Introduction
Cervical cancer is one of the most serious diseases
that threaten the physical and mental health of women. The
morbidities and mortalities of cervical cancer rank first in China
with >150,000 newly diagnosed cases annually, accounting for
~38% of the total number of cases worldwide (1,2). Aetiology
studies have demonstrated that the incidence of cervical cancer is
multi-factorial, involving multiple steps, and that the risk
factors for cervical cancer may be associated with behavioural
factors, biological factors and genetic susceptibility factors
(3,4).
Therefore, it is very important for the clinical diagnosis and
treatment of cervical cancer to evaluate the associated mechanisms
of the occurrence and development of cervical cancer.
The occurrence and development of tumours are
associated with the pathological proliferation of cells and
abnormal apoptosis (5,6). Once the balance between promoting
apoptotic genes and inhibiting apoptotic genes is broken, tumours
inevitably develop (6,7). Mitochondrial dysfunction is one of the
key factors for apoptosis (8).
Therefore, the present study investigated the role of mitochondrial
dysfunction in the development of cervical cancer cells.
Understanding the complex process of the regulation of a variety of
signalling networks in the development and progression of cervical
cancer is of great importance. As one of the key genes promoting
apoptosis, tumour protein P53 (p53) serves an important role in
controlling the cell cycle, inhibiting tumour formation,
maintaining cell genome integrity and responding to various
cellular stresses (9–12). In recent years, accumulating evidence
has demonstrated that the p53 protein may translocate from the
cytoplasm to the mitochondria and activate mitochondria-related
apoptotic pathways. However, the p53-regulated genes that serve a
major role in cervical cancer have not yet been identified.
Haematopoietic cell-specific protein 1-associated
protein X-1 (HAX-1) is a mitochondrial membrane protein with
complex physiological functions and is also a potential oncogene
(13). HAX-1 genes are overexpressed
in numerous types of cancer and are involved in the tumorigenesis,
growth, progression, invasion and metastasis of a number of human
malignancies (14). The present study
aimed to comprehensively determine the role of the p53 gene in
HAX-1-induced biological behaviour of cervical cancer cells and the
associated mechanism of mitochondrial function.
Materials and methods
Chemicals and reagents
The human cervical cancer SiHa cell line and the
HeLa cell line were provided by the Hangzhou Hibio Bio-tech Co.,
Ltd (Hangzhou, China; http://www.hi-bio.cn/). The human cervical epithelial
CRL2614cell line was purchased from American Type Culture
Collection (ATCC, Manassas, VA, USA; http://www.atcc.org/Products/All/CRL-2614.aspx). The
Lipofectamine® 2000 transfection reagent was purchased
from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
A water-soluble tetrazolium salt (WST-1) kit, H2DCFDAkit
and JC-1kit were purchased from Invitrogen; Thermo Fisher
Scientific, Inc. Antibodies againstHAX-1 (cat no. sc-166845), p53
and β-actin were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Carbonyl cyanide-p-trifluoro
methoxyphenylhydrazone (FCCP) was obtained from Tianjin Kaitong
Chemical Reagent Co., Ltd. (Tianjing, China). The plasmids pCB6 +
p53 encoding wild-type (wt) p53 and pCB6 + p53 173L encoding
mutantp53 (mt) were supplied by Hangzhou Hibio Bio-tech Co., Ltd.
The wild-type amino acid sequence prior to mutation for pCB6 + p53
173L was KQSQHMTEV (164–172). Transwell chambers were obtained from
Invitrogen; Thermo Fisher Scientific, Inc. HAX-1 and β-actin gene
primers were synthesized by Shanghai Boya Biotechnology Co., Ltd.
(Shanghai, China; http://hkjum1210485.51sole.com/).
Tissue procurement and
preparation
The present study was approved by the Ethics
Committee of the Chinese Academy of Sciences and the Nanjing
Maternity and Child Health Care Hospital (Nanjing, China) and
written informed consent was obtained from all participants.
Subsequently, the specimens were organized and case data were
collected. Between December 2013 and October 2015, specimens of 24
cases (median age of 43 years, age range of 25–56 years) of
cervical cancer and adjacent non-cancerous cervical tissues were
collected at the Nanjing Maternity and Child Care Center Affiliated
to Nanjing Medical University (Nanjing, China). All specimens were
confirmed by pathological examination. The research specimens were
divided into two groups: i) There were 24 cases of human cervical
cancer tissues (T), the pathological diagnoses of which were
definite, and ii) 24 cases of surrounding non-cancerous cervical
tissues (N), with no tumour cell infiltration, confirmed by
pathology. For cervical tissue analysis, the exclusion criteria was
as follows: Positivity for Chlamydia trachomatis, Neisseria
gonorrhoeae, Gardnerella vaginalis, Mycoplasma genitalium,
Trichomonas vaginalis, Mycoplasma pneumoniae and Herpes simplex
virus type 2 (HSV-2) sexually transmitted pathogens.
Immunohistochemical analysis
Immunohistochemistry for HAX-1in cervical cancer and
adjacent non-cancerous cervical tissues was performed as follows:
Dewaxing in fresh xylene (30 min at 55°C) and rehydration using an
ethanol gradient (100, 95, 80 and 70%) for 2 min each at room
temperature for antigen retrieval, the slides (2–3-µm-thick
sections) were heated in a microwave oven in 0.02 M citrate buffer
at pH 6.0. Subsequent to cooling, the sections were incubated in 3%
perhydrol solution for 15 min at room temperature to block the
endogenous peroxidase reaction. Non-specific binding was blocked
for 1 h by incubation in 5% bovine serum albumin (Sigma Aldrich;
Merck KGaA, Darmstadt, Germany) at room temperature. Then, the
sections were incubated with mouse anti-human HAX-1 monoclonal
antibody (dilution, 1:200; Santa Cruz Biotechnology, Inc. Dallas,
TX, USA; cat. no. sc-166845) overnight at 4°C. The slides were then
incubated with horseradish peroxidase-conjugated goat anti-mouse
secondary antibody (dilution, 1:2,000; Santa Cruz Biotechnology,
Inc.; cat. no. sc-2005) for 30 min at 37°C and
3,3′-diaminobenzidine Sigma-D8001 staining kit (Sigma Aldrich;
Merck KGaA) staining was used for evaluation. The positive (brown)
staining indicates the presence of the HAX-1 protein, as detected
by light microscopy (magnification, ×200).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cervical tissue using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The RNA was quantified by absorption at 260 nm. The isolated
RNA was then DNase-treated and reverse-transcribed according to
manufacturer's recommended protocol. To detect HAX-1, the following
primer sets were used: Forward, 5′-CGCGGATCCAGTACGGGAATGAGCCCT-3′
and reverse, 5′-ACGCGTCGACTTAACAAGGCTACCGGGA-3′. According to the
HAX-1 mutated vector, a single point mutation for D148A
(GAT->GCT) and D159A (GAC->GCC) was introduced to full-length
HAX-1 that overlaps with the specific Asp residue that was changed
to Ala. The mutant forward and reverse primers were as follows:
D148A forward, 5′-TTGGAGAGTGCTGCAAGAAGTGAATCCCCCCAA-3′ and reverse
5′-ACTTCTTGCAGCACTCTCCAAGACCCCCCCAAA-3′; and D159A forward,
5′-CCAGCACCAGCCTGGGGCTCCCAGAGGCCATTT-3′ and reverse,
5′-GGAGCCCCAGGCTGGTGCTGGTTGGGGGGATTC-3′. The sequence analysis was
performed to confirm Asp to Ala conversions. Briefly, mRNA were
reverse transcribed using a PrimeScript reverse transcription kit,
miScript SYBR Green PCR kit and miScript primer assays according to
the manufacturer's protocol (Qiagen, Inc., Valencia, CA, USA).
