Introduction
Glioblastoma multiforme (GBM) is a glial cancer
classified by WHO as a grade IV astrocytoma. Depending on mutation
of the isocitrate dehydrogenase (IDH) gene, three main GM subsets
can be identified: IDH-wild type, that represent ~90% of the cases,
IDH-mutated, generally observed in younger patients with prior
lower grade gliomas and NOS (no otherwise specified), where
evaluation of the IDH gene cannot be performed (1).
Treatment of first choice is surgery, coupled with
chemotherapy and radiotherapy. Since no change in overall survival
(OS) has occurred after the introduction in 2005 of the STUPP
regimen (radiotherapy or chemotherapy in association with
temozolomide), a better understanding of the molecular mechanisms
related to GBM proliferation and recurrence could suggest
alternative avenues for the development of novel therapies and
prognostic markers.
The macrophage migration inhibition factor (MIF) is
an uncommon cytokine with multiple biological functions and
pleiotropic effects (2,3). MIF binds the HLA class II
histocompatibility antigen gamma chain, CD74. The phosphorylation
of CD74, following MIF binding, leads to the recruitment of CD44
and the activation of its downstream signaling, through the
ERK-MAPK pathway (2,3). MIF is also a non-cognate ligand for the
chemokine receptors, CXCR2 and CXCR4. Beside MIF, the MIF
superfamily includes the recently identified homolog D-dopachrome
tautomerase, D-DT (also known as MIF-2), located on chromosome
22q11.23 (4,5). Similarly to MIF, D-DT possesses
enzymatic binding pockets with tautomerase activity for the
D-dopachrome and phenylpyruvate substrates. However, the
end-products of the dopachrome substrates are different for the two
genes. Also, D-DT binds the CD74 ectodomain, with an about 3-fold
higher acid dissociation constant and a 11-fold higher dissociation
rate as compared to MIF. Interestingly, D-DT lacks the motif that
allows MIF binding to the chemokine receptor, CXCR2 (4,5). Studied
for its role in immunity, MIF has been primarily identified as an
inflammatory mediator involved in the regulation of macrophage
activity, chemotaxis, and cytokine secretion and has been indicated
as a major player in the pathogenesis of immune-inflammatory and
autoimmune diseases including multiple sclerosis, Guillain-Barrè
syndrome, type 1 diabetes, systemic lupus erythematosus and
rheumatoid arthritis (2,6–9).
However, MIF activity is not limited to the immune
system: it can act as a hormone, by exerting glucocorticoid
antagonism; as an enzyme, by catalyzing the tautomerization of the
D-dopachrome in 5,6-Dihydroxyindole-2-carboxylic Acid (DHICA); as a
cell differentiation factor, by activating the Wnt/β-catenin
pathway in neurons. MIF is also involved in many tumor processes,
including deregulation of cell cycle, angiogenesis and metastasis
formation (10).
A significant increase in MIF expression has been
observed in several types of cancer, including cervical cancer,
breast, prostate, liver, lung cancer, neuroblastoma, colorectal
cancer, pancreatic, renal carcinomas and lymphocytic leukemia
(10).
There are several biological pathways triggered by
MIF that may contribute to tumorigenesis. Indeed, upon endocytosis,
MIF interacts with c-Jun activation domain-binding protein-1 (JAB1)
and deactivates it. JAB1 is a negative regulator of p27KIP1, that
controls cell cycle progression at the G1 phase (11). MIF also promotes tumor growth by
activating the MAPK/PI3K/Akt pathways, by inhibiting p53-dependent
apoptosis, by promoting angiogenesis via up-regulated secretion of
vascular endothelial growth factor (VEGF) (10), and by promoting immune escape via
inhibition of Natural Killer cell lysis and recruitment of Myeloid
Derived Suppressor Cells (MDSCs). Moreover, MIF favors tumor
invasion by facilitating epithelial-to-mesenchymal transition (EMT)
(12,13) and by increasing the expression of
matrix metalloproteinases (MMPs) (12).
