Introduction
Hepatic blood flow is abundant due to the hepatic
artery and portal vein double blood supply. Cancer cells
originating from systemic organs are able to metastasize to the
liver through the circulatory system. In general, ~50% of patients
with colorectal cancer develop liver metastases, and 80–90% of
these liver metastases are contraindicated for radical resection
(1–4).
Liver metastasis is also a significant factor contributing to the
high mortality rate in patients with colorectal cancer (5). The median survival time of patients with
untreated liver metastases was 6.9 months, and the 5-year survival
rate of the patients with unresectable liver metastases was close
to 0% (6,7).
Liver metastases seriously influence patient
survival. Percutaneous radiofrequency ablation (PRFA) converts
radiofrequency waves into heat energy, which leads to tissue
dehydration and eventually results in the coagulative necrosis of
cells. This efficacious technique has been used for >20 years,
and evidence has demonstrated that the short-term and long-term
efficacies of RFA have no statistical difference compared with that
of surgery in hepatocellular carcinoma with a tumor diameter of
<5 cm (8). As for colorectal liver
metastases, research has revealed that although the rate of
recurrence-free survival in RFA is lower compared with that in
resection, the 5-year overall survival rate is as high as 48%,
which is similar to that of surgical resection (9,10).
Compared with surgery, open RFA and PRFA both demonstrated to
increase the number of circulating tumor cells (CTCs) in peripheral
circulation (11). However, such
reports included a small number of patients with hepatocellular
carcinoma and/or colorectal liver metastases, and did not the
explore changes in the phenotypes of CTC and other associated
factors. The present study focuses on liver metastases of multiple
cancer types. To the best of our knowledge, the present study has
demonstrated, for the first time, the association between factors
in the performance of PRFA and changes in the CTC level, including
changes in CTC phenotype.
CTCs are tumor cells in the circulatory system that
originate from solid tumor lesions, including hepatocellular
carcinoma lesions and metastatic tumor lesions. They enter the
circulatory system as a result of diagnostic or therapeutic
surgery, or spontaneous tumor shedding. The majority of CTCs are
subjected to apoptosis or phagocytosis in the circulatory system,
and only a few survive and develop into metastatic lesions due to
the unique microenvironment in the circulating system. CTCs are
able to significantly improve the rate of patient mortality.
Various reports confirmed that CTCs are a significant prognostic
indicator (12,13) and are even more accurate compared with
imaging modalities to a certain extent (14). The changes in the numbers and
phenotypes of CTCs reflect the tumor status and effect of treatment
in real time. Methods for detecting CTCs have significantly
approved in the recent years, including immune-magnetic separation
(15) and quantitative polymerase
chain reaction-based assays (16). In
the present study, CTCs were examined using the second-generation
CanPatrol CTCs detection technology (17), which allows for the monitoring of
changes in the numbers and phenotypes, including the epithelial
phenotype, mesenchymal phenotype and mixed epithelial/mesenchymal
phenotype, of CTCs.
The present study focused on the impact of liver
tumor PRFA on the quantity of CTCs, and also the CTC phenotypes and
other blood parameters in patients with different tumors, including
the following: Colorectal cancer liver metastases, hepatocellular
carcinoma, gastric cancer liver metastases, nasopharyngeal
carcinoma liver metastases, small intestine cancer liver
metastases, ovarian cancer liver metastases, breast ductal
carcinoma liver metastases, and ampullary carcinoma liver
metastases; and treatment backgrounds (chemotherapy and targeted
therapy). Changes in the quantity and phenotypes of CTCs, blood
count, and liver function indexes were evaluated in total patients,
while immune cell subsets and tumor markers were evaluated in only
a portion of the patient population. Given that RFA has been
reported to affect the immune system (18,19),
lymphocyte subsets were also evaluated the current study. As for
PRFA surgery, the target tumor burden, number of ablation points,
puncture times, treatment time and ablation location (liver left
lobe and/or right lobe) were included in the analysis. The target
tumor burden was calculated as the sum of the longest diameter of
the target tumor. The impact of liver tumor PRFA on CTCs and other
blood indexes in patients with different backgrounds was
comprehensively assessed.
Patients and methods
Patient characteristics
Between May 2016 and August 2017, 43 patients (28
male; 15 female) with a mean age of 53 years (range, 22–73 years)
who had been diagnosed with any type of malignant liver tumor,
including hepatocellular carcinoma and liver metastases, via
definite pathological and imaging modalities were recruited. The
present study was approved by the medical ethics committee of
Nanfang Hospital, Southern Medical University (Guangzhou, China),
and written informed consent was obtained from all patients. After
all patients underwent comprehensive enhanced computed tomography
(CT) or magnetic resonance imaging (MRI) and multidisciplinary
treatment, no patient in the current study was identified to be
eligible for surgical resection of liver metastases. Consequently,
individualized RFA plans were created based on the status of the
liver tumors that were evaluated using the last enhanced CT or
MRI.
Flow cytometric analysis of human
blood cells
Routine blood test (complete blood count) and liver
function indexes were assessed in all patients 30 min before and 3
days after PRFA. The total number and classification count of
leukocytes were measured by flow cytometry in a Sysmex XE2100
automatic blood analyzer (Sysmex Corporation, Kobe, Japan). In
brief, blood samples (200 µl blood per sample) were mixed with
Stromatolyser-4DL (Sysmex Corporation) and treated with
Stromatolyser-4DS (Sysmex Corporation) at 41°C for 22 sec,
containing 0.002% polymethine dye to stain the nucleus and
organelles. Then, the samples were irradiated with a semiconductor
laser, whereby the dye produces different intensities of
fluorescence. Each cell detected three different scattering angles:
Forward scattered light (representing cell volume), lateral
scattered light (representing contents of a cell, including nucleus
or granules) and lateral fluorescence (representing contents of DNA
and RNA). Using this automatic blood analyzer and flow cytometry
method, monocyte and lymphocyte counts were obtained. Furthermore,
studies using the same automatic blood analyzer and method have
also been published previously (20–22). In
addition, immune cell subsets and tumor markers were assessed in
certain patients 30 min before and 3 days after PRFA. Immune cell
subsets were detected by flow cytometry. In brief, 50 µl
anticoagulant whole blood sample was incubated for 20 min at room
temperature with 20 µl of the following labelled antibodies:
Cluster of differentiation (CD)4-fluorescein isothiocyanate
(FITC)/CD8-PE/CD3-PerCP (cat no. 340298; BD Biosciences, Franklin
Lakes, NJ, USA), IOTEST CD3-FITC/CD (16+56)-PE (cat no. 340300;
Beckman Coulter, Inc., Brea, CA, USA). Then, 300 µl hemolysin (BD
Biosciences) was added to the test tube, and incubated for 20 min
at room temperature for full hemolysis to occur. Next, 1 ml sheath
(Jinan Xisenmeikang Medical Electronic Co., Ltd., Jinan, China) was
added and the mixture was centrifuged at 180 × g for 5 min at 4°C.
