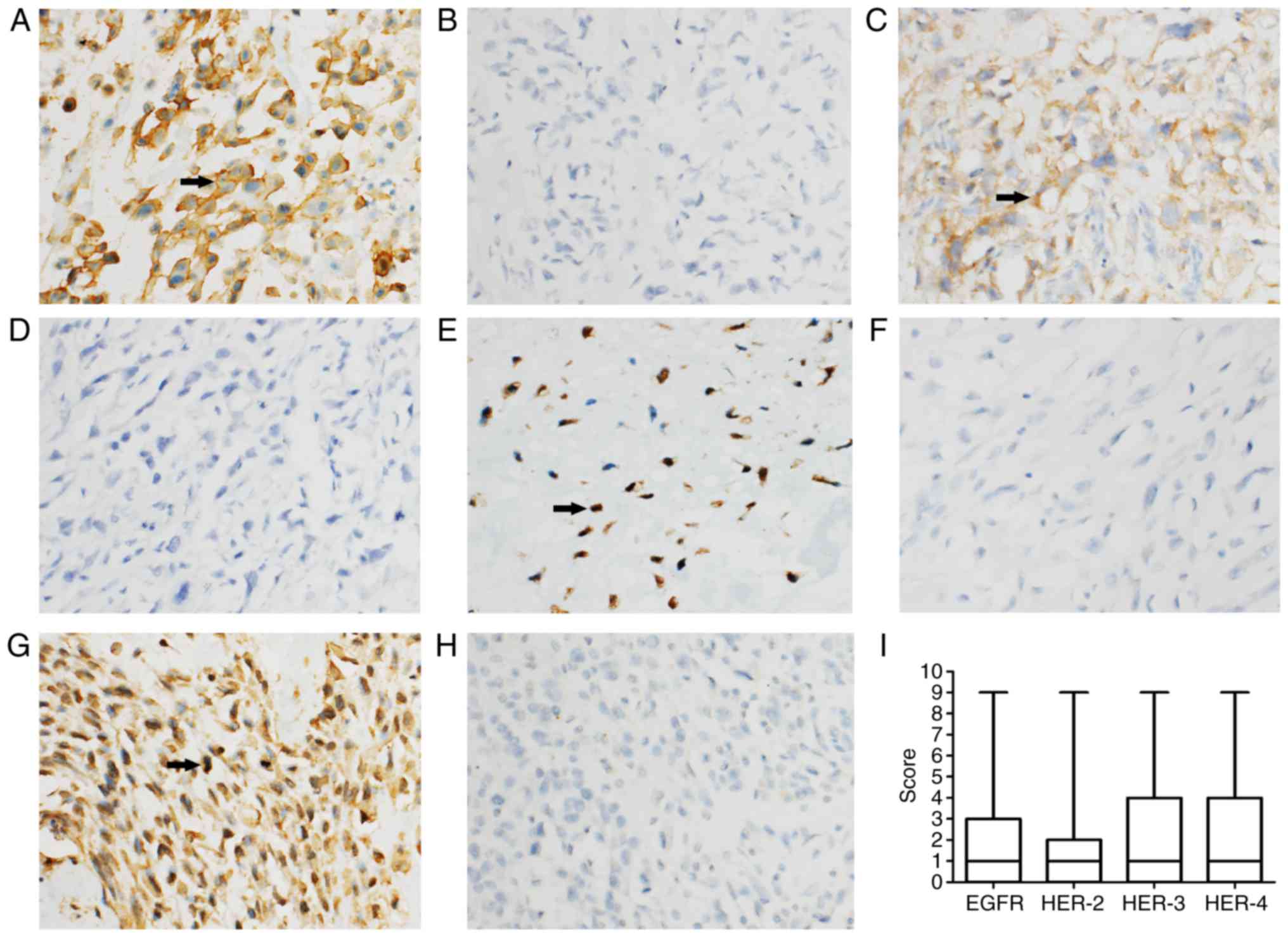
were significantly higher than those of osteochondroma (Table I). EGFR was predominantly membranous,
with some cytoplasmic staining. HER-2 demonstrated a cytoplasmic
staining pattern. HER-3 and HER-4 demonstrated nuclear and
cytoplasmic immunostaining (Fig. 1).
Fig. 1I shows the median score of HER
expression (the horizontal line), the inter-quartile range (the
box), and the minimum and maximum values (the whiskers) (24). Since the final scores, including 0, 1,
2, 3, 4, 6 and 9, were of skewed distribution, large error bars are
present in this figure. There was no significant difference among
the expression levels of the HER family members (P>0.05,
Friedman test). The present study investigated the co-expression of
2, 3 and all 4 members of the HER family in osteosarcoma cases,
which were not found in osteochondroma cases. Among which, the
expression rates of EGFR/HER-2, EGFR/HER-3, HER-2/HER-3 and
HER-3/HER-4 between the two groups were statistically different.
EGFR, HER-2, HER-3 and HER-4 were all expressed together in only 1
case of osteosarcoma (Table I). There
was no correlation among the expression levels of the HER family
members, as determined using the Spearman's rank correlation in
this study (Table II).
 | Table I.Comparisons of HER family expression
in osteosarcoma (n=60) and corresponding osteochondroma (n=60)
cases. |
Table I.