Increasing body of data suggests an important
pathogenic role for MIF in the progression of gliomas (14). Immunohistological analysis of GBM
samples has shown that MIF strongly accumulates in proximity of
necrotic areas and in cancer cells adjacent to the blood vessels
(15). It was also shown that MIF
immunoreactivity increases with tumor grade (15). Moreover, expression of the CD74 in GBM
seems to be involved in the resistance to temozolomide (16). In addition, an association between MIF
expression and tumor recurrence and poor prognosis of glioma
patients has been reported (17). It
has also been reported that MIF enhances autophagy by regulating
ROCK1 activity and contributes to the escape of dendritic cell
surveillance in GBM (18).
In agreement with these data, a study in primary GBM
cells has shown that inhibition of MIF with ISO-1, an inhibitor of
its D-dopachrome tautomerase site, reduced the growth rate of
primary GBM cells in a dose-dependent manner (19). MiR-608 has also been shown to inhibit
the migration and invasion of glioma stem cells by targeting
macrophage migration inhibitory factor (20).
The aim of this study was to evaluate the expression
of MIF and of its functionally related genes, in different grade
gliomas, and to highlight the potential role of MIF in GBM, as
biomarker or therapeutic target.
Materials and methods
Characterization of MIF and
functionally-related genes in GBM
In order to evaluate the expression levels of MIF
and related genes in gliomas, RNA Seq data from the TCGA datasets
were downloaded through the cBioportal web-based utility
(http://www.cbioportal.org). Selected
genes were MIF, D-DT, CD74, CD44, CXCR2, CXCR4 and JAB1. Complete
clinical data of the patients were retrieved and only data from
primary tumors, with no neoadjuvant therapy prior to excision, were
selected. Data were subjected to Kolmogorov-Smirnov test,
D'Agostino and Person Omnibus test and Shapiro-Wilk normality test.
Accordingly to the results from the normality tests, the
Kruskal-Wallis test followed by Dunn's Post Test was applied to
assess statistical significance for the differences among cancer
types. Overall, this study comprised 153 patients with GBM, 31 with
Oligoastrocytoma, 22 with Oligodendroglioma, 15 with Astrocytomas,
29 with Anaplastic Astrocytoma and 16 patients with Anaplastic
Oligoastrocytomas.
In order to evaluate how the genes of interest are
similar in their expression pattern, a Gene Distance Matrix was
built using the Multi Experiment Viewer software (mev.tm4.org). The distance between two genes, which
represents the inverse of similarity, was calculated using Pearson
correlation. Values can vary from −1 to 1. Correlations near 1
indicate a strong positive correlation, while values closer to −1
indicate a negative correlation.
Identification of genetic aberrations of the genes
of interest has been performed on a cohort of 281 and 283
DNA-sequenced GBM and low-grade glioma patients, respectively, as
available in the TGCA database.
Correlation between the selected genes and the OS of
GBM patients were performed using linear regression analysis and
Pearson's test.
Effect of neoadjuvant therapy on MIF
expression
Microarray transcriptomic data of 497 tumor samples
from patients who underwent tumor excision without neoadjuvant
therapy and from 20 patients treated with neoadjuvant therapy
before cancer resection were also obtained from the cBioportal
web-based utility. No information regarding the type of neoadjuvant
therapy has been disclosed. Unpaired Student's t-test was applied
to assess statistical significance of the mean difference in MIF
levels between the two groups.