After discarding the supernatant and adding 500 µl sheath into the
tube, flow cytometry was performed using a FACScalibur (BD
Biosciences) to detect different tumor markers (23–25),
including carcinoembryonic antigen (cat no. 401–10; CanAg
Diagnostics Co., Ltd., Beijing, China), CA19-9 (cat no. 120-10;
CanAg Diagnostics Co., Ltd.), CA72-4 (cat no. EIA-5071; DRG
Diagnostics GmbH; Marburg, Germany), CA24-2 (cat no. 101-10; CanAg
Diagnostics Co., Ltd.) and α-fetoprotein (cat no. 600-10; CanAg
Diagnostics Co., Ltd.) using the corresponding tumor marker kits
(CanAg Diagnostics, Co., Ltd., Goteborg, Sweden).
PRFA treatment
In the present study, all patients had signed
informed consent prior to PRFA treatment. All patients received
PRFA treatment under conscious sedation with pethidine and local
anesthesia with 2% lidocaine. The procedures were performed under
ultrasound guidance. The mean PRFA treatment time was 26.6 min
(range, 2.5–66.1 min).
CTC isolation and classification
Blood samples of 5 ml each were collected from the
peripheral circulation 30 min before and 3 days after PRFA. The
CanPatrol™ CTC enrichment technique was used to isolate
and classify the CTCs as previously described (17). In brief, the CTCs were first isolated
by size using a filter-based equipment comprising a filtration tube
(Surexam, Guangzhou, China) with calibrated membranes with
8-µm-diameter pores (EMD Millipore, Billerica, MA, USA), manifold
vacuum plate with valve setting (Surexam), an E-Z96 vacuum manifold
(Omega Bio-Tek, Inc., Norcross, GA, USA), and a vacuum pump.
Tri-color RNA in situ hybridization (RNA-ISH), which is
based on the branched DNA signal amplification technology, was then
performed to classify the different phenotype of CTCs according to
epithelial-mesenchymal transition (EMT) biomarkers. After treating
with a protease (Qiagen GmbH, Hilden, Germany) at 25°C for 1 h, the
cells on the membrane were hybridized with capture probes
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
specific for the epithelial biomarkers EpCAM and CK 8/18/19, two
mesenchymal biomarkers vimentin and twist, and the leukocyte
biomarker CD45 (Table I). Then,
4′,6-diamidino-2-phenylindole (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used to stain the CTCs, and an Olympus BX53
fluorescence microscope (Olympus Corporation, Tokyo, Japan) was
used for enumeration and analysis at a magnification of ×100. The
red and green fluorescent signal represented epithelial CTCs and
mesenchymal CTCs, respectively. Mixed epithelial/mesenchymal
phenotype CTCs were recognized as red and green fluorescent signal.
EpCAM and CK are commonly used for epithelial CTC detection
(26–28), but one report revealed that the
epithelial marker-based enrichment method results in failure of CTC
detection due to the downregulation of EpCAM have been published
(29). The CanPatrol CTC enrichment
technique classifies CTCs that do not express EpCAM, including the
brain glioma cell line U118MG (17).
Furthermore, papers using the CanPatrol CTC enrichment technique
have also been published recently (30–32).
 | Table I.Probe sequences for the EpCAM,
CK8/18/19, vimentin, twist and CD45 genes. |
Table I.
Probe sequences for the EpCAM,
CK8/18/19, vimentin, twist and CD45 genes.
| Gene | Sequences
(5′→3′) | Gene | Sequences
(5′→3′) |
|---|
| EpCAM |
TGGTGCTCGTTGATGAGTCA | Vimentin |
GAGCGAGAGTGGCAGAGGAC |
|
|
AGCCAGCTTTGAGCAAATGA |
|
CTTTGTCGTTGGTTAGCTGG |
|
|
AAAGCCCATCATTGTTCTGG |
|
CATATTGCTGACGTACGTCA |
|
|
CTCTCATCGCAGTCAGGATC |
|
GAGCGCCCCTAAGTTTTTAA |
|
|
TCCTTGTCTGTTCTTCTGAC |
|
AAGATTGCAGGGTGTTTTCG |
|
|
CTCAGAGCAGGTTATTTCAG |
|
GGCCAATAGTGTCTTGGTAG |
| CK8 |
CGTACCTTGTCTATGAAGGA | Twist |
ACAATGACATCTAGGTCTCC |
|
|
ACTTGGTCTCCAGCATCTTG |
|
CTGGTAGAGGAAGTCGATGT |
|
|
CCTAAGGTTGTTGATGTAGC |
|
CAACTGTTCAGACTTCTATC |
|
|
CTGAGGAAGTTGATCTCGTC |
|
CCTCTTGAGAATGCATGCAT |
|
|
CAGATGTGTCCGAGATCTGG |
|
TTTCAGTGGCTGATTGGCAC |
|
|
TGACCTCAGCAATGATGCTG |
|
TTACCATGGGTCCTCAATAA |
| CK18 |
AGAAAGGACAGGACTCAGGC | CD45 |
TCGCAATTCTTATGCGACTC |
|
|
GAGTGGTGAAGCTCATGCTG |
|
TGTCATGGAGACAGTCATGT |
|
|
TCAGGTCCTCGATGATCTTG |
|
GTATTTCCAGCTTCAACTTC |
|
|
CAATCTGCAGAACGATGCGG |
|
CCATCAATATAGCTGGCATT |
|
|
AAGTCATCAGCAGCAAGACG |
|
TTGTGCAGCAATGTATTTCC |
|
|
CTGCAGTCGTGTGATATTGG |
|
TACTTGAACCATCAGGCATC |
| CK19 |
CTGTAGGAAGTCATGGCGAG |
|
|
|
|
AAGTCATCTGCAGCCAGACG |
|
|
|
|
CTGTTCCGTCTCAAACTTGG |
|
|
|
|
TTCTTCTTCAGGTAGGCCAG |
|
|
|
|
CTCAGCGTACTGATTTCCTC |
|
|
|
|
GTGAACCAGGCTTCAGCATC |
|
|
Statistical analysis
Paired-sample t-tests were used to analyze the
significant differences between matched data. Independent-sample
t-tests and one-way analysis of variance followed by least
significant difference and Dunnett's post-hoc tests were performed
to test the significant differences among groups of data. Bivariate
correlation analysis was also used to explore the correlativity
between target data and changes in the value of CTCs, followed by
Spearman correlation coefficient. Data are presented as mean ±
standard deviation (n=43), and P<0.05 was considered to indicate
a statistically significant difference. All data analyses were
performed using IBM SPSS Statistics 22 (IBM Corp., Armonk, NY,
USA).
Results
Effect of the general background and
tumor background on CTC level
Age, sex, primary tumor type, T stage, N stage,
number of distant metastases, and the time interval between the
diagnosis of liver tumors and the PRFA were included in the
analysis. The average age of patients was 53 years (range, 22–73
years), and the male-to-female ratio was 28:15. The cohort
comprised 27 cases of colorectal cancer liver metastases, 7
hepatocellular carcinoma, 3 gastric cancer liver metastases, 2
nasopharyngeal carcinoma liver metastases, 1 small intestine cancer
liver metastases, 1 ovarian cancer liver metastases, 1 breast
ductal carcinoma liver metastases and 1 ampullary carcinoma liver
metastases (Table II). The distant
metastases included liver, lung, bone, brain, adrenal gland, ovary
and distant lymph nodes. Distant metastasis was then divided into
two groups: Liver metastases only and liver metastases with
extrahepatic metastases, such as distant organ and regional lymph
node metastases. The time interval between the diagnosis of liver
tumors and the PRFA was also divided into two groups according to
the median time interval of 5.6 months. All P-values were >0.05,
and the results of the analysis demonstrated that the general
background and tumor background of patients did not affect the CTC
level following PRFA (Tables II and
III).
 | Table II.Patient general background and tumor
background. |
Table II.
