Introduction
Prostate cancer (PCa) is the most commonly diagnosed
cancer in men. In 2016, there were an estimated 1,685,210 incident
cases of PCa in the USA (1). The
annual change in prostate cancer incidence was 4.7% between
2005–2011 in China (2). PCa is
clinically divided into different risk groups, based on the levels
of serum prostate specific antigen (PSA), clinical
tumor-node-metastasis (TNM) (3) and
Gleason score (3). PSA has been used
as a biomarker in the diagnosis of PCa for almost 30 years
(4). However, the false-positive or
false-negative rates of PSA-based diagnosis restrict its use as a
sole criterion (4). It was
demonstrated that 49.6% patients with PCa were misdiagnosed using
PSA only (5). Therefore, there is an
urgent requirement to identify a highly specific and accurate
biomarker for PCa diagnosis and prognosis.
Targeting protein for Xenopus kinesin-like protein 2
(TPX2) belongs to the Microtubule-associated protein family. All
members of this family contain a conserved TPX2 motif that
interacts with microtubules (6). The
interaction regulates microtubule dynamics or certain microtubule
functions, including the maintenance of cell morphology and
proliferation (6). Overexpression of
TPX2 induces the amplification of the centrosome and leads to DNA
polyploidy (7). Its expression is
tightly regulated by the cell cycle, and this protein is detected
during the G1-S stage and disappears following the completion of
mitosis (7). During the early stage
of mitosis, TPX2 is released in a Ran GTP-dependent manner and
serves an important role in mitotic spindle formation and proper
segregation of chromosomes during cell division (7). TPX2 may promote the
centromere-associated microtubule formation and increase the rate
of centrosome-associated microtubule assembly (7).
Previous evidence indicated that increased TPX2
expression was observed in different cancer types: It has been
demonstrated that high TPX2 expression was associated with tumor
progression and poor survival rate of gastric cancer (8,9). Previous
studies have also revealed the upregulation of TPX2 expression in
breast and pancreatic cancer, hepatocellular carcinoma, colon
cancer and other types of cancer (10–13).
Furthermore, TPX2 serves as an oncogenic protein and promotes the
expression of matrix metalloproteinases (MMPs) through the
activation of the phosphatidylinositol 3-kinase (PI3K)/Protein
kinase B (Akt) signaling pathway in colon cancer (14). Depletion of TPX2 with TPX2 small
interfering RNA weakens the ability of invasion and proliferation
in SMMC-7721 and HepG2 cells (14).
However, the role of TPX2 in PCa remains unclear. According to the
results of one previous study, TPX2 functions as a novel
co-regulator on the MYC pathway (15). Inhibiting the Aurora kinase A
(AURKA)/TPX2 axis may be a novel synthetic lethal therapeutic
approach for MYC-driven types of cancer (15). Furthermore, TPX2 overexpression
increases the mortality in patients with breast cancer (16). Therefore, the present study
investigated whether the overexpression of TPX2 in PCa cells
contributed to tumor progression and was able to predict poor
prognosis in patients with PCa.
Materials and methods
Patients and tissue samples
These procedures were approved by the Research
Ethics Committee of The Third Affiliated Hospital of Guangzhou
Medical University (Guangzhou, China). A tissue microarray (TMA)
containing 73 primary PCa tissues and 7 adjacent noncancerous
prostate tissues was purchased from Alenabio (cat no. PR803c;
Xi'an, China). None of the patients from which the samples were
taken had undergone chemotherapy or radiotherapy prior to surgery.
The Taylor dataset (Gene Expression Omnibus accession no.,
GSE21032), an online PCa dataset that included 150 primary PCa
tissues and 29 adjacent noncancerous prostate tissues, was
downloaded from http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi. The
primary criterion was biochemically-measured recurrence-free
survival time of serum PSA levels. An additional analysis criterion
was the overall survival time. All patients who succumbed to
diseases other than PCa or unexpected events were excluded. The
clinical features of the patients used in the TMA (age range, 20–87
years old) and Taylor datasets (age range, 37–83 years old) are
summarized in Table I.
 | Table I.Clinical features of patients. |
Table I.
Clinical features of patients.
|
| Dataset |
|---|
|
|
|
|---|
| Clinical
features | TMAa | Taylor dataset |
|---|
| Benign tissue,
cases | 7 | 29 |
| Prostate cancer,
cases | 73 | 150 |
| Age, years |
|
Mean | 68.29±10.20 | 58.33±7.10 |
|
<66 | 23 | 124 |
|
≥66 | 44 | 26 |
| Serum PSA |
|
<4 | – | 21 |
|
4–10 | – | 81 |
|
≥10 | – | 42 |
| Gleason score |
| ≤6 | – | 41 |
| 7 | – | 76 |
| ≥8 | – | 22 |
| Pathological
stage |
|
I–II | 43 | 86 |
|
III–IV | 27 | 55 |
| TNM stage |
|
≤T2A | 45 | 135 |
|
≥T2B | 27 | 10 |
Western blotting
Proteins of LNCap, PC3 and DU145 cells were
extracted by RIPA lysis buffer (cat no. P0013B; Beyotime Institute
of Biotechnology, Shanghai, China) for 30 min at 4°C, using the
protease inhibitor phenylmethylsulfonyl fluoride (100 mM; cat no.
ST506; Beyotime Institute of Biotechnology) and the supernatant
liquid was centrifuged at 13780 × g. Protein quantification was
performed using BCA (cat no. 23227; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and 15 µg protein/lane was separated using 10%
SDS-PAGE and transferred to nitrocellulose membranes. Subsequent to
blocking with 10% skim milk (Kang Long Group, Inc., Inwood, NY,
USA) for 1 h at room temperature, the membrane was incubated with
the TPX2 polyclonal rabbit antibody (dilution, 1:100; cat no.
HPA005487; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
β-actin antibody (dilution, 1:200; cat no. BM0627; Boster
Biological Technology, Pleasanton, CA, USA) at 4°C overnight.
Subsequent to washing three times with Tris-buffered saline with
0.25% Tween-20, the membrane was incubated with a horseradish
peroxidase (HRP)-conjugated secondary antibody (goat anti-rabbit
IgG; dilution, 1:400; cat no. BA1060; Boster Biological Technology)
for a further 2 h at room temperature. Finally, the immunoreactive
protein bands were detected by a Chemiluminescence imaging analysis
system (5200; Tanon Science and Technology Co., Ltd., Shanghai,
China). The relative density of protein expression was quantified
using Image J software 1.46 (National Institutes of Health,
Bethesda, MD, USA).
Immunohistochemistry analysis
Immunohistochemistry staining of the tissue
microarray slice was conducted with the DAKO EnVision system (Dako
Diagnostics, Zug, Switzerland) on Dako automated immunostaining
instruments, according to the protocol of our previous studies
(17) The primary antibodies against
TPX2 (Anti-TPX2 antibody, rabbit polyclonal antibody; cat no.
