Introduction
Lung cancer has become the leading cause of
cancer-associated mortality worldwide (1). Small cell lung cancer (SCLC) is the main
subtype of lung cancer and accounts for 15–20% of all lung cancer
cases (2). The incidence of
cancer-associated mortality has increased from 28 to 50% among
females with SCLC based on the surveillance, epidemiology and end
results database (2). SCLC is
characterized by a rapid growth rate and a positive response to
treatment, but relapse often occurs (3). Consequently, the overall survival (OS)
is poor, with a 5-year survival rate of 20% for limited-stage SCLC
and 9.1% for extensive-stage SCLC, despite the sensitivity of SCLC
to chemoradiotherapy (4,5). The role of surgery in SCLC remains
controversial, even though surgery could prolong survival in a
subpopulation of individuals with SCLC (6). In addition, the risk of distant
metastasis increases with prolonged survival. The brain is the most
common organ of distant metastasis in patients with SCLC. The
prevalence of brain metastasis (BM) ranges between 10 and 24% at
the time of diagnosis and increases to 50% at 2 years
post-diagnosis. Therefore, the prognosis of patients with BM is
poor, with a median survival time (MST) of 4–6 months (7–9).
Prophylactic cranial irradiation (PCI) decreases the risk of BM but
increases survival in patients with surgically resected SCLC,
excluding pathological-stage I patients (10). PCI of unselected patients not only is
a waste of medical resources but also negatively impacts patient
quality of life with a low cost-efficacy. Therefore, an effective
biomarker with high sensitivity or specificity that can predict BM
in SCLC is urgently required.
Programmed death-ligand 1 (PD-L1, also known as
B7-H1 or CD274) is present in a variety of tumor cells, including
malignant melanoma, non-small cell lung cancer and SCLC (11–13). PD-L1
binds with PD-1 on tumor cells, competitively inhibiting the
binding of B7 and CD28 and suppressing the ability of activated T
cells to promote immune escape and tolerance (14,15). A
number of studies have investigated the distribution of PD-L1 in
SCLC and investigated the correlation between PD-L1 expression and
clinical outcomes, particularly in patients with BM (16–22).
Increased expression of PD-L1 in a subset of SCLC tumors caused by
focal amplification of CD274 suggested that the PD-L1 axis may be a
novel therapeutic target for SCLC (23). A retrospective study revealed that
PD-L1 was positively expressed in 51.8% of SCLC specimens (n=83)
and demonstrated that the MST was significantly longer in patients
with PD-L1(+) tumors than in those with PD-L1(−) expression (17.0
vs. 9.0 months, P=0.018) (20).
Another retrospective study revealed that PD-L1 was highly
expressed in SCLC (15–45%) and that PD-L1(+) expression was
correlated with improved disease-free survival, compared with
PD-L1(−) expression [hazard ratio (HR)=0.268, P=0.003] (16). Additionally, PD-L1 expression in tumor
cells was observed in 75.0% (24/32) of BM specimens obtained from
patients with SCLC (21).
Furthermore, high levels of PD-L1 expression were negatively
correlated with BM size, which indicated that anti-PD-L1
potentially reduced the incidence of BM (24). Based on the aforementioned evidence,
PD-L1 expression may predict BM in patients with SCLC and targeting
PD-L1 may prevent BM in patients with SCLC.
To date, the prevalence of PD-L1 expression in SCLC
and the correlation between PD-L1 expression and BM in SCLC remains
controversial. Therefore, a retrospective study was conducted to
detect PD-L1 expression in postoperative SCLC specimens and to
further investigate the role of PD-L1 expression in BM and OS. The
results demonstrated that PD-L1 was highly expressed in SCLC
(65.0%, 52/80) and that PD-L1 expression was associated with
improved OS and a lower risk of BM.
Patients and methods
Patients
Postoperative specimens were collected from 80
patients who had undergone complete resection between January 2010
and December 2012 at The Affiliated Hospital of Weifang Medical
University (Shandong, China) and were stained with hematoxylin and
eosin to identify SCLC cells. Prior to surgery, standardized
evaluations were performed, including constant thoracic and
abdominal computed tomography (CT), brain magnetic resonance
imaging (MRI) and bone radionuclide imaging. Positron emission
tomography (PET)-CT was performed on certain patients. All patients
were staged according to the criteria of the American Joint
Committee on Cancer (AJCC) 7th edition (25). Pathological node 0–2 (pN0-2) and
p-stage I–III were used accordingly. Of the 80 patients, 45 (56.3%)
were male and 35 (43.7%) were female. The median age for the whole
cohort at diagnosis was 54 years, with a range of 34–72 years.
Surgical procedures consisted of lobectomy or pneumonectomy with
mediastinal nodal dissection. Postoperative chemotherapy (POCT, 4–6
cycles) with cisplatin/etoposide (EP) or carboplatin/etoposide (CE)
was administrated. For postoperative thoracic irradiation (PORT), a
total dose of 50–60 Gy was administered using a three-dimensional
conformal radiotherapy or intensity-modulated radiotherapy
technique for 5–6 weeks with 1.8–2.0 Gy per fraction for 5 days per
week. For patients without BM identified by brain MRI prior to PCI,
a total dose of 30 Gy with 3.0 Gy per fraction, or a total dose of
25 Gy with 2.5 Gy per fraction was administrated. PCI was delivered
concurrently or sequentially with chemoradiotherapy.
The present study was approved by the institutional
review board and ethics committee of the Affiliated Hospital of
Weifang Medical University and Shanghai Changhai Hospital
(Shanghai, China). Written informed consent was obtained from every
patient and/or their legal guardian prior to analysis.
Immunohistochemistry (IHC)
Immunohistochemistry was performed to determine
PD-L1 expression in resected specimens. Formalin-fixed (formalin
concentration, 10%; 12–24 h at room temperature), paraffin-embedded
sections (4-µm thick) were air-dried at room temperature overnight.
Sections were deparaffinized with 100% xylene (25°C) and rehydrated
through a grade ethanol series (100, 95 and 70% ethanol) at 100°C
for 15 min. Following this, antigen retrieval (190°C for 5 min) was
performed in a high-pressure cooker following washing of the slides
3 times with PBS. Peroxidase activity was quenched by 3% hydrogen
peroxide at room temperature for 5 min, then the tissues were
incubated for 15 min in 5% fetal bovine serum at room temperature
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) blocking
buffer. As no antibodies were approved for NSCLC detection when the
present study took place, the primary antibody SP142 (cat. no.