Patient general background and tumor
background.
| Characteristic | No. of patients
(%) | Mean difference in
CTCsa |
P-valueb |
|---|
| Age (years) |
|
| 0.940 |
|
<60 | 28 (65) |
3.96 |
|
|
≥60 | 15 (35) |
3.73 |
|
| Sex |
|
| 0.516 |
|
Male | 28 (65) |
4.57 |
|
|
Female | 15 (35) | 2.6 |
|
| Tumor types |
|
| 0.232 |
|
Colorectal cancer liver
metastasis | 27 (63) |
4.12 |
|
|
Hepatocellular carcinoma | 7
(16) |
1.71 |
|
| Gastric
cancer liver metastases | 3 (7) | 13.33 |
|
|
Nasopharyngeal carcinoma liver
metastasis | 2 (5) | −1 |
|
| Small
intestine cancer liver metastasis | 1 (2) | 6 |
|
| Ovarian
cancer liver metastasis | 1 (2) | 4 |
| Breast
ductal carcinoma liver metastases | 1 (2) | −1 |
|
|
Ampullary carcinoma liver
metastases | 1 (2) | −1 |
|
| T stage |
|
| 0.649 |
| 2 | 1 (2) | 2 |
|
| 3 | 9
(21) |
1.89 |
|
| 4 | 26 (61) |
5.46 |
|
| N stage |
|
| 0.560 |
| 0 | 9
(21) |
4.33 |
|
| 1 | 11 (26) |
2.27 |
|
| 2 | 12 (28) | 7 |
|
 | Table III.Liver tumor background. |
Table III.
Liver tumor background.
| Characteristic | No. of patients
(%) | Mean difference in
CTCsa |
P-valueb |
|---|
| Number of distant
metastases |
|
| 0.137 |
| Liver
metastasis only | 18 (42) | 1.67 |
|
| Liver
metastasis with extrahepatic metastasis | 25 (58) | 5.48 |
|
| Time interval
between the diagnosis of liver tumor |
|
| 0.313 |
| and PRFA,
months |
|
|
|
|
<12 | 32 (74) | 3.03 |
|
|
≥12 | 11 (26) | 6.36 |
|
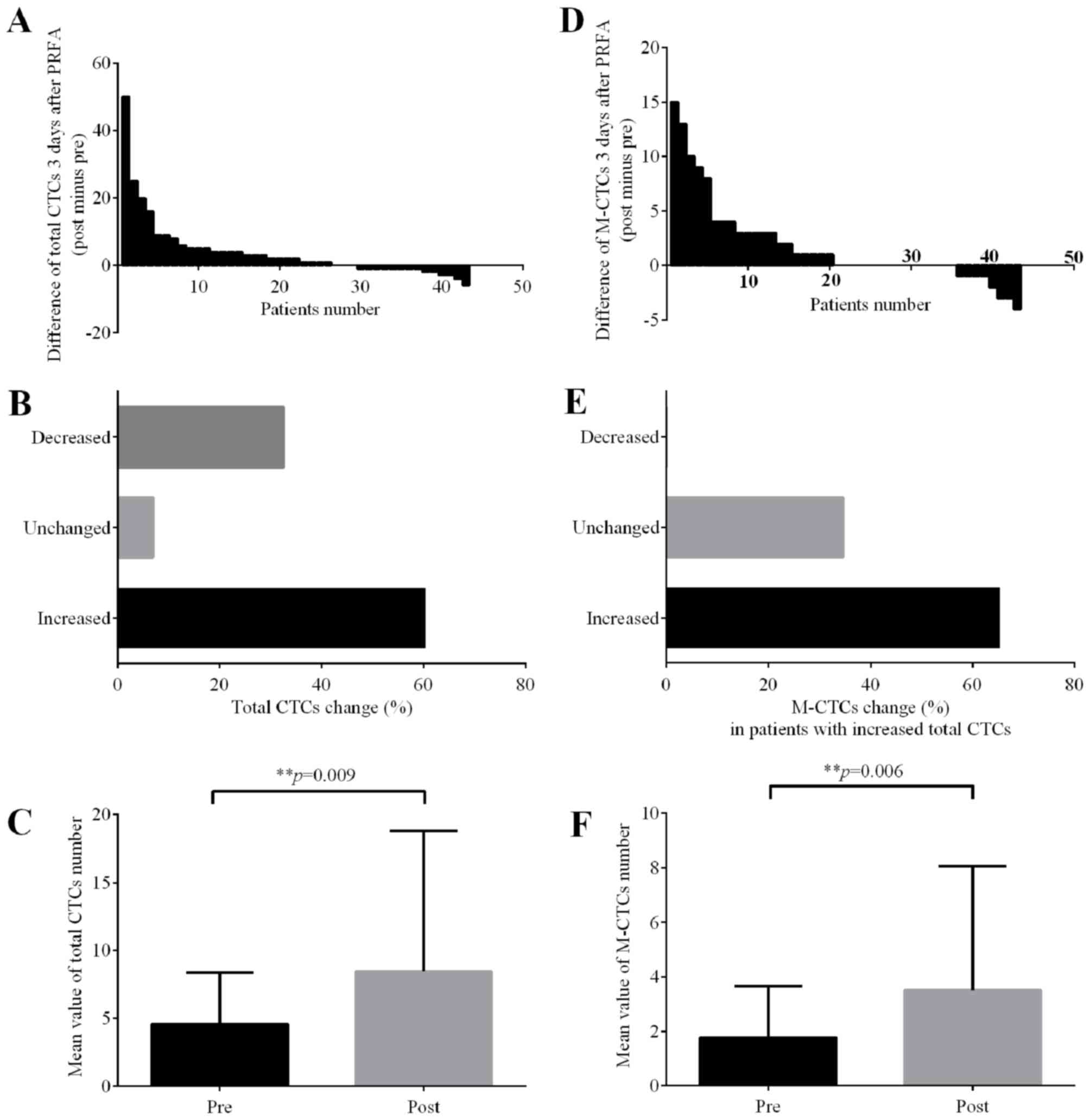
Total CTC level increases following
PRFA, particularly the mesenchymal phenotype CTCs
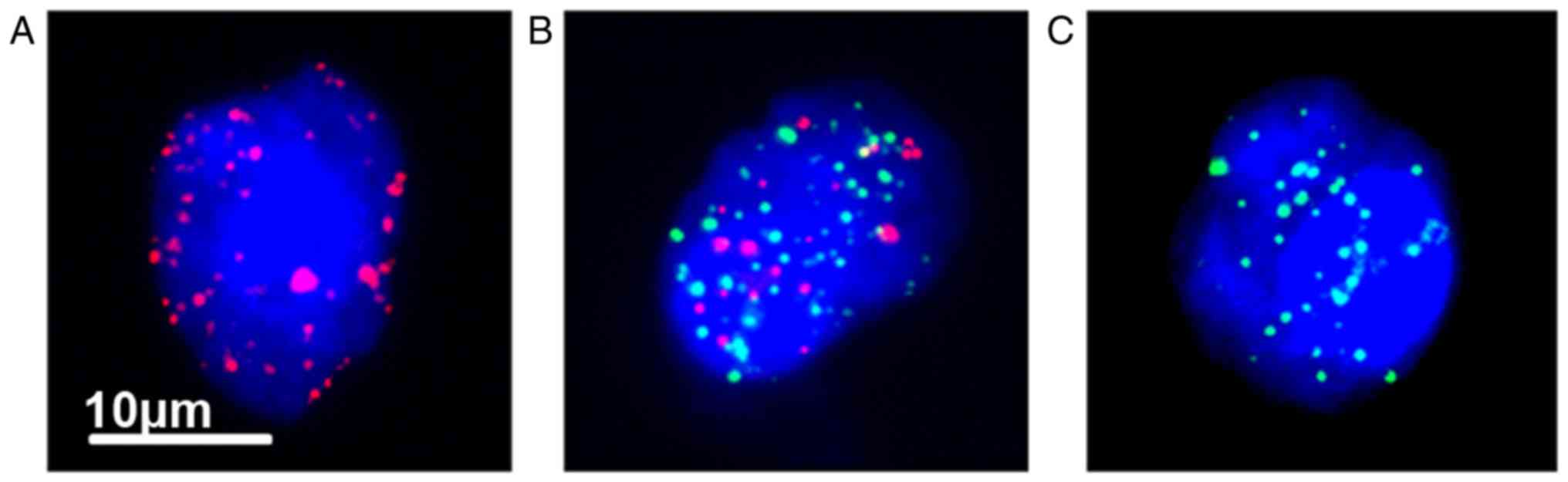
After counting the three phenotype of CTCs
separately (Fig. 1), it was revealed
that the CTC-positive rate prior to PRFA was 88.4% (38/43), while
that 3 days after PRFA was 97.7% (42/43). Of the 43 patients with
liver tumors, the CTC level increased in 26 patients (60.5%)
following PRFA (Fig. 2A and B).