Statistical analysis
Expression data are presented as dot plots with line
at median value. GraphPad Prism 5 (GraphPad Software, La Jolla, CA,
USA) was used for all the statistical analysis. Data were subjected
to a Kolmogorov-Smirnov test, D'Agostino and Person Omnibus test
and Shapiro-Wilk normality test. According to the results from the
normality tests, the Kruskal-Wallis test followed by Dunn's post
hoc test were applied to assess the statistical significance for
the differences among cancer types. The association between the
selected genes and the OS of GBM patients was evaluated using
linear regression analysis and Pearson's test. An unpaired
Student's t-test was applied to assess statistical significance of
the mean difference in MIF levels between the group of patients who
underwent neoadjuvant therapy and patients without neoadjuvant
therapy. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of MIF and its
functionally-related genes in GBM
A marked upregulation of MIF expression was observed
in GBM as compared to all of the other lower grade Gliomas
(Fig. 1A). Similarly to MIF,
significant higher levels of D-DT were found in GBM as compared to
Oligoastrocytoma and Anaplastic Astrocytoma (Fig. 1B). As regards CD74 and the
co-receptor, CD44, significant higher levels were observed in GBM
when compared to all of the other lower grade Gliomas (Fig. 1C and D). Along the same lines, the
non-cognate receptors CXCR2 and CXCR4 were also significantly
overexpressed in GBM samples as compared to all of the other lower
grade gliomas (Fig. 1E, F). In
addition, in GBM samples significantly higher expression levels of
JAB1 were also observed with respect to lower grade gliomas
(Fig. 1G). A significant positive
correlation in the expression levels was observed between MIF and
D-DT (P<0.0001 by Spearman test), CD74 (P=0.0422 by Spearman
test), CXCR4 (P<0.0001 by Spearman test) and JAB1 (P<0.0001
by Spearman test) (Fig. 1H).
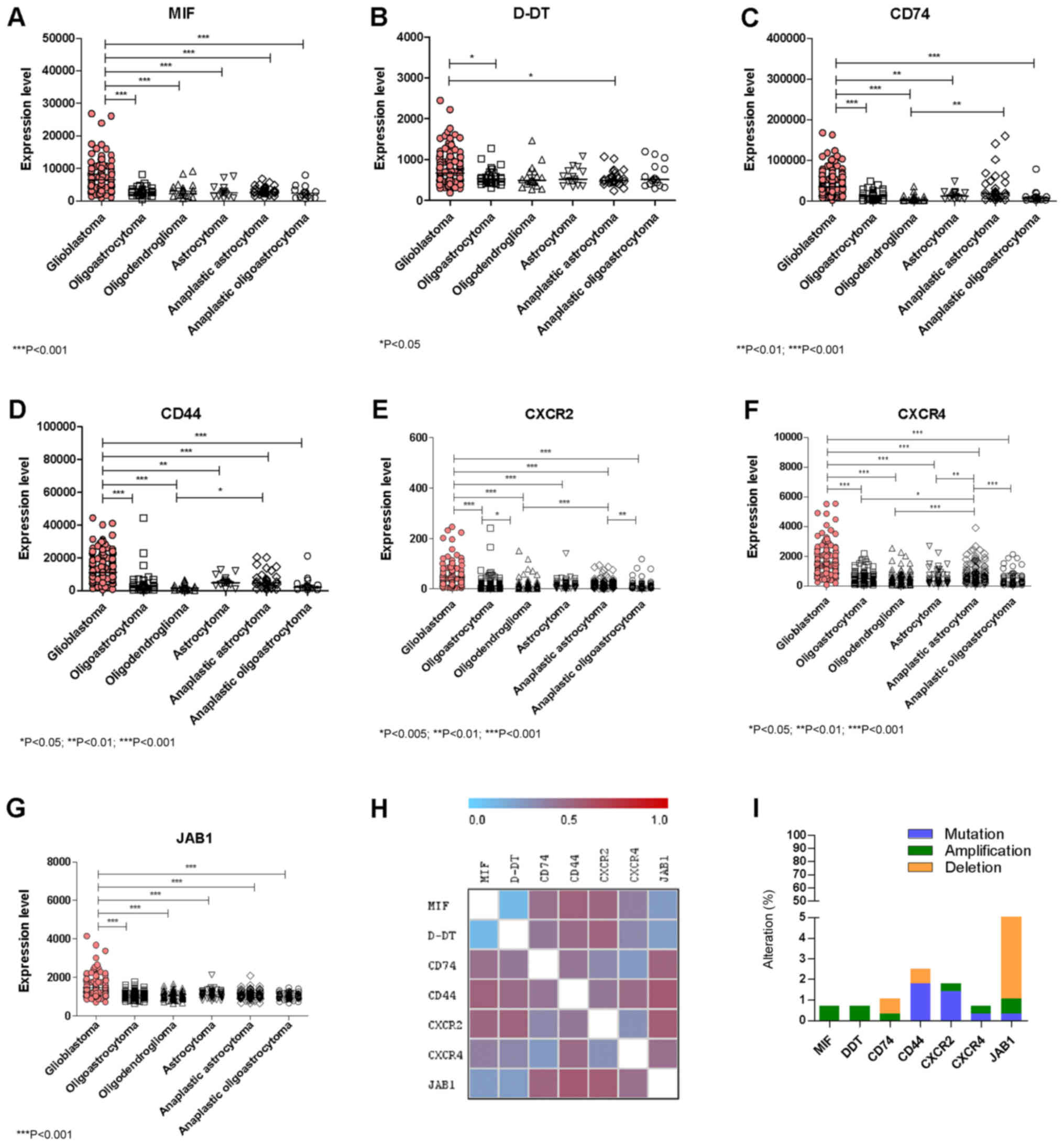 | Figure 1.Evaluation of the expression levels of