07309554001; 1:100; Spring Bioscience Corporation, Pleasanton, CA,
USA) was utilized and incubated for 1 h at 25°C, followed by
incubation with the secondary HRP-labeled anti-rabbit antibody
(cat. no. K4003; 1:100; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA) for 30 min at room temperature. Following
incubation in 3,3′-diaminobenzidine (cat. no. DAB-0031; Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China), the slides were
counterstained with hematoxylin (15 sec at 25°C) followed by
dehydration and mounting with a coverslip. The Olympus BX51 light
microscope was used to observe the staining status (magnification,
×40). In concordance with a previous study, human placenta
(obtained from Changhai Hospital of Shanghai in July, 2014) was
used as a positive control specimen for PD-L1 IHC (26). Written informed consent was obtained
at the time the placenta samples were collected.
Evaluation of PD-L1 expression was performed by at
least two pathologists who were blinded to the clinical data. PD-L1
expression at the cell membrane was evaluated by combining staining
intensity and distribution scores. Staining intensity was scored as
follows: 0, no staining; 1+, weak; 2+, moderate and 3+, strong. The
final histoscore was formulated as follows: [weak (1+) ×1] ×
percentage + [moderate (2+) ×2] × percentage + [strong (3+) ×3] ×
percentage. Weak [1+] × percentage refers to the percentage of
tumor cells with weak staining. Moderate [2+]/strong [3+] staining
was also included into this formula. According to the references
cited in this study, PD-L1 expression ≥1, ≥5 or ≥10% was mostly
defined as PD-L1(+). However, no significant difference in survival
was observed between the PD-L1(−) group and the PD-L1(+) group when
PD-L1(+) was defined as PD-L1 expression ≥1 or ≥10%, respectively,
therefore PD-L1(+) expression was defined as PD-L1 expression
≥5%.
Follow-up
Patient follow-up occurred every 3 months for up to
2 years, every 6 months for the following 3 years and annually
thereafter. The standard follow-up included a medical history and
physical examination, chest CT, abdominal CT or ultrasound and
bloodwork as clinically indicated. PET/CT and brain MRI were not
routinely recommended, but for patients with suspected BM, an
enhanced MRI or CT scan of the brain was performed.
Statistical analysis
SPSS 22.0 statistical analysis software (IBM Corp.,
Armonk, NY, USA) was used to analyze all data in the present study.
A χ2 test was performed to compare the patients'
clinical characteristics. Measurement data and enumeration data are
presented as median number and percentage (%), respectively. All
statistics were calculated at least three times by two independent
authors in a double-blind situation. OS and the time of BM were
calculated from the date of surgery to the date of BM diagnosis or
to the last day of follow-up. The Kaplan-Meier method and log-rank
test were used to evaluate patient survival and the cumulative risk
of developing BM. Multivariate analyses for OS and BM were
performed using Cox regression, and a backward-forward stepwise
method was selected. Tests were two-sided, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Between January 2010 and December 2013, 293 patients
were diagnosed with SCLC, including 112 patients who underwent
surgery. Among those 112 patients, 9 were excluded due to R1/R2
resections. An additional 12 patients were lost to follow-up, and
11 patients underwent segmentectomies. Consequently, a total of 80
patients were recruited for the current study. Karnofsky
performance statuses (27) following
surgery were as follows: 86.3% (69/80) of patients had scores of
80–90, and 13.7% (11/80) of patients had a score of 70. The
clinical features of the patients are summarized in Table I. The median follow-up period was 52
months (range, 6–71 months). At the end of follow-up, 21.3% (17/80)
of the patients remained alive. BM was detected in a total of 27
(33.8%) patients. According to the criteria of the AJCC 7th
edition, no T4 or N3 patients were present in our patient
population.
 | Table I.Clinical features of 80 patients with
completely resected small cell lung cancer. |
Table I.
Clinical features of 80 patients with
completely resected small cell lung cancer.
| Variable | n | % |
|---|
| Sex |
|
|
| Male | 45 | 56.2 |
|
Female | 35 | 43.8 |
| Age, years |
|
|
| ≥60 | 26 | 32.5 |
|
<60 | 54 | 67.5 |
| pN stage |
|
|
|
N0-1 | 55 | 68.7 |
| N2 | 25 | 31.3 |
| p-stage |
|
|
| I | 25 | 31.3 |
| II | 23 | 28.7 |
|
III | 32 | 40 |
| POCT |
|
|
|
Yes | 62 | 77.5 |
| No | 18 | 22.5 |
| PORT |
|
|
|
Yes | 71 | 88.7 |
| No | 9 | 11.3 |
| PCI |
|
|
|
Yes | 51 | 63.7 |
| No | 29 | 36.3 |
A total of 74 patients (92.5%) underwent lobectomy,
and 6 patients (7.5%) underwent pneumonectomy. POCT was
administered to 77.5% (62/80) of the patients, and 88.8% (71/80) of
the patients underwent PORT. Nine patients did not undergo PORT for
the following reasons: 4 had poor pulmonary function and 5 had poor
performance status. As PCI was not routinely recommended for
postoperative patients during that period in The Department of
Medical Oncology, Affiliated Hospital of Weifang Medical
University, only 63.8% (51/80) of the patients underwent PCI.