RT-qPCR was performed using an ABI PRISM 7300 sequence detection
system. Thermocycling parameters were 2 min at 50°C and 10 min at
95°C, followed by a total of 40 cycles of 15 s at 95°C and 1 min at
60°C. All of the reactions were performed in triplicate. The gene
expression Cq values of mRNA were calculated by normalizing with
the internal control of β-actin. The relative amounts of mRNA were
calculated using the 2−ΔΔCq method (15).
Electron microscopy
The cultured SiHa cells were trypsinized and
pelleted at 800 × g for 15 min at 4°C. Following supernatant
removal, the cell mass was post-fixed in 1% OsO4 for 1 h
at room temperature and stained with 1% uranyl acetate for 2 h at
room temperature. Next, the cell mass was dehydrated an acetone
series (50, 70, 80, 90 and 100%) for 15 min each at room
temperature, and flat-embedded in Durcupan (Fluka Chemie AG, Buchs,
Switzerland) and sectioned to 60–70 nm thickness on 300 mesh copper
slot grids. Finally, ultrathin sections were examined at
magnifications of ×3,700 and ×12,500 and photographs were taken
using a Zeiss 109 electron microscope (Carl Zeiss AG,
Oberkochen, Germany).
Western blot analysis
The cultured SiHa cells were treated with
pcDNA3.1-HAX-1 vector, pcDNA3.1-HAX-1 mutated vector and pcDNA3.1
vector for 72 h at 37°C. Total protein of SiHa cells was extracted
using radioimmunoprecipitation assay protein lysis buffer
containing 150 mM NaCl, 1 mM Na3VO4, 50 mM
NaF, 1% Triton X-100, 1mM EDTA, 1 mM PMSF, 10% glycerol, 20 mM
Tris-HCl (pH 7.5) and protease inhibitors for 30 min on ice. An
equal quantity of total protein (~20-30 µg, with the concentration
of protein having been determined by a BCA protein assay, was
subjected to 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked for 1 h in 5% skimmed milk dissolved in PBST
(PBS containing 0.05% Tween 20) at room temperature. Following
blocking, primary antibodies againstHAX-1 (dilution, 1:400; cat.
no. sc-166845), p53 (dilution, 1:500; cat. no. sc-47698) and
β-actin (dilution, 1:500; cat. no. sc-69879) were applied to the
membranes overnight at 4°C. The proteins were visualized with
horseradish peroxidase-conjugated goat anti-mouse secondary
antibodies (dilution, 1:2,000; cat. no. sc-2005) for 1 h at room
temperature using the enhanced chemiluminescence western detection
system (Cell Signalling Technology, Inc., Danvers, MA, USA),
including a biotinylated protein ladder, 20× LumiGLO reagent and
20× peroxide.
Cell survival analysis
The cultured SiHa cells were treated with
pcDNA3.1-HAX-1 vector, pcDNA3.1-HAX-1 mutated vector and pcDNA3.1
vector for 72 h and examined for cell survival using a WST-1 assay.
The WST-1 (10 µl/well) was added to each well and incubated at 37°C
for 4 h. The cell cultures were terminated and the culture
supernatant was discarded. Dimethyl sulfoxide (150 µl) was added to
the cell culture and incubated for 10 min to fully dissolve the
reaction product. The absorbance was measured at an optical density
(OD) of 490 nm using an automated microplate reader (Elx808; BioTek
Instruments, Inc., Winooski, VT, USA).
SiHa cell migration analysis
The effect of different treatments on SiHa cell
migration was measured by Transwell experiments. Prior to the test,
the cultured SiHa cells (7.5×106 cells/ml) were starved
for 24 h and placed in the upper well of a 6.5-mm Transwell chamber
with 8-µm pores (Corning Incorporated, Corning, NY, USA). The upper
chamber was filled with 100 µl serum-free DMEM and the lower
chamber was filled with 500 µl culture medium containing 20% foetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 1%
nonessential amino acids and 2 mM glutamine. After 4 h of
incubation, the migratory ability of SiHa cells was calculated by
staining the adherent cells directly with 4% paraformaldehyde for
30 min at room temperature. The migrated cells were counted under
the light microscope (magnification, ×400) in five different fields
per filter. The experiments were repeated 3–5 times.
Cell proliferation assay
The SiHa cells were treated with pcDNA3.1-HAX-1
vector (2 µg/ml), pcDNA3.1-HAX-1 mutated vector (2 µg/ml) and
pcDNA3.1 vector (2 µg/ml) for 72 h and were serum starved for 24 h.
DNA synthesis was measured by 3H-thymidine incorporation
(3H-TdR; Hibio Bio-tech Co.) during the final 18–24 h.
The cells were digested and collected onto glass fibre filter
paper. The radioactivity retained on the dried filters was placed
in liquid scintillation vial (a control vial was also used)
containing 5 ml scintillation solution (Hibio Bio-tech Co.) and
counted in a TopCount NxT scintillation counter (LKB Instruments,
Mount Waverly, Victoria, Australia).
Assay of intracellular reactive oxygen
species (ROS)
A H2DCFDA fluorescent probe kit was used
to estimate ROS generation. The SiHa cells were treated with
pcDNA3.1-HAX-1 vector (2 µg/ml), pcDNA3.1-HAX-1 mutated vector (2
µg/ml) and pcDNA3.1 vector (2 µg/ml) for 72 h and incubated with 10
µM H2DCFDA at 37°C for 20 min in the dark. The
production of intracellular ROS was detected by inverted
fluorescence microscopy (magnification, ×200) with excitation at
488 nm and emission at 530 nm. The increase in fluorescence
intensity with respect to normoxic untreated controls was
calculated by subtracting the basal fluorescence levels.