HPA005487; Sigma-Aldrich; Merck KGaA) were used at a dilution of
1:200. HRP-labeled antibodies and alkaline-phosphatase-labeled
antibodies [dilution, 1:200; UltraSensitive SP (Mouse/Rabbit) IHC
kit] were employed to detect the specifically-bound primary
antibodies. Immunostaining was scored by 2 independent experienced
pathologists (The Third Affiliated Hospital of Guangzhou Medical
University, Guangzhou Medical University, Guangzhou, China) who
were blinded to the clinicopathological data and clinical outcomes
of the patients. The scores of the 2 pathologists were compared,
and any discrepant scores were resolved through re-examining the
staining by the pathologists to achieve a consensus score. The
number of stained cells in 10 representative microscopic fields was
counted and the percentage of positive cells was calculated using a
light microscope (Nikon Corporation, Tokyo, Japan). Due to the
homogeneity of the staining of the target proteins, tumor specimens
were scored in a semi-quantitative manner. The percentage of
immunoreactive cells was separated into five groups as follows: 0,
0%; 1, 1–10%; 2, 11–50%; 3, 51–80%; and 4, >80%. The staining
intensity was visually scored and stratified as follows: 0,
negative; 1, weak; 2, moderate; and 3, strong. Final
immunoreactivity scores were obtained for each case by multiplying
the percentage and the intensity score.
Cell culture and stable cell line
generation
The three human PCa DU145, PC3 (CVCL_0035) and LNCaP
cell lines used in the present study were purchased from the
American Type Culture Collection (Manassas, VA, USA) and were
maintained in the high-glucose Dulbecco's modified Eagle's medium
(DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). The cells were cultured at 37°C in a 5%
CO2 humidified incubator. The TPX2 coding sequence
(Genebank: NM_012112.4) was synthesized and cloned into
lentivirus-vector by Shanghai Generay Biotech Co., Ltd. (Shanghai,
China), and the plasmid was verified by sequencing. The lentivirus
of TPX2-overexpresion and blank vector were produced by Guangzhou
HYY Medical Science Co., Ltd. (Guangzhou, China). Subsequently, the
LNCaP, DU145 and PC3 cell lines were transfected by the lentivirus
of TPX2-overexpresion and blank vector, as the negative control,
and finally the stable cell line was produced through puromycin
treatment.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) assay (cat. no.,
C0038; Beyotime Institute of Biotechnology, Shanghai, China) was
used to evaluate the proliferation of PCa cells. A total of
~2×103 LnCap and PC3 cells/well were seeded into 96-well
plates and cultured for 24, 48, and 72 h at 37°C. Cells were then
incubated with 10 µl CCK-8 for 2 h at 37°C. The absorbance at a
wavelength of 450 nm was measured with a spectrophotometer
(Multiskan™ MK3, Thermo Fisher Scientific, Inc.). Data
are presented as mean ± standard deviation (SD) of three
independent experiments.
Apoptosis
Apoptosis was analyzed using an Annexin V-
allophycocyanin (APC)/7-aminoactinomyocin (7-AAD) Apoptosis
Detection kit (BD Pharmingen; BD Biosciences, Franklin Lakes, NJ,
USA). The cultured cells were collected and suspended in 1× Binding
Buffer (from the apoptosis detection kit) followed by incubation
with Annexin V-APC/7AAD for 15 min at room temperature (Annexin
V-APC/7AAD kit, cat no. 4224750). Apoptotic analyses were conducted
on a BD FACSCalibur flow cytometer (BD Biosciences), and a minimum
of 1×106 cell counts were used for each experimental
sample. Docetaxel (10 nmol; Shanghai Yu Yan Biotech Co, Ltd,
Shanghai, China) was added to the PC3 cells for 48 h at 37°C and
the experiments were repeated. Only PC3 cells were used due to
patients with androgen-dependent prostate cancer primarily
receiving emasculation therapy, with patients with
non-androgen-dependent prostate cancer being treated with
chemotherapy. LNCap is an androgen-dependent cell and PC3 is a
non-androgen-dependent cell (18);
therefore, only PC3 cells were treated with chemotherapy.
Scratch wound healing assay
The scratch wound healing assay was performed to
evaluate the migratory ability of the PCa cells. The transfections
with the TPX2 or negative control vectors were performed when the
cells (LnCap and PC3) reached 80–90% confluence using Lipofectamine
2000 reagent (cat no. 11668019; Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
24 h transfection, a scratch was made with a 10 µl pipette tip. The
cells were then returned to the incubator until the indicated times
(24, 48 and 72 h) at 37°C. Representative images were captured, and
the distance that the cells that had migrated from the wound edge
were counted at each time point use ImageJ Pro-Plus 6.0 (National
Institutes of Health, Bethesda, MD, USA). Data are presented as
mean ± SD of three independent experiments.
Transwell invasion assay
The CytoSelect Cell Migration and Invasion kit (Cell
Biolabs, Inc., San Diego, CA, USA) was used according to the
manufacturer's protocol. After 24 h transfection, PCa cells were
re-suspended in a serum-free RPMI-1640 medium (cat no. sh30809.01B;
HyClone; GE Healthcare Life Sciences) to a density of
25×104/ml. The suspended cells (200 µl) were seeded in
the upper chambers, and the lower chamber contained 10% fetal
bovine serum as a chemoattractant. Following incubation at 37°C for
48 h, the cells that had migrated through the membrane were stained
by 0.1% crystal violet (cat no. BS234b; Biosharp, Hefei, China) at
room temperature for 15 min and quantified by counting 9
independent symmetrical visual fields under a light microscope
(Nikon Corporation). Data are presented as mean ± SD of three
independent experiments.
