Introduction
Gastric cancer is a common malignancy with high
morbidity and mortality in men and women (1). The 5-year relative survival rate of
patients with gastric cancer is only ~20% worldwide (2). In 2012, ~952,000 individuals were
diagnosed with gastric cancer and ~723,000 succumbed to the disease
(3). The conventional method of
treating gastric cancer is surgery, and, when accompanied by
adjuvant chemotherapy and radiotherapy, the prognosis of patients
with gastric cancer may be markedly improved (4). Chemotherapy is a successful method of
decreasing pain and prolonging the life expectancy of patients with
gastric cancer (5). However, owing to
side effects and drug resistance, the clinical efficacy of
chemotherapeutic agents is limited (6,7). Owing to
a small number of side effects and the wide range of targets of
plant-derived components, a number of previous studies have focused
on the function of plant-derived agents in antitumor treatment
(8–10).
Resveratrol (3,4,5-trihydroxystilbene) is a natural
phytoalexin product, and is widely present in a variety of plants,
including grapes, berries and the Chinese medicine plant
Polygonum cuspidatum (Japanese knotweed) (11). As an important component of red wine,
resveratrol has long been hypothesized to exhibit cardioprotective
effects, and it is well-known for its phytoestrogenic and
antioxidant properties (12–15). In addition, previous studies have
identified that resveratrol was able to prolong lifespan and resist
cancer. For instance, feeding fish with resveratrol resulted in an
increase in median and maximum lifespan by 33 and 27%,
respectively, compared with fish that were fed without resveratrol
supplementation (16). Furthermore,
injection of resveratrol into mice led to a significant inhibition
of the proliferation of breast cancer stem cell-like cells by
suppressing the Wnt/β-catenin signaling pathway (17). Although these studies demonstrated the
antitumor effect of resveratrol, the precise underlying molecular
mechanisms remain unclear.
In the present study, the gastric cancer cell line
SGC-7901 was used to investigate the effects and acting mechanisms
of resveratrol on cell viability and apoptosis. The results may
provide an improved understanding of the effects of resveratrol in
the treatment of gastric cancer.
Materials and methods
Chemicals and reagents
Resveratrol (Chemical Abstracts Service identifier,
501-36-0; purity ≥99%), MTT, acridine orange (AO), ethidium bromide
(EB) and propidium iodide (PI) were purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany). Resveratrol was dissolved in
dimethyl sulfoxide (DMSO) to form a 100 mM stock solution. MTT was
dissolved in PBS to form a 5 mg/ml working solution. RPMI-1640
medium was purchased from HyClone; GE Healthcare (Chicago, IL, USA)
and fetal bovine serum (FBS) was purchased from Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd. (Hangzhou, China). All
other chemicals and reagents used in the present study were of
analytical grade.
Cell culture
SGC-7901 cells were purchased from the China Center
for Type Culture Collection (Wuhan, China) and cultured in
RPMI-1640 medium supplemented with 10% FBS and antibiotics (100
U/ml streptomycin and 100 U/ml penicillin) in 25-cm2
culture flasks at 37°C in a humidified atmosphere containing 5%
CO2. When the SGC-7901 cells reached exponential growth
phase, the cells were subcultured and the experiments were
performed on the subcultured cells.
MTT assay
The anti-proliferative effect of resveratrol against
SGC-7901 cells was determined using the colorimetric MTT assay as
described previously (18). The
SGC-7901 cells were seeded on 96-well culture plates with RPMI-1640
medium at a density of 1×104 cells/ml. Following
incubation for 24 h at 37°C, the cells were treated with different
concentrations of resveratrol (0, 10, 50, 100, 200 and 400 µM) for
24, 36 and 48 h. Subsequently, 10 µl MTT (5 mg/ml) was separately
added to each well, and the cells were cultured at 37°C for an
additional 3 h. Finally, 150 µl DMSO was separately added to each
well and the optical density (OD) was determined at 490 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The inhibition rate of resveratrol against the SGC-7901 cells was
determined using the equation: Inhibition rate
(%)=(ODcontrol-ODtreatment)/ODcontrol
×100.
AO/EB dual staining assay
The apoptosis of SGC-7901 cells induced by
resveratrol was examined using an AO/EB dual-fluorescence staining
assay as described previously (19).
Sterile round coverslips were placed on the bottom of the wells of
a 12-well plate onto which the SGC-7901 cells were seeded with
RPMI-1640 medium at a density of 1×104 cells/ml. After
24 h of incubation at 37°C, the cells were treated with different
concentrations of resveratrol (0, 50, 200 and 400 µM) for 24 h.
Subsequently, the round coverslips were removed. Dual-fluorescence
staining solution (10 µl) containing 100 µg/ml AO and 100 µg/ml EB
was added to each suspension prior to being covered with a
coverslip. The morphology of cells was examined in each sample
within 20 min using a fluorescence microscope (Nikon 80i; Nikon
Corporation, Tokyo, Japan).
Apoptosis assay
Cell apoptosis was determined using flow cytometry
following treatment as described previously (20). SGC-7901 cells were seeded in 6-well
culture plates with RPMI-1640 medium and left to attach overnight
at 37°C at a density of 1×104 cells/ml. Following
treatment with resveratrol (0, 50, 200 and 400 µM) for 24 h, the
cells were harvested and prepared as cell suspensions. Adherent
cells were digested with EDTA-free trypsin and washed three times
with ice-cold PBS. Subsequently, the cells (1×106
cells/ml) were resuspended in 200 µl staining buffer. Annexin
V-fluorescein isothiocyanate staining solution (2 µl) was then
added to the cell suspension. The mixture was gently mixed and
incubated in the dark at 2–8°C for 15 min. Subsequently, 4 µl PI
staining solution was added to the cell suspension. The mixture was
mixed and incubated in the dark at 2–8°C for 5 min. The apoptotic
cells were quantified immediately using a flow cytometer (BD Accuri
C6; BD Biosciences, Franklin Lakes, NJ, USA) and the results were
analyzed using FlowJo software (version 7.6; FlowJo LLC, Ashland,
OR, USA).
Cell cycle assay
Cell cycle distribution was detected using a flow
cytometer following drug treatment as described previously
(21). SGC-7901 cells were seeded in
6-well culture plates at a density of 1×104 cells/ml
with RPMI-1640 medium and left to attach overnight at 37°C.