Measurement of the mitochondrial
membrane potential (Δψm)
The loss of mitochondrial membrane potential (Δψm)
was measured in SiHa cells using the fluorescent cationic dye JC-1
(Molecular Probes; Thermo Fisher Scientific, Inc.), which is a
mitochondria-specific fluorescent dye. According to the
manufacturer's protocols, treated SiHa cells were collected and
stained with 10 µM JC-1 for 15 min at room temperature. Changes in
JC-1 monomers were detected at the excitation wavelength 485 nm and
the emission wavelength 530 nm under fluorescence microscopy
(magnification, ×200). The data are representative of 10 individual
experiments.
Statistical analysis
SPSS 18 (SPSS, Inc., Chicago, IL, USA) and GraphPad
Prism (GraphPad Software, Inc., La Jolla, CA, USA) were used for
data analysis. The data are presented as the mean ± standard error
of the mean. Quantitative data were analysed by one-way analysis of
variance, followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
HAX-1 gene expression in human
cervical tissues
The results demonstrated that the level of HAX-1
mRNA and protein in cervical cancer tissues was significantly
higher than that in adjacent cervical tissues (P<0.001; Fig. 1A and B). The location of HAX-1 protein
was detected by IHC. Fig. 1C
demonstrates that HAX-1 protein (brown staining) is predominantly
expressed in the cytoplasm of cervical squamous cell carcinoma
tissues, the positive ratio for these samples was ~75% of the
immunohistochemical staining. The expression of HAX-1 protein was
detected in the endoplasmic reticulum (ER), nuclear (Nu) and
mitochondrial (Mt) fractions in SiHa cells, but was primarily
localized to the mitochondria (Fig.
1D). These data indicated that the expression of the HAX-1 gene
may be associated with the occurrence of cervical cancer.
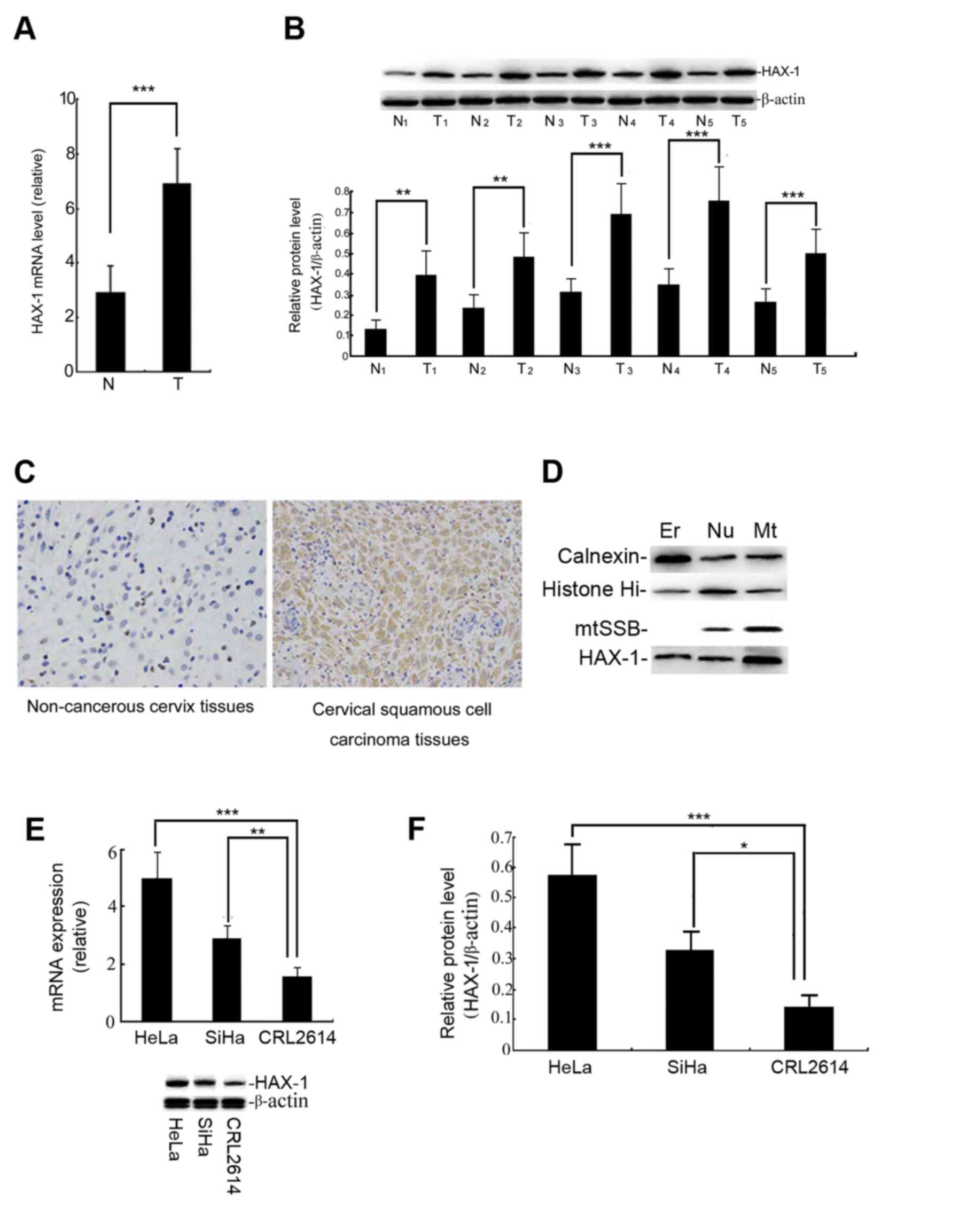 | Figure 1.Expression level of the HAX-1 gene in
human cervical tissues. (A) The relative HAX-1 gene levels in the T
and N tissues. The level of HAX-1 mRNA was measured by qPCR. The
results are presented as the mean ± SEM of three independent
experiments, each conducted in triplicate. ***P<0.001. (B) The
HAX-1 protein level was measured by western blot analysis. The
graph was grey-scale scanned, and the target protein HAX-1 level
was quantified and normalised to β-actin. The results are expressed
as the mean ± SEM of three separate experiments. T vs. N.