Comparisons of HER family expression
in osteosarcoma (n=60) and corresponding osteochondroma (n=60)
cases.
| HER family
member | Osteosarcoma, n
(%) | Osteochondroma, n
(%) | P-value |
|---|
| EGFR | 18 (30) | 2 (3) | <0.001 |
| HER-2 | 13 (22) | 1 (0) | 0.002 |
| HER-3 | 23 (38) | 7 (13) | 0.004 |
| HER-4 | 19 (32) | 2 (3) | <0.001 |
| EGFR/HER-2 | 6 (10) | 0 (0) | 0.027 |
| EGFR/HER-3 | 10 (17) | 0 (0) | 0.001 |
| EGFR/HER-4 | 5 (8) | 0 (0) | NS |
| HER-2/HER-3 | 6 (10) | 0 (0) | 0.027 |
| HER-2/HER-4 | 4 (7) | 0 (0) | NS |
| HER-3/HER-4 | 7 (12) | 0 (0) | 0.013 |
|
EGFR/HER-2/HER-3 | 3 (5) | 0 (0) | NS |
|
EGFR/HER-2/HER-4 | 1 (2) | 0 (0) | NS |
|
EGFR/HER-3/HER-4 | 2 (3) | 0 (0) | NS |
|
HER-2/HER-3/HER-4 | 3 (5) | 0 (0) | NS |
|
EGFR/HER-2/HER-3/HER-4 | 1 (2) | 0 (0) | NS |
 | Table II.Correlations among HER family members
for patients with osteosarcoma. |
Table II.
Correlations among HER family members
for patients with osteosarcoma.
| EGFR | HER-3 | HER-4 |
|---|
|
|
|
|
|---|
| HER family
member | Correlation
coefficient | P-value | Correlation
coefficient | P-value | Correlation
coefficient | P-value |
|---|
| HER-2 | 0.185 | 0.156 | 0.085 | 0.520 | −0.010 | 0.939 |
| HER-3 | 0.232 | 0.075 | – | – | −0.021 | 0.874 |
| HER-4 | −0.055 | 0.678 | −0.021 | 0.874 | – | – |
Association between HER family member
expression and clinicopathological characteristics
The results of the associations between HER family
member expression and clinicopathological characteristics are
summarized in Table III. The high
expression of EGFR (P=0.027) and HER-4 (P=0.013) were associated
with distant metastasis. HER-3 high expression was significantly
associated with an advanced Enneking stage (P=0.029) and distant
metastasis (P=0.013). Other statistically significant associations
between HER family member expression and the remaining
clinicopathological parameters were not found in this study.
 | Table III.Associations between HER family
member expression and clinicopathological characteristics. |
Table III.
Associations between HER family
member expression and clinicopathological characteristics.
|
|
| EGFR | HER-2 | HER-3 | HER-4 |
|---|