Compared with the CTC level prior to PRFA, the CTC level 3 days
after PRFA was significantly increased (P=0.009) (Fig. 2C). Meanwhile, of the 26 patients who
exhibited high total CTC levels following PRFA, 17 (65.4%) had
increased mesenchymal phenotype CTCs, while the number of
mesenchymal phenotype CTCs remained unchanged in 9 patients (34.6%)
(Fig. 2D and E). It was demonstrated
that an increasing number of mesenchymal phenotype CTCs (P=0.006)
significantly contributed to the total increase in CTCs (Fig. 2F). In addition, CTC was not detected
in 5 patients prior to PRFA, but 4 (80%) of them exhibited high CTC
levels in the peripheral circulation 3 days after PRFA (Table IV). In addition to the CTC level, the
changes in different CTC phenotypes following PRFA were evaluated.
These results indicated that the level of CTCs, particularly the
mesenchymal phenotype CTCs, is significantly increased following
PRFA of liver tumors.
 | Table IV.CTC level increase in patients who
did not find CTC prior to PRFA. |
Table IV.
CTC level increase in patients who
did not find CTC prior to PRFA.
| Patients | CTC number prior to
PRFA | CTC number
following PRFA |
|---|
| No. 04 | 0 | 0 |
| No. 07 | 0 | 4 |
| No. 16 | 0 | 1 |
| No. 17 | 0 | 25 |
| No. 18 | 0 | 4 |
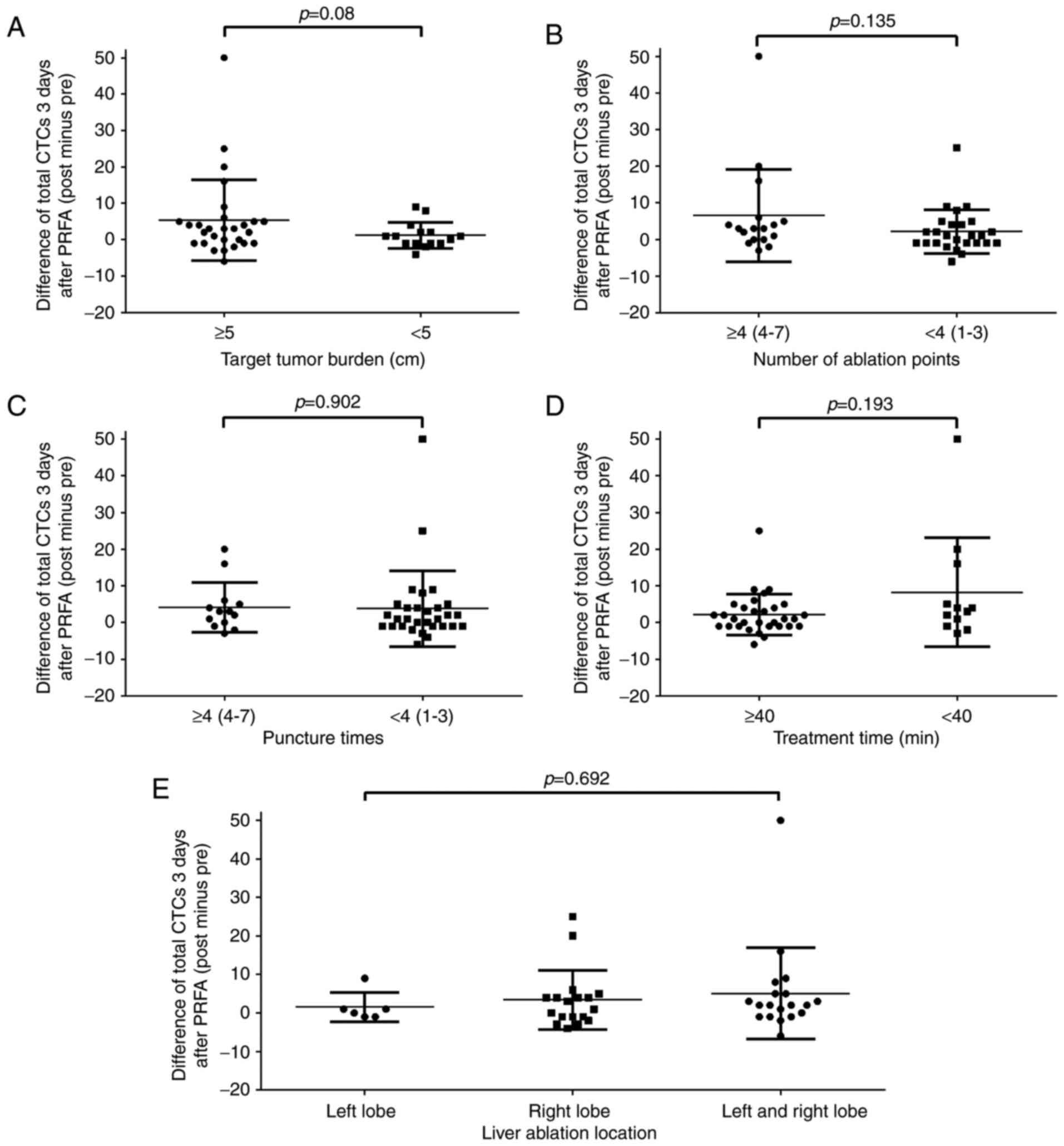
Relevance between the changes of CTCs
and PRFA factors
PRFA has been revealed to increase CTC levels, but
the factors contributing to such occurrences remain unknown. As
such, the present study focused on the following factors in the
performance of PRFA: Target tumor burden, numbers of ablation
points, puncture times, treatment time and ablation location (left
lobe and/or right lobe of the liver) (Fig. 3). The sum of the longest diameter of
the target tumor was used as the target tumor burden. Aside from
t-tests, bivariate correlation analysis was also conducted between
PRFA factors and changes in the number of CTCs to determine whether
the aforementioned factors affected the changes in CTC levels.