Following treatment with resveratrol (0, 50, 200 and 400 µM), cells
were incubated further with the compounds for 24 h at 37°C before
being harvested, washed twice with PBS and resuspended in 2 ml
ice-cold PBS. The cells were fixed with cold (−20°C) 70% ethanol
overnight at 4°C. Following three PBS washes, the cells were
resuspended in 2 ml PI/RNase staining solution and incubated for 1
h at 4°C. Cells were quantified immediately using a flow cytometer
(BD Accuri C6) and the results were analyzed using ModFitLT™
software (version 5.1; Verity Software House, Inc., Topsham, ME,
USA). The PI fluorescence signal at FL2-A peak versus counts was
used to determine cell cycle distribution.
Western blot analysis
Following treatment, proteins in SGC-7901 cells were
extracted as described previously (22). Following treatment with resveratrol
(0, 50, 200 and 400 µM) for 24 h, total protein was extracted with
cell lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China), centrifuged at 13,4000 × g for 10 min at 4°C. Nuclear and
cytosolic proteins were extracted using a Nuclear and Cytoplasmic
Protein Extraction kit (Applygen Technologies, Inc., Beijing,
China), according to the manufacturer's protocol. The cell lysate
was mixed with 2 mM Na3VO4, as a
phosphorylation protective agent. Protein concentrations were
determined using a Bicinchoninic Acid Protein Assay kit (Wuhan
Boster Biological Technology, Ltd., Wuhan, China), according to the
manufacturer's protocol. Total protein (40 µg) was separated using
SDS-PAGE (12% gel) and transferred onto a nitrocellulose membrane
(EMD Millipore, Billerica, MA, USA). Following blocking with
Tris-buffered saline containing 1% Tween-20 (TBST) and 5% fat-free
milk powder, the membrane was incubated with primary antibodies
against β-actin (cat. no. bs-0061R, 1:1,000; BIOSS, Beijing,
China), histone H3 (cat. no. bs-0349R, 1:1,000; BIOSS), B-cell
lymphoma 2 (Bcl-2; cat. no. bs-20351R, 1:200; BIOSS),
Bcl-2-associated X protein (Bax; cat. no. bs-0127R, 1:200; BIOSS),
cleaved caspase-3 (cat. no. BA2885-2, 1:200; Wuhan Boster
Biological Technology, Ltd.), cleaved caspase-8 (cat. no. BA3971,
1:200; Wuhan Boster Biological Technology, Ltd.), pro-caspase-3
(cat. no. BM3954, 1:200; Wuhan Boster Biological Technology, Ltd.),
pro-caspase-8 (cat. no. BM4423, 1:200; Wuhan Boster Biological
Technology, Ltd.), nuclear factor κB (NF-κB) (p65) (cat. no.
10745-1-AP, 1:1,000; ProteinTech Group, Inc., Chicago, IL, USA) and
phospho-NF-κB (p65) (cat. no. bs-0982R, 1:1,000; BIOSS) overnight
at 4°C. The membrane was then washed with TBST and incubated with
the corresponding horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) in TBST for 2 h at room temperature. Following a
further rinse, all proteins were detected using chemiluminescence
reagent (ECL Plus reagent; Beyotime Institute of Biotechnology).
The result was analyzed using Image Lab™ software (version 5.0; MCM
DESIGN, Hillerød, Denmark). β-actin was used as a loading control
for whole cell and cytoplasmic proteins. Histone H3 was used as an
internal control for detection of nuclear proteins.
Statistical analysis
Each experiment was performed at least three times.
Results are presented as the mean ± standard deviation. The results
were analyzed by one-way analysis of variance followed by a least
significant difference post hoc test using SPSS (version 19.0; IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Resveratrol inhibits the viability of
SGC-7901 cells
SGC-7901 cells were treated with different
concentrations of resveratrol for 24, 36 and 48 h, respectively
(Fig. 1A-C). Cell viability was
analyzed by MTT assay. As presented in Fig. 1D, the inhibition of cell viability was
significantly increased in SGC-7901 cells in response to
resveratrol in a dose- and time-dependent manner compared with the
control group (0 µM resveratrol) (P<0.05).
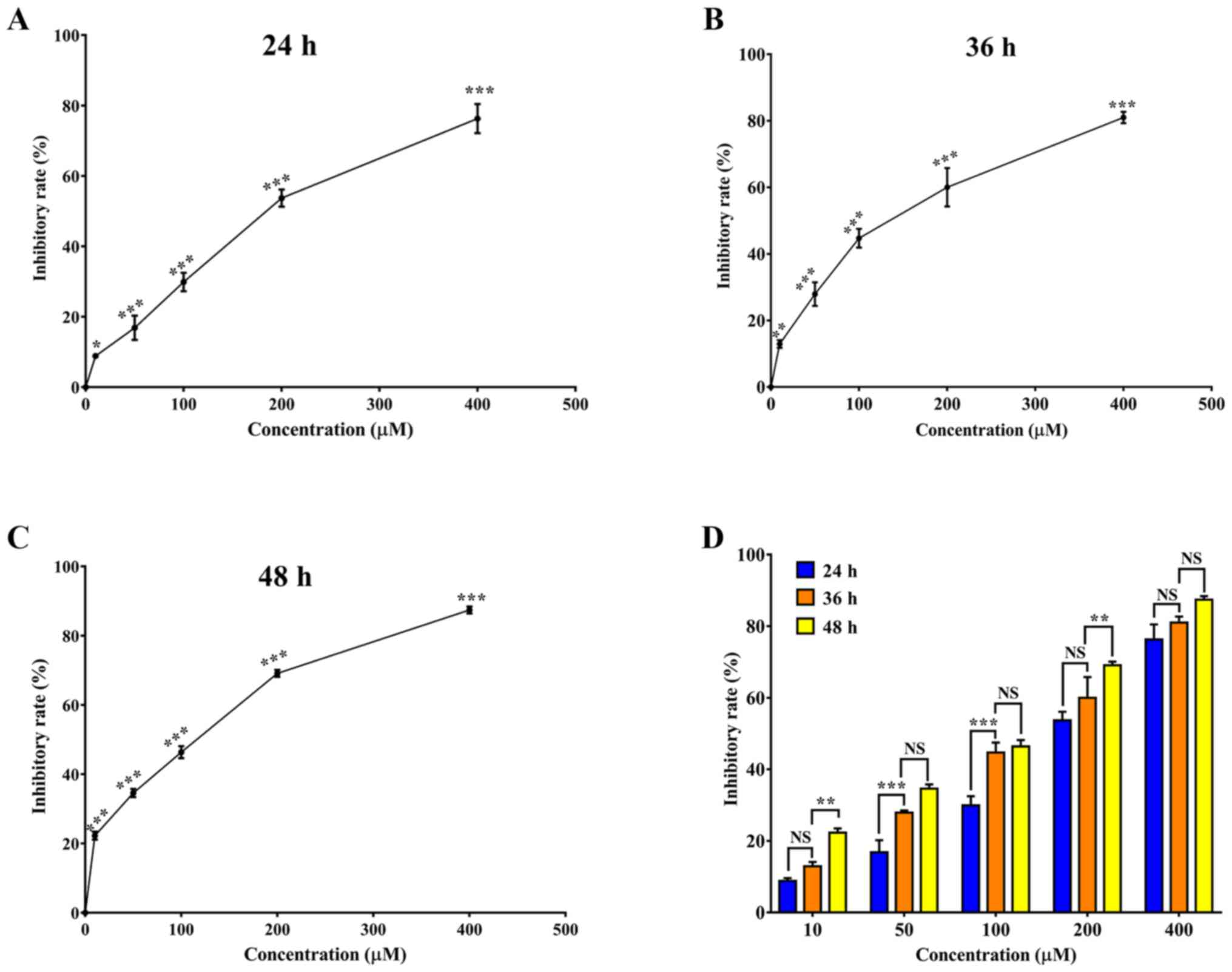 | Figure 1.Resveratrol inhibits the viability of
SGC-7901 cells. The cell viability inhibitory rate was determined
using an MTT assay. SGC-7901 cells were treated with 0, 10, 50,
100, 200 and 400 µM resveratrol for (A) 24, (B) 36 and (C) 48 h.