|
|
|
|
|
|
|
|---|
| Clinicopathological
data | Cases, n | HE (n=18) | LE (n=42) | P-value | HE (n=13) | LE (n=47) | P-value | HE (n=23) | LE (n=37) | P-value | HE (n=19) | LE (n=41) | P-value |
|---|
| Sex |
|
Male | 39 | 9 | 30 | NS | 9 | 30 | NS | 13 | 26 | NS | 11 | 28 | NS |
|
Female | 21 | 9 | 12 | | 4 | 17 | | 10 | 11 | | 8 | 13 |
|
| Age, years |
|
<18 | 26 | 9 | 17 | NS | 5 | 21 | NS | 11 | 15 | NS | 7 | 19 | NS |
|
≥18 | 34 | 9 | 25 | | 8 | 26 | | 12 | 22 | | 12 | 22 | |
| Tumor size, cm |
|
<5 | 25 | 6 | 19 | NS | 4 | 21 | NS | 9 | 16 | NS | 5 | 20 | NS |
| ≥5 | 35 | 12 | 23 | | 9 | 26 | | 14 | 21 | | 14 | 21 | |
| Tumor location |
|
Tibia/Femur | 40 | 9 | 31 | NS | 9 | 31 | NS | 16 | 24 | NS | 13 | 27 | NS |
| Other
location | 20 | 9 | 11 | | 4 | 16 | | 7 | 13 | | 6 | 14 |
|
| Histological
subtype |
|
Conventional | 55 | 16 | 39 | NS | 12 | 43 | NS | 21 | 34 | NS | 18 | 37 | NS |
|
Special | 5 | 2 | 3 | | 1 | 4 | | 2 | 3 | | 1 | 4 |
|
| Surgical stage |
|
I–IIA | 16 | 4 | 12 | NS | 3 | 13 | NS | 3 | 13 | 0.029 | 4 | 12 | NS |
|
IIB | 44 | 14 | 30 | | 10 | 34 | | 20 | 24 | | 15 | 29 | |
| Distant
metastasis |
|
Yes | 27 | 12 | 15 | 0.027 | 9 | 18 | NS | 15 | 12 | 0.013 | 13 | 14 | 0.013 |
| No | 33 | 6 | 27 | | 4 | 29 | | 8 | 25 | | 6 | 27 | |
Impact of HER family member expression
on osteosarcoma patient survival
To evaluate whether the prognostic ability of HER
family members was affected by clinicopathological features,
univariate and multivariate analyses were performed. Results among
stage I–IIB patients (n=60) showed that high expression of EGFR
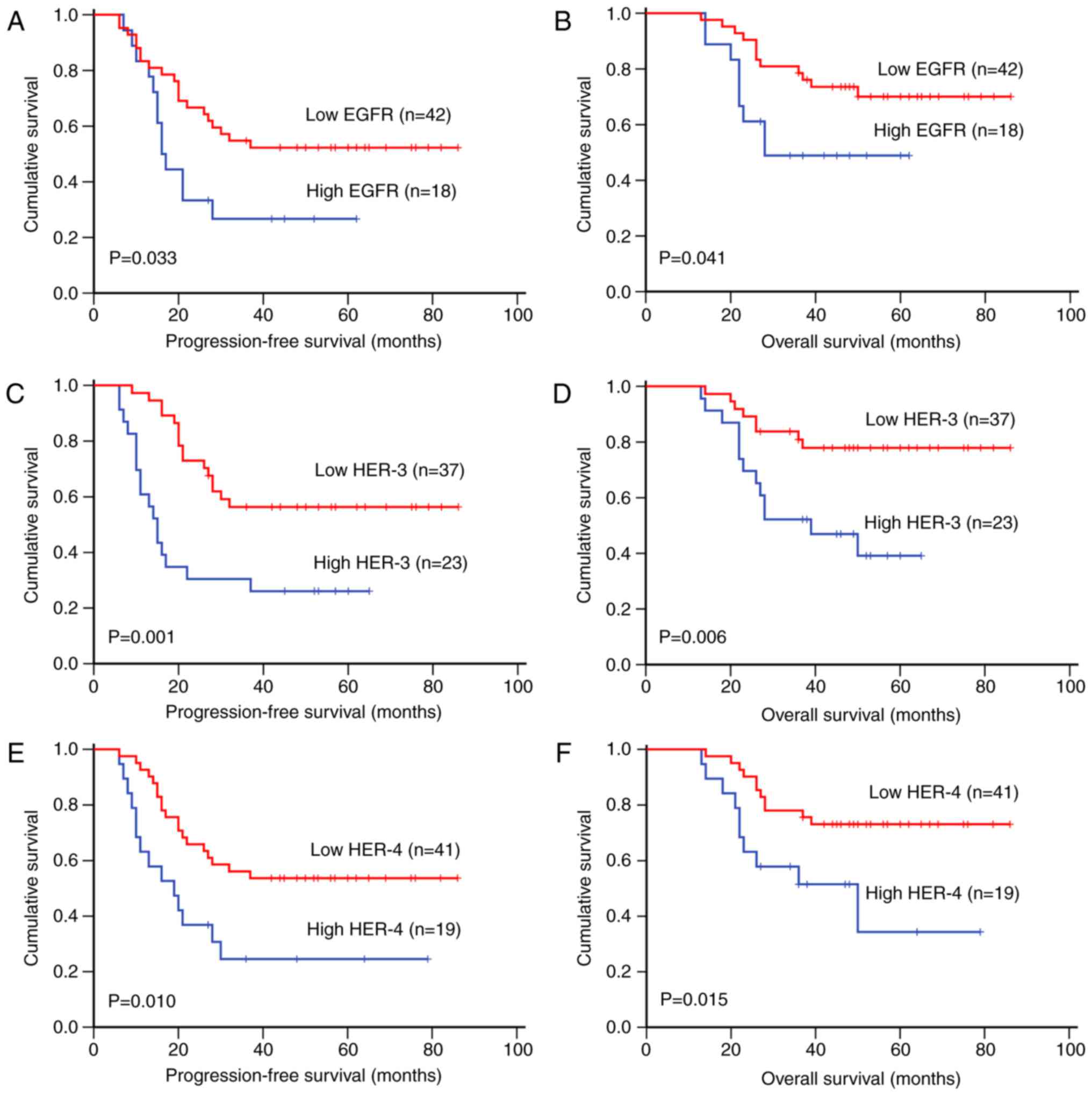
(PFS, P=0.033; OS, P=0.041; Fig. 2A and
B), HER-3 (PFS, P=0.001; OS, P=0.006; Fig. 2C and D), HER-4 (PFS, P=0.010; OS,
P=0.015; Fig. 2E and F), EGFR/HER-3
(PFS, P=0.010; OS, P=0.003), EGFR/HER-4 (PFS, P=0.033; OS,
P=0.033), HER-2/HER-4 (PFS, P=0.001; OS, P=0.003) and HER-3/HER-4
(PFS, P<0.001; OS, P<0.001), in addition to tumor size,
surgical stage and distant metastasis, were associated with short
PFS and OS time upon univariate analysis (Table IV). Upon multivariate analysis with
adjustment for tumor size, surgical stage and distant metastasis,