Statistical analysis
The software of SPSS 13.0 for Windows (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. Continuous
variables are presented as mean ± SD. The Kaplan-Meier method was
used for the survival analysis, and a log-rank test was used to
analyze the difference between survival times. The Student's t-test
was used for data analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of TPX2 is associated
with the clinicopathological characteristics of PCa and tumor
recurrence time in patients with PCa
To assess TPX2 protein expression in PCa, a prostate
tissue chip containing 7 benign prostate tissues and 73 prostate
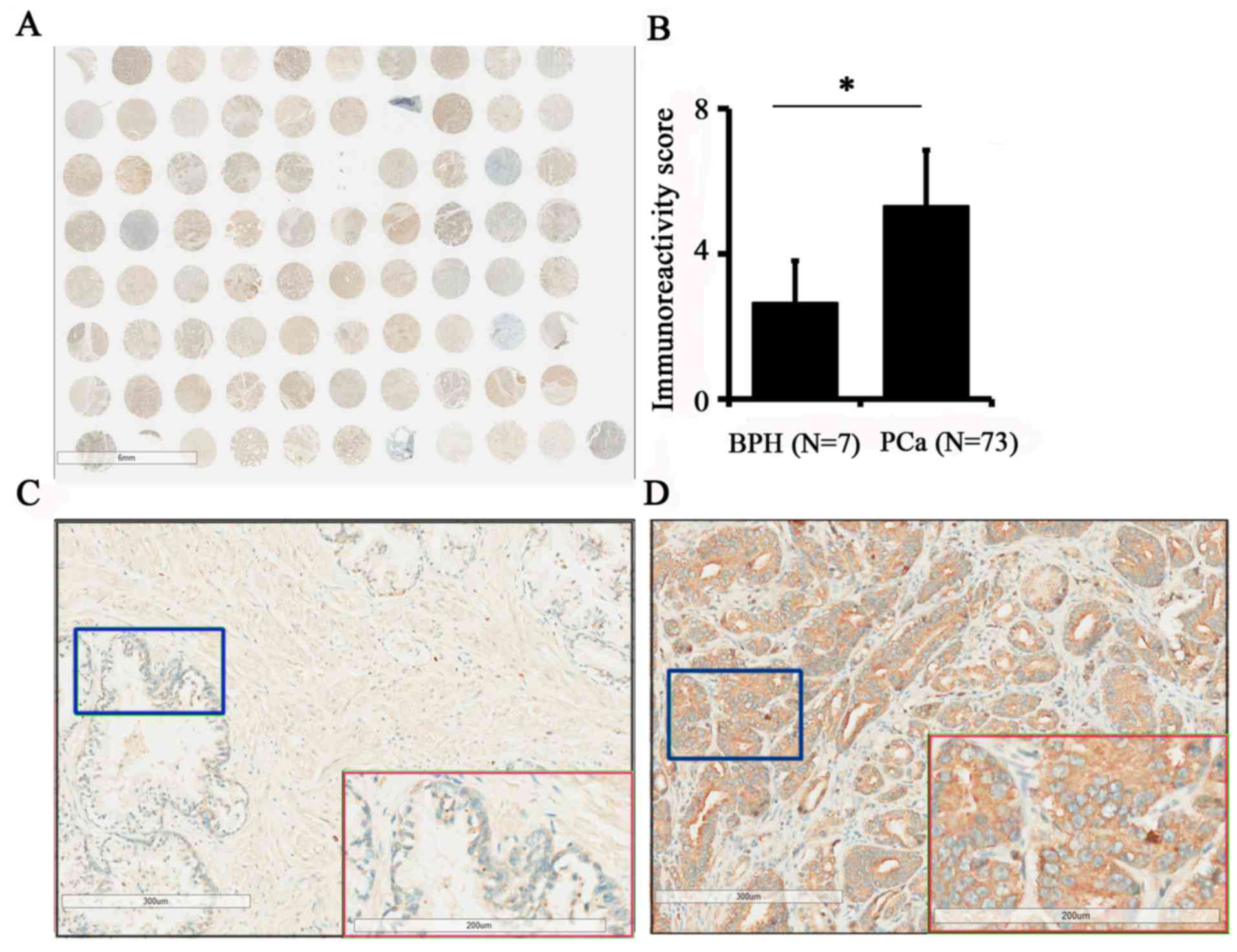
cancer tissues was evaluated by immunohistochemistry (Fig. 1A). The results demonstrated that the
expression of TPX2 in PCa was increased compared with benign
tissues (5.12±2.91 vs. 2.43±1.72; P=0.019; Fig. 1B). In addition, the expression of TPX2
protein was positively associated with pathological stage (3) and TNM stage (3) (Table II).
Additionally, TPX2 was primarily located in the nucleus and
cytoplasm, indicating that the expression of TPX2 was positively
associated with the progression of PCa. Images of TPX2 staining in
non-cancer tissues and PCa are presented in Fig. 1C and D, respectively.
 | Table II.Association of TPX2 expression with
clinicopathological characteristics of prostate cancer. |
Table II.
Association of TPX2 expression with
clinicopathological characteristics of prostate cancer.
|
| Dataset |
|---|
|
|
|
|---|
|
| TMA
dataseta | Taylor dataset |
|---|
|
|
|
|
|---|
| Clinical
features | Case | Mean ± SD | P-value | Case | Mean ± SD | P-value |
|---|
| Tissue |
|
Benign | 7 | 2.43±1.72 | 0.019 | 29 | 5.79±0.16 | <0.001 |
|
Cancer | 73 | 5.12±2.91 | – | 150 | 6.20±0.61 | – |
| Age |
|
<66 | 24 | 5.17±2.88 | 0.930 | 12 | 56.18±0.57 | 0.421 |
|
≥66 | 49 | 5.10±2.95 | – | 25 | 6.29±0.75 | – |
| Serum PSA |
|
<10 | – | – | – | 105 | 6.08±0.47 | 0.009 |
|
≥10 | – | – | – | 42 | 6.37±0.63 | – |
| Gleason score |
| ≤7 | – | – | – | 117 | 6.05±0.44 | 0.004 |
| ≥8 | – | – | – | 22 | 6.59±0.77 | – |
| Pathological
stage |
|
I–II | 43 | 4.26±2.37 | 0.002 | 86 | 6.03±0.41 | 0.007 |
|
III–IV | 27 | 6.63±3.25 | – | 55 | 6.30±0.66 | – |
| TNM stage |
|
≤T2A | 45 | 4.16±2.25 | 0.001 | 135 | 6.14±0.55 | 0.028 |
|
≥T2B | 27 | 6.78±3.22 | – | 10 | 6.55±0.76 | – |
| Metastasis |
| No | – | – | – | 122 | 6.02±0.37 | <0.001 |
|
Yes | – | – | – | 28 | 6.96±0.81 | – |
| PSA rise following
treatment |
|
Negative | – | – | – | 104 | 6.01±0.37 | 0.001 |
|
Positive | – | – | – | 36 | 6.46±0.73 | – |
| Overall
survival |
|
Alive | – | – | – | 131 | 6.46±0.73 | 0.002 |
|
Succumbed | – | – | – | 19 | 6.90±0.96 | – |
As the tissue chip data did not contain additional
clinicopathological parameters, including metastasis, PSA level or
biochemical recurrence time, the Taylor dataset, an online PCa
dataset, was used for the analysis of survival and prognosis. The
results of the analysis of the Taylor dataset revealed that PCa at
later pathological stages (III–IV) demonstrated increased TPX2
expression compared with PCa in earlier pathological stages (I–II).
In addition, statistical analyses of the Taylor dataset also
indicated that PCa with a Gleason Score ≥8 exhibited a higher
expression of TPX2 compared with those with a Gleason Score <8
(P=0.004) at the mRNA level (Table
II).