(D) SGC-7901 cells were treated with 0, 10, 50, 100, 200 and 400 µM
resveratrol for 24, 36 and 48 h, and the inhibitory rate at each
concentration at different times was compared. Results are
presented as the mean ± standard deviation (n=3). *P<0.05,
**P<0.01 and ***P<0.001 vs. control group. |
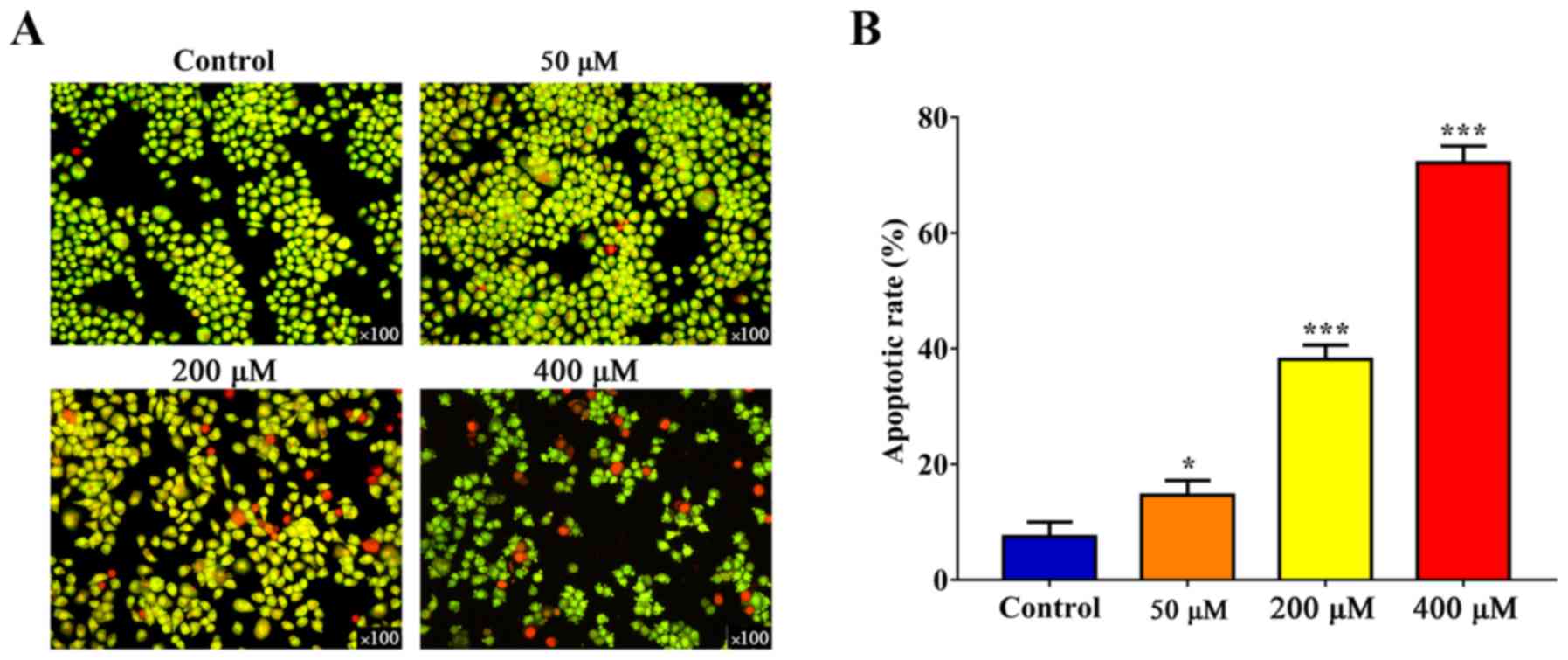
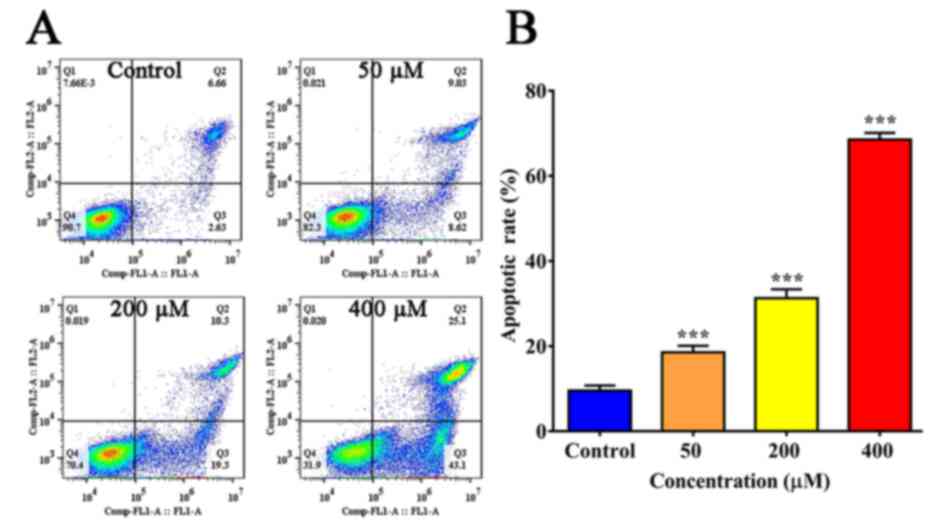
Resveratrol induces the apoptosis of
SGC-7901 cells
SGC-7901 cells were treated with different doses of
resveratrol for 24 h, labeled using AO/EB and examined using a
fluorescence microscope (Fig. 2A).