However, no significant correlation was identified between changes
in CTC levels and all the PRFA factors, particularly the treatment
time and the number of ablation points, which were initially
considered to be associated with changes in CTC levels (Table V). Notably, the number of ablation
points demonstrated a partial correlation with the changes in the
number of CTCs (Spearman correlation coefficient, 0.303; P=0.048).
Although this correlation coefficient is not satisfactory, further
studies using a larger sample size are warranted.
 | Table V.PRFA surgery and CTC changes. |
Table V.
PRFA surgery and CTC changes.
|
Characteristics | No. of patients
(%) | Mean difference in
CTCsa |
P-valueb |
r-valuec |
|---|
| Target tumor burden
(cm) |
|
| 0.08 | 0.200 |
| ≥5 | 28 (65) | 5.32 |
|
|
|
<5 | 15 (35) | 1.20 |
|
|
| Number of ablation
points |
|
| 0.135 | 0.303 |
| ≥4
(4–7) | 17 (40) | 6.53 |
|
|
| <4
(1–3) | 26 (60) | 2.15 |
|
|
| Puncture times |
|
| 0.902 | 0.185 |
| ≥4
(4–7) | 13 (30) | 4.15 |
|
|
| <4
(1–3) | 30 (70) | 3.77 |
|
|
| Treatment time
(min) |
|
| 0.193 | 0.218 |
|
≥40 | 12 (28) | 8.25 |
|
|
|
<40 | 31 (72) | 2.19 |
|
|
| Ablation
location |
|
| 0.692 | 0.127 |
| Left
lobe | 6
(14) | 1.5 |
|
|
| Right
lobe | 18 (42) | 3.39 |
|
|
| Left
and right lobe | 19 (44) | 5.11 |
|
|
CTCs increases significantly in
patients with reduced lymphocyte levels
To thoroughly analyze the factors that may affect
the CTC level, blood indexes, including that in routine blood test
and liver function indexes, were investigated in all patients,
while immune cell subsets and tumor markers were evaluated in
certain patients (Table VI). Tumor
markers including CEA, CA19-9, CA24-2 (for colorectal cancer liver
metastases patients), CA72-4 (for gastric cancer liver metastases
patients) and AFP (for hepatocellular carcinoma patients).
Following statistical analysis, it was determined that of the 23
patients, 12 patients exhibited elevated tumor markers (1 patient
with elevated CA72-4 level, 1 patient with elevated AFP level and
10 patients with elevated CEA/CA19-9/CA24-2 levels); however, 11
patients exhibited decreased or unchanged levels of tumor markers
(4 patients with decreased CEA/CA19-9/CA24-2 levels and 7 patients
with unchanged CEA/CA19-9/CA24-2 levels). No significant
differences were determined between the mean differences of CTCs in
the two groups. Considering the possibility of bone marrow
suppression as the majority of patients had received chemotherapy
in the past month, changes in the levels of white blood cells,
neutrophils, erythrocytes and platelets could not be analyzed.
Analysis using t-tests demonstrated that the number of lymphocytes
following PRFA was significantly lower compared with that prior to
PRFA (P=0.007; Fig. 4A), and the CTC
level in the decreased lymphocyte group was significantly higher
compared with those in the increased lymphocyte group (Fig. 4B). This result revealed that a
decrease in lymphocytes may be beneficial for the survival of tumor
cells in the peripheral circulatory system. The immune system is
known to inhibit the metastasis of cancer cells, and PRFA may
affect the immune system, thus decreasing the number of
lymphocytes, in turn decreasing the inhibitory effect on cancer
cells. In terms of liver function indexes, it was demonstrated that
alanine transaminase (ALT) and aspartate transaminase (AST) were
significantly elevated (P<0.001) in patients following RFA
(Fig. 4C and D), but their elevation
was not significantly associated with changes in the CTC level
(Table VII; Fig. 4E and F), and may instead be associated
with the heat and mechanical damage of the liver cells during PRFA.
Such damages to normal tissue may not lead to high CTC levels. No
significant correlations were identified between immune cell
subsets, tumor markers and changes in CTC levels.
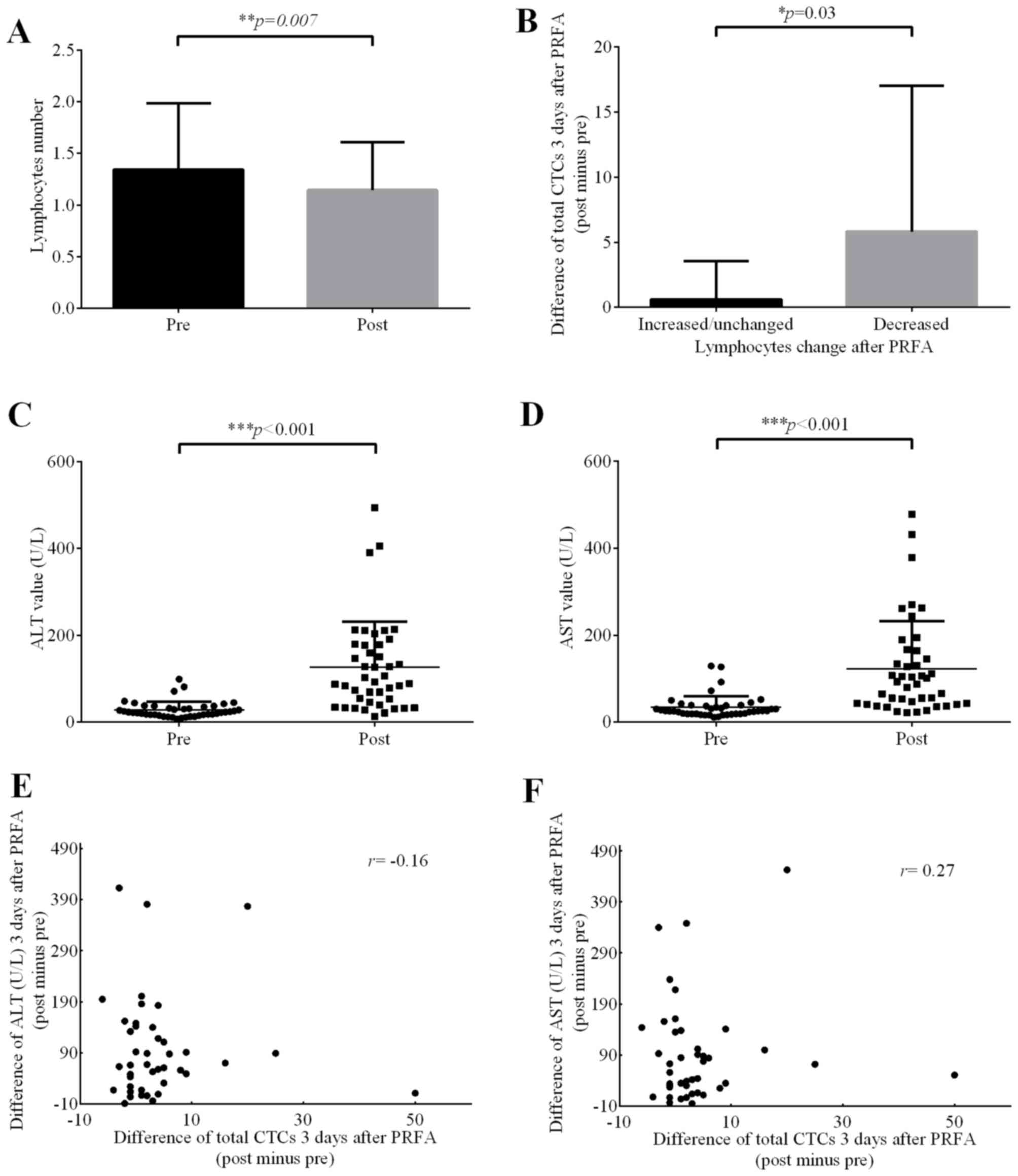 | Figure 4.Effect of PRFA on lymphocyte numbers
and ALT/AST value. (A) Significant difference in lymphocyte numbers
between the pre-PRFA and post-PRFA group (P=0.007, paired sample
t-test). (B) After dividing the difference in total CTCs (post PRFA
minus pre) into two groups (increased and decreased lymphocyte
numbers), a significant increase was identified in decreased
lymphocyte group (P=0.03, independent sample t-test). (C) ALT and
(D) AST levels increased significantly following PRFA (both
P<0.001, paired sample t-test). Bivariate correlation analysis
of changes in (E) ALT and (F) AST (post minus pre) vs. total CTC
change (post minus pre), no correlation was identified (Spearman
correlation coefficient, r=−0.16 and r=0.27, respectively). PRFA,
percutaneous radiofrequency ablation; CTCs, circulating tumor
cells; ALT, alanine transaminase; AST, aspartate transaminase. |
 | Table VI.Blood indexes of partial patients and
CTC changes mean value. |
Table VI.