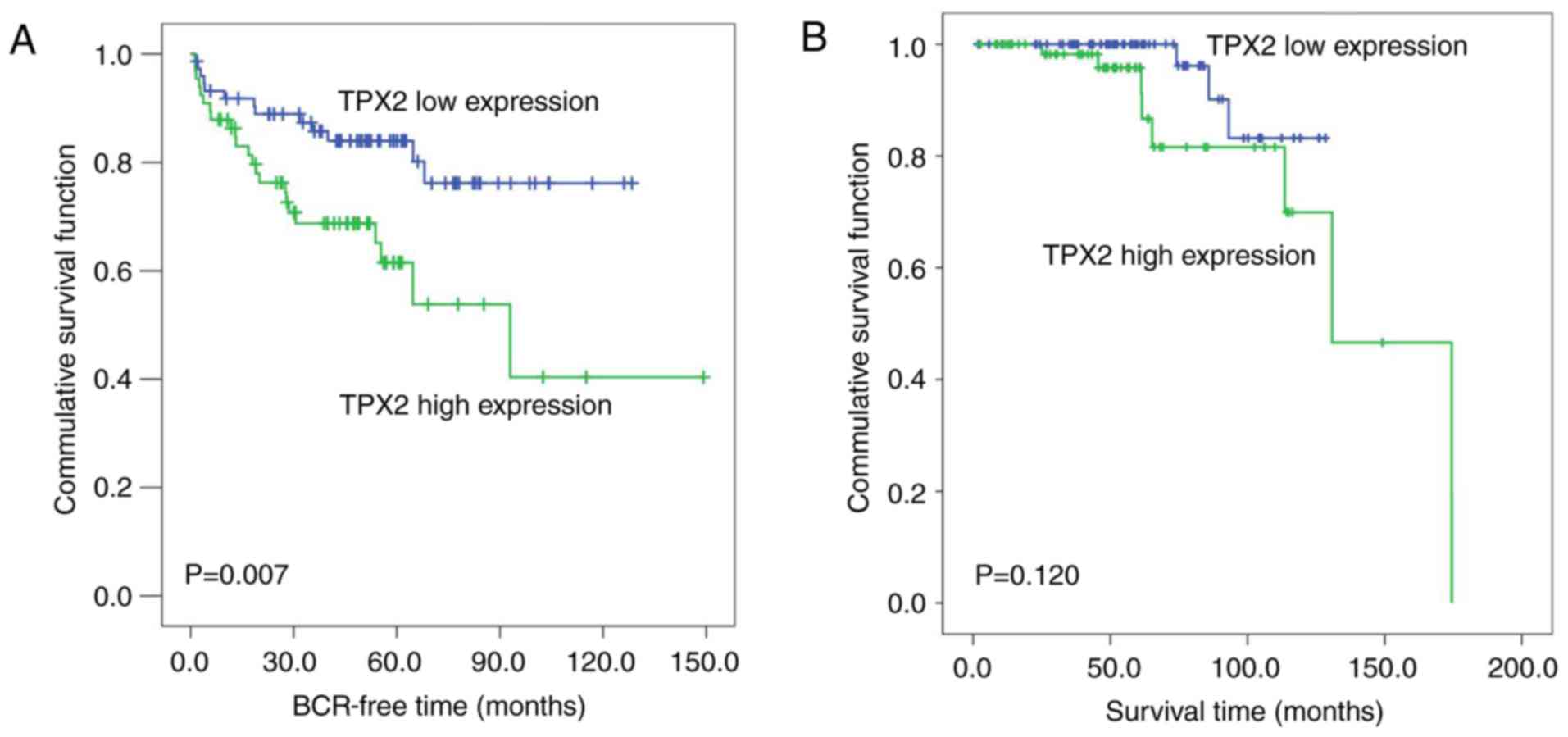
The Kaplan-Meier method was then used to analyze the
association of TPX2 expression levels with the biochemical
recurrence-free time and the overall survival time of patients with
PCa in the Taylor dataset. The median TPX2 expression (~6.03) in
all PCa patients was used as the cutoff to divide all PCa tissues
into high (n=74) and low (n=74) TPX2 expression groups. As
demonstrated in Fig. 2A, the
biochemical recurrence-free time of the high TPX2 group was shorter
compared with the low group (P=0.007; Fig. 2A). However, no significant association
between the overall survival time and TPX2 expression was observed
in patients with PCa (Fig. 2B).
Overexpression of TPX2 enhances the
proliferation of PCa cells
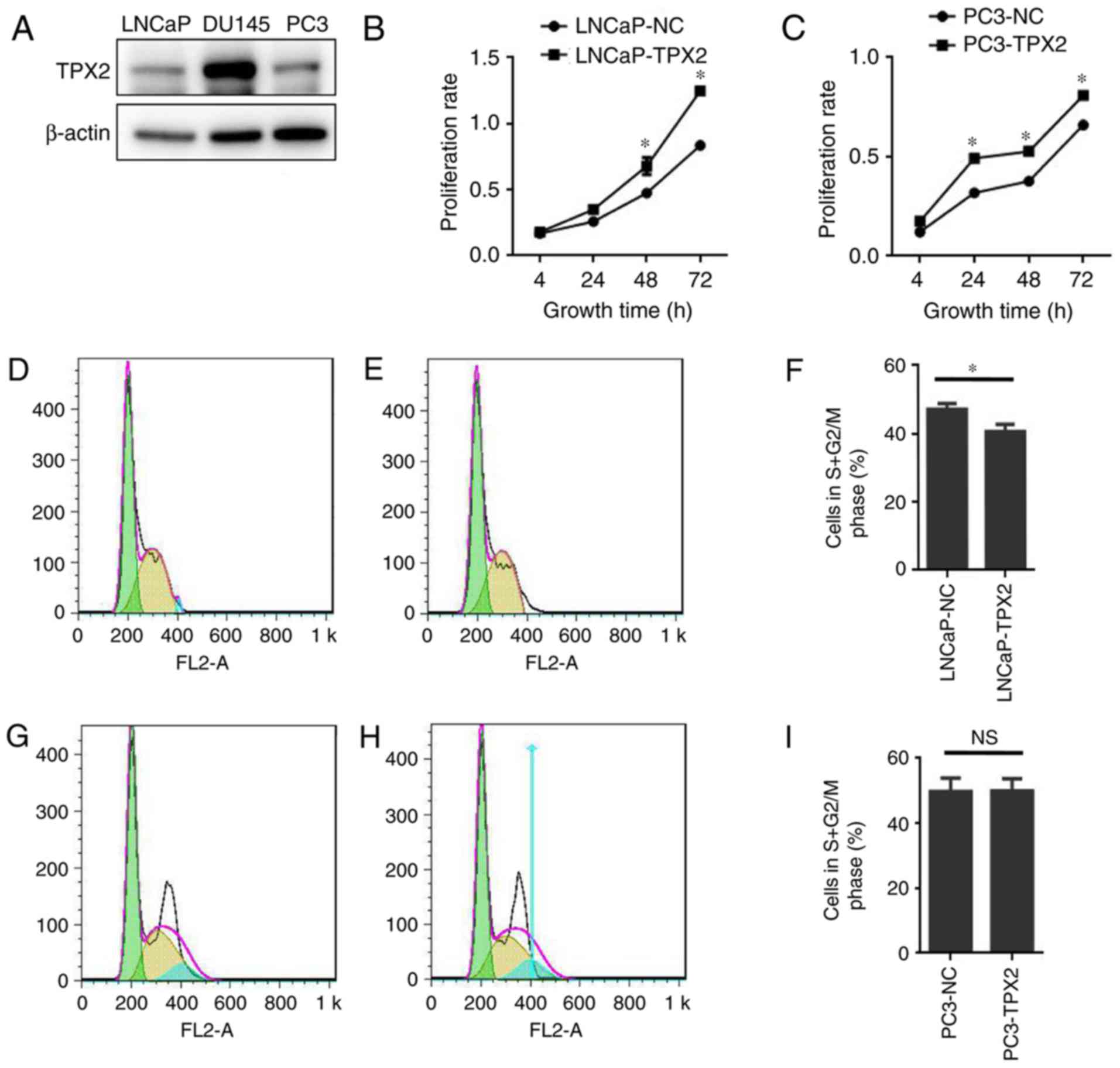
To assess the expression of TPX2 in human PCa cells,
the expression of TPX2 in three established PCa cell lines: LNCaP;
DU145; and PC3, was first assessed. Notably, TPX2 was highly
expressed in the DU145 cell line, and weakly expressed in the LNCaP
and PC3 cell lines (Fig. 3A). To
determine the activity of TPX2 in PCa cells, LNCaP and PC3 cells
were forced to express TPX2 by stable transfection. The CCK8 assay
demonstrated that the LNCaP-TPX2 and PC3-TPX2 cell lines exhibited
an increased proliferation rate compared with the empty
vector-transfected cells (Fig. 3B and
C). To determine whether overexpression affected cell
proliferation, FACS analysis was used to examine the cell cycle.