the levels of EGFR, HER-3, HER-4, EGFR/HER-3, EGFR/HER-4 and
HER-3/HER-4 were found to be independent predictors of poor PFS and
OS time of osteosarcoma patients (Table
IV). In patients with stage IIB disease only (n=44), which was
the predominant surgical stage of primary osteosarcoma, univariate
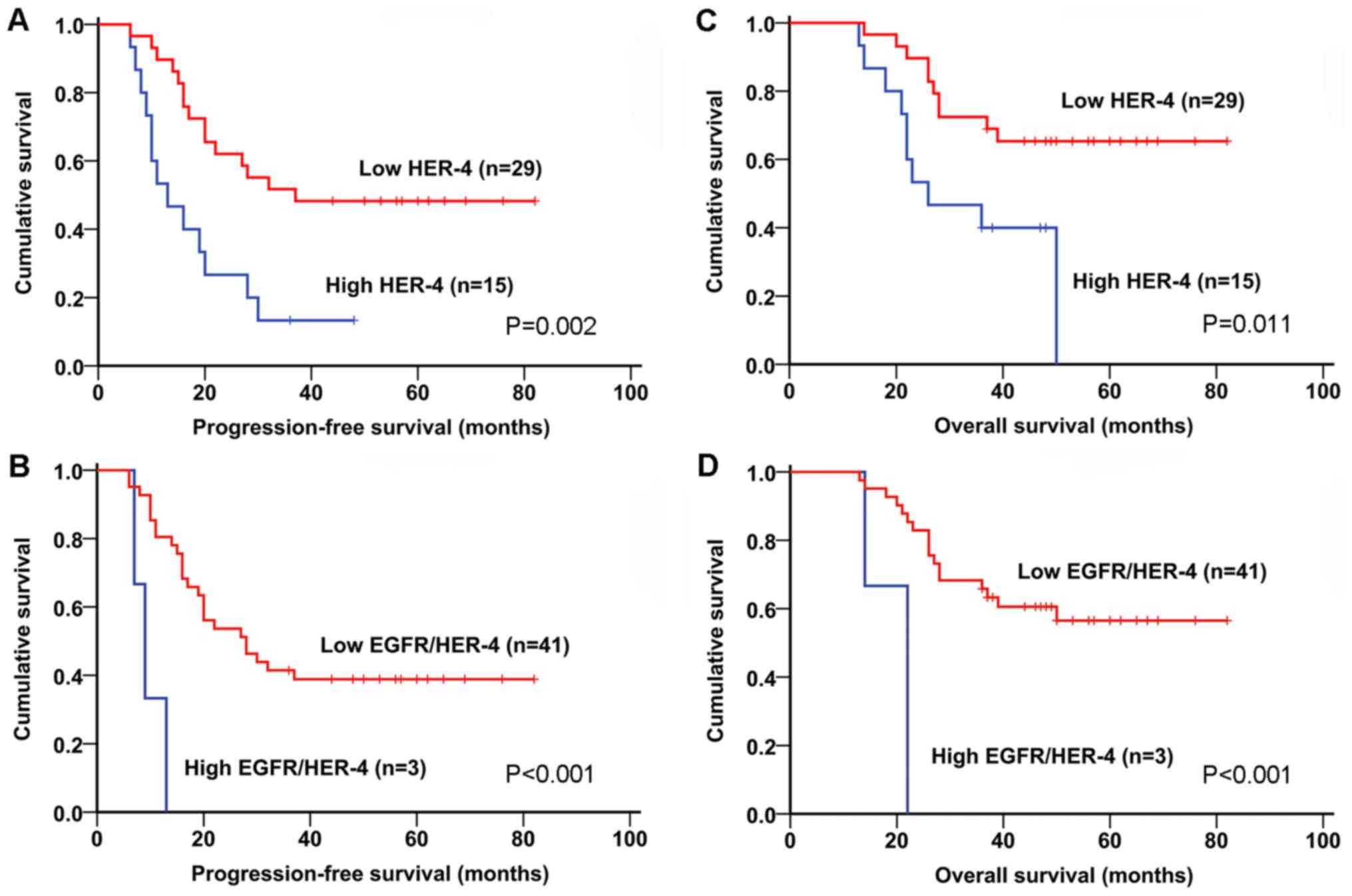
analysis demonstrated that EGFR (P<0.001), HER-3 (P=0.002),
HER-4 (P=0.002; Fig. 3A), EGFR/HER-3
(P<0.001), EGFR/HER-4 (P<0.001; Fig. 3B), HER-2/HER-4 (P=0.007) and
HER-3/HER-4 (P<0.001), as well as tumor size and distant
metastasis were associated with worse PFS time. Finally, upon
multivariate analysis, EGFR, HER-4, EGFR/HER-3 and EGFR/HER-4
showed greater effects on PFS time in osteosarcoma patients with
stage IIB than stage I–IIB (Table
IV). With regard to OS time, EGFR (P=0.001), HER-4 (P=0.011;
Fig. 3C), EGFR/HER-3 (P=0.004),
EGFR/HER-4 (P<0.001; Fig. 3D),
HER-2/HER-4 (P=0.022) and HER-3/HER-4 (P=0.003) were also
significant predictors in univariate analysis. Multivariate
analysis suggested that HER-4, EGFR/HER-4, and HER-3/HER-4 had
greater effects on OS time in osteosarcoma patients with IIB than
stage I–IIB (Table IV). Therefore,
expression of HER-4 and EGFR/HER-4 demonstrated greater impacts on
PFS and OS time in patients with stage IIB osteosarcoma. In this
study, HER-2, EGFR/HER-2 and HER-2/HER-3 demonstrated no prognostic
significance among stage I–IIB and stage IIB patients upon
univariate analysis (data not shown), thus were not included in
multivariate analysis demonstrated in Table IV. The potential disadvantage of this
approach was that variables with P>0.05 in univariate analysis
may finally be significant in the further multivariate analysis, as
demonstrated by a previous study (25). However, the three aforementioned
receptors were verified to exhibit no statistical significance upon
a different multivariate analysis including all variables (data not
shown). Consequently, the survival analyses did not cause bias to
the results.
 | Table IV.Univariate and multivariate analyses
of variables with regard to survival of osteosarcoma patients. |
Table IV.