Blood indexes of partial patients and
CTC changes mean value.
| Characteristic | No. of patients
(%) | Mean difference in
CTCsa |
P-valueb |
|---|
| Tumor markers |
|
|
|
|
Increase | 12 (30) | 3.58 | 0.968 |
|
Decrease | 11 (26) | 3.45 |
|
| Monocytes |
|
|
|
|
Increase | 30 (70) | 3.6 | 0.766 |
|
Decrease | 13 (30) | 4.54 |
|
| Lymphocytes |
|
|
|
|
Increase | 16 (37) | 0.63 | 0.03 |
|
Decrease | 27 (63) | 5.81 |
|
| Lymphocytes
percentage |
|
|
|
|
Increase | 17 (40) | 3.06 | 0.645 |
|
Decrease | 26 (60) | 4.42 |
|
| Total number of T
cells |
|
|
|
|
Increase | 15 (35) | 2.8 | 0.165 |
|
Decrease | 5 (12) | 8.6 |
|
| CD3+CD4+ T
cells |
|
|
|
|
Increase | 15 (35) | 3.6 | 0.709 |
|
Decrease | 5 (12) | 5.2 |
|
| CD3+CD8+T
cells |
|
|
|
|
Increase | 12 (28) | 0.83 | 0.073 |
|
Decrease | 8 (19) | 8.75 |
|
| NK cells |
|
|
|
|
Increase | 12 (28) | 1.42 | 0.158 |
|
Decrease | 8 (19) | 7.88 |
|
 | Table VII.Liver function indexes ALT and AST
change. |
Table VII.
Liver function indexes ALT and AST
change.
| Characteristic | No. of patients
(%) | Mean difference in
ALT/ASTa |
P-valueb |
r-valuec |
|---|
| ALT |
| 98.1 | <0.001 | −0.16 |
|
Increase | 43 (100) |
|
|
|
|
Decrease | 0 (0) |
|
|
|
| AST |
| 88.8 | <0.001 | 0.27 |
|
Increase | 43 (100) |
|
|
|
|
Decrease | 0 (0) |
|
|
|
Effect of treatment history on the
change of CTCs following PRFA
In addition to PRFA factors, the patient's treatment
history was analyzed, which included chemotherapy history and
targeted therapy history (Table
VIII). The following factors were taken into consideration:
Chemotherapy or targeted therapy taken in the past month, the line
of chemotherapy chosen, and the chemotherapy evaluation (partial
response, stable disease, or progressive disease). In addition,
whether chemotherapy regimens in patients with colorectal cancer
affected CTC levels following PRFA were investigated. It was
revealed that none of the aforementioned chemotherapy factors were
significantly correlated with changes in CTC levels. As for
targeted therapy, the use of targeted therapy within the past month
did not affect the changes in CTC levels.
 | Table VIII.Effect of drug treatment on the
changes in CTCs following PRFA. |
Table VIII.
Effect of drug treatment on the
changes in CTCs following PRFA.
| Characteristic | No. of patients
(%) | Mean difference in
CTCsa |
P-valueb |
|---|
| Chemotherapy
history |
|
| 0.522 |
| No
chemotherapy taken in the past month | 10 (23) | 4.32 |
|
|
Chemotherapy taken in the past
month | 33 (77) | 2.2 |
|
| Treatment |
|
|
|
| 5-FU +
oxaliplatin | 16 (37) | 3.63 | 0.596 |
| 5-FU +
irinotecan | 10 (23) | 6.0 |
|
| Chemotherapy
line |
|
| 0.388 |
|
Maintenance chemotherapy | 2 (5) | 0.5 |
|
|
First-line chemotherapy | 11 (26) | 5.56 |
|
|
Second-line chemotherapy | 16 (37) | 0.82 |
|
|
Third-line chemotherapy | 4 (10) | 13.25 |
|
| Last therapeutic
evaluation |
|
| 0.185 |
| PR | 4 (10) | 10.5 |
|
| SD | 9 (21) | −0.67 |
|
| PD | 15 (35) | 5.6 |
|
| Targeted
therapy |
|
| 0.845 |
| Not
used in the past month | 9 (21) | 3.33 |
|
| Used in
the past month | 34 (79) | 4.03 |
|
Discussion
The levels of CTCs have been reported to increase
following RFA (33); however, no
further studies have been performed to identify the factors that
cause such increases. To the best of our knowledge, the present
study is the first to investigate the association between liver
tumor PRFA and changes in CTC levels. In addition, the current
study is the first to investigate the variation in CTC phenotypes
following liver tumor PRFA. Furthermore, it was confirmed that
liver PRFA reduced the total number of lymphocytes, which may
reduce the immune surveillance and killing function of tumor cells.
This may be among the causes of elevated levels of total CTC in
peripheral circulation. Further experiments are required to explore
the association between changes in CTC levels and the immune
system.
The CTC classification method used in the present
study was based on RNA-ISH, which provides sufficient information
at the transcriptional level. However, there may be certain
limitations that exist at the protein level. Generally, compared
with the post-transcriptional results of western blot analysis,
RNA-ISH is more reliable.