The staining of early-stage apoptotic cells was marked by
crescent-shaped or granular yellow-green AO nuclear staining, and
orange nuclear EB staining was asymmetrically localized in
late-stage apoptotic cells. In the control group, no obvious
apoptotic changes were identified by flow cytometric analysis.
However, the number of apoptotic cells was significantly increased
following resveratrol treatment in a dose-dependent manner
(Figs. 2B and 3).
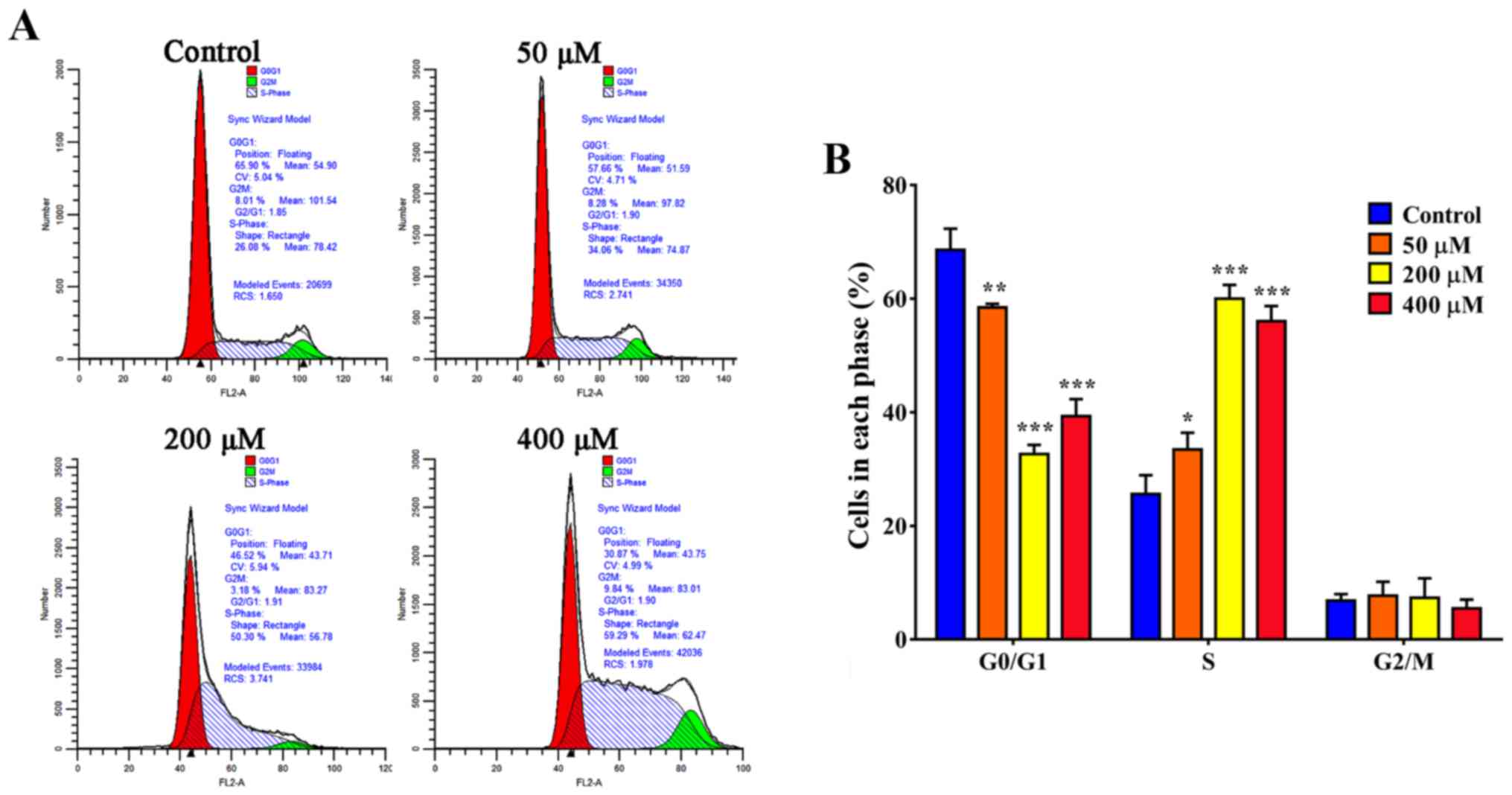
Resveratrol promotes S-phase arrest in
SGC-7901 cells
Cell cycle distribution was observed by flow
cytometric analysis. As presented in Fig.
4A, the proportion of cells in S-phase increased to 34.06% in
the presence of resveratrol (50 µM) compared with 26.08% in the
control group (P<0.05). When treated with 200 µM resveratrol,
the proportion of SGC-7901 cells in S-phase was 59.29%; however,
the proportion of SGC-7901 cells in S-phase was 50.3% when treated
with 400 µM resveratrol (Fig. 4).