***P<0.001, **P<0.01. (C) The results of immunohistochemical
staining (magnification, ×200). The positive (brown) staining
indicates the presence of the HAX-1 protein in human cervical
tissues. (D) The subcellular localization of HAX-1 was detected
following cellular fractionation. The SiHa cells were separated
into ER, Nu and Mt fractions. Calnexin, histone Hi and mtSSB were
detected by immunoblotting as markers for the ER, Nu and Mt
fractions, respectively. (E) Relative HAX-1 gene expression levels
were observed in the cervical squamous carcinoma cell line, HeLa,
SiHa and the human cervical epithelial CRL2614 cell line. The
different expression levels of HAX-1 were analysed by qPCR. (F) The
expression of the HAX-1 protein was measured by western blot
analysis. The graph depicts the relative HAX-1 protein levels
normalised to β-actin. The results are expressed as the mean ± SEM
of three separate experiments. ***P<0.001, **P<0.01,
*P<0.05 vs. CRL2614 cells. HAX-1, haematopoietic cell-specific
protein 1-associated protein X-1; SEM, standard error of the mean;
qPCR, quantitative polymerase chain reaction; T, human cervical
squamous cell carcinoma tissue; N, non-cancerous tissue; ER,
endoplasmic reticulum; Nu, nuclear; Mt, mitochondrial. |
The basal level of HAX-1 in HeLa and SiHa cells is
higher than in human cervical epithelial CRL2614 cells, but the
basal level of HAX-1 in SiHa cells is markedly lower than in HeLa
cells (Fig. 1E and F). The SiHa cells
were therefore used as the primary subject in subsequent
experiments.
Effect of overexpression of HAX-1 gene
on the biological behaviour of SiHa cells
The results demonstrated that the level of HAX-1
protein in the pcDNA3.1-HAX-1 vector group was significantly higher
than that in the pcDNA3.1 vector group (P<0.01; Fig. 1A). The results of the WST-1 assay
demonstrated that HAX-1 was able to increase the survival of SiHa
cells (Fig. 2B). To determine whether
HAX-1 is a regulator of SiHa cell migratory ability, Transwell
experiments were performed, which indicated that the number of
HAX-1-mediated migrated SiHa cells increased markedly (Fig. 2C). The present study further utilized
the 3H-TdR incorporation assay to test whether HAX-1 is
a regulator of SiHa cell proliferation. As demonstrated in Fig. 2D, the number of HAX-1-mediated
proliferating SiHa cells increased significantly.
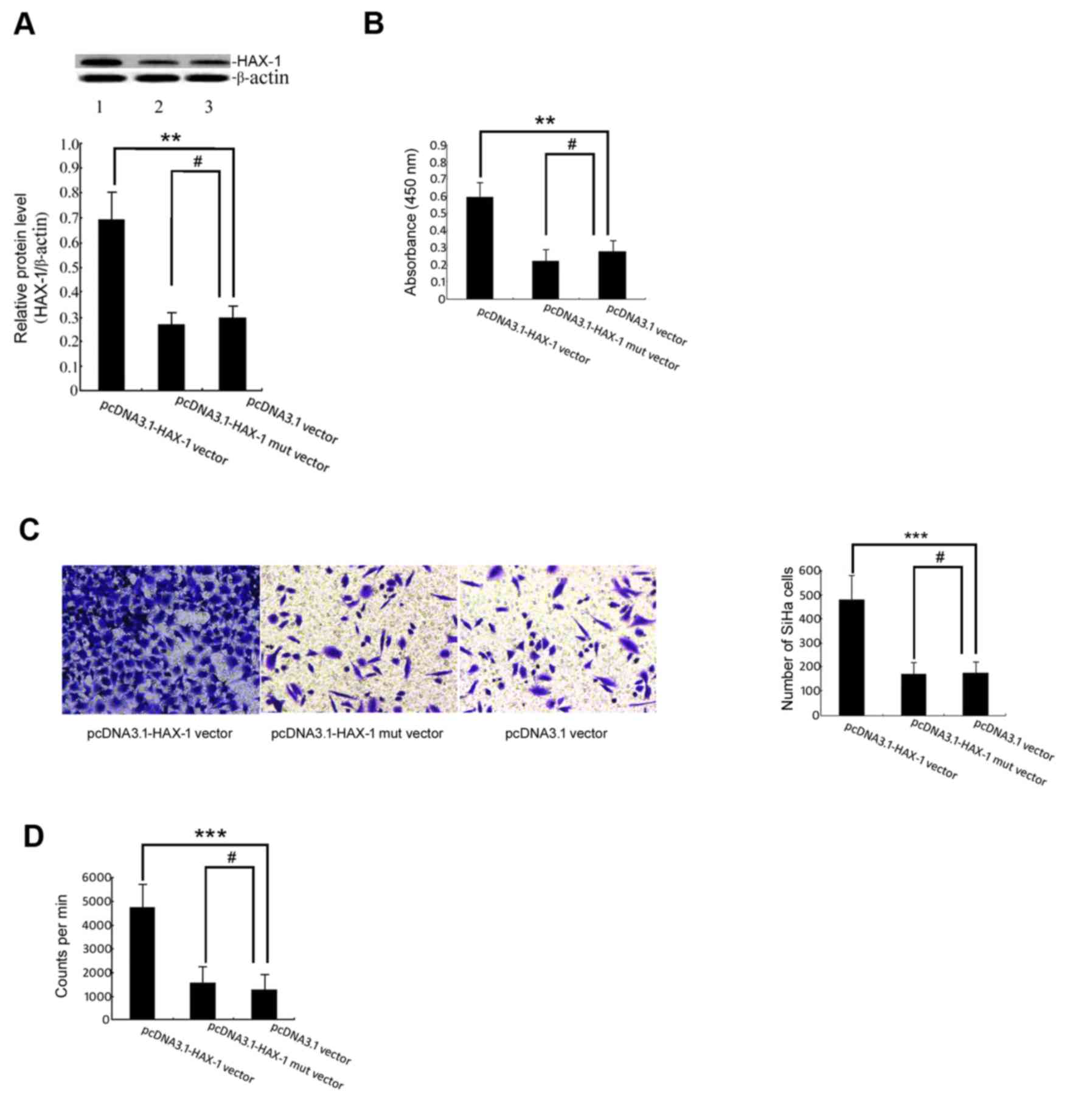 | Figure 2.Effect of the HAX-1 gene on the
biological function of SiHa cells. (A) The HAX-1 protein level was
measured by western blot analysis. The graph was grey-scale
scanned, and the target protein HAX-1 level was quantified and
normalised to β-actin. The results are expressed as the mean ± SEM
of three separate experiments. **P<0.01, #P>0.05
vs. pcDNA3.1 vector groups. (B) WST-1 cell proliferation and
viability. The SiHa cells were transfected with pcDNA3.1-HAX-1
vector (2 µg/ml), pcDNA3.1-HAX-1 mutated vector (2 µg/ml) and
pcDNA3.1 null carrier plasmids (2 µg/ml) for 72 h before performing
the WST-1 assay. The results are presented as the mean ± SEM of
three independent experiments, each conducted in triplicate.