A comprehensive analysis of various factors
demonstrated that the PRFA factors did not correlate with the
changes in CTC levels. Incomplete liver tumor ablation increases
the risk for local recurrence and distant recurrence (34). To develop an efficacious PRFA plan,
the ablation points, puncture times and treatment time should be
decided based on the situation of individual patients. Certain
doctors may doubt that increased puncture time or high number of
ablation sites would further increase the CTC level. Particular
cases of needle tract seeding following percutaneous biopsy have
been reported (35). In theory,
physical factors, including heat injury and mechanical damage may
also cause metastasis. RFA is a thermal ablation technique during
which heat injury and mechanical damage to tumor tissue may lead to
the shedding of CTCs from solid tumor and enter the circulatory
system. A larger tumor burden comes with greater heat injury and
mechanical damage, resulting in a higher possibility of CTC
shedding into the circulatory system. Therefore, target tumor
burden, numbers of ablation points, puncture times, treatment time
and ablation location were all included in the present study. The
evidence presented in the current study may aid in individually
adjusting the PRFA factors to maximize the tumor ablation effect
and decrease the risk of CTC level increase. The total number of
CTCs increase following PRFA, but appropriate puncture times or
tumor ablation points reduce any further increases to CTC
levels.
In addition, no correlation was identified between
elevated liver function indices, including ALT and AST, and
elevated CTC levels. The liver function index may be used to
determine the degree of damage to normal liver cells as liver PRFA
inevitably damages normal liver tissue. However, damage to normal
liver tissue adjacent to tumor tissue do not result in changes to
CTC levels. This may aid clinicians in creating a more
comprehensive plan for PRFA of tumors with irregular shapes.
One previous study reported that the total number of
CTCs increase following RFA (11).
The current study further analyzed the changes in CTC phenotypes
and confirmed that the increase in the total number of CTCs was
primarily based on the mesenchymal phenotype of CTCs. Among the 43
patients included in the present study, 26 (60.5%) exhibited an
increase in CTCs following PRFA. Among these 26 patients, 17
(65.4%) had more mesenchymal phenotype CTCs. PRFA leads to an
elevated number of total CTC, and also to elevated mesenchymal
phenotype CTCs. Epithelial CTCs transform into mesenchymal CTCs
through EMT, and mesenchymal phenotype CTCs have an increased
invasion capability (36). The
mechanism by which mesenchymal phenotype CTCs increase following
PRFA is unclear, but the EMT of CTC has been confirmed in the
circulatory system (37), which may
be one possible explanation. In addition, the second time point
(day 3) by which blood samples were collected from the peripheral
circulation was also a limitation. The EMT process of CTCs takes
time, that is to say, different detection time points should
indicate different CTC phenotypes. CTCs need to overcome several
obstacles to colonize distant organs (38) and this process takes time. Thus, we
hypothesized that a considerable proportion of epithelial phenotype
CTCs on day 0 may transform into mesenchymal phenotype CTCs by day
3 via EMT. Thus, the total number of mesenchymal phenotype CTCs
significantly increased on day 3. To depict the timeline of EMT of
CTCs, phenotypes may be detected at different time points in
follow-up studies.
Immunization has been demonstrated to inhibit
certain malignant tumors (39).
CD8+ T-cells and natural killer (NK) cells have been
proven to have significant effect on anti-metastatic immune
surveillance (40). The current study
focused on liver tumors and NK cells are abundant in the liver. One
previous study has indicated that NK cell depletion in mice
increase the occurrence of hepatic metastasis (41). In the analysis of the blood indexes in
the present study, lymphocyte numbers were significantly lower 3
days after PRFA. Furthermore, the CTC level was significantly
elevated in patients with reduced lymphocyte numbers. However, the
P-values for CD3+8+ T cells and NK cells in
certain patients were 0.073 and 0.158, respectively, and no
statistical significance was identified. This may be associated
with the small number of patients (14/43) tested for immune cell
subsets.
Collectively, these results indicate that the
increased number of CTCs following PRFA may be due to the decreased
lymphocyte numbers, and more experiments are required to confirm
this inference. Fewer lymphocytes mean a decrease in immune
surveillance and killing function, which may lead to survival of
more tumor cells in the circulatory system. In addition, EMT may
result in a significant increase in the number of mesenchymal
cells, which enhances the potential metastatic capability of
cancers. Larger sample size studies as well as further studies
investigating the mechanisms underlying this phenomenon are
required. The results of the present study may aid clinicians in
understanding the implications of elevated CTCs during liver tumor
PRFA.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81502535), the Natural
Science Foundation of Guangdong Province (grant no.
2015A030310039), the Natural Science Foundation of Guangdong
Province (grant no. 2015A030310085), and the National Natural
Science Foundation of China (grant no. 81772580).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL, MS and YL designed and conceived this study. NH,
CW and LL helped to collect patients' clinical data. HM and MZ
helped to perform the statistical analysis, put together the
figures and edit the manuscript. ZH and LS made substantial
contributions to acquisition of data and drafting the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the medical ethics
committee of Nanfang Hospital, Southern Medical University
(Guangzhou, China), and written informed consent was obtained from
all patients.
Consent for publication
Consent for publication was received from
patients.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Vibert E, Canedo L and Adam R: Strategies
to treat primary unresectable colorectal liver metastases. Semin
Oncol. 32 6 Suppl 8:33–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kemeny N: Management of liver metastases
from colorectal cancer. Oncology (Williston Park). 20:1161–1176.
2006.PubMed/NCBI
|
|
3
|
Lau WY and Lai EC: Hepatic resection for
colorectal liver metastases. Singapore Med J. 48:635–639.
2007.PubMed/NCBI
|
|
4
|
Taniai N, Akimaru K, Yoshida H and Tajiri
T: Surgical treatment for better prognosis of patients with liver
metastases from colorectal cancer. Hepatogastroenterology.
54:1805–1809. 2007.PubMed/NCBI
|
|
5
|
Foster JH: Treatment of metastatic disease
of the liver: A skeptic's view. Semin Liver Dis. 4:170–179. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma S, Camci C and Jabbour N:
Management of hepatic metastasis from colorectal cancers: An
update. J Hepatobiliary Pancreat Surg. 15:570–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zervoudakis A, Boucher T and Kemeny NE:
Treatment options in colorectal liver metastases: Hepatic arterial
infusion. Visc Med. 33:47–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH,
Zhang YQ, Lin XJ and Lau WY: A prospective randomized trial
comparing percutaneous local ablative therapy and partial
hepatectomy for small hepatocellular carcinoma. Ann Surg.
243:321–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gillams A, Goldberg N, Ahmed M, Bale R,
Breen D, Callstrom M, Chen MH, Choi BI, de Baere T, Dupuy D, et al:
Thermal ablation of colorectal liver metastases: A position paper
by an international panel of ablation experts, The Interventional
Oncology Sans Frontières meeting 2013. Eur Radiol. 25:3438–3454.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He N, Jin QN, Wang D, Yang YM, Liu YL,
Wang GB and Tao KX: Radiofrequency ablation vs. hepatic resection
for resectable colorectal liver metastases. J Huazhong Univ Sci
Technolog Med Sci. 36:514–518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiao LR, Apostolopoulos C, Jacob J, Szydlo
R, Johnson N, Tsim N, Habib NA, Coombes RC and Stebbing J: Unique
localization of circulating tumor cells in patients with hepatic
metastases. J Clin Oncol. 27:6160–6165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cristofanilli M, Hayes DF, Budd GT, Ellis
MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC,
et al: Circulating tumor cells: A novel prognostic factor for newly
diagnosed metastatic breast cancer. J Clin Oncol. 23:1420–1430.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Budd GT, Cristofanilli M, Ellis MJ,
Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV,
Terstappen LW and Hayes DF: Circulating tumor cells versus
imaging-predicting overall survival in metastatic breast cancer.