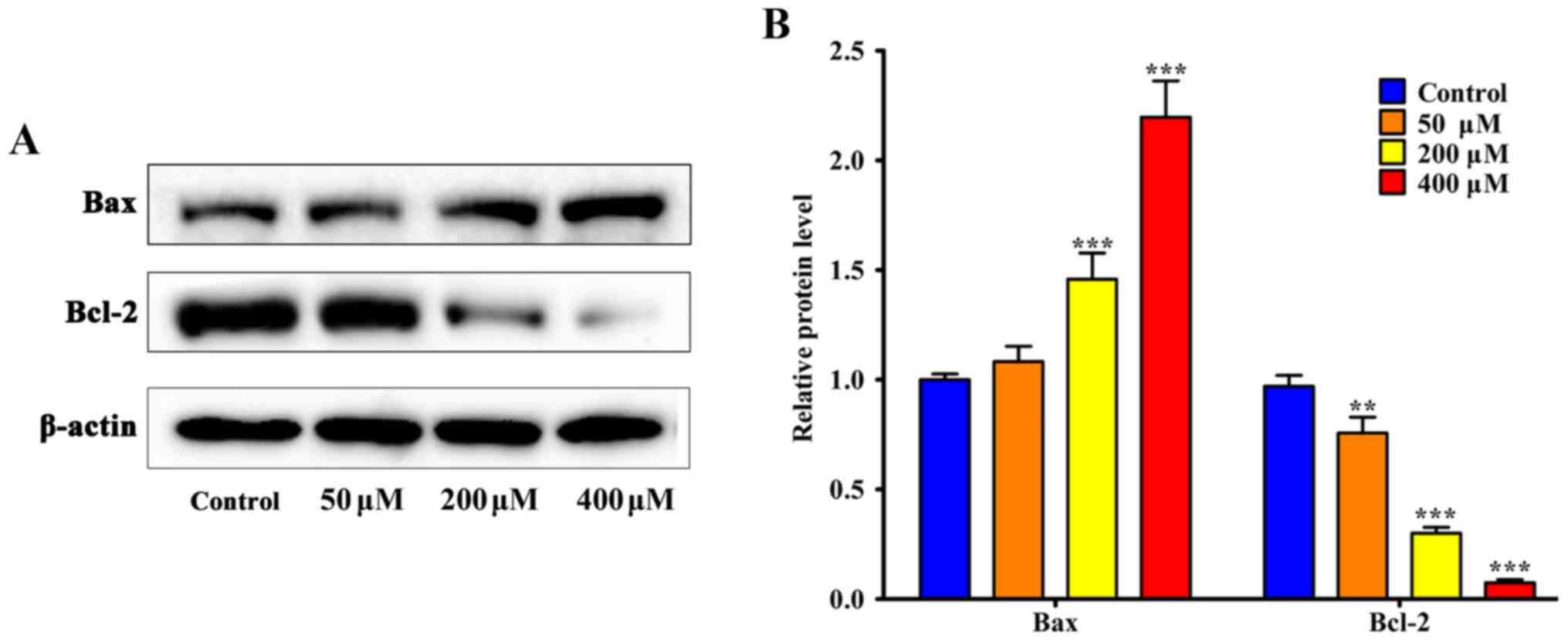
Effect of resveratrol on NF-κB
expression
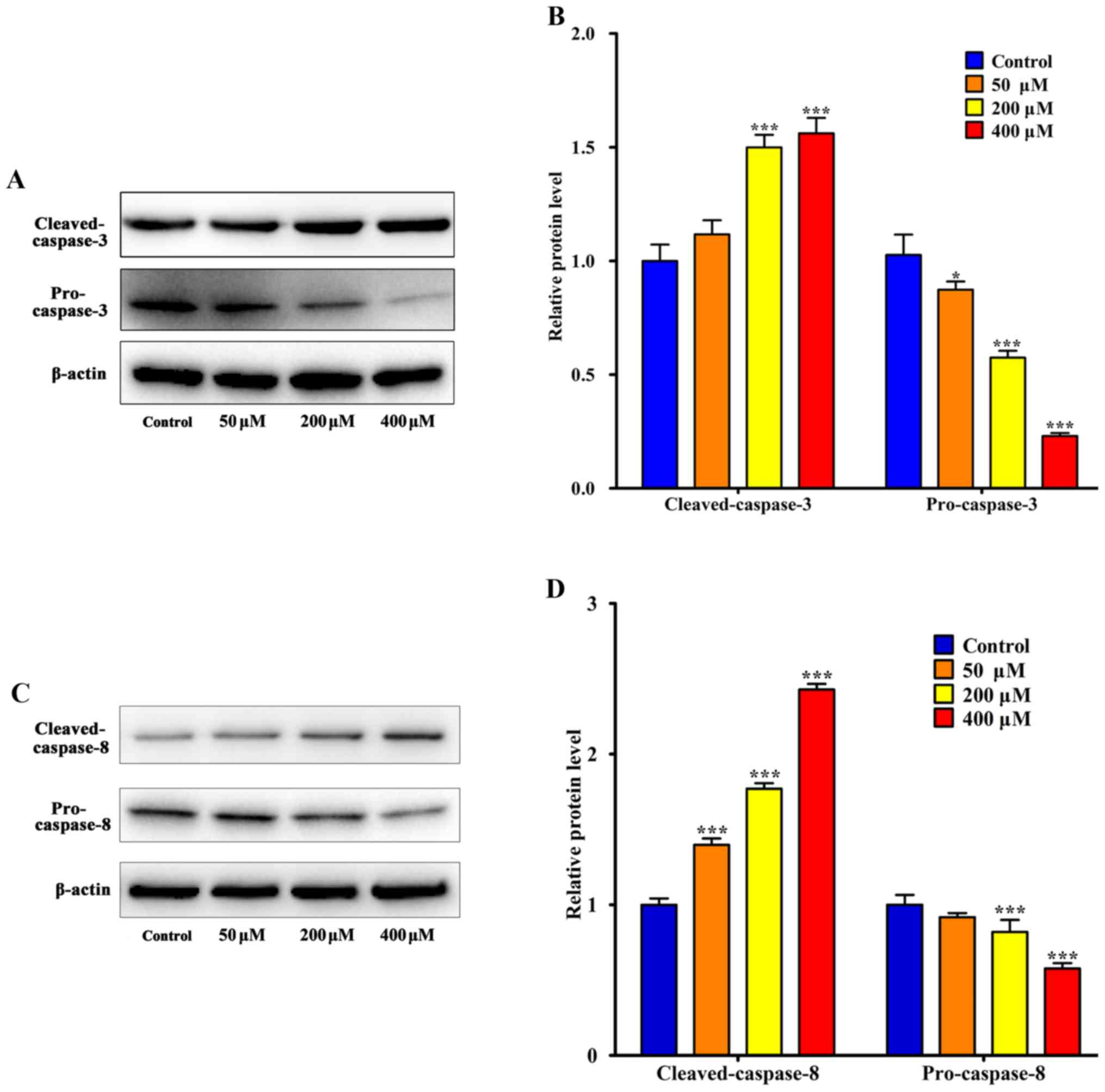
The apoptosis-associated proteins Bax and Bcl-2,
caspase-3 and caspase-8 were detected using western blotting. The
results indicated that 200 µM resveratrol induced significant
upregulation of Bax and cleaved caspase-3 compared with the control
group, respectively (P<0.001; Figs.