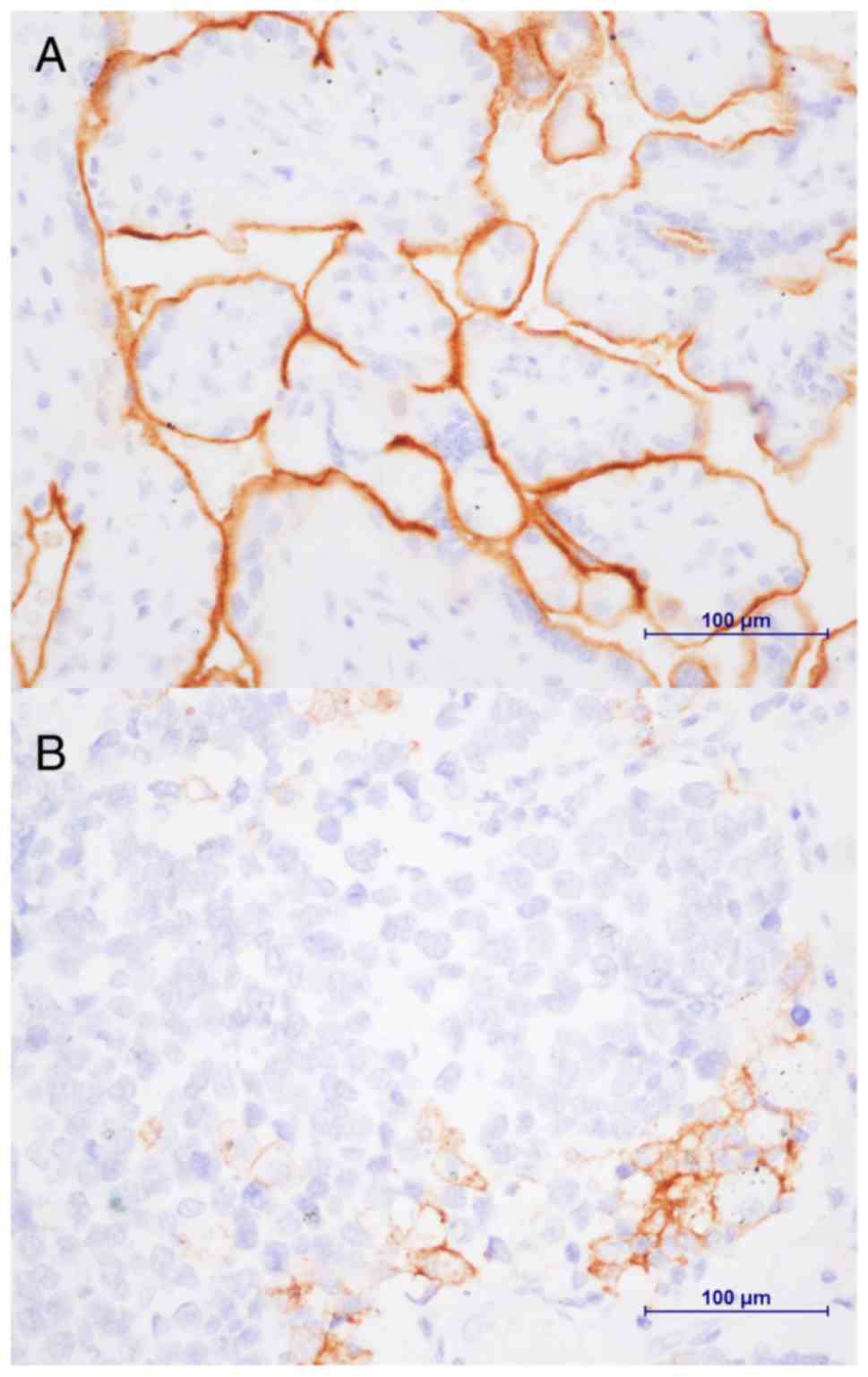
PD-L1 expression
PD-L1 expression in 80 formalin-fixed
paraffin-embedded specimens was evaluated (Fig. 1). Among the cohort, staining
intensities of 1+, 2+ and 3+ were observed in 15.0% (12/80), 28.7%
(23/80) and 56.3% (45/80) of the patients, respectively. In total,
PD-L1 was expressed in specimens from 52 patients (65.0%). Among 27
patients with BM, a 3+ staining intensity was observed in 15
patients (55.6%), while a 1+ intensity was observed in 4 patients
(14.8%) and a 2+ intensity was observed in 8 patients (29.63%).
Additionally, positive PD-L1 expression was detected in 16 patients
(59.3%) with BM. PD-L1 was mainly expressed in patients with pN0-1
(51.25%, 41/80) and p-stage I–II disease (45.0%, 36/80). The
expression levels of PD-L1 in subgroups of patients with SCLC is
outlined in Table II.
 | Table II.PD-L1 expression in patients with
completely resected small cell lung cancer. |
Table II.
PD-L1 expression in patients with
completely resected small cell lung cancer.
| Variable | Cases (n=80) | PD-L1 ≥5% | PD-L1 <5% | P-value |
|---|
| Sex |
|
|
| 0.35 |
|
Male | 45 | 27 | 18 |
|
|
Female | 35 | 25 | 10 |
|
| Age, years |
|
|
| 0.21 |
|
≥60 | 26 | 14 | 12 |
|
|
<60 | 54 | 38 | 16 |
|
| pN stage |
|
|
| 0.02 |
|
N0-1 | 56 | 41 | 15 |
|
| N2 | 24 | 11 | 13 |
|
| p-stage |
|
|
| 0.02 |
| I | 25 | 16 | 9 |
|
| II | 23 | 20 | 3 |
|
|
III | 32 | 16 | 16 |
|
| BM status |
|
|
| 0.47 |
|
Yes | 27 | 16 | 11 |
|
| No | 53 | 36 | 17 |
|
Survival
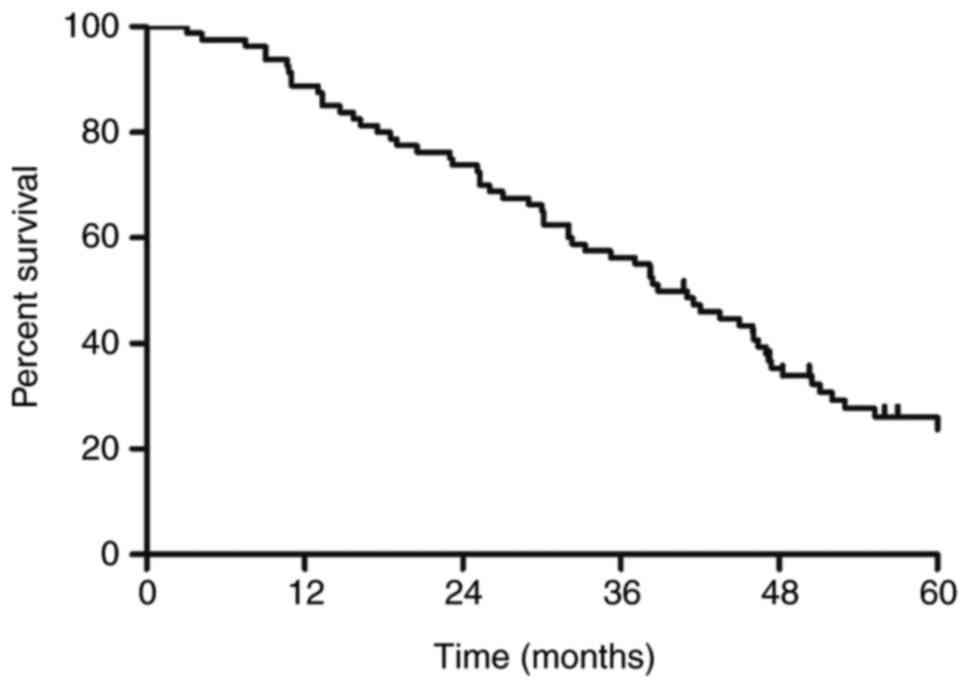
The survival curve for the entire group is presented
in Fig. 2. The MST was 38.8 months.