Univariate and multivariate analyses
of variables with regard to survival of osteosarcoma patients.
|
|
| Progression-free
survival in months | Overall survival in
months |
|---|
|
|
|
|
|
|---|
|
|
|
| Multivariate
analysis |
| Multivariate
analysis |
|---|
|
|
|
|
|
|
|
|---|
| Variables | Comparison | Univariate
P-value | Hazard ratio | 95% CI | P-value | Univariate
P-value | Hazard ratio | 95% CI | P-value |
|---|
| Stages I–IIB cases
(n=60) |
|
EGFR | High/low | 0.033 | 2.7 | 1.2–5.9 | 0.015 | 0.041 | 3.2 | 1.3–8.2 | 0.014 |
|
HER-3 | High/low | 0.001 | 3.5 | 1.7–7.3 | 0.001 | 0.006 | 2.7 | 1.1–6.7 | 0.032 |
|
HER-4 | High/low | 0.010 | 2.3 | 1.1–4.8 | 0.022 | 0.015 | 2.7 | 1.1–6.5 | 0.033 |
|
EGFR/HER-3 | High/low | 0.010 | 3.3 | 1.4–8.1 | 0.008 | 0.003 | 3.6 | 1.4–9.5 | 0.009 |
|
EGFR/HER-4 | High/low | 0.033 | 3.7 | 1.1–12.7 | 0.035 | 0.033 | 7.1 | 1.7–30.0 | 0.007 |
|
HER-2/HER-4 | High/low | 0.001 | 2.5 | 0.8–7.5 | 0.113 | 0.003 | 2.2 | 0.6–8.0 | 0.214 |
|
HER-3/HER-4 | High/low | <0.001 | 18.6 | 5.4–63.9 | <0.001 | <0.001 | 4.1 | 1.5–10.9 | 0.005 |
| Tumor size, cm | <5/≥5 | 0.025 | – | – | – | 0.037 | – | – | – |
| Surgical stage | I–IIA/IIB | 0.034 | – | – | – | 0.009 | – | – | – |
| Distant
metastasis | Yes/no | <0.001 | – | – | – | <0.001 | – | – | – |
| Stages IIB cases
only (n=44) |
|
EGFR | High/low | <0.001 | 3.3 | 1.4–7.8 | 0.006a | 0.001 | 3.2 | 1.2–8.3 | 0.017 |
|
HER-3 | High/low | 0.002 | 4.1 | 1.8–9.1 | 0.001 | 0.085 | – | – | – |
|
HER-4 | High/low | 0.002 | 2.6 | 1.2–5.8 | 0.015a | 0.011 | 3.0 | 1.2–7.4 | 0.016a |
|
EGFR/HER-3 | High/low | <0.001 | 4.4 | 1.6–12.2 | 0.004a | 0.004 | 3.1 | 1.1–8.6 | 0.029 |
|
EGFR/HER-4 | High/low | <0.001 | 9.3 | 2.2–39.1 | 0.002a | <0.001 | 7.6 | 1.9–30.8 | 0.004a |
|
HER-2/HER-4 | High/low | 0.007 | 2.4 | 0.8–7.5 | 0.121 | 0.022 | 2.3 | 0.6–8.2 | 0.210 |
|
HER-3/HER-4 | High/low | <0.001 | 18.2 | 5.0–66.8 | <0.001 | 0.003 | 4.1 | 1.6–11.0 | 0.004a |
| Tumor size, cm | <5/≥5 | 0.017 | – | – | – | 0.055 | – | – | – |
| Distant
metastasis | Yes/no | 0.001 | – | – | – | 0.004 | – | – | – |
Discussion
Previous studies on EGFR and HER-2 expression in
osteosarcoma have created controversy over their roles in
osteosarcoma survival (9,10,14,15).
Furthermore, no previous study has simultaneously investigated the
associations between the expression of all four members of the HER
family and their prognostic significance in osteosarcoma. In the
present study, the high expression levels of EGFR, HER-2, HER-3 and
HER-4, and the co-expression of 2, 3 and all 4 members of the HER
family were identified. Furthermore, EGFR, HER-3 and HER-4
expression, as well as the expression of EGFR/HER-3, EGFR/HER-4 and
HER-3/HER-4 were found to be independent prognostic factors of poor
PFS and OS time in stage I–IIB osteosarcoma. The expression of
HER-4 and EGFR/HER-4 had more significant impacts on PFS and OS
time in osteosarcoma patients with stage IIB disease than in those
with stage I–IIB.
The present results showed high EGFR expression with
membranous and some cytoplasmic staining, which was significantly
associated with poor survival, indicating the involvement of EGFR
in the development of osteosarcoma. Probably due to the small
sample size and selection bias, studies by Do et al
(8) and Lee et al (9) did not find a similar association.
Kersting et al (10) observed
an association between EGFR expression and a favorable clinical
outcome. However, the efficacy of EGFR-targeting agents (cetuximab)
in the management of osteosarcoma does not support this conclusion
(26). Interplay of heterodimers of
the HER may not explain the unexpected finding either, since
EGFR/HER-3 and EGFR/HER-4 were found to be associated with the poor
survival of patients with osteosarcoma in the present study. Tumors
initially sensitive to anti-EGFR agent, such as cetuximab, often
develop resistance. Compensatory HER-3/PI3K/AKT signaling has been
confirmed as vital in the development of acquired resistance to
EGFR inhibitors (27). Dimerization
of HER-4 with EGFR is a crucial step to stimulate the HER-4
receptor. Interactions between EGFR and HER-4 regulate
stretch-induced differentiation of fetal lung cells via the ERK
pathway (28). In addition, a
previous study showed that co-expression of EGFR and HER-4
contributed to neoplastic transformation, and was demonstrated to
predict the invasion and poor clinical outcomes of oral squamous
cell carcinoma (29). The present
results supported the potential of EGFR within dual targeting
therapy, such as combined treatment with MEHD7945A (27), for refractory osteosarcoma in the
future.