**P<0.01, #P>0.05 vs. pcDNA3.1 vector groups. (C)
The effects of the HAX-1 gene on cell migration were measured by
Transwell migration assay. ***P<0.001, #P>0.05 vs.
pcDNA3.1 vector groups. (D) The proliferation of SiHa cells was
detected by 3H-TdR incorporation. The absolute CPM value
indicated the proliferation of SiHa cells (cpm/106
cells). ***P<0.001, #P>0.05 vs. pcDNA3.1 vector
groups. HAX-1, haematopoietic cell-specific protein 1-associated
protein X-1; SEM, standard error of the mean; WST-1, water-soluble
tetrazolium salt; CPM, counts per minute. |
Effect of overexpression of the HAX-1
gene on mitochondrial function
Mitochondrial function was assessed based upon the
changes in mitochondrial ROS content and Δψm. The experimental data
demonstrated that the ROS production mediated by the HAX-1 gene in
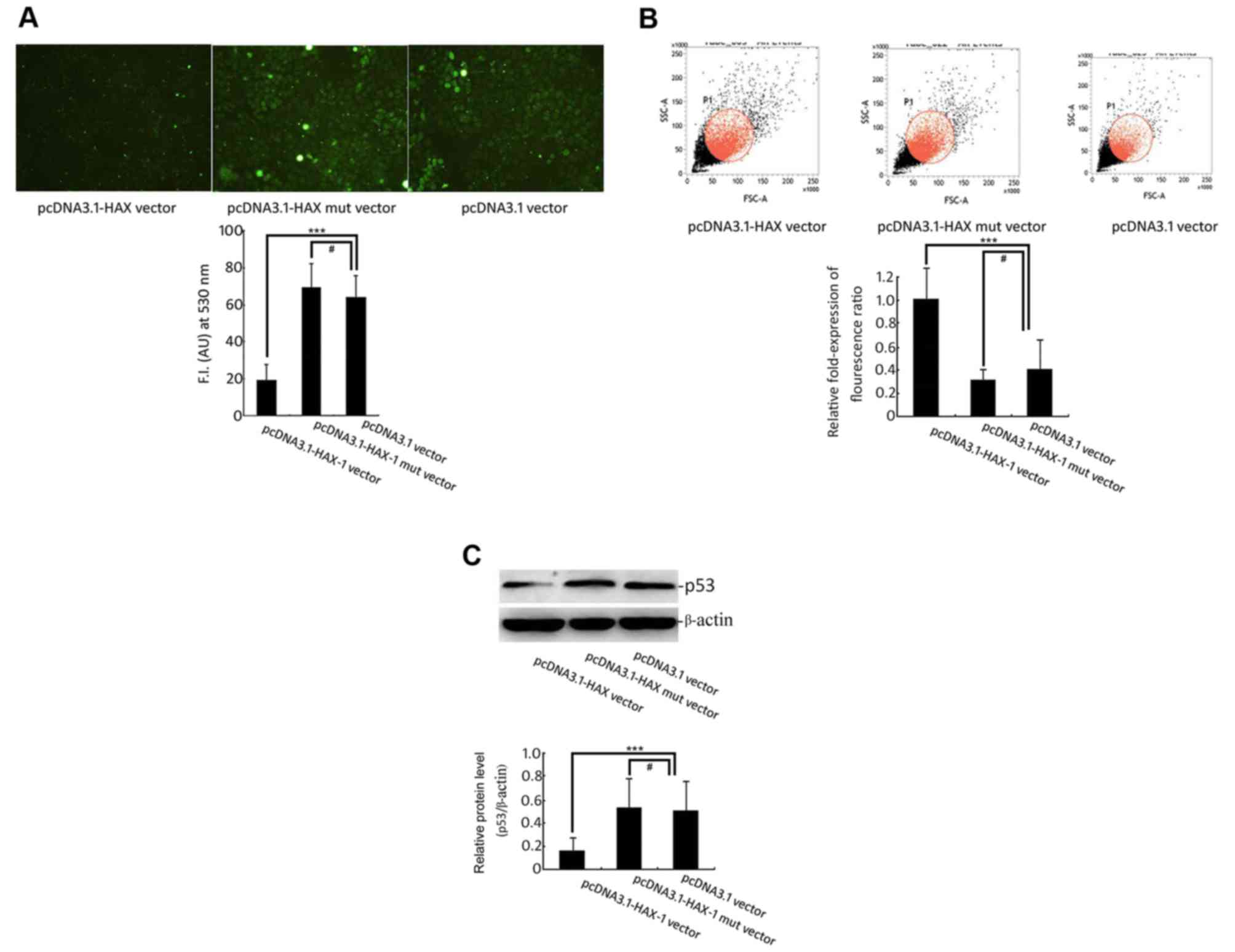
SiHa cells was significantly reduced (P<0.001; Fig. 3A). Additionally, the Δψm of SiHa cells
induced by the HAX-1 gene did not change and maintained complete
membrane potential (Fig. 3B).
However, the ROS production exhibited a significant increase, and
the Δψm decreased significantly in the pcDNA 3.1-HAX-1 mutated
vector group and the pcDNA3.1-null plasmid group.
The experimental western blot analysis results
indicated that overexpression of the HAX-1 gene significantly
inhibited the expression of p53 (Fig.
3C) and that the HAX-1 mutant did not change the expression of
the p53 protein. These data indicated that the HAX-1 gene might be
an upstream regulatory factor of pro-apoptotic protein p53,
inhibiting the rise in its expression level.
Effect of the p53 gene on
HAX-1-induced biological function in SiHa cells
These results demonstrated that HAX-1 was able to
significantly increase the cell survival (Fig. 4A), migration ability (Fig. 4B) and proliferation ability (Fig. 4C) of SiHa cells, but these effects
could be reversed by overexpression of the wild-type p53 gene or
FCCP-induced mitochondrial dysfunction. At the same time, these
results indicated that in the pcDNA3.1-HAX-1 + wild-type p53 vector
group and the pcDNA3.1-HAX-1 + FCCP group, the intracellular ROS
increased significantly compared with the pcDNA3.1-HAX-1 group
(P<0.01, P<0.05; Fig. 4D).
However, Δψm of SiHa cells was significantly decreased in the
pcDNA3.1-HAX-1 + wild-type p53 vector group and the pcDNA3.1-HAX-1
+ FCCP group (P<0.01; Fig. 4E).
The mitochondrial morphology indicated the presence of crista
breaks that disappeared, and the stroma presented a loose state.