The 1-, 3- and 5-year survival rates were 88.8, 56.3 and 26.2%,
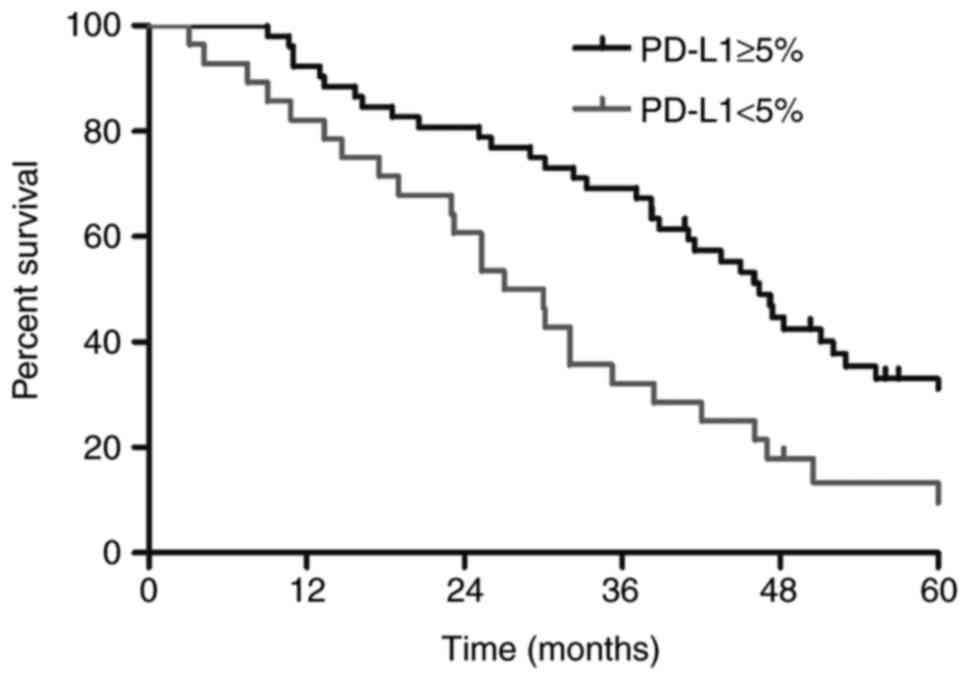
respectively. As presented in Fig. 3,
the MST and 1-, 3- and 5-year survival rates were 46.4 months,
92.3, 69.2 and 33.1%, respectively, among patients who were
PD-L1(+) and 28.5 months, 82.1, 32.1 and 13.4%, respectively, among
patients who were PD-L1(−) (P=0.002). Univariate analysis revealed
that sex (P=0.034), N stage (P=0.012), pathological stage
(P<0.01), POCT (P=0.003), PORT (P=0.027), PCI (P<0.01), PD-L1
expression (P=0.001) and BM (P<0.001) were associated with an
increased MST of patients with SCLC. Multivariate analysis
indicated that POCT (HR=0.476, P=0.023), PCI (HR=0.229, P<0.01),
PD-L1 expression (HR=0.485, P=0.011) and BM (HR=1.900, P=0.026)
were independent prognostic factors for OS (Table III).
 | Table III.Univariate and multivariate analysis
of the effect of prognostic factors on OS in patients with
completely resected small cell lung cancer. |
Table III.
Univariate and multivariate analysis
of the effect of prognostic factors on OS in patients with
completely resected small cell lung cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | 1-year OS, % | 3-year OS, % | χ2 | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
| 0.896 |
|
Male | 86.7 | 46.7 | 4.52 | 0.034 | 0.963 | 0.543–1.707 |
|
|
Female | 91.4 | 68.6 |
|
|
|
|
|
| Age, years |
|
|
|
|
|
|
|
|
≥60 | 92.3 | 46.2 | 0.065 | 0.798 |
|
|
|
|
<60 | 87.0 | 61.1 |
|
|
|
|
|
| pN stage |
|
|
|
|
|
| 0.301 |
|
N0-1 | 91.1 | 64.3 | 6.285 | 0.012 | 0.594 | 0.222–1.594 |
|
| N2 | 83.3 | 37.5 |
|
|
|
|
|
| p-stage |
|
|
|
|
|
| 0.181 |
| I | 96.0 | 76.0 | 16.53 | 0.000 | 1.266 | 0.896–1.788 |
|
| II | 91.3 | 65.2 |
|
|
|
|
|
|
III | 78.1 | 34.4 |
|
|
|
|
|
| POCT |
|
|
|
|
|
| 0.023 |
|
Yes | 90.3 | 59.7 | 8.83 | 0.003 | 0.476 | 0.251–0.904 |
|
| No | 83.3 | 44.4 |
|
|
|
|
|
| PORT |
|
|
|
|
|
| 0.116 |
|
Yes | 91.5 | 60.6 | 4.88 | 0.027 | 0.508 | 0.219–1.181 |
|
| No | 66.7 | 22.2 |
|
|
|
|
|
| PCI |
|
|
|
|
|
| <0.001 |
|
Yes | 96.1 | 78.4 | 30.01 | <0.001 | 0.229 | 0.121–0.443 |
|
| No | 75.9 | 17.2 |
|
|
|
|
|
| PD-L1
expression |
|
|
|
|
|
| 0.011 |
|
<5% | 82.1 | 32.1 | 10.39 | 0.001 | 0.485 | 0.279–0.845 |
|
|
≥5% | 92.3 | 69.2 |
|
|
|
|
|
| BM |
|
|
|
|
|
| 0.026 |
|
Yes | 81.5 | 25.9 | 20.12 | <0.001 | 1.900 | 1.078–3.348 |
|
| No | 92.5 | 71.7 |
|
|
|
|
|
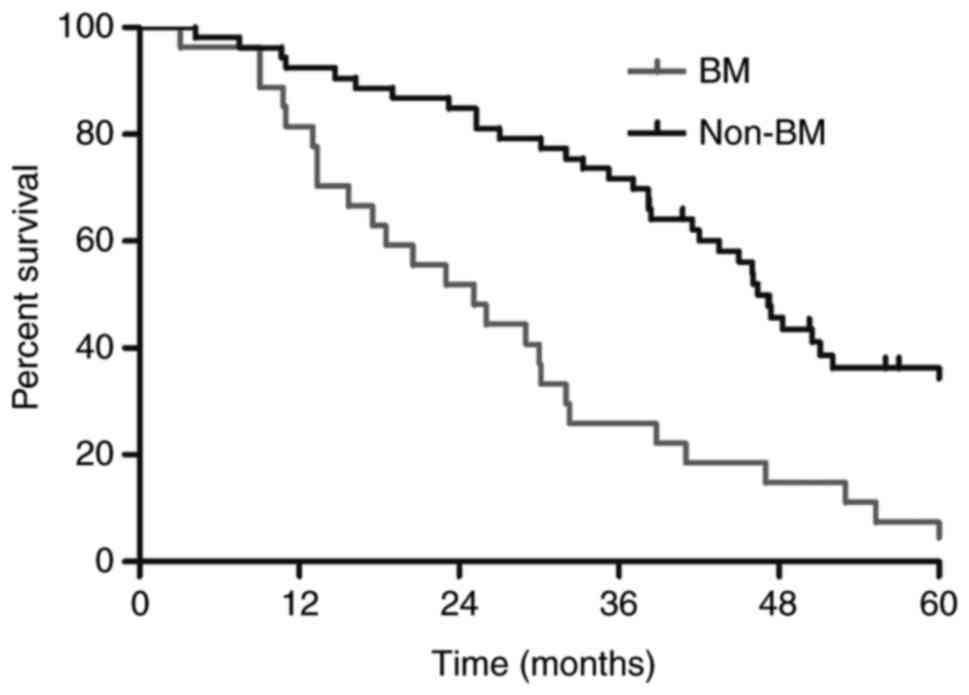
In addition, significantly longer survival times
were observed among patients without BM than among patients with BM
(MST; 46.4 vs. 25.1 months; P<0.01; Fig. 4). Among the 27 patients with BM, the
survival rates between the PD-L1(+) and PD-L1 (−) groups were not
significantly different (P=0.55, data not presented).