Clin Cancer Res. 12:6403–6409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith BM, Slade MJ, English J, Graham H,
Lüchtenborg M, Sinnett HD, Cross NC and Coombes RC: Response of
circulating tumor cells to systemic therapy in patients with
metastatic breast cancer: Comparison of quantitative polymerase
chain reaction and immunocytochemical techniques. J Clin Oncol.
18:1432–1439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stathopoulou A, Vlachonikolis I, Mavroudis
D, Perraki M, Kouroussis Ch, Apostolaki S, Malamos N, Kakolyris S,
Kotsakis A, Xenidis N, et al: Molecular detection of
cytokeratin-19-positive cells in the peripheral blood of patients
with operable breast cancer: Evaluation of their prognostic
significance. J Clin Oncol. 20:3404–3412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu S, Liu S, Liu Z, Huang J, Pu X, Li J,
Yang D, Deng H, Yang N and Xu J: Classification of circulating
tumor cells by epithelial-mesenchymal transition markers. PLoS One.
10:e01239762015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schneider T, Hoffmann H, Dienemann H,
Herpel E, Heussel CP, Enk AH, Ring S and Mahnke K: immune response
after radiofrequency ablation and surgical resection in nonsmall
cell lung cancer. Semin Thorac Cardiovasc Surg. 28:585–592. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Napoletano C, Taurino F, Biffoni M, De
Majo A, Coscarella G, Bellati F, Rahimi H, Pauselli S, Pellicciotta
I, Burchell JM, et al: RFA strongly modulates the immune system and
anti-tumor immune responses in metastatic liver patients. Int J
Oncol. 32:481–490. 2008.PubMed/NCBI
|
|
20
|
Wang XY, Yu HY, Zhang YY, Wang YP, Feng
XH, Li ZP, Du XJ and Gao W: Serial changes of mean platelet volume
in relation to Killip Class in patients with acute myocardial
infarction and primary percutaneous coronary intervention. Thromb
Res. 135:652–658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mazumdar R, Evans P, Culpin R, Bailey J
and Allsup D: The automated monocyte count is independently
predictive of overall survival from diagnosis in chronic
lymphocytic leukaemia and of survival following first-line
chemotherapy. Leuk Res. 37:614–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang YH, Yang SH, Wang TF, Lin TY, Yang
KL and Chen SH: Complete blood count reference values of cord blood
in Taiwan and the influence of gender and delivery route on them.
Pediatr Neonatol. 52:155–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Dai W, Li Y, Xu Y, Li X and Cai S:
Nomograms for predicting the prognostic value of serological tumor
biomarkers in colorectal cancer patients after radical resection.
Sci Rep. 7:463452017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson PJ: The role of serum
alpha-fetoprotein estimation in the diagnosis and management of
hepatocellular carcinoma. Clin Liver Dis. 5:145–159. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ychou M, Duffour J, Kramar A, Gourgou S
and Grenier J: Clinical significance and prognostic value of CA72-4
compared with CEA and CA19-9 in patients with gastric cancer. Dis
Markers. 16:105–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge F, Zhang H, Wang DD, Li L and Lin PP:
Enhanced detection and comprehensive in situ phenotypic
characterization of circulating and disseminated heteroploid
epithelial and glioma tumor cells. Oncotarget. 6:27049–27064. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu YK, Hu BS, Li ZL, He X, Li Y and Lu
LG: An improved strategy to detect the epithelial-mesenchymal
transition process in circulating tumor cells in hepatocellular
carcinoma patients. Hepatol Int. 10:640–646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Si Y, Lan G, Deng Z, Wang Y, Lu Y, Qin Y,
Huang B, Yang Y, Weng J, Han X, et al: Distribution and clinical
significance of circulating tumor cells in nasopharyngeal
carcinoma. Jpn J Clin Oncol. 46:622–630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gorges TM, Tinhofer I, Drosch M, Röse L,
Zollner TM, Krahn T and von Ahsen O: Circulating tumour cells
escape from EpCAM-based detection due to epithelial-to-mesenchymal
transition. BMC Cancer. 12:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang S, Wu T, Peng X, Liu J, Liu F, Wu S,
Liu S, Dong Y, Xie S and Ma S: Mesenchymal phenotype of circulating
tumor cells is associated with distant metastasis in breast cancer
patients. Cancer Manag Res. 9:691–700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guan X, Ma F, Liu S, Wu S, Xiao R, Yuan L,
Sun X, Yi Z, Yang H and Xu B: Analysis of the hormone receptor
status of circulating tumor cell subpopulations based on
epithelial-mesenchymal transition: A proof-of-principle study on
the heterogeneity of circulating tumor cells. Oncotarget.
7:65993–66002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li TT, Liu H, Li FP, Hu YF, Mou TY, Lin T,
Yu J, Zheng L and Li GX: Evaluation of epithelial-mesenchymal
transitioned circulating tumor cells in patients with resectable
gastric cancer: Relevance to therapy response. World J
Gastroenterol. 21:13259–13267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hinz S, Tepel J, Röder C, Kalthoff H and
Becker T: Profile of serum factors and disseminated tumor cells
before and after radiofrequency ablation compared to resection of
colorectal liver metastases-a pilot study. Anticancer Res.
35:2961–2967. 2015.PubMed/NCBI
|
|
34
|
Horiike N, Iuchi H, Ninomiya T, Kawai K,
Kumagi T, Michitaka K, Masumoto T and Onji M: Influencing factors
for recurrence of hepatocellular carcinoma treated with
radiofrequency ablation. Oncol Rep. 9:1059–1062. 2002.PubMed/NCBI
|
|
35
|
Berger-Richardson D and Swallow CJ: Needle
tract seeding after percutaneous biopsy of sarcoma: Risk/benefit
considerations. Cancer. 123:560–567. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Serrano-Gomez SJ, Maziveyi M and Alahari
SK: Regulation of epithelial-mesenchymal transition through
epigenetic and post-translational modifications. Mol Cancer.
15:182016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Massagué J and Obenauf AC: Metastatic
colonization by circulating tumour cells. Nature. 529:298–306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Galati D and Zanotta S: Hematologic
neoplasms: Dendritic cells vaccines in motion. Clin Immunol.
183:181–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eyles J, Puaux AL, Wang X, Toh B, Prakash
C, Hong M, Tan TG, Zheng L, Ong LC, Jin Y, et al: Tumor cells
disseminate early, but immunosurveillance limits metastatic
outgrowth, in a mouse model of melanoma. J Clin Invest.
120:2030–2039. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takeda K, Hayakawa Y, Smyth MJ, Kayagaki
N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H and Okumura K:
Involvement of tumor necrosis factor-related apoptosis-inducing
ligand in surveillance of tumor metastasis by liver natural killer
cells. Nat Med. 7:94–100. 2001. View
Article : Google Scholar : PubMed/NCBI
|