The results indicated that TPX2 arrested the cell cycle in G0 phase
(Fig. 3D-F) in the LNCaP cells, but
not in the PC3 cells (Fig. 3G-I).
Overexpression of TPX2 suppresses
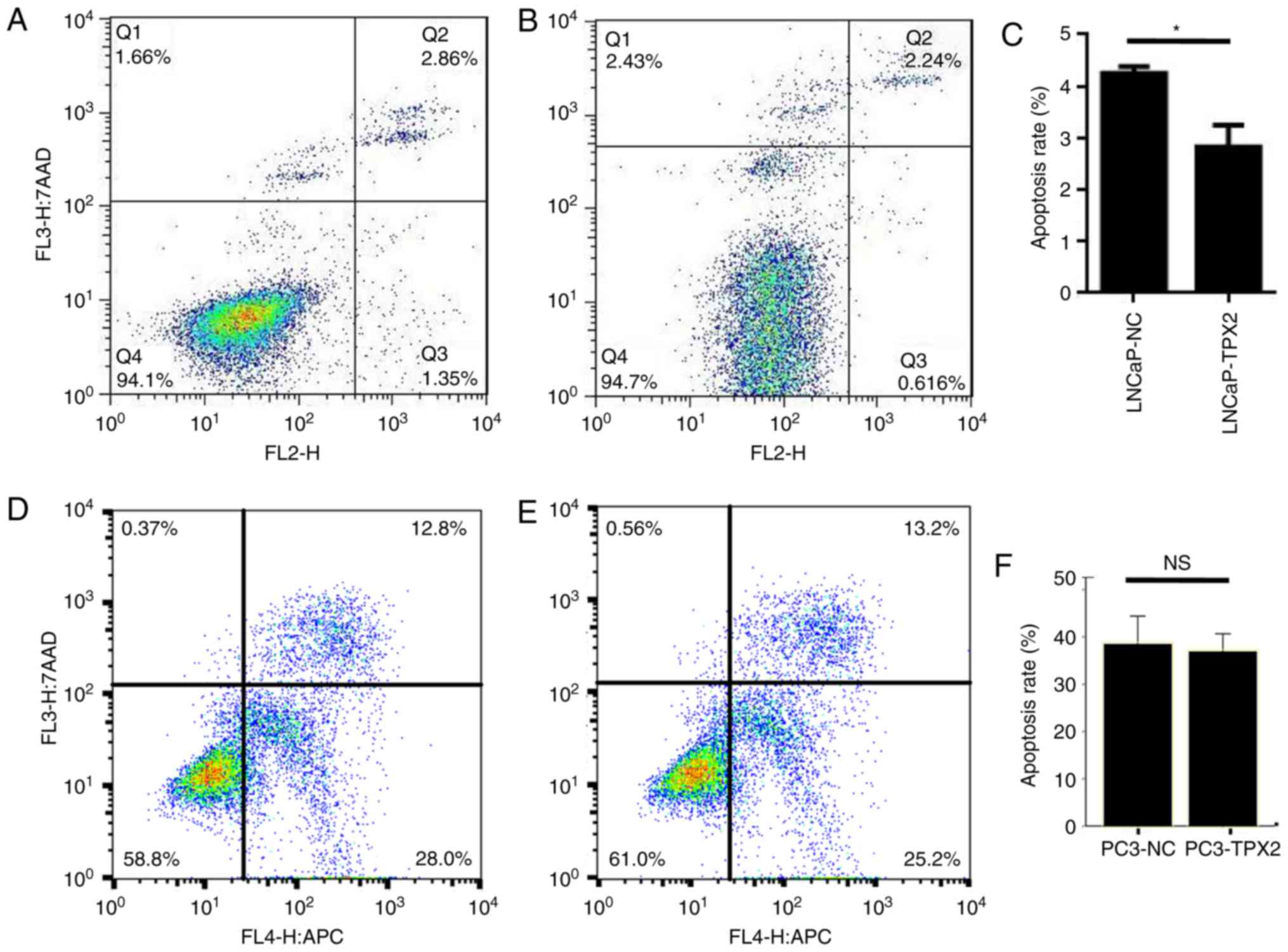
apoptosis in LNCaP cells
The data indicated that the LNCaP-TPX2 cells
exhibited fewer apoptotic cells compared with the
vector-transfected LNCaP cells (Fig.
4A-C). Docetaxel is a potent apoptosis inducer (19). Therefore, docetaxel-induced apoptosis
in PC3 cells with or without forced expression of TPX2 was
determined by Annexin V staining. Notably, there was no significant
difference in apoptosis between PC3-TPX2 and PC3-NC cells when
treated with docetaxel (36.92±1.49 vs. 38.49±2.36, respectively;
P=0.383; Fig. 4D-F), suggesting that
the anti-apoptotic activity of TPX2 was attenuated by docetaxel
treatment.