Risk factors for BM
For the whole patient cohort, the risk of BM at 1, 3
and 5 years was 6.4, 27.5 and 40.6%, respectively. Clinical and
pathological features were evaluated to determine their predictive
values for developing BM (Table IV).
The risk of developing BM was associated with PD-L1 expression. As
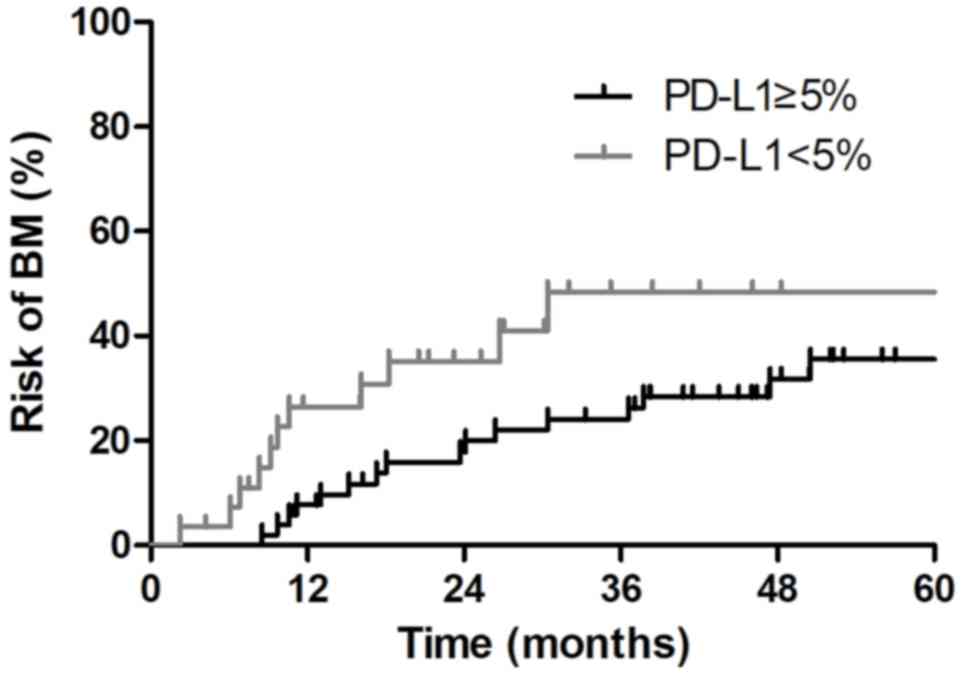
presented in Fig. 5, the risk of
developing BM at 1 and 3 years was 7.7 and 24.1%, respectively,
among PD-L1(+) patients, which was significantly lower than the
26.5 and 48.4% risk, respectively, among PD-L1(−) patients
(P=0.046). Multivariate analysis revealed that pathological stage
(HR=2.139, P=0.022), PCI (HR=0.186, P<0.001) and PD-L1
expression (HR=0.335, P=0.024) were independent factors associated
with the incidence of BM. The risk curves for the development of BM
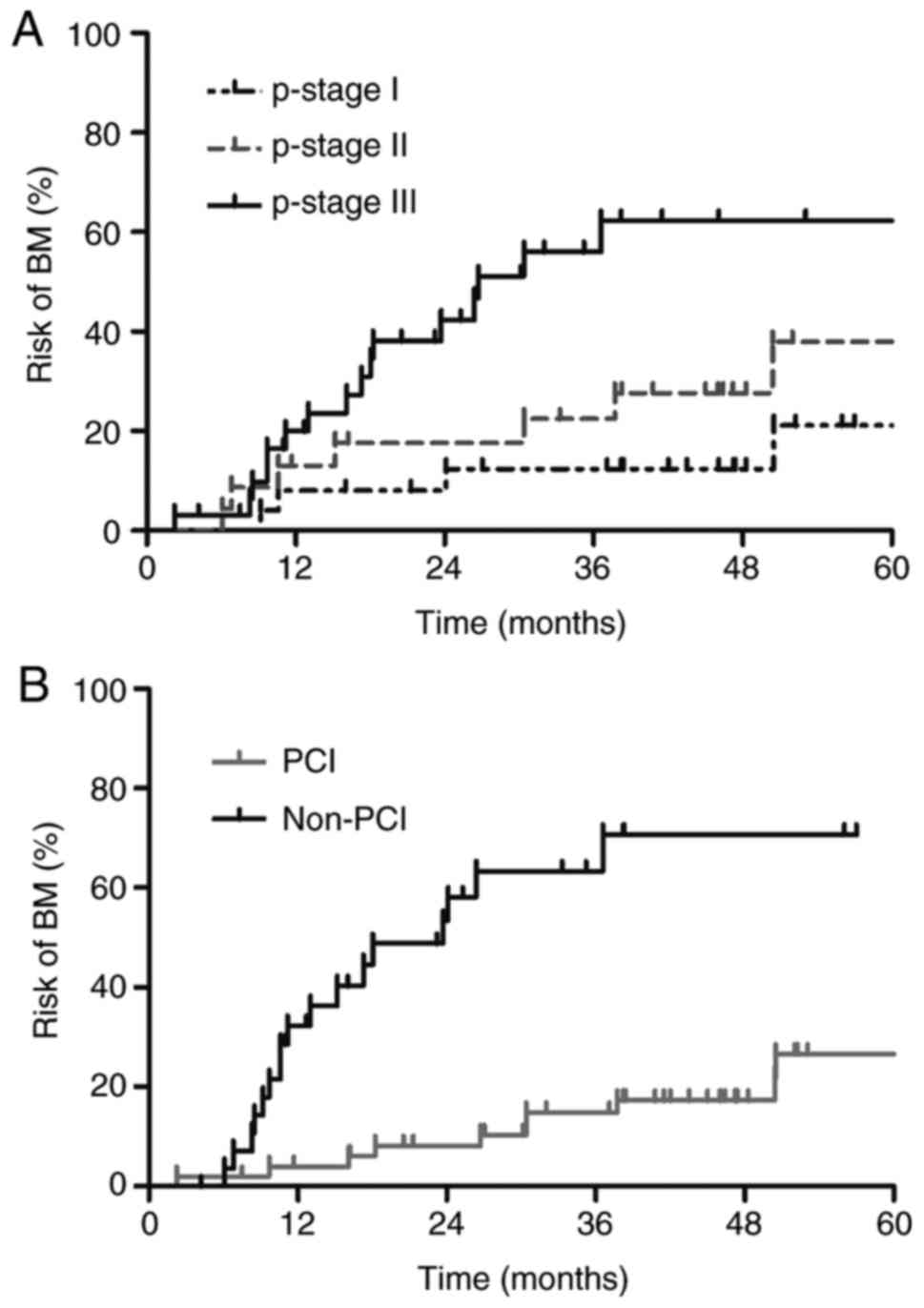
based on PCI and p-stage are presented in Fig. 6.