5 and 6). Additionally, Bcl-2 was
significantly downregulated following treatment with 50 µM
resveratrol (P<0.01; Fig, 5). As
presented in Fig. 6, SGC-7901 cells
that were treated with 50 µM resveratrol exhibited an increase in
the expression of activated cleaved caspases compared with the
control group. In addition, resveratrol treatment decreased the
levels of pro-caspase-3 and pro-caspase-8. Following resveratrol
treatment, the activation of caspase-3 and caspase-8 increased.
These results suggested that resveratrol-induced cell death is
associated with the death receptor pathway. In addition, western
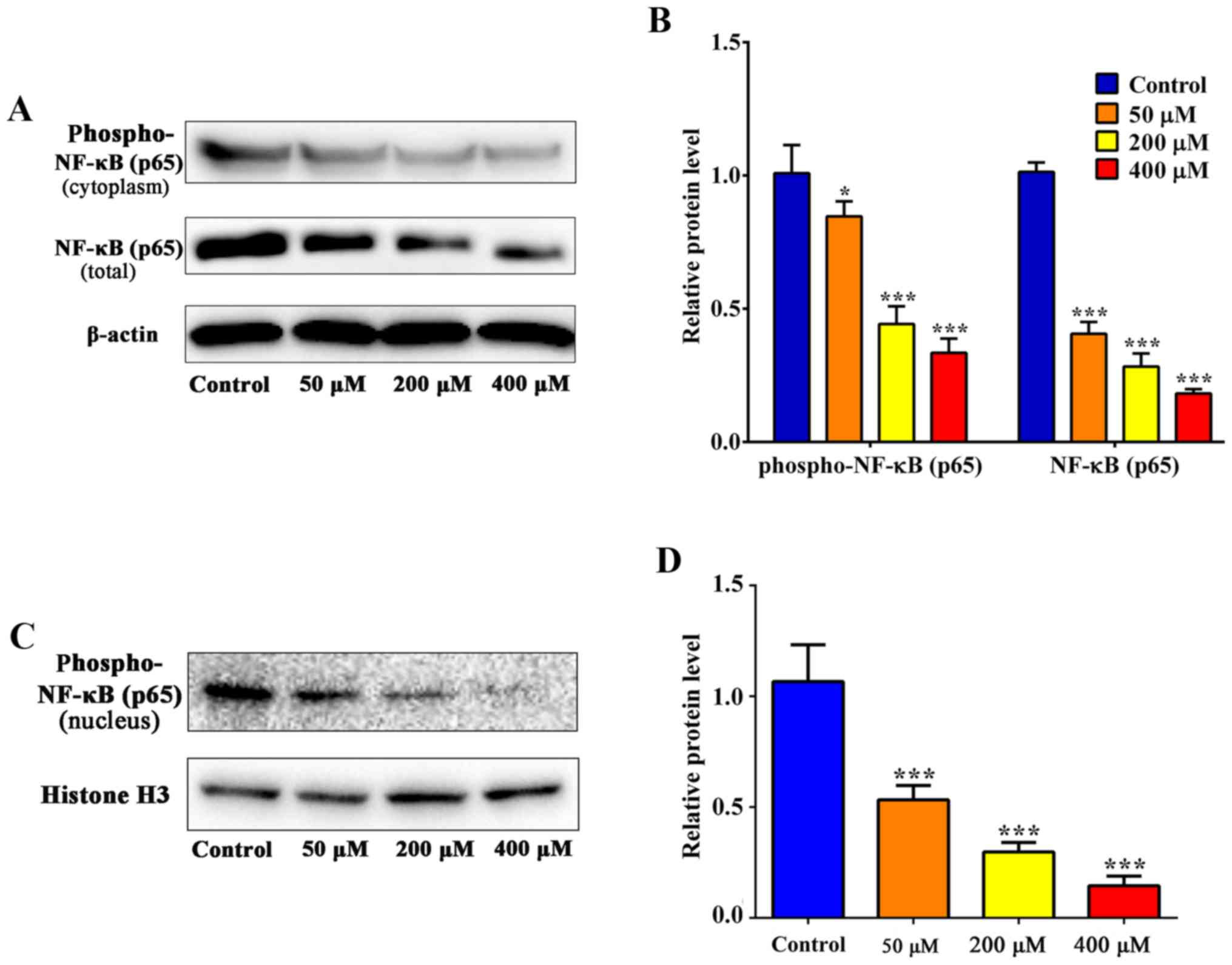
blot analysis was used to determine the levels of NF-κB (p65) and
phospho-NF-κB (p65) (Fig. 7A). The
level of NF-κB (p65) expression was significantly decreased
following treatment with 50 µM resveratrol compared with the
control (P<0.001; Fig. 7B), which
suggests that the inhibition of the NF-κB signaling pathway was
involved in resveratrol-induced apoptosis in SGC-7901 cells. As
presented in Fig. 7B and D,
phospho-NF-κB (p65) expression decreased in the cytoplasm and in
the nucleus.
Discussion
The results of the present study indicated that
resveratrol was able to significantly inhibit the viability and
induce the apoptosis of SGC-7901 cells in a dose- and
time-dependent manner within a certain range, which is consistent
with the results of a previous study on pancreatic cancer (23).
By analyzing the results of the MTT assay, it was
identified that when the concentration of resveratrol was ≥50 µM
(except 200 µM), the inhibition rates of cell viability at 36 h
were not significantly different from those at 48 h at the same
concentration. Additionally, when the concentration of resveratrol
was ≥200 µM, the inhibition rates of cell viability were not
significantly different between 24 and 36 h at the same
concentration. We hypothesize that, once the concentration had
increased to a certain point, prolonging the duration of drug
treatment did not make a difference to the inhibition of cell
viability following resveratrol treatment.