Certain mitochondria were markedly swollen, and certain
mitochondria even appeared vacuolated (Fig. 4F).
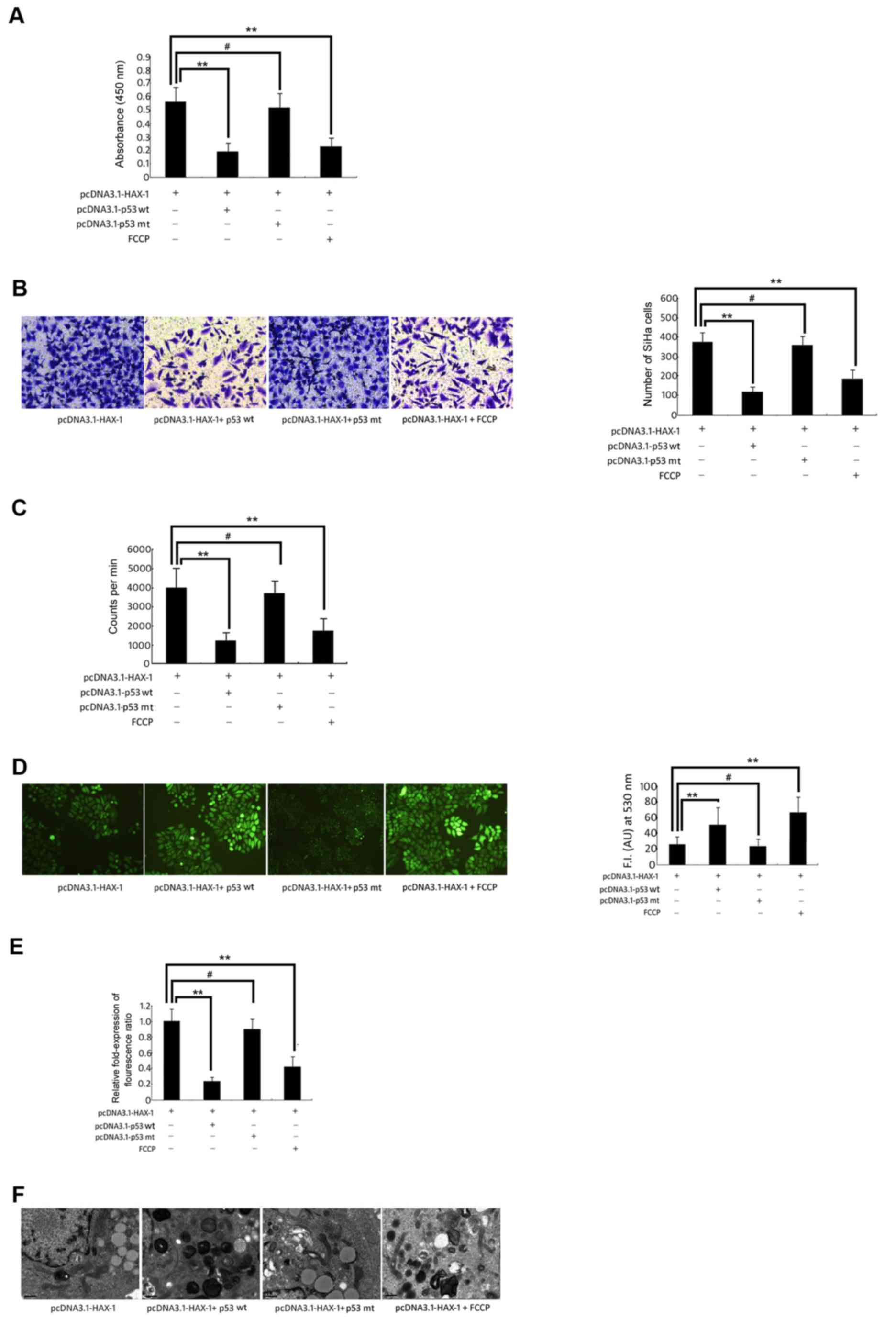 | Figure 4.Change in biological function of SiHa
cells. SiHa cells were co-transfected with pcDNA3.1-HAX-1 vector (2
µg/ml), pcDNA3.1-p53 wt vector (100 ng) orpcDNA3.1-p53 mut vector
(100 ng), and treated with FCCP (50 µM) for 72 h. (A) The
proliferative activity of SiHa cells was measured by WST-1 assay.
**P<0.01, #P>0.05 vs. pcDNA3.1-HAX-1 vector
groups. (B) Transwell assay was used to measure the migration of
SiHa cells. **P<0.01, #P>0.05 vs. pcDNA3.1-HAX-1
vector groups. (C) The proliferation of SiHa cells was detected by
3H-TdR incorporation. The cpm/106 cells
represents the proliferative ability of SiHa cells. **P<0.01,
#P>0.05 vs. pcDNA3.1-HAX-1 vector groups. (D)
Reactive oxygen species generation was quantified using the
H2DCFDA fluorescence probe.**P<0.01, *P<0.05,
#P>0.05 vs. pcDNA3.1-HAX-1 vector groups. (E) The
fluorescence of JC-1 (590 nm: 527 nm fluorescence ratio) was used
to calculate the relative change in mitochondrial potential value.
**P<0.01, #P>0.05 vs. pcDNA3.1-HAX-1 vector
groups. (F) The morphological changes of mitochondria in SiHa cells
were observed by transmission electron microscopy (magnification,
×12,500). HAX-1, haematopoietic cell-specific protein 1-associated
protein X-1; WST-1, water-soluble tetrazolium salt; FCCP, carbonyl
cyanide-p-trifluoro methoxyphenylhydrazone. |
Discussion
In recent years, novel therapies for targeting
receptor, gene and signal transduction in tumour formation have
been emerging (16,17). Among them, the inhibition of tumour
cell migration and proliferation is a popular research topic. A
potential strategy for tumour gene therapy is to transfect
apoptotic genes into tumour cells and induce their apoptosis
(18), which may become a novel
approach for the treatment of cervical cancer.
HAX-1 is a mitochondrial protein, which prevents the
accumulation of B-cell lymphoma 2-associated X protein and
apoptosis regulator (Bax), thereby inhibiting the mitochondrial
apoptosis pathway (19,20). Studies have demonstrated that HAX-1 is
overexpressed in cancer. The results of the present study also
indicated that the HAX-1 protein is predominantly expressed in the
mitochondria of cervical squamous cells (Fig. 1D). Overexpression of HAX-1 inhibits
mitochondrial collapse and the subsequent release of
mitochondria-derived apoptotic molecules, including cytochrome c.
Apoptosis, which is usually induced by mitochondrial dysfunction,
is an important target of cancer treatment (21). Mitochondrial damage results in the
opening of a non-specific pore in the inner mitochondrial membrane,
known as the mitochondrial permeability transition pore (mPTP).