(A) MIF, (B) D-DT, (C) CD74, (D) CD44, (E) CXCR2, (F) CXCR4 and (G)
JAB1 in glioblastoma and lower grade glioma samples. (H) Gene
Distance Matrix was built using the Multi Experiment Viewer
software (mev.tm4.org/), in order to evaluate how
the genes of interest are similar in their expression pattern. (I)
Percentage of genetic aberrations in the genes of interest has been
performed on a cohort of 281 and 283 DNA-sequenced glioblastoma and
low-grade glioma patients, respectively. Data were retrieved from
the The Cancer Genome Atlas dataset through the cBioportal
web-based utility (cbioportal.org). *P<0.05, **P<0.01 and
***P<0.001, as indicated. MIF, macrophage migration inhibitory
factor; D-DT, D-dopachrome tautomerase; CD, cluster of
differentiation; CXCR, C-X-C Motif Chemokine Receptor; JAB1, c-Jun
activation domain-binding protein-1. |
Characterization of MIF and related
genes in GBM
Only a small percentage of samples presented genetic
aberrations in the studied genes (Fig.
1I). These aberrations included: 2 cases (over 283 samples)
among lower grade gliomas of MIF and D-DT gene amplification; 1
amplification and 2 deletions for CD74 in lower grade glioma
samples; 2 cases of CD44 deletion among glioma samples and 2
missense mutations in glioma samples, as well as, 1 missense and 2
nonsense mutations among GBM samples (over 273 samples); 1 case of
amplification and 1 missense mutation of the CXCR2 gene in GBM
samples, as well as 3 missense mutations among glioma samples; 1
missense mutation of CXCR4 and 1 gene amplification among glioma
and GBM samples, respectively; 1 case of missense mutation of JAB1
and 13 cases of deletions among gliomas and 2 deletions among GBM
patient (Fig. 1I).
No significant correlations among the selected genes
and the OS of the GBM patients could be observed, although a trend
of positive correlation was observed for MIF (Fig. 2A-G). Also, neoadjuvant therapy was
associated to a moderate, although significant (P=0.0315), increase
in MIF expression (Fig. 2H).