Overexpression of TPX2 enhances cell
migration in PCa cells
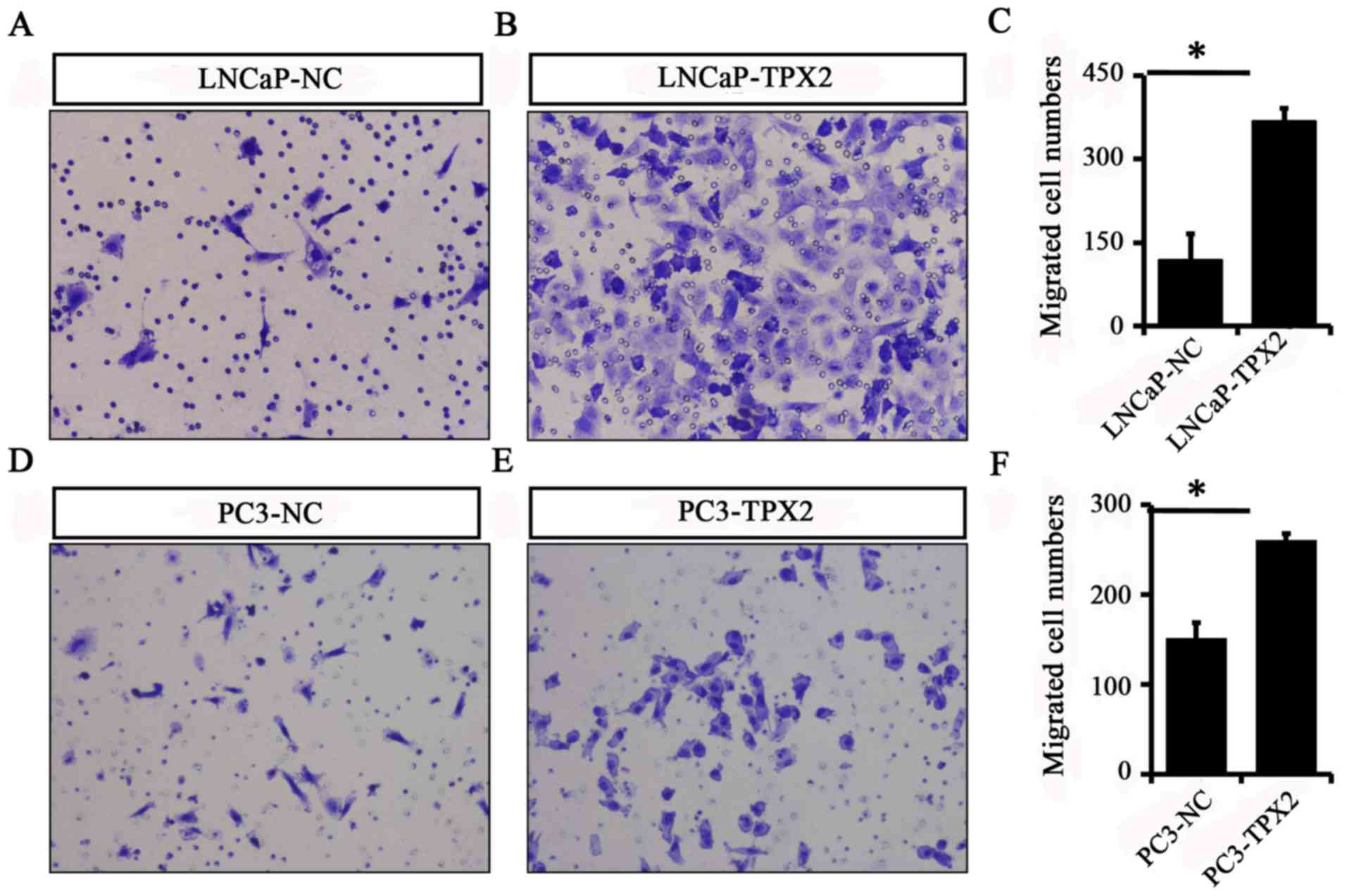
To identify whether TPX2 promoted cell mobility, A
Transwell assay was used to assess the migration activity of LNCaP
and PC3 cells with or without forced expression of TPX2. The
results indicated that the migratory ability of the LNCaP-Tpx2
cells was increased compared with the LNCaP-NC cells (Fig. 5A-C). Similarly, the PC3-Tpx2 cells
also exhibited increased migratory activity compared with the
PC3-NC cells (Fig. 5D-F).
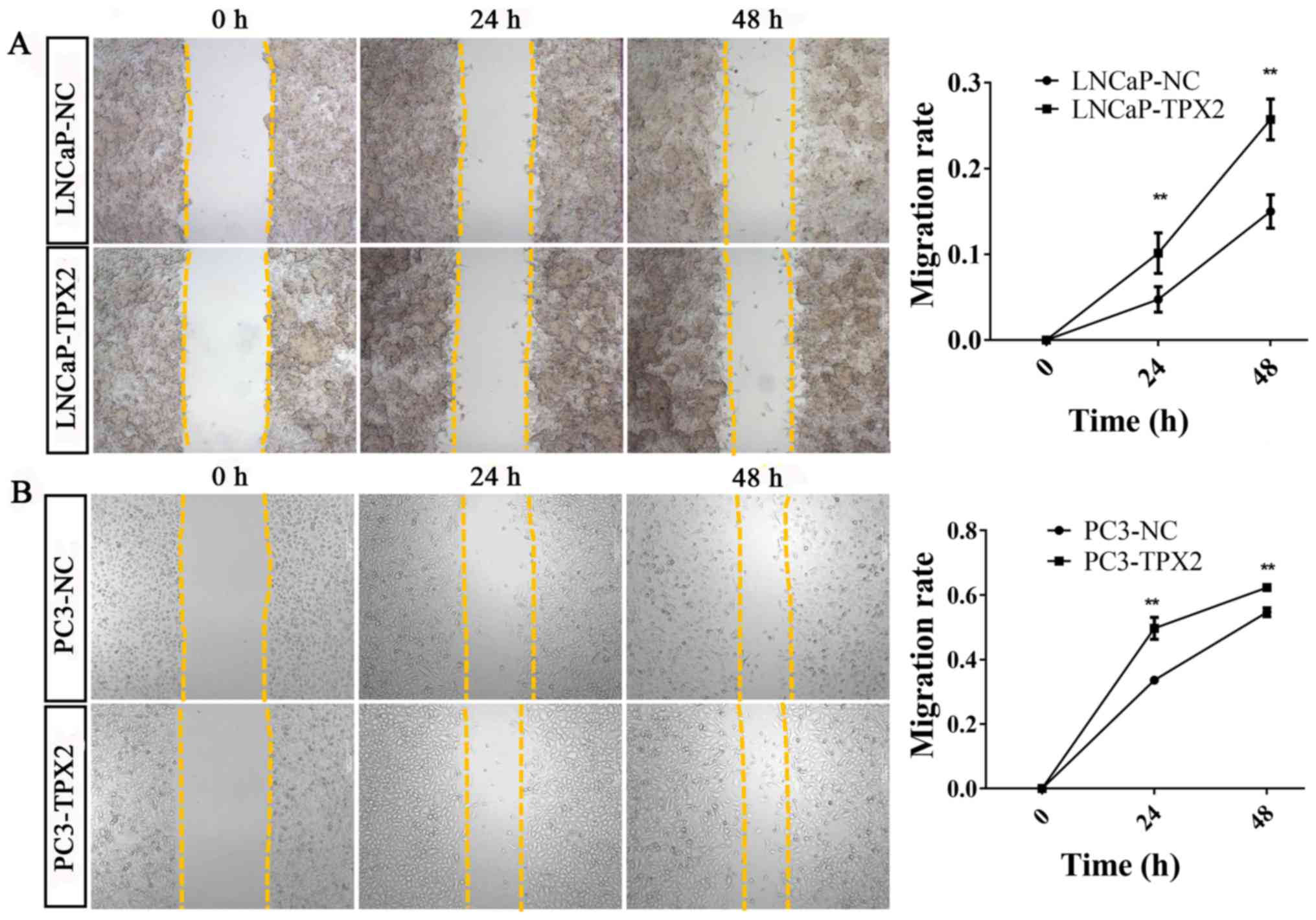
Furthermore, the wound-healing assay revealed similar results to
the migration assay (Fig. 6).