Opening of the mPTP causes the release of mitochondrial components
into the cytoplasm (22). Among these
components are ROS, which are key inducers of mitochondrial
apoptosis (23). Therefore, the
present study aimed to comprehensively determine the role of
p53-induced apoptosis in the HAX-1-induced biological behaviour of
cervical cancer cells and the associated mechanism of mitochondrial
collapse and ROS generation.
HAX-1 acts as an anti-apoptotic protein by
inhibiting the accumulation of Bax, thereby interrupting the
mitochondrial apoptosis pathway (24). Previous studies have revealed that the
expression of HAX-1 varies in different tumour types, including
oesophageal squamous cell carcinoma (25), lung cancer, leukaemia, myeloma, breast
cancer and hepatoma (26–28). Studies have reported that
overexpression of HAX-1 is associated with a poor prognosis in
patients with oesophageal squamous cell carcinoma (29). Therefore, HAX-1 may serve different
biological roles in different tissues by modulating specific
proteins and specific signalling pathways (30). In order to understand the association
between the HAX-1 gene and the biological behaviour of cervical
cancer cells, the cell survival rate, migration rate and
proliferation rate were monitored. Following transfection of the
HAX-1 expression vector into SiHa cells, the migration rate and the
survival rate increased significantly. These data confirmed that
HAX-1 serves an important role in promoting the survival and
migration ability of cervical cancer cells. Furthermore, the HAX-1
gene may significantly reduce the accumulation of intracellular
ROS, protect the mitochondrial membrane potential, and maintain the
integrity of the mitochondrial structure and morphology.
As a key factor in inhibiting tumour formation, the
p53 gene may be either wild-type or mutant type. The wild-type p53
gene may act as a tumour suppressor gene by repairing damaged DNA,
blocking cell cycle, inhibiting cell proliferation and promoting
apoptosis (31–34). However, the mutant-type p53 promotes
cell proliferation through negative regulation of the wild-type p53
protein, thereby protecting cells from apoptosis and promoting
tumour development. Therefore, mutant-type p53 exerts a cancer gene
function (35). Over 50% of cancer
cell lines evade apoptosis by mutant p53 and the other cancer cell
lines also inhibit the activity of wild-type p53 by other
mechanisms (36). The results of the
present study demonstrated that the HAX-1 gene might significantly
inhibit the p53 protein. The HAX-1 gene may be regarded as a
potential upstream regulator of p53. The p53 signalling pathway has
been involved in HAX-1-induced cervical cancer cell migration and
proliferation. In the present study, the HAX-1 gene and wild-type
p53 gene were co-transfected into cervical cancer cells. It was
revealed that wild-type p53 is able to reverse the HAX-1-mediated
inhibition of the production of ROS and mitochondrial membrane
potential. At the same time, the wild-type p53 gene also abolished
the HAX-1-induced survival and migration ability of cervical cancer
cells. The aforementioned effects of wild-type p53 interfere with
the HAX-1-induced biological changes, in a similar way to the
effects of FCCP-induced mitochondrial dysfunction. Therefore, HAX-1
may induce the biological behaviour of cervical cancer cells
through inhibiting the p53-dependent pathway.
Taken together, the results of the present study
provide primary evidence that HAX-1 induced the survival, migration
and proliferation of human cervical squamous carcinoma cells by
inhibiting its downstream regulatory factor p53 in SiHa cells.
HAX-1 therefore holds promise as a prognostic biomarker and
potential therapeutic target for the treatment of cervical
cancer.
Acknowledgements
The authors would like to thank Dr Wenqu Li, Dr Rong
Huang, and Dr Rongbing Shi, from the Department of Gynaecology, the
Affiliated Obstetrics and Gynaecology Hospital of Nanjing Medical
University (Nanjing, China) for their critical reading of the
manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81571437), the
National Natural Science Foundation of Jiangsu Province (grant no.
BK20151078) and the Science and Technology Commission Foundation of
Nanjing (grant no. YKK16292).
Availability of data and materials
The datasets produced during and/or analysed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
LJG and RS conceived and designed the experiments.
BQ performed the experiments. FT, LJZ and BQ analyzed the data and
contributed to the interpretation of results obtained and
manuscript construction. LJG and RS edited the final draft
manuscript. All authors read and approved the final manuscript.
Ethics statement and consent to
participate
The present study was approved by the Ethics
Committee of the Chinese Academy of Sciences and the Nanjing
Maternity and Child Health Care Hospital (Nanjing, China), and
written informed consent was obtained from all participants.
Consent for publication
The patient and healthy subjects provided written
informed consent for the publication of any associated data and
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karageorgopoulou S, Kostakis ID, Gazouli
M, Markaki S, Papadimitriou M, Bournakis E, Dimopoulos MA and
Papadimitriou CA: Prognostic and predictive factors in patients
with metastatic or recurrent cervical cancer treated with
platinum-based chemotherapy. BMC Cancer. 17:4512017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sodhani P, Gupta S, Gupta R and Mehrotra
R: Bacterial vaginosis and cervical intraepithelial neoplasia: Is
there an association or is co-existence incidental? Asian Pac J
Cancer Prev. 18:1289–1292. 2017.PubMed/NCBI
|
|
3
|
Kwon MJ, Kang SY, Nam ES, Cho SJ and Rho
YS: HIPK2 overexpression and its prognostic role in human
papillomavirus-positive tonsillar squamous cell carcinoma. Biomed
Res Int. 2017:10564272017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borcoman E and Tourneau CL: Pembrolizumab
in cervical cancer: latest evidence and clinical usefulness. Ther
Adv Med Oncol. 9:431–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karan D, Tawfik O and Dubey S: Expression
analysis of inflammasome sensors and implication of NLRP12
inflammasome in prostate cancer. Sci Rep. 7:43782017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosania R, Varbanova M, Wex T, Langner C,
Bornschein J, Giorgio F, Ierardi E and Malfertheiner P: Regulation
of apoptosis is impaired in atrophic gastritis associated with
gastric cancer. BMC Gastroenterol. 17:842017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen S, Li W, Ouyang MA and Wang J:
Structure-activity relationship of triterpenes and derived
glycosides against cancer cells and mechanism of apoptosis
induction. Nat Prod Res. 32:654–661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paech F, Bouitbir J and Krähenbühl S:
Hepatocellular toxicity associated with tyrosine kinase inhibitors:
Mitochondrial damage and inhibition of glycolysis. Front Pharmacol.