 | Table IV.Risk factors for developing brain
metastasis in patients with completely resected small cell lung
cancer. |
Table IV.
Risk factors for developing brain
metastasis in patients with completely resected small cell lung
cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | 1-year OS,% | 3-year OS, % | χ2 | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
Male | 16.14 | 36.74 | 1.427 | 0.232 |
|
|
|
|
Female | 11.43 | 24.51 |
|
|
|
|
|
| Age, years |
|
|
|
|
|
|
|
|
≥60 | 19.23 | 32.90 | 0.04 | 0.838 |
|
|
|
|
<60 | 11.48 | 30.75 |
|
|
|
|
|
| pN stage |
|
|
|
|
|
|
|
|
N0-1 | 12.64 | 24.53 | 4.072 | 0.044 | 0.529 | 0.172–1.630 | 0.267 |
| N2 | 17.62 | 56.80 |
|
|
|
|
|
| p-stage |
|
|
|
| 2.139 | 1.113–4.108 | 0.022 |
| I | 8.00 | 12.38 | 10.69 | 0.001 |
|
|
|
| II | 13.04 | 22.47 |
|
|
|
|
|
|
III | 19.97 | 56.04 |
|
|
|
|
|
| POCT |
|
|
|
|
|
|
|
|
Yes | 16.13 | 33.70 | 0.438 | 0.508 |
|
|
|
| No | 6.25 | 22.12 |
|
|
|
|
|
| PORT |
|
|
|
|
|
|
|
|
Yes | 12.844 | 28.71 | 2.440 | 0.118 |
|
|
|
| No | 23.81 | 59.37 |
|
|
|
|
|
| PCI |
|
|
|
|
|
|
|
|
Yes | 3.96 | 14.88 | 23.43 | <0.001 | 0.186 | 0.080–0.436 | <0.001 |
| No | 32.33 | 63.36 |
|
|
|
|
|
| PD-L1
expression |
|
|
|
|
|
|
|
|
<5% | 26.5 | 48.4 | 3.970 | 0.046 | 0.335 | 0.130–0.864 | 0.024 |
|
≥5% | 7.7 | 24.1 |
|
|
|
|
|
Discussion
In the current study, PD-L1 expression was detected
by IHC with the SP142 antibody in 80 postoperative specimens from
patients with SCLC. As indicated in Fig.
1, PD-L1 expression in ≥5% of tumor cells was observed in 65.0%
(52/80) of the patients. The majority of PD-L1(+) tumor cells were
detected in patients with p-stage I–II (45%) and pN0-1 disease
(51.3%). The correlation of PD-L1 expression with survival was
investigated and the predictive value of PD-L1 expression for BM
development was evaluated in Tables
III and IV and Figs. 3 and 4.
PD-L1(+) expression was a top factor associated with improved
survival and a lower risk of developing BM. As depicted in Fig. 3, the MST in the PD-L1(+) group was
46.4 months, which was longer than the MST of 28.5 months in the
PD-L1(−) group (P=0.002). Fig. 5
depicted the risk of developing BM at 3 years was significantly
lower in PD-L1(+) patients than in PD-L1(−) patients (24.1% vs.
48.4%, P=0.046). As depicted in Tables
III and IV, multivariate
analysis demonstrated that PD-L1 expression was an independent
factor for survival (HR=0.485, P=0.011) and BM (HR=0.335,
P=0.024).
The PD-L1/PD-1 pathway is a negative costimulatory
signal that limits effector T cell responses and promotes tumor
evasion and intolerance (15). PD-L1
is not only expressed on the membranes of tumor cells but also in
immune cells. Whether PD-L1 expression on tumor cells or immune
cells influences tumor progression remains controversial. Recent
evidence has suggested that PD-L1 expression on tumor cells may
control tumor growth (28,29). PD-L1 expression in SCLC remains
unclear. A retrospective analysis of PD-L1 expression in 61
pulmonary and 33 extrapulmonary cases of SCLC revealed no positive
PD-L1 staining in tumor cells (19).
Another study was conducted to detect PD-L1 expression in four SCLC
cell lines which revealed that PD-L1 was weakly expressed on the
cell surface of all four cell lines (18). However, a recent study including 249
patients with SCLC demonstrated that PD-L1 was positively expressed
in 16.5% (41/249) of the tumor samples, as detected by two
antibodies (SP142 and Dako 28–8) with the threshold of PD-L1
positivity set as ≥1% cell staining (13). Additionally, a study including 102
SCLC specimens indicated 71.6% of tumor cells were PD-L1(+), which
was significantly associated with limited disease SCLC (17). PD-L1(+) expression was also observed
in 51.83% (n=83) SCLC specimens (20). The differences among the samples
obtained, the antibodies used, the defined cut-off values and the
evaluation system in these studies may contribute to the
inconsistent results. Takada et al (30) evaluated PD-L1 expression in 40
surgically resected SCLC specimens by IHC using three different
antibodies (clones E1L3 N, 28-8 and SP142) and three different
evaluation methods [Allred score with range 0–8 (A, Intensity 0–3;
B, proportion of PD-L1 + cells 0–5; A+B=Allred score); ≥1% cut-off
and ≥5% cut-off]. The results revealed that PD-L1 was positively
expressed on the membranes of tumor cells, but expression levels
were different (Allred score, 22.5–35%; 1% cut-off, 20–32.5%, 5%
cut-off, 15%). In the current study, IHC was performed with the
SP142 antibody to detect PD-L1 expression in postoperative
specimens. The results revealed that with a cut-off of ≥5%
staining, PD-L1 was positively expressed in 65.0% of SCLC samples.