The expression rate of HER-2 ranges from its
detection in 4 to 71.9% of patients (12,15,30) to the
absence of expression (31), as
determined by previous studies. These conflicting results may be
due to differences in technical method, specimen treatment,
antibodies used or results interpretation. The present results
revealed a HER-2 cytoplasmic staining pattern in 13 (22%)
osteosarcoma samples, with no prognostic significance, which is
supported by previous studies (13,32).
Scotlandi et al (33) observed
no therapeutic effectiveness for trastuzumab-driven therapy in
osteosarcoma. Since trastuzumab targets the extracellular domain of
HER-2, incomplete membranous immunoreactivity for HER-2 may induce
resistance to trastuzumab-driven therapy (34). Lapatinib potently and reversibly binds
to the intracellular domains of HER-2, and has been proven to alter
the malignant phenotype of osteosarcoma cells (35). Thus, lapatinib is supported as a
promising chemotherapeutic agent for the treatment of osteosarcoma.
Nevertheless, the correlation between the expression of HER-2 and
cancer regulation molecules, including tumor protein p53 or
retinoblastoma protein, and associated signaling pathways should be
confirmed in future studies.
In the present study, HER-3 demonstrated high
expression in 23 cases (38%), with a nuclear and cytoplasmic
staining pattern, which is different from the majority of previous
studies observing negative expression for HER-3 in osteosarcoma
cell lines and tumor specimens (18,19). The
unfavorable prognostic role of HER-3 expression in osteosarcoma
investigated in the present study may be explained by the two
following mechanisms. Firstly, downregulation of HER-3 contributes
to early osteoblast differentiation of mesenchymal stem cells
through increased Wnt/β-catenin signaling (36). However, the absence of nuclear
β-catenin staining and Wnt-luciferase activity have been found in
the biopsies and cell lines of osteosarcoma. Therefore, HER-3
overexpression-induced inactivation of the Wnt/β-catenin pathway
activity, which is required for osteoblast differentiation, may
contribute to osteosarcoma development (37). Secondly, overexpression of microRNA
(miR)-3928, which targets the HER-3 gene, induces cell apoptosis
and inhibits tumor growth. HER-3 overexpression induced by the
downregulated expression of miR-3928 in osteosarcoma may be another
factor promoting cell proliferation and tumor growth (38). HER-3/HER-4 expression was also found
to be significantly associated with worse PFS and OS time in the
present study. HER-3 and HER-4 were significantly co-expressed in a
large fraction of tumors (39) and
the co-expression pattern may represent a crucial intracellular
molecular switch in breast carcinogenesis (40). Agents trapping HER-3 in the inactive
conformation may provide an effective therapeutic strategy for
osteosarcoma patients.
Nuclear localization of HER-4 antigen in
osteosarcoma tumor specimens in the present study was generally
concordant with the protein expression measured by Hughes et
al (19). The cleavage of HER-4
by ligand binding leads to a Mr 80,000 fragment of HER-4
translocation to the nucleus (41).
The nuclear pattern of HER-4 expression observed by the present
study suggested an activated state. Upregulated HER-4 expression in
non-adherent tumor spheroids of osteosarcoma contributes to the
tolerance to anoikis, serum starvation and chemotherapy resistance
via regulating the survival pathway of osteosarcoma under various
cellular stress conditions (20).
HER-4 signaling is the key backup mechanism contributing to
acquired resistance to HER-targeted therapies, and knockdown of
HER-4, but not HER1-3, led to apoptosis in resistant breast cancer
cells (42). The noticeable adverse
effect of HER-4 expression on the survival of osteosarcoma patients
with stage I–IIB and stage IIB disease investigated in the present
study confirms the significant contribution of HER-4 to
osteosarcoma pathogenesis and outcome. Thus, pan-HER, particularly
HER-4-targeted inhibitors, may provide an insight into the
treatment of resistant osteosarcoma.
Certain limitations should be considered in the
present study. First, due to the retrospective nature of this
study, selection bias may have existed when collecting patient
information; a possibly randomized and prospective study would
further clarify the prognostic role of HER receptors in
osteosarcoma. Second, the application of immunohistochemistry and
semi-quantitative measures for the assessment of HER expression is
somewhat subjective and imposes limitations. The present findings
should promote further investigation into the quantitative analysis
of the receptors and associated molecular mechanisms using fresh
osteosarcoma specimens or cell lines. Third, the study cohort
included a relatively small number of patients, and the follow-up
to evaluate the patient survival was relatively short; further
large-scale research and a longer follow-up time would offer more
convincing evidence in the future.
In conclusion, the present study demonstrated that
the expression of EGFR, HER-3 and HER-4 together with the
heterodimerization of EGFR/HER-3, EGFR/HER-4 and HER-3/HER-4 may
contribute significantly to the unfavorable clinical outcome of
osteosarcoma patients. Therapies targeting EGFR, HER-3 and HER-4
may provide promising strategies for treating primary osteosarcoma,
if these results are confirmed by larger multicenter clinical
studies.
Acknowledgements
The authors would like to thank Dr Chen Yupeng and
Dr Zhang Zhenzhen from Department of Pathology, The First
Affiliated Hospital of Fujian Medical University for providing
instruction with regard to the immunohistochemistry protocols.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 31571292), the Joint Funds
for the Innovation of Science and Technology of Fujian Province
(grant no. 2016Y9019) and the Outstanding Youth Fund of Fujian
Province (grant no. 2017J06017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SLW and GXZ conducted the experiment and drafted the
manuscript. XWW and FQY contributed to statistical analysis and
manuscript writing. DFW and XXW participated in collecting data.