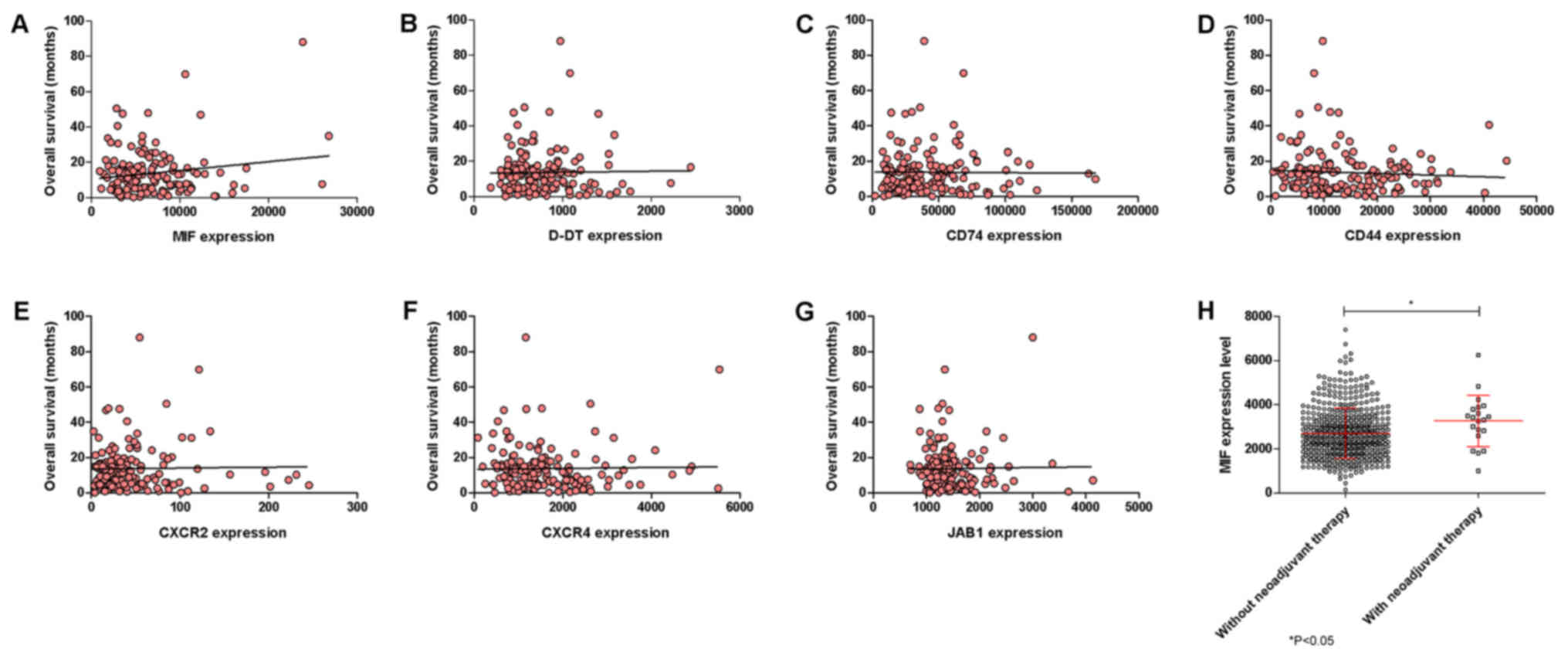 | Figure 2.Correlation between overall survival
and expression levels (A) MIF, (B) D-DT, (C) CD74, (D) CD44, (E)
CXCR2, (F) CXCR4 and (G) JAB1 was performed using linear regression
analysis and Pearson's test on glioblastoma samples. (H)
Differences in MIF expression levels were calculated in
glioblastoma samples from drug-naïve patients and patients who
underwent neoadjuvant therapy. Data were retrieved from the The
Cancer Genome Atlas dataset through the cBioportal web-based
utility (cbioportal.org). *P<0.05, as
indicated. MIF, macrophage migration inhibitory factor; D-DT,
D-dopachrome tautomerase; CD, cluster of differentiation; CXCR,
C-X-C Motif Chemokine Receptor; JAB1, c-Jun activation
domain-binding protein-1. |
Discussion
The results presented herein partially confirm the
previous observations on the modulation of MIF and CD74 in central
nervous system (CNS) tumors (15–17) and
expand the current knowledge on their most functionally related
genes. These data demonstrate that a local over-expression of MIF,
D-DT, CD44, CD74, CXCR2, CXCR4 and JAB1 occurs in invasive and
malignant forms of GBM, as compared to other lower grade CNS
tumors. To the best of our knowledge this is the first paper to
demonstrate the overexpression of the MIF related genes, CD44,
CXCR2, CXCR4 and JAB1 and D-DT in GBM.