It was noted that one study investigating PD-L1 expression in BM
specimens revealed that 34.4% (11/32) of patients demonstrated
PD-L1 expression in the membrane of tumor cells using a cut-off of
≥5% staining (21). At the Department
of Medical Oncology, Affiliated Hospital of Weifang Medical
University, where the present study was undertaken, BM diagnosis
was based on imaging findings or lumbar puncture. Therefore, the
prevalence of PD-L1 expression in BM tissues could not be
accurately assessed.
SCLC treatment continues to rely on conventional
chemotherapy, and there is not yet an effective target to improve
survival. The breakthrough of anti-PD-1/PD-L1 treatment for
non-small cell lung cancer and melanoma has shed light on its
potential as a treatment for SCLC. Several studies have
demonstrated that PD-L1(+) expression is a novel biomarker that
could improve prognosis prediction among patients with SCLC
(16,17,20). A
retrospective study including 40 patients with resected SCLC
indicated an improved disease-free survival among patients with
PD-L1(+) expression (16). The MST
among patients with SCLC with PD-L1(+) tumors was longer than those
with PD-L1(−) tumors (16.3 vs. 7.3 months, P<0.001), and PD-L1
was an independent factor for survival, as demonstrated by
multivariate analyses (HR=0.435, P=0.008) (17). Another study detected PD-L1 expression
in 83 SCLC specimens and revealed an improved MST in patients with
PD-L1(+) tumors (17.0 vs 9.0 months, P=0.018) (20). Despite differences in PD-L1
antibodies, tumor tissue specimens obtained from biopsy or surgery
and other clinical features among those studies, PD-L1(+)
expression was consistently identified as a predictor of improved
OS. In the current study, all specimens were obtained by surgery,
and the prolonged survival in PD-L1(+) group further demonstrated
the predictive value of PD-L1 for survival. However, no data was
available to investigate the effect of PD-L1 expression on drug
treatment outcome. As the patients recruited to the present study
had all undergone complete resection between 2010 and 2012, no
specimens were available for secondary biopsy following
chemotherapy. Moreover, no checkpoint inhibitor therapy was
available in China at the time of the present study.
The brain is the most common metastatic site for
SCLC, and BM further reduces patient survival. As indicated in a
previous study, patients with pathological stage I do not benefit
from PCI following surgery (10);
therefore, routine administration of PCI to all patients with
resected SCLC may not be clinically advisable. Meanwhile,
conventional chemotherapy and targeted drugs have difficulty
penetrating the brain due to the blood-brain barrier. Although the
correlation of PD-L1 expression has been less studied in BM from
patients with SCLC, it has been initially demonstrated to be of
value (24). In a large mixed cohort
of 252 BM specimens, the correlation between PD-L1 expression and
BM size was investigated, and the results revealed a strong
negative correlation between PD-L1 expression and BM size
(P=0.0016) (24). However, only 9
SCLC specimens were included in Harter's study. Another study
including 32 SCLC specimens was conducted to detect PD-L1
expression and revealed that 75.0% of BM specimens expressed PD-L1,
with 34.4% of cases demonstrating PD-L1 expression in ≥5% of the
tumor cells; furthermore, PD-L1 expression in tumor cells was not
correlated with survival (P=0.662) (21). In the present study, the risk of
developing BM in the PD-L1(+) group was significantly lower than
that in the PD-L1(−) group. Regardless of the variations between
studies, the data suggested that the PD-L1 pathway serves an
important role in BM in SCLC and reveal a positive effect of PD-L1
positivity on clinical outcomes.
Several limitations can be attributed to this
retrospective study. As previously mentioned, only 38.2% (112/293)
of patients with SCLC underwent surgery within four years at the
Department of Medical Oncology, Affiliated Hospital of Weifang
Medical University, where the present study was undertaken.
According to the criteria of enrolment, the number of eligible
patients was limited to 80. For this retrospective study however,
the data from this small cohort is sufficient to explain certain
findings. In addition, there were no approved antibodies for
detecting PD-L1 expression at the time of the study. On the other
hand, SP142 was more readily available than other antibodies. In
addition, as the present study was a preliminary exploratory study,
research funding was identified as a limiting factor. Furthermore,
the initial objective was to investigate the expression of PD-L1 in
resectable SCLC. Following completion of the preliminary IHC result
analysis, the findings of the present study were presented. We have
performed animal experiments to establish a SCLC brain metastasis
model and investigated the role of PD-L1/PD-1 and other
immune-related molecules in survival and response to radiotherapy
and checkpoint inhibitor treatment.
PD-L1 is commonly expressed on the membranes of SCLC
cells. PD-L1(+) expression is associated with reduced risk of BM
and is predictive of improved survival, which suggests that
checkpoint inhibition therapy at an optimal time in the course of
the disease may further prolong survival. Considering these results
and the small sample size in this retrospective study, further
large-scale and/or randomized studies are urgently required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific
Research Project of Weifang Health Bureau to Jin Liu (grant no.
2014013).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JL performed the statistical analysis and drafted
the manuscript, ZL helped collect the data and revise the
manuscript, XS designed the study, JL collected the data and WW
performed the statistical analysis and revised the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board or Ethics Committee of the Affiliated Hospital of
Weifang Medical University. Written informed consent was obtained
from every patient and/or their legal guardian prior to inclusion
in the present study.
Consent for publication
Consent for publication was obtained from all
participants and/or their legal guardian.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SCLC
|
small cell lung cancer
|
|
OS
|
overall survival
|
|
BM
|
brain metastasis
|
|
MST
|
median survival time
|
|
PCI
|
prophylactic cranial irradiation
|
|
PD-L1
|
programmed death ligand 1
|
|
HR
|
hazard ratio
|
|
MRI
|
magnetic resonance imaging
|
|
CT
|
computed tomography
|
|
PET
|
positron emission tomography
|
|
POCT
|
postoperative chemotherapy
|
|
PORT
|
postoperative irradiation
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bayman NA, Sheikh H, Kularatne B, Lorigan
P, Blackhall F, Thatcher N and Faivre-Finn C: Radiotherapy for
small-cell lung cancer-Where are we heading? Lung Cancer.