JHL conceived the present study and helped to revise the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of The First Affiliated Hospital of Fujian Medical
University, and the protocols conformed to the ethical guidelines
of the Declaration of Helsinki. All participants involved in the
present study provided written informed consent.
Consent for publication
Patient, parent or next of kin provided written
informed consent for the publication of any associated data and
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou W, Hao M, Du X, Chen K, Wang G and
Yang J: Advances in targeted therapy for osteosarcoma. Discov Med.
17:301–307. 2014.PubMed/NCBI
|
|
3
|
De Luca A, Carotenuto A, Rachiglio A,
Gallo M, Maiello MR, Aldinucci D, Pinto A and Normanno N: The role
of the EGFR signaling in tumor microenvironment. J Cell Physiol.
214:559–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendelsohn J and Baselga J: Epidermal
growth factor receptor targeting in cancer. Semin Oncol.
33:369–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Renoir JM, Marsaud V and Lazennec G:
Estrogen receptor signaling as a target for novel breast cancer
therapeutics. Biochem Pharmacol. 85:449–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siu MK, Abou-Kheir W, Yin JJ, Chang YS,
Barrett B, Suau F, Casey O, Chen WY, Fang L, Hynes P, et al: Loss
of EGFR signaling-regulated miR-203 promotes prostate cancer bone
metastasis and tyrosine kinase inhibitors resistance. Oncotarget.
5:3770–3784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu L, Wu H, Jiang C, Wang H, Gao B, Yan S,
Qi Y and Zhou S: Dacomitinib, a new pan-EGFR inhibitor, is
effective in killing ovarian cancer cells. Discov Med. 22:297–309.
2016.PubMed/NCBI
|
|
8
|
Do SI, Jung WW, Kim HS and Park YK: The
expression of epidermal growth factor receptor and its downstream
signaling molecules in osteosarcoma. Int J Oncol. 34:797–803.
2009.PubMed/NCBI
|
|
9
|
Lee JA, Ko Y, Kim DH, Lim JS, Kong CB, Cho
WH, Jeon DG, Lee SY and Koh JS: Epidermal growth factor receptor:
Is it a feasible target for the treatment of osteosarcoma? Cancer
Res Treat. 44:202–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kersting C, Gebert C, Agelopoulos K,
Schmidt H, van Diest PJ, Juergens H, Winkelmann W, Kevric M,
Gosheger G, Brandt B, et al: Epidermal growth factor receptor
expression in high-grade osteosarcomas is associated with a good
clinical outcome. Clin Cancer Res. 13:2998–3005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baxevanis CN, Sotiropoulou PA, Sotiriadou
NN and Papamichail M: Immunobiology of HER-2/neu oncoprotein and
its potential application in cancer immunotherapy. Cancer Immunol
Immunother. 53:166–175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abdou AG, Kandil M, Asaad NY, Dawoud MM,
Shahin AA and Eldayem Abd AF: The prognostic role of Ezrin and
HER2/neu expression in osteosarcoma. Appl Immunohistochem Mol
Morphol: AIMM. 24:355–363. 2016. View Article : Google Scholar
|
|
13
|
Yalcin B, Gedikoğlu G, Kutluk T, Varan A,
Akyüz C and Büyükpamukçu M: C-erbB-2 expression and prognostic
significance in osteosarcoma. Pediatric Blood Cancer. 51:222–227.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Q, Liu F, Wang B, Li Z, Zhou D, Yang
Q, Dong J and Li J: HER-2 expression in biopsy and surgical
specimen on prognosis of osteosarcoma: A systematic review and
meta-analysis of 16 studies. Medicine. 95:e36612016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akatsuka T, Wada T, Kokai Y, Kawaguchi S,
Isu K, Yamashiro K, Yamashita T, Sawada N, Yamawaki S and Ishii S:
ErbB2 expression is correlated with increased survival of patients
with osteosarcoma. Cancer. 94:1397–1404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geller DS and Gorlick R: HER-2 targeted
treatment of osteosarcoma: The challenges of developing targeted
therapy and prognostic factors for rare malignancies. Exp Opin
Pharmacother. 11:51–61. 2010. View Article : Google Scholar
|
|
17
|
Ma J, Lyu H, Huang J and Liu B: Targeting
of erbB3 receptor to overcome resistance in cancer treatment. Mol
Cancer. 13:1052014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hassan SE, Bekarev M, Kim MY, Lin J,
Piperdi S, Gorlick R and Geller DS: Cell surface receptor
expression patterns in osteosarcoma. Cancer. 118:740–749. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hughes DP, Thomas DG, Giordano TJ, Baker
LH and McDonagh KT: Cell surface expression of epidermal growth
factor receptor and Her-2 with nuclear expression of Her-4 in
primary osteosarcoma. Cancer Res. 64:2047–2053. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hua Y, Yang Y and Hughes D: Abstract 197:
Multi-cellular tumor spheroids in vitro model reveals a critical
role of ERBB4 in survival of osteosarcoma. Cancer Res. 71:197.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 106–120. 1980.PubMed/NCBI
|
|
22
|
Srirajaskanthan R, Shah T, Watkins J,
Marelli L, Khan K and Caplin ME: Expression of the HER-1–4 family
of receptor tyrosine kinases in neuroendocrine tumours. Oncol Rep.