However, in spite of these multiple evidence
pointing to a pro-oncogenic role of MIF in GBM, the net biological
significance of our present findings is hampered by the observation
that the over-expression of MIF does not correlate with a lower OS
but rather exhibits an inverse trend to increased OS. In addition,
the biological significance of this trend is strengthened by the
observation that neoadjuvant therapy is associated to a significant
increase in MIF expression. The potential protective role of MIF in
GBM may concur with recent findings that CD74 is restricted to
microglia/macrophages associated with an M1-polarized immune milieu
and prolonged patient survival in gliomas (21). Favoring the beneficial anti-oncogenic
role of endogenous MIF in GBM, it is also the observation that
bevacizumab resistance in GBM is driven by reduced MIF at the tumor
edge, causing proliferative expansion of M2 macrophages, which in
turn promotes tumor growth (22).
In contrast, other Authors have reported high levels
of CD74 expression in high grade gliomas and have proposed this
expression as a mechanism involved in temozolomide resistance
(16). Also, Wang and collaborators
have shown that in patients with WHO grade III and IV gliomas the
survival time was significantly shorter in patients with high
expression of MIF or IL-8 in high-grade tumors than those with
protein low expression in their tumors (17). However, in multivariate analysis, only
histological grade and MIF expression in gliomas were independently
associated with survival (17). In
particular, the discrepancy of these data with ours can be due to
the fact that we analyze MIF expression at the transcriptomic level
and not at the protein level. Also, as pointed out by Verjans et
al (23), cellular localization
of MIF can be associated to different biological effects in cancer.
In particular, Verjans et al, have shown that when MIF is
localized within breast cancer cells, it acts as a favorable
prognostic marker, while it plays a pro-oncogenic role, by
promoting breast cancer cells-stroma interactions, when it is
localized in the extracellular space (23).
Taken as a whole, these data highlight a complex,
pleiotropic and eventually even dichotomous role of MIF in GBM
maintenance and progression. The pleiotropism of MIF, as well as of
most cytokines, in biological systems is well known. MIF is clearly
endowed with pro-inflammatory properties that include induction of
nitric oxide, cyclooxygenase 2 (24)
and Toll-like receptor 4 (25), the
production of pro-inflammatory cytokines (26) and glucocorticoid antagonism (27). Nonetheless, in other settings, MIF
acts as anti-inflammatory cytokine that recruits myeloid derived
suppressor cells and tumor associated macrophages (28,29) and
inhibits CD8+ T- and NK cells-mediated cytotoxicity (30).
The major limitation of our data is that only the
transcriptional levels of MIF and related genes have been
investigated. Therefore, no direct comparison can be performed
between the above mentioned studies and our results. Further
analysis on the cellular localization of these proteins will
eventually shed light on their role in GBM development, progression
and prognosis. Also, it should be pointed out that MIF undergoes
post-translational modifications, including carbamylation of the
Pro-2; cysteinylation at Cys-60; S-nitrosilation and
phosphorylation of Cys-81 and Ser-91 (31), that may alter MIF activity at specific
sites, especially where oxidative events occur.
Although the eventual concentration and
dose-dependency of the effects of MIF (and D-DT) in the context of
GBM remains to be defined, it seems possible to hypothesize that
different environmental conditions, the local concentration and/or
protein localization may ultimately dictate the effects of MIF in
GBM. The exact definition of the precise role of MIF in GBM could
lead to important diagnostic and therapeutic achievements in terms
of prognosis and identification of biomarkers that could be
associated to either beneficial or detrimental effects and
predictors of therapeutic responses. In particular, therapeutic
approaches based on either MIF-D-DT agonism or antagonisms could be
envisaged in a tailored fashion for at least certain subsets of GBM
patients, once the protective or pathogenic role of these cytokine
homologs is more clearly understood.
Acknowledgements
Not applicable.
Funding
The present study was supported by current research
funds 2016 of IRCCS ‘Centro Neurolesi ‘Bonino Pulejo’,
Messina-Italy.
Availability of data and materials
All data used in this paper are available for
download from the cBioportal website (cbioportal.org).
Authors' contributions
FN and PF conceived and designed the study. MP, EM,
MSB and MCP collected and analyzed the data. AB, GC and PB
conducted the statistical analysis, interpreted the data and wrote
the manuscript. All authors read and revised the manuscript, and
approved the final version to be published.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stosic-Grujicic S, Stojanovic I and
Nicoletti F: MIF in autoimmunity and novel therapeutic approaches.