63:307–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turrisi AT III, Kim K, Blum R, Sause WT,
Livingston RB, Komaki R, Wagner H, Aisner S and Johnson DH:
Twice-daily compared with once-daily thoracic radiotherapy in
limited small-cell lung cancer treated concurrently with cisplatin
and etoposide. N Engl J Med. 340:265–271. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeremic B, Shibamoto Y, Nikolic N, Milicic
B, Milisavljevic S, Dagovic A, Aleksandrovic J and
Radosavljevic-Asic G: Role of radiation therapy in the
combined-modality treatment of patients with extensive disease
small-cell lung cancer: A randomized study. J Clin Oncol.
17:2092–2099. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Veronesi G, Bottoni E, Finocchiaro G and
Alloisio M: When is surgery indicated for small-cell lung cancer?
Lung Cancer. 90:582–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Socha J and Kępka L: Prophylactic cranial
irradiation for small-cell lung cancer: How, when and for whom?
Expert Rev Anticancer Ther. 12:505–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seute T, Leffers P, ten Velde GP and
Twijnstra A: Neurologic disorders in 432 consecutive patients with
small cell lung carcinoma. Cancer. 100:801–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Komaki R, Cox JD and Whitson W: Risk of
brain metastasis from small cell carcinoma of the lung related to
length of survival and prophylactic irradiation. Cancer Treat Rep.
65:811–814. 1981.PubMed/NCBI
|
|
10
|
Zhu H, Guo H, Shi F, Zhu K, Luo J, Liu X,
Kong L and Yu J: Prophylactic cranial irradiation improved the
overall survival of patients with surgically resected small cell
lung cancer, but not for stage I disease. Lung Cancer. 86:334–338.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sunshine JC, Nguyen PL, Kaunitz GJ,
Cottrell TR, Berry S, Esandrio J, Xu H, Ogurtsova A, Bleich KB,
Cornish TC, et al: PD-L1 expression in melanoma: A quantitative
immunohistochemical antibody comparison. Clin Cancer Res.
23:4938–4944. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He Y, Rozeboom L, Rivard CJ, Ellison K,
Dziadziuszko R, Yu H, Zhou C and Hirsch FR: PD-1, PD-L1 protein
expression in non-small cell lung cancer and their relationship
with tumor-infiltrating lymphocytes. Med Sci Monit. 23:1208–1216.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu H, Batenchuk C, Badzio A, Boyle TA,
Czapiewski P, Chan DC, Lu X, Gao D, Ellison K, Kowalewski AA, et
al: PD-L1 expression by two complementary diagnostic assays and
mRNA in situ hybridization in small cell lung cancer. J Thorac
Oncol. 12:110–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toyokawa G, Takada K, Haratake N, Takamori
S, Akamine T, Katsura M, Fujishita T, Shoji F, Okamoto T, Oda Y and
Maehara Y: Favorable disease-free survival associated with
programmed death ligand 1 expression in patients with surgically
resected small-cell lung cancer. Anticancer Res. 36:4329–4336.
2016.PubMed/NCBI
|
|
17
|
Ishii H, Azuma K, Kawahara A, Yamada K,
Imamura Y, Tokito T, Kinoshita T, Kage M and Hoshino T:
Significance of programmed cell death-ligand 1 expression and its
association with survival in patients with small cell lung cancer.
J Thorac Oncol. 10:426–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamane H, Isozaki H, Takeyama M, Ochi N,
Kudo K, Honda Y, Yamagishi T, Kubo T, Kiura K and Takigawa N:
Programmed cell death protein 1 and programmed death-ligand 1 are
expressed on the surface of some small-cell lung cancer lines. Am J
Cancer Res. 5:1553–1557. 2015.PubMed/NCBI
|
|
19
|
Schultheis AM, Scheel AH, Ozretić L,
George J, Thomas RK, Hagemann T, Zander T, Wolf J and Buettner R:
PD-L1 expression in small cell neuroendocrine carcinomas. Eur J
Cancer. 51:421–426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miao L, Lu Y, Xu Y, Zhang G, Huang Z, Gong
L and Fan Y: PD-L1 and c-MET expression and survival in patients
with small cell lung cancer. Oncotarget. 8:53978–53988.
2017.PubMed/NCBI
|
|
21
|
Berghoff AS, Ricken G, Wilhelm D, Rajky O,
Widhalm G, Dieckmann K, Birner P, Bartsch R and Preusser M: Tumor
infiltrating lymphocytes and PD-L1 expression in brain metastases
of small cell lung cancer (SCLC). J Neurooncol. 130:19–29. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan F, Pang J, Peng Y, Molina JR, Yang P
and Liu S: Elevated cellular PD1/PD-L1 expression confers acquired
resistance to cisplatin in small cell lung cancer cells. PLoS One.
11:e01629252016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
George J, Saito M, Tsuta K, Iwakawa R,
Shiraishi K, Scheel AH, Uchida S, Watanabe SI, Nishikawa R, Noguchi
M, et al: Genomic amplification of CD274 (PD-L1) in small-cell lung
cancer. Clin Cancer Res. 23:1220–1226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harter PN, Bernatz S, Scholz A, Zeiner PS,
Zinke J, Kiyose M, Blasel S, Beschorner R, Senft C, Bender B, et
al: Distribution and prognostic relevance of tumor-infiltrating
lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain
metastases. Oncotarget. 6:40836–40849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kligerman S and Abbott G: A radiologic
review of the new TNM classification for lung cancer. AJR Am J
Roentgenol. 194:562–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang SC, Latchman YE, Buhlmann JE,
Tomczak MF, Horwitz BH, Freeman GJ and Sharpe AH: Regulation of
PD-1, PD-L1 and PD-L2 expression during normal and autoimmune
responses. Eur J Immunol. 33:2706–2716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lau J, Cheung J, Navarro A, Lianoglou S,
Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J, et
al: Tumour and host cell PD-L1 is required to mediate suppression
of anti-tumour immunity in mice. Nat Commun. 8:145722017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Juneja VR, McGuire KA, Manguso RT, LaFleur
MW, Collins N, Haining WN, Freeman GJ and Sharpe AH: PD-L1 on tumor
cells is sufficient for immune evasion in immunogenic tumors and
inhibits CD8 T cell cytotoxicity. J Exp Med. 214:895–904. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takada K, Toyokawa G, Okamoto T, Akamine
T, Takamori S, Katsura M, Fujishita T, Shoji F, Oda Y and Maehara
Y: An immunohistochemical analysis of PD-L1 protein expression in
surgically resected small cell lung cancer using different
antibodies and criteria. Anticancer Res. 36:3409–3412.
2016.PubMed/NCBI
|