23:909–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Q, Hao L, Lou Z, Gao X, Gong H, Hong
Y, Fu C and Zhang W: Survival time and prognostic factors of
patients with initial noncurative colorectal liver metastases.
Medicine (Baltimore). 96:e88312017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu H, Sharp GC, Zhao Q, Shirato H and
Jiang SB: Statistical analysis and correlation discovery of tumor
respiratory motion. Phys Med Biol. 52:4761–4774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishikawa H, Nishijima N, Arimoto A,
Inuzuka T, Kita R, Kimura T and Osaki Y: Prognostic factors in
patients with hepatitis B virus-related hepatocellular carcinoma
undergoing nucleoside analog antiviral therapy. Oncol Lett.
6:1213–1218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pahl JH, Ruslan SE, Buddingh EP, Santos
SJ, Szuhai K, Serra M, Gelderblom H, Hogendoorn PC, Egeler RM,
Schilham MW and Lankester AC: Anti-EGFR antibody cetuximab enhances
the cytolytic activity of natural killer cells toward osteosarcoma.
Clin Cancer Res. 18:432–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang S, Li C, Armstrong EA, Peet CR,
Saker J, Amler LC, Sliwkowski MX and Harari PM: Dual targeting of
EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR
inhibitors and radiation. Cancer Res. 73:824–833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Z, Wang Y, Nayak PS, Dammann CE and
Sanchez-Esteban J: Stretch-induced fetal type II cell
differentiation is mediated via ErbB1-ErbB4 interactions. J Biol
Chem. 287:18091–18102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silva SD, Alaoui-Jamali MA, Hier M, Soares
FA, Graner E and Kowalski LP: Cooverexpression of ERBB1 and ERBB4
receptors predicts poor clinical outcome in pN+ oral squamous cell
carcinoma with extranodal spread. Clin Exp Metastasis. 31:307–316.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anninga JK, van de Vijver MJ,
Cleton-Jansen AM, Kristel PM, Taminiau AH, Nooij M, Egeler RM and
Hogendoorn PC: Overexpression of the HER-2 oncogene does not play a
role in high-grade osteosarcomas. Eur J Cancer. 40:963–970. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maitra A, Wanzer D, Weinberg AG and Ashfaq
R: Amplification of the HER-2/neu oncogene is uncommon in pediatric
osteosarcomas. Cancer. 92:677–683. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kilpatrick SE, Geisinger KR, King TS,
Sciarrotta J, Ward WG, Gold SH and Bos GD: Clinicopathologic
analysis of HER-2/neu immunoexpression among various histologic
subtypes and grades of osteosarcoma. Mod Pathol. 14:1277–1283.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scotlandi K, Manara MC, Hattinger CM,
Benini S, Perdichizzi S, Pasello M, Bacci G, Zanella L, Bertoni F,
Picci P and Serra M: Prognostic and therapeutic relevance of HER2
expression in osteosarcoma and Ewing's sarcoma. Eur J Cancer.
41:1349–1361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshida R, Tazawa H, Hashimoto Y, Yano S,
Onishi T, Sasaki T, Shirakawa Y, Kishimoto H, Uno F, Nishizaki M,
et al: Mechanism of resistance to trastuzumab and molecular
sensitization via ADCC activation by exogenous expression of
HER2-extracellular domain in human cancer cells. Cancer Immunol
Immunother. 61:1905–1916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Long XH, Zhang GM, Peng AF, Luo QF, Zhang
L, Wen HC, Zhou RP, Gao S, Zhou Y and Liu ZL: Lapatinib alters the
malignant phenotype of osteosarcoma cells via downregulation of the
activity of the HER2-PI3K/AKT-FASN axis in vitro. Oncol Rep.
31:328–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jullien N, Maudinet A, Leloutre B, Ringe
J, Häupl T and Marie PJ: Downregulation of ErbB3 by Wnt3a
contributes to wnt-induced osteoblast differentiation in
mesenchymal cells. J Cell Biochem. 113:2047–2056. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai Y, Mohseny AB, Karperien M, Hogendoorn
PC, Zhou G and Cleton-Jansen AM: Inactive Wnt/beta-catenin pathway
in conventional high-grade osteosarcoma. J Pathol. 220:24–33. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu H, Liu X and Zhao J: Down-regulation of
miR-3928 promoted osteosarcoma growth. Cell Physiol Biochem.
33:1547–1556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bianchi S, Palli D, Falchetti M, Saieva C,
Masala G, Mancini B, Lupi R, Noviello C, Omerovic J, Paglierani M,
et al: ErbB-receptors expression and survival in breast carcinoma:
A 15-year follow-up study. J Cell Physiol. 206:702–708. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Karamouzis MV, Badra FA and Papavassiliou
AG: Breast cancer: The upgraded role of HER-3 and HER-4. Int J
Biochem Cell Biol. 39:851–856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Citri A and Yarden Y: EGF-ERBB signalling:
Towards the systems level. Nat Rev Mol Cell Biol. 7:505–516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Canfield K, Li J, Wilkins OM, Morrison MM,
Ung M, Wells W, Williams CR, Liby KT, Vullhorst D, Buonanno A, et
al: Receptor tyrosine kinase ERBB4 mediates acquired resistance to
ERBB2 inhibitors in breast cancer cells. Cell Cycle. 14:648–655.
2015. View Article : Google Scholar : PubMed/NCBI
|