Discussion
Although early detection of PCa through serum
testing of PSA, and improved procedures of surgical intervention
and radiation therapy have significantly decreased the number of
fatalities, the prognosis of late-stage PCa remains poor (17). The current diagnostic biomarkers fail
to predict the progression of PCa (17). Therefore, it is of importance to
develop novel biomarkers for estimating the recurrence and
metastatic potential of PCa (17).
The TPX2 gene is located at chromosome 20q11.2, upstream of B-cell
lymphoma 2. The kinase domain at the N-terminal region that
connects to AURKA and the C-terminal region that anchors to the
microtubules are the two important domains of TPX2 (20). Previous studies have suggested that
the overexpression of TPX2 promoted the development of different
types of tumors by enhancing microtubule assembly forming the
spindle through activation of Ran-GTP pathway and the AURKA,
followed by phosphorylation of Hepatoma upregulated protein,
Cytoskeleton-associated protein 5A and Kinesin-like protein KIF2C
(20,21). Furthermore, TPX2 was also associated
with chromosomal abnormalities and DNA damage (22).
Aberrant activity of TPX2 has been confirmed in
several types of cancer (23).
Overexpression of TPX2 significantly enhanced the phosphorylation
of Akt and increased the expression of cyclin D1 and matrix
metalloproteinase (MMP)-9 in glioma cells (23). In contrast, TPX2 knockdown inhibited
cell proliferation and invasion through decreased Akt
phosphorylation and expression of MMP-9 and cyclin D1 (23). Expression of TPX2 was significantly
upregulated in renal cell carcinoma (RCC) and promoted the
proliferative and invasive ability of RCC cells (24). In addition, it has been proposed that
Absent in melanoma 1, Endoplasmic reticulum-Golgi intermediate
compartment protein 1, Transmembrane emp24 domain-containing
protein 3 and TPX2 are potential drug targets in PCa (25). TPX2 expression was associated with an
increase in PSA following prostate cancer treatment, while
silencing reduced the expression of PSA (25).
Chemotherapy has served a pivotal role in the
treatment of metastatic castration-resistant prostate cancer
(mCRPC) since 2004, when docetaxel-based chemotherapy first
exhibited modest improvement in survival time compared with
mitoxantrone-based therapy as a first-line chemotherapy (26,27). While
novel drugs have been developed, docetaxel remains one of the
standard initial systemic therapies for patients with mCRPC. It has
been recommended as the first-line drug in the 2015 National
Comprehensive Cancer Network guidelines (28). However, a substantial proportion of
patients with mCRPC treated with docetaxel eventually become
refractory and progress due to the development of drug resistance
(28). Docetaxel binding stabilizes
microtubules, preventing the normal formation of mitotic spindles
and their disassembly (29), while
TPX2 promotes spindle assembly. Additional clarification of whether
TPX2 overexpression reduces the function of docetaxel in promoting
apoptosis is required.
In conclusion, the present study suggested that the
overexpression of TPX2 improved the proliferative, invasive and
migratory abilities and inhibited apoptosis of PCa cell lines.
Overexpression of TPX2 was more frequently observed in high TNM and
clinicopathological staging. Statistical analyses of the online
Taylor dataset revealed that high TPX2 levels were associated with
high Gleason scores, metastasis and PSA rising again following
treatment, indicating that high-level TPX2 in PCa tissues indicated
poor prognosis. Therefore, the preliminary conclusion of the
present study is that the overexpression of TPX2 serves as a
potential biomarker for PCa diagnosis and prognosis.
Previous studies have demonstrated that the
proliferation and invasion of prostate cancer cells may be promoted
by inhibiting the expression of TPX2 (30). The apoptosis results of the present
study are in agreement with the published literature (30). The apoptotic rate in the PC3-TPX2 and
PC3-NC groups were similar (36.92±1.49 vs. 38.49±2.36,
respectively; P=0.383) when treated with docetaxel, which was in
agreement with previous studies where the rate of apoptosis in the
control groups of PC3 cells was 20–40% following docetaxel
treatment (17,31). The apoptosis results obtained in the
present study using docetaxel were not significant. Therefore, a
different drug should be employed in future studies. In addition,
to study the function of a gene, besides overexpression, knockdown
of its expression is also important. DU145 cells exhibited high
TPX2 expression in the present study, and therefore may considered
a good model for conducting future experiments.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Guangzhou Health Science and Technology General Guidance project
[Health Science and technology (2017) 6], Guangzhou Medical
University Fund (grant no., 2015C26), Natural Science Foundation of
Guangdong Province (grant no., 2014A030310088), the Zhejiang Open
Foundation of the Most Important (grant no., YFKJ003), the
Fundamental Research Funds for the Central Universities (grant
nos., 2017PY023 and 2017MS127).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WDZ supervised the entire study, participated in
study design and coordination. JZ, RYH, FNJ, DXC, CW, ZDH and YXL
performed most of the experiments and statistical analyses, and
drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
These procedures were approved by the Research
Ethics Committee of The Third Affiliated Hospital of Guangzhou
Medical University (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TPX2
|
targeting protein for Xenopus
kinesin-like protein 2
|
|
PCa
|
prostate cancer
|
|
PSA
|
prostate specific antigen
|
|
TNM
|
clinical tumor-node-metastasis
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 1:7–30. 2016. View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 2:115–132. 2016. View Article : Google Scholar
|
|
3
|
Partin AW, Kattan MW, Subong EN, Walsh PC,
Wojno KJ, Oesterling JE, Scardino PT and Pearson JD: Combination of
prostate-specific antigen, clinical stage, and Gleason score to
predict pathological stage of localized prostate cancer. A
multi-institutional update. JAMA. 277:1445–1451. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stamey TA, Caldwell M, McNeal JE, Nolley
R, Hemenez M and Downs J: The prostate specific antigen era in the
United States is over for prostate cancer: What happened in the
last 20 years? J Urol. 172:1297–1301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zare-Mirzaie A, Balvayeh P, Imamhadi MA
and Lotfi M: The frequency of latent prostate carcinoma in
autopsies of over 50 years old males, the Iranian experience. Med J
Islam Repub Iran. 2:73–77. 2012.