Decreased cell viability is a comprehensive
response, which reflects the functional state of cells in a number
of aspects. Inhibition of cell proliferation, induction of
apoptosis or autophagy, and cytotoxic necrosis all decrease cell
viability. Yu et al (24)
demonstrated that resveratrol decreases cell viability by way of
the induction of apoptosis and G2/M-phase cell cycle
arrest. However, Opipari et al (25) identified that resveratrol induces cell
death in ovarian cancer A2780 cells via a mechanism distinct from
apoptosis. Neither apoptotic pathways associated with Bcl-2 and
Bcl-xL nor activation of caspase-9 were demonstrated to be required
for the resveratrol-induced death of A2780 cells. Furthermore,
decreased cell viability associated with the induction of autophagy
was observed in breast cancer stem-like cells following treatment
with resveratrol (17).
The cell cycle is a basic process common to all
living organisms. The cell cycle can be divided into two major
phases: Mitosis phase (M-phase) and interphase. On the basis of DNA
synthesis, interphase is divided into G1-, S- and
G2-phases. It has been identified that the effect of
resveratrol on the cell cycle is primarily via two mechanisms: i)
Decreasing the proportion of G0/G1-phase
cells; and ii) blocking S-phase cells, namely the G1-S
and S-G2 transitions, thereby inhibiting tumor cell
proliferation (26). In the present
study, cell cycle assay indicated that resveratrol was able to
arrest SGC-7901 cells at S-phase, suggesting that blocking S-phase
cells may be associated with resveratrol-induced apoptosis in
SGC-7901 cells.
Apoptosis, an efficient cell death program, is
primarily mediated via the intrinsic or the extrinsic pathway in
response to different stimuli in various cell types. Dysregulated
apoptosis is a distinguishing feature of human cancer. The
initiation and execution of endogenous and exogenous apoptosis are
regulated by Bcl-2 and caspase family proteins (27,28). It is
well-known that Bcl-2 is an anti-apoptotic protein, and Bax is a
pro-apoptotic protein (29). The
balance of these two types of protein has a key function in
regulating the sensitivity of cells to apoptosis (30). In the present study, the results
indicated that the Bax/Bcl-2 ratio increased with resveratrol
treatment, which suggested that resveratrol may inhibit the
proliferation of gastric cancer cells via downregulating
anti-apoptotic proteins and upregulating pro-apoptotic proteins.
Abnormal caspase expression and activation have been involved in
various types of cancer (31,32). In the present study, western blot
analysis indicated that the levels of cleaved caspase-3 protein in
SGC-7901 cells significantly increased following resveratrol
treatment. By contrast, expression of pro-caspase-3 and
pro-caspase-8 was markedly decreased in the experimental groups
following resveratrol treatment. Furthermore, this resulted in a
higher ratio of cleaved caspase-3/pro-caspase-3 (8). Therefore, increased caspase-3 and
caspase-8 activation in the presence of resveratrol may contribute
to resveratrol-induced apoptosis of SGC-7901 cells.
The NF-κB family of transcription factors is known
for its function in immunity and inflammation (33), and its abnormal upregulation has been
observed in a number of types of cancer (34–37). In
the present study, the expression of NF-κB was negatively
associated with the viability and survival of SGC-7901 cells. The
presence of an NF-κB-binding site in the Bcl-2 promoter has been
identified. Transactivation of transcription by NF-κB from the
Bcl-2 p2 promoter is mediated via the Bcl-2 p2 site 1 (38). In certain cancer cell lines, the
activation of NF-κB is triggered by chemotherapeutic drugs and
ionizing radiation that accompany the activation of the Bcl-2
family of proteins (39). Sun et
al (40) identified that
resveratrol treatment was able to arrest cells at G1-
and S-phases. NF-κB was also downregulated, which resulted in
decreased expression of anti-apoptotic proteins, including Bcl-2,
Bcl-2-like protein 1 and X-linked inhibitor of apoptosis. In
myeloma, caspase-3 activation and loss of mitochondrial
transmembrane potential were observed to be associated with
resveratrol-induced apoptosis (41).
In line with these results, it is possible that NF-κB has a key
function in resveratrol-induced apoptosis in SGC-7901 cells. In the
present study, resveratrol was able to downregulate the expression
and activation of NF-κB in cancer cells, which regulated the
expression of apoptotic-associated proteins and also modulated cell
cycle distribution.
In conclusion, the results of the present study
indicated that resveratrol was able to inhibit the viability and
induce the apoptosis of SGC-7901 cancer cells by inhibiting NF-κB
activation. Therefore, a potential application of resveratrol may
be as an anticancer drug for the treatment of gastric cancer.
Acknowledgements
The authors thank Dr Zhigang Wang and Dr Jianghuan
Hua (School of Basic Medical Sciences, Hubei University of Chinese
Medicine, Wuhan, China), for assisting in the editing of the
manuscript before submission.
Funding
The present study was supported by the Nature
Science Foundation of Hubei Province, China (grant nos. 2013CFB067
and 2013CFB068).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QFY, GZ and XXW conceived and designed the study.
XXW, QL, YDX and BRZ performed the experiments. XXW and GZ wrote
the paper. GZ, QFY, XXW, YDX, BRZ and QL reviewed and edited the
paper. All authors read and approved the paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham SC, Kamangar F, Kim MP, Hammoud
S, Haque R, Maitra A, Montgomery E, Heitmiller RE, Choti MA,
Lillemoe KD, et al: Survival after gastric adenocarcinoma
resection: Eighteen-year experience at a single institution. J
Gastrointest Surg. 9:718–725. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart B and Wild CP: World Cancer Report
2014. Health. 2017.
|
|
4
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nobili S, Lippi D, Witort E, Donnini M,
Bausi L, Mini E and Capaccioli S: Natural compounds for cancer
treatment and prevention. Pharmacol Res. 59:365–378. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers (Basel). 3:1351–1371.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
James MI, Iwuji C, Irving G, Karmokar A,
Higgins JA, Griffin-Teal N, Thomas A, Greaves P, Cai H, Patel SR,
et al: Curcumin inhibits cancer stem cell phenotypes in ex vivo
models of colorectal liver metastases, and is clinically safe and
tolerable in combination with FOLFOX chemotherapy. Cancer Lett.