Autoimmun Rev. 8:244–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bloom J, Sun S and Al-Abed Y: MIF, a
controversial cytokine: A review of structural features,
challenges, and opportunities for drug development. Expert Opin
Ther Targets. 20:1463–1475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Merk M, Zierow S, Leng L, Das R, Du X,
Schulte W, Fan J, Lue H, Chen Y, Xiong H, et al: The D-dopachrome
tautomerase (DDT) gene product is a cytokine and functional homolog
of macrophage migration inhibitory factor (MIF). Proc Natl Acad
Sci. 108:E577–E585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merk M, Mitchell RA, Endres S and Bucala
R: D-dopachrome tautomerase (D-DT or MIF-2): Doubling the MIF
cytokine family. Cytokine. 59:10–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Connelly KL, Kandane-Rathnayake R, Hoi A,
Nikpour M and Morand EF: Association of MIF, but not type I
interferon-induced chemokines, with increased disease activity in
Asian patients with systemic lupus erythematosus. Sci Rep.
6:299092016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lang T, Foote A, Lee JP, Morand EF and
Harris J: MIF: Implications in the pathoetiology of systemic lupus
erythematosus. Front Immunol. 6:5772015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benedek G, Meza-Romero R, Jordan K, Zhang
Y, Nguyen H, Kent G, Li J, Siu E, Frazer J, Piecychna M, et al: MIF
and D-DT are potential disease severity modifiers in male MS
subjects. Proc Natl Acad Sci USA. 114:E8421–E8429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicoletti F, Créange A, Orlikowski D,
Bolgert F, Mangano K, Metz C, Di Marco R and Al Abed Y: Macrophage
migration inhibitory factor (MIF) seems crucially involved in
Guillain-Barré syndrome and experimental allergic neuritis. J
Neuroimmunol. 168:168–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nobre CC, de Araújo JM, Fernandes TA,
Cobucci RN, Lanza DC, Andrade VS and Fernandes JV: Macrophage
migration inhibitory factor (MIF): Biological activities and
relation with cancer. Pathol Oncol Res. 23:235–244. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burger-Kentischer A, Finkelmeier D, Thiele
M, Schmucker J, Geiger G, Tovar GE and Bernhagen J: Binding of
JAB1/CSN5 to MIF is mediated by the MPN domain but is independent
of the JAMM motif. FEBS Lett. 579:1693–1701. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Babu SN, Chetal G and Kumar S: Macrophage
migration inhibitory factor: A potential marker for cancer
diagnosis and therapy. Asian Pac J Cancer Prev. 13:1737–1744. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Funamizu N, Hu C, Lacy C, Schetter A,
Zhang G, He P, Gaedcke J, Ghadimi MB, Ried T, Yfantis HG, et al:
Macrophage migration inhibitory factor induces epithelial to
mesenchymal transition, enhances tumor aggressiveness and predicts
clinical outcome in resected pancreatic ductal adenocarcinoma. Int
J Cancer. 132:785–794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bach JP, Deuster O, Balzer-Geldsetzer M,
Meyer B, Dodel R and Bacher M: The role of macrophage inhibitory
factor in tumorigenesis and central nervous system tumors. Cancer.