|
|
6
|
Du P, Kumar M, Yao Y, Xie Q, Wang J, Zhang
B, Gan S, Wang Y and Wu AM: Genome-wide analysis of the TPX2 family
proteins in Eucalyptus grandis. BMC Genomics. 1:9672016. View Article : Google Scholar
|
|
7
|
Tulu US, Fagerstrom C, Ferenz NP and
Wadsworth P: Molecular requirements for kinetochore-associated
microtubule formation in mammalian cells. Curr Biol. 16:536–541.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shao C, Duan C, Wang J, Luan S, Gao Y, Jin
D, Wang D, Li Y and Xu L: Expression of microtubule-associated
protein TPX2 in human gastric carcinoma and its prognostic
significance. Cancer Cell Int. 16:792016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomii C, Inokuchi M, Takagi Y, Ishikawa T,
Otsuki S, Uetake H, Kojima K and Kawano T: TPX2 expression is
associated with poor survival in gastric cancer. World J Surg
Oncol. 1:142017. View Article : Google Scholar
|
|
10
|
Yang Y, Li DP, Shen N, Yu XC, Li JB, Song
Q and Zhang JH: TPX2 promotes migration and invasion of human
breast cancer cells. Asian Pac J Trop Med. 12:1064–1070. 2015.
View Article : Google Scholar
|
|
11
|
Miwa T, Kokuryo T, Yokoyama Y, Yamaguchi J
and Nagino M: Therapeutic potential of targeting protein for Xklp2
silencing for pancreatic cancer. Cancer Med. 4:1091–1100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Y, Guo W and Kan H: TPX2 is a
prognostic marker and contributes to growth and metastasis of human
hepatocellular carcinoma. Int J Mol Sci. 15:18148–18161. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei P, Zhang N, Xu Y, Li X, Shi D, Wang Y,
Li D and Cai S: TPX2 is a novel prognostic marker for the growth
and metastasis of colon cancer. J Transl Med. 11:3132013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Yang P, Tu K, Zhang H, Zheng X and
Yao Y: TPX2 knockdown suppressed hepatocellular carcinoma cell
invasion via inactivating AKT signaling and inhibiting MMP2 and
MMP9 expression. Chin J Cancer Res. 26:410–417. 2014.PubMed/NCBI
|
|
15
|
Takahashi Y, Sheridan P, Niida A, Sawada
G, Uchi R, Mizuno H, Kurashige J, Sugimachi K, Sasaki S, Shimada Y,
et al: The AURKA/TPX2 axis drives colon tumorigenesis cooperatively
with MYC. Ann Oncol. 26:935–942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aushev VN, Lee E, Zhu J, Gopalakrishnan K,
Li Q, Teitelbaum SL, Wetmur J, Esposti Degli D, Hernandez-Vargas H,
Herceg Z, et al: Novel predictors of breast cancer survival derived
from miRNA activity analysis. Clin Cancer Res. 24:581–591. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu X, Chen G, Cai ZD, Wang C, Liu ZZ, Lin
ZY, Wu YD, Liang YX, Han ZD, Liu JC and Zhong WD: Overexpression of
BUB1B contributes to progression of prostate cancer and predicts
poor outcome in patients with prostate cancer. Onco Targets Ther.
9:2211–2220. 2016.PubMed/NCBI
|
|
18
|
Zeng J, Liu W, Fan YZ, He DL and Li L:
PrLZ increases prostate cancer docetaxel resistance by inhibiting
LKB1/AMPK-mediated autophagy. Theranostics. 8:109–123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dávila-González D, Choi DS, Rosato RR,
Granados-Principal SM, Kuhn JG, Li WF, Qian W, Chen W, Kozielski
AJ, Wong H, et al: Pharmacological inhibition of NOS activates
ASK1/JNK pathway augmenting docetaxel-mediated apoptosis in
triple-negative breast cancer. Clin Cancer Res. 24:1152–1162. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garrido G and Vernos I: Non-centrosomal
TPX2-dependent regulation of the aurora A kinase: Functional
implications for healthy and pathological cell division. Front
Oncol. 6:882016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu J, Bian M, Xin G, Deng Z, Luo J, Guo X,
Chen H, Wang Y, Jiang Q and Zhang C: TPX2 phosphorylation maintains
metaphase spindle length by regulating microtubule flux. J Cell
Biol. 10:373–383. 2015. View Article : Google Scholar
|
|
22
|
de Castro Perez I and Malumbres M: Mitotic
stress and chromosomal instability in cancer: The case for TPX2.
Genes Cancer. 3:721–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu JJ, Zhang JH, Chen HJ and Wang SS: TPX2
promotes glioma cell proliferation and invasion via activation of
the AKT signaling pathway. Oncol Lett. 12:5015–5022. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen QI, Cao B, Nan N, Wang YU, Zhai XU,
Li Y and Chong T: TPX2 in human clear cell renal carcinoma:
Expression, function and prognostic significance. Oncol Lett.
5:3515–3521. 2016. View Article : Google Scholar
|
|
25
|
Vainio P, Mpindi JP, Kohonen P, Fey V,
Mirtti T, Alanen KA, Perala M, Kallioniemi O and Lljin K:
High-throughput transcriptomic and RNAi analysis identifies AIM1,
ERGIC1, TMED3 and TPX2 as potential drug targets in prostate
cancer. PLoS One. 7:e398012012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lohiya V, Aragon-Ching JB and Sonpavde G:
Role of chemotherapy and mechanisms of resistance to chemotherapy
in metastatic castration-resistant prostate cancer. Clin Med
Insights Oncol. 10 Suppl 1:S57–S66. 2016.
|
|
27
|
Antonarakis ES and Armstrong AJ: Evolving
standards in the treatment of docetaxel-refractory
castration-resistant prostate cancer. Prostate Cancer Prostatic
Dis. 14:192–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamashita S, Kohjimoto Y, Iguchi T, Koike
H, Kusumoto H, Iba A, Kikkawa K, Kodama Y, Matsumura N and Hara I:
Prognostic factors and risk stratification in patients with
castration-resistant prostate cancer receiving docetaxel-based
chemotherapy. BMC Urol. 16:132016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang RC, Chen X, Parissenti AM, Joy AA,
Tuszynski J, Brindley DN and Wang Z: Sensitivity of
docetaxel-resistant MCF-7 breast cancer cells to
microtubule-destabilizing agents including vinca alkaloids and
colchicine-site binding agents. PLoS One. 12:e01824002017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pan HW, Su HH, Hsu CW, Huang GJ and Wu TT:
Targeted TPX2 increases chromosome missegregation and suppresses
tumor cell growth in human prostate cancer. Onco Targets Ther.
10:3531–3543. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Banerjee S, Singh SK, Chowdhury I, Lillard
JW Jr and Singh R: Combinatorial effect of curcumin with docetaxel
modulates apoptotic and cell survival molecules in prostate cancer.
Front Biosci (Elite Ed). 9:235–245. 2017.PubMed/NCBI
|