364:135–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He B, Wei W, Liu J, Xu Y and Zhao G:
Synergistic anticancer effect of curcumin and chemotherapy regimen
FP in human gastric cancer MGC-803 cells. Oncol Lett. 14:3387–3394.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tran KQ, Tin AS and Firestone GL:
Artemisinin triggers a G1 cell cycle arrest of human Ishikawa
endometrial cancer cells and inhibits Cyclin Dependent Kinase-4
promoter activity and expression by disrupting NF-kB
transcriptional signaling. Anticancer Drugs. 25:270–281. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Novelle MG, Wahl D, Diéguez C, Bernier M
and de Cabo R: Resveratrol supplementation: Where are we now and
where should we go? Ageing Res Rev. 21:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du RH, Dai T, Cao WJ, Lu M, Ding JH and Hu
G: Kir6.2-containing ATP-sensitive K(+) channel is required for
cardioprotection of resveratrol in mice. Cardiovasc Diabetol.
13:352014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inglés M, Gambini J, Miguel MG,
Bonet-Costa V, Abdelaziz KM, El Alami M, Viña J and Borrás C: PTEN
mediates the antioxidant effect of resveratrol at nutritionally
relevant concentrations. Biomed Res Int. 2014:5808522014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Labinskyy N, Csiszar A, Veress G, Stef G,
Pacher P, Oroszi G, Wu J and Ungvari Z: Vascular dysfunction in
aging: Potential effects of resveratrol, an anti-inflammatory
phytoestrogen. Curr Med Chem. 13:989–996. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valenzano DR, Terzibasi E, Genade T,
Cattaneo A, Domenici L and Cellerino A: Resveratrol prolongs
lifespan and retards the onset of age-related markers in a
short-lived vertebrate. Curr Biol. 16:296–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu Y, Chang H, Peng X, Bai Q, Yi L, Zhou
Y, Zhu J and Mi M: Resveratrol inhibits breast cancer stem-like
cells and induces autophagy via suppressing Wnt/β-catenin signaling
pathway. PLoS One. 9:e1025352014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan SL, Wei YQ, Wang XJ, Xiao F, Li SF
and Zhang J: Growth inhibition and apoptosis induction of
tanshinone II-A on human hepatocellular carcinoma cells. World J
Gastroenterol. 10:2024–2028. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arora S, Jain J, Rajwade J and Paknikar K:
Interactions of silver nanoparticles with primary mouse fibroblasts
and liver cells. Toxicol Appl Pharmacol. 236:310–318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Zhao G, Wang F, Gao F, Luo H, Wang
Y, Du Y, Chen X, Xue C, Dong Z and Song G: Upregulation of miR-513b
inhibits cell proliferation, migration, and promotes apoptosis by
targeting high mobility group-box 3 protein in gastric cancer.
Tumor Biol. 35:11081–11089. 2014. View Article : Google Scholar
|
|
21
|
Guo H, Xu YM, Ye ZQ, Yu JH and Hu XY:
Curcumin induces cell cycle arrest and apoptosis of prostate cancer
cells by regulating the expression of IkappaBalpha, c-Jun and
androgen receptor. Pharmazie. 68:431–434. 2013.PubMed/NCBI
|
|
22
|
Zhu P, Zhang J, Zhu J, Shi J, Zhu Q and
Gao Y: MiR-429 induces gastric carcinoma cell apoptosis through
Bcl-2. Cell Physiol Biochem. 37:1572–1580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding XZ and Adrian TE: Resveratrol
inhibits proliferation and induces apoptosis in human pancreatic
cancer cells. Pancreas. 25:e71–e76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu XD, Yang JL, Zhang WL and Liu DX:
Resveratrol inhibits oral squamous cell carcinoma through induction
of apoptosis and G2/M phase cell cycle arrest. Tumour Biol.
37:2871–2877. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Opipari AW Jr, Tan L, Boitano AE, Sorenson
DR, Aurora A and Liu JR: Resveratrol-induced autophagocytosis in
ovarian cancer cells. Cancer Res. 64:696–703. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim AL, Zhu Y, Zhu H, Han L, Kopelovich L,
Bickers DR and Athar M: Resveratrol inhibits proliferation of human
epidermoid carcinoma A431 cells by modulating MEK1 and AP-1
signalling pathways. Exp Dermatol. 15:538–546. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the nomenclature committee on cell
death 2012. Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer Inst. 98:1777–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: Apoptosomes or mitochondria? Genes to cells.
3:697–707. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Estrov Z, Thall PF, Talpaz M, Estey EH,
Kantarjian HM, Andreeff M, Harris D, Van Q, Walterscheid M and
Kornblau SM: Caspase 2 and caspase 3 protein levels as predictors
of survival in acute myelogenous leukemia. Blood. 92:3090–3097.
1998.PubMed/NCBI
|
|
32
|
Hopkins-Donaldson S, Bodmer JL, Bourloud
KB, Brognara CB, Tschopp J and Gross N: Loss of caspase-8
expression in highly malignant human neuroblastoma cells correlates
with resistance to tumor necrosis factor-related apoptosis-inducing
ligand-induced apoptosis. Cancer Res. 60:4315–4319. 2000.PubMed/NCBI
|
|
33
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gasparini C, Celeghini C, Monasta L and
Zauli G: NF-κB pathways in hematological malignancies. Cell Mol
Life Sc. 71:2083–2102. 2014. View Article : Google Scholar
|
|
35
|
Pacifico F and Leonardi A: NF-kappaB in
solid tumors. Biochem Pharmacol. 72:1142–1152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X,
Lin L, Yao H, Su F, Li D, et al: A Cytoplasmic NF-κB interacting
long noncoding RNA Blocks IκB Phosphorylation and suppresses breast
cancer metastasis. Cancer Cell. 27:370–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Catz SD and Johnson JL: Transcriptional
regulation of bcl-2 by nuclear factor kappa B and its significance
in prostate cancer. Oncogene. 20:7342–7351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li F and Sethi G: Targeting transcription
factor NF-kappaB to overcome chemoresistance and radioresistance in
cancer therapy. Biochim Biophys Acta. 1805:167–180. 2010.PubMed/NCBI
|
|
40
|
Sun C, Hu Y, Liu X, Wu T, Wang Y, He W and
Wei W: Resveratrol downregulates the constitutional activation of
nuclear factor-kappaB in multiple myeloma cells, leading to
suppression of proliferation and invasion, arrest of cell cycle,
and induction of apoptosis. Cancer Genet Cytogenet. 165:9–19. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shimizu T, Nakazato T, Xian MJ, Sagawa M,
Ikeda Y and Kizaki M: Resveratrol induces apoptosis of human
malignant B cells by activation of caspase-3 and p38 MAP kinase
pathways. Biochem Pharmacol. 71:742–750. 2006. View Article : Google Scholar : PubMed/NCBI
|