115:2031–2040. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bacher M, Schrader J, Thompson N, Kuschela
K, Gemsa D, Waeber G and Schlegel J: Up-regulation of macrophage
migration inhibitory factor gene and protein expression in glial
tumor cells during hypoxic and hypoglycemic stress indicates a
critical role for angiogenesis in glioblastoma multiforme. Am J
Pathol. 162:11–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitange GJ, Carlson BL, Schroeder MA,
Decker PA, Morlan BW, Wu W, Ballman KV, Giannini C and Sarkaria JN:
Expression of CD74 in high grade gliomas: A potential role in
temozolomide resistance. J Neurooncol. 100:177–186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang XB, Tian XY, Li Y, Li B and Li Z:
Elevated expression of macrophage migration inhibitory factor
correlates with tumor recurrence and poor prognosis of patients
with gliomas. J Neurooncol. 106:43–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu S, Guo X, Gao X, Xue H, Zhang J, Guo X,
Qiu W, Zhang P and Li G: Macrophage migration inhibitory factor
enhances autophagy by regulating ROCK1 activity and contributes to
the escape of dendritic cell surveillance in glioblastoma. Int J
Oncol. 49:2105–2115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baron N, Deuster O, Noelker C, Stüer C,
Strik H, Schaller C, Dodel R, Meyer B and Bacher M: Role of
macrophage migration inhibitory factor in primary glioblastoma
multiforme cells. J Neurosci Res. 89:711–717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Xue Y, Wang P, Zhu J and Ma J:
MiR-608 inhibits the migration and invasion of glioma stem cells by
targeting macrophage migration inhibitory factor. Oncol Rep.
35:2733–2742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeiner PS, Preusse C, Blank AE, Zachskorn
C, Baumgarten P, Caspary L, Braczynski AK, Weissenberger J, Bratzke
H, Reiß S, et al: MIF receptor CD74 is restricted to
microglia/macrophages, associated with a M1-polarized immune milieu
and prolonged patient survival in gliomas. Brain Pathol.
25:491–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Castro BA, Flanigan P, Jahangiri A,
Hoffman D, Chen W, Kuang R, De Lay M, Yagnik G, Wagner JR,
Mascharak S, et al: Macrophage migration inhibitory factor
downregulation: A novel mechanism of resistance to anti-angiogenic
therapy. Oncogene. 36:3749–3759. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verjans E, Noetzel E, Bektas N, Schütz AK,
Lue H, Lennartz B, Hartmann A, Dahl E and Bernhagen J: Dual role of
macrophage migration inhibitory factor (MIF) in human breast
cancer. BMC Cancer. 9:2302009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jde Rosado D and Rodriguez-Sosa M:
Macrophage migration inhibitory factor (MIF): A key player in
protozoan infections. Int J Biol Sci. 7:1239–1256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gregory JL, Leech MT, David JR, Yang YH,
Dacumos A and Hickey MJ: Reduced leukocyte-endothelial cell
interactions in the inflamed microcirculation of macrophage
migration inhibitory factor-deficient mice. Arthritis Rheum.
50:3023–3034. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Onodera S, Nishihira J, Koyama Y, Majima
T, Aoki Y, Ichiyama H, Ishibashi T and Minami A: Macrophage
migration inhibitory factor up-regulates the expression of
interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid
arthritis patients: common transcriptional regulatory mechanism
between interleukin-8 and interleukin-1beta. Arthritis Rheum.
50:1437–1447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fingerle-Rowson G, Koch P, Bikoff R, Lin
X, Metz CN, Dhabhar FS, Meinhardt A and Bucala R: Regulation of
macrophage migration inhibitory factor expression by
glucocorticoids in vivo. Am J Pathol. 162:47–56. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Ye YL, Li MX, Ye SB, Huang WR,
Cai TT, He J, Peng JY, Duan TH, Cui J, et al: CXCL2/MIF-CXCR2
signaling promotes the recruitment of myeloid-derived suppressor
cells and is correlated with prognosis in bladder cancer. Oncogene.
36:2095–2104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yaddanapudi K, Putty K, Rendon BE, Lamont
GJ, Faughn JD, Satoskar A, Lasnik A, Eaton JW and Mitchell RA:
Control of tumor-associated macrophage alternative activation by
macrophage migration inhibitory factor. J Immunol. 190:2984–2993.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krockenberger M, Dombrowski Y, Weidler C,
Ossadnik M, Hönig A, Häusler S, Voigt H, Becker JC, Leng L, Steinle
A, et al: Macrophage migration inhibitory factor contributes to the
immune escape of ovarian cancer by down-regulating NKG2D. J
Immunol. 180:7338–7348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schindler L, Dickerhof N, Hampton MB and
Bernhagen J: Post-translational regulation of macrophage migration
inhibitory factor: Basis for functional fine-tuning. Redox Biol.
15:135–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
