8:3672017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Wang YX, Dong MQ, Zhang B, Luo Y,
Niu W and Li ZC: Reoxygenation reverses hypoxic pulmonary arterial
remodeling by inducing smooth muscle cell apoptosis via reactive
oxygen species-mediated mitochondrial dysfunction. J Am Heart
Assoc. 6(pii): e0056022017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frumovitz M, Burzawa JK, Byers LA, Lyons
YA, Ramalingam P, Coleman RC and Brown J: Sequencing of mutational
hotspots in cancer-related genes in small cell neuroendocrine
cervical cancer. Gynecol Oncol. 141:588–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan JY, Liu LY, Wang P, Li ZF, Ni L, Wang
A, Xiao SX, Song TS and Huang C: Small-interfering RNA-mediated
silencing of the MAPK p42 gene induces dual effects in HeLa cells.
Oncol Lett. 1:649–655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SA, Kim JW, Roh JW, Choi JY, Lee KM,
Yoo KY, Song YS and Kang D: Genetic polymorphisms of GSTM1, p21,
p53 and HPV infection with cervical cancer in Korean women. Gynecol
Oncol. 93:14–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You B, Cao X, Shao X, Ni H, Shi S, Shan Y,
Gu Z and You Y: Clinical and biological significance of HAX-1
overexpression in nasopharyngeal carcinoma. Oncotarget.
7:12505–12524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Huo X, Cao Z, Xu H, Zhu J, Qian L,
Fu H and Xu B: HAX-1 is overexpressed in hepatocellular carcinoma
and promotes cell proliferation. Int J Clin Exp Pathol.
8:8099–8106. 2015.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Q, Shen F and Wang C: TUC338 promotes
cell migration and invasion by targeting TIMP1 in cervical cancer.
Oncol Lett. 13:4526–4532. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen AH, Qin YE, Tang WF, Tao J, Song HM
and Zuo M: MiR-34a and miR-206 act as novel prognostic and therapy
biomarkers in cervical cancer. Cancer Cell Int. 17:632017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishi K, Luo H, Ishikura S, Doi K,
Iwaihara Y, Wills L, Baillie GS, Sakata T, Shirasawa S and Tsunoda
T: Apremilast induces apoptosis of human colorectal cancer cells
with mutant KRAS. Anticancer Res. 37:3833–3839. 2017.PubMed/NCBI
|
|
19
|
Suzuki Y, Demoliere C, Kitamura D,
Takeshita H, Deuschle U and Watanabe T: HAX-1, a novel
intracellular protein, localized on mitochondria, directly
associates with HS1, a substrate of Src family tyrosine kinases. J
Immunol. 158:2736–2744. 1997.PubMed/NCBI
|
|
20
|
Chao JR, Parganas E, Boyd K, Hong CY,
Opferman JT and Ihle JN: Hax1-mediated processing of HtrA2 by Parl
allows survival of lymphocytes and neurons. Nature. 452:98–102.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Halestrap AP, Clarke SJ and Javadov SA:
Mitochondrial permeability transition pore opening during
myocardial reperfusion-a target for cardioprotection. Cardiovasc
Res. 61:372–385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu JY, Li M, Cao LJ, Sun ML, Chen D, Ren
HG, Xia Q, Tao ZT, Qin ZH, Hu QS and Wang GH: Protease Omi cleaving
Hax-1 protein contributes to OGD/R-induced mitochondrial damage in
neuroblastoma N2a cells and cerebral injury in MCAO mice. Acta
Pharmacol Sin. 36:1043–1052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li M, Tang Y, Zang W, Xuan X, Wang N, Ma
Y, Wang Y, Dong Z and Zhao G: Analysis of HAX-1 gene expression in
esophageal squamous cell carcinoma. Diagn Pathol. 8:472013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Trebinska A, Rembiszewska A, Ciosek K,
Ptaszynski K, Rowinski S, Kupryjanczyk J, Siedlecki JA and
Grzybowska EA: HAX-1overexpression, splicing and cellular
localization in tumors. BMC Cancer. 10:762010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun X, Li Y, Zheng M, Zuo W and Zheng W:
MicroRNA-223 increases the sensitivity of triple-negative breast
cancer stem cells to TRAIL-induced apoptosis by targeting HAX-1.
PLoS One. 11:e01627542016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banerjee A, Saito K, Meyer K, Banerjee S,
Ait-Goughoulte M, Ray RB and Ray R: Hepatitis C virus core protein
and cellular protein HAX-1 promote 5-fluorouracil-mediated
hepatocyte growth inhibition. J Virol. 83:9663–9671. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun SJ, Feng L, Zhao GQ and Dong ZM: HAX-1
promotes the chemoresistance, invasion, and tumorigenicity of
esophageal squamous carcinoma cells. Dig Dis Sci. 57:1838–1846.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirasaka K, Mills EM, Haruna M, Bando A,
Ikeda C, Abe T, Kohno S, Nowinski SM, Lago CU, Akagi K, et al: UCP3
is associated with Hax-1 in mitochondria in the presence of calcium
ion. Biochem Biophys Res Commun. 472:108–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia C, Shui L, Lou G, Ye B, Zhu W, Wang J,
Wu S, Xu X, Mao L, Xu W, et al: 0404 inhibits hepatocellular
carcinoma through a p53/miR-34a/SIRT1 positive feedback loop. Sci
Rep. 7:43962017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hoffman S, Martin D, Meléndez A and
Bargonetti J: C. elegans CEP-1/p53 and BEC-1 are involved in DNA
repair. PLoS One. 9:e888282014.
|
|
33
|
Ai G, Dachineni R, Kumar DR, Marimuthu S,
Alfonso LF and Bhat GJ: Aspirin acetylates wild type and mutant p53
in colon cancer cells: Identification of aspirin acetylated sites
on recombinant p53. Tumour Biol. 37:6007–6016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Delbridge AR, Pang SH, Vandenberg CJ,
Grabow S, Aubrey BJ, Tai L, Herold MJ and Strasser A: RAG-induced
DNA lesions activate proapoptotic BIM to suppress lymphomagenesis
in p53-deficient mice. J Exp Med. 213:2039–2048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iwanicki MP, Chen HY, Iavarone C,
Zervantonakis IK, Muranen T, Novak M, Ince TA, Drapkin R and Brugge
JS: Mutant p53 regulates ovarian cancer transformed phenotypes
through autocrine matrix deposition. JCI Insight. 1:e868292016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang M, Zhang Y, Wang T, Zhang J, Zhou Z,
Sun Y, Wang S, Shi Y, Luan X, Zhang Y, et al: The USP7 inhibitor
P5091 induces cell death in ovarian cancers with different P53
status. Cell Physiol Biochem. 43:1755–1766. 2017. View Article : Google Scholar : PubMed/NCBI
|