Introduction
Lung cancer remains the leading cause of
cancer-related mortality worldwide with estimated 160,000 death
cases/year, and most frequently in developing countries (1). Non-small cell lung cancer (NSCLC)
accounts for 85% of the total lung cancer burden and includes the
pathologically distinct sub-types: Adenocarcinoma, squamous cell
carcinoma and large cell carcinoma (2). Currently, platinum combined with
taxanes, vinorelbine, gemcitabine, or pemetrexed are the standard
care of advanced NSCLC (3). Despite
advances of techniques in diagnosis, staging and surgery and new
protocols in chemotherapy and radiotherapy, the overall five-year
survival rate of NSCLC is still only about 15% (4). NSCLC cells with strong metastasis
capability is capable of evading the regulation in division and
apoptosis, which directly leads to treatment failure. Therefore,
finding effective therapeutic approaches to inhibit the unlimited
proliferation and metastasis of NSCLC cells are expected to reduce
mortality of NSCLC.
Bolbostemma paniculatum (Maxim) Franquet
(Cucurbitaceae) is a traditional Chinese medicinal plant
widely used in China for thousands of years for its extensively
anti-inflammatory, antiviral and immunosuppressive effects
(5). Tubeimoside-1 (TBMS1) is a
triterpenoid saponin isolated from the tuber of Bolbostemma
paniculatum (Maxim) Franquet (6),
which sugar chains are connected with 3-hydroxy-3-methylglutaric
acid to form a unique macro cyclic structure (7). Both in vivo and in vitro
studies reported that TBMS1 exerted potent anti-tumor activity with
low toxicity. TBMS1 could suppress proliferation and promote
apoptosis in various cancers, including lung cancer (8,9), gastric
cancer, liver cancer, nasopharyngeal carcinoma and glioma cancer
(5,10–12). TBMS1
also inhibited the migration and invasion of colorectal cancer and
breast cancer cells (7,13). Apart from that, Gu et al
pointed out that TBMS1 suppressed tumor angiogenesis by stimulation
of proteasomal VEGFR2 and Tie2 degradation in a NSCLC xenograft
model (6). However, neither the roles
of TBMS1 in the migration and invasion of NSCLC cells nor the
potential mechanisms of the anti-tumor effects of TBMS1 has been
substantiated.
In the present study, NCI-H1299 cells were incubated
with 10 µmol/l TBMS1 for different h to evaluate the proliferation
and confirm the perfect time, then flow cytometry, wound healing
and Transwell invasion assays were employed to explore the effect
of TBMS1 on the apoptosis, migration and invasion of NCI-H1299
cells. Further 14 cases of NSCLC tissues and 14 cases of normal
adjacent tissues were collected to compare the expression of
miR-126-5p in NCI-H1299 cells and tissues with or without TBMS1
administration respectively, then miR-126-5p targeted downstream
pathway was detected. We found that the cytostatic and
anti-metastatic effects of TBMS1 was associated with overexpression
of miR-126-5p repressed VEGF-A/VEGFR2/ERK pathway.
Materials and methods
Cell culture
Human non small cell lung cancer cell line NCI-H1299
was obtained from Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences. Cells were cultured in Roswell Park
Memorial Institute-1640 (RPMI-1640; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
and streptomycin/penicillin (100 U/ml) at 37°C in an atmosphere of
5% CO2. The suspension was decanted and replaced with
fresh medium every 2 to 3 days. When reached 80% confluences,
NCI-H1299 cells were digested for subsequent experiments.
Drug treatment
TBMS1 (≥97%; PureOne Biotechnology, Shanghai, China)
was dissolved in ddH2O, and its structure is shown in
http://www.pureonebio.com/products/tubeimoside-a-102040-03-9-p588.html.
NCI-H1299 cells were exposed to TBMS1 of an ascending concentration
range (0, 2.5, 5, 10, 25, 50 µM) for 48 h followed by CCK-8 assay
to find the optimum concentration, and incubated with 10 µM TBMS1
for gradient increased h (0, 12, 24, 48 and 72 h) to find the
optimum time. For other experiments, NCI-H1299 cells were
pre-incubated with 10 µmol/l TBMS1 for 48 h. The untreated cells
and 8 µΜ 5-Fluorouracil (5-FU) treated NCI-H1299 cells were
experimented in parallel as positive control.
Patients
We recruited tumor tissues from 14 patients who
underwent thoracoscopic lobectomy surgery for non small cell lung
cancer between May 2013 and January 2016 at The Third Affiliated
Hospital of Qiqihar Medical University, Heilongjiang, China, and 14
paraneoplastic lung tissue samples (>5 cm away from tumors) were
taken as healthy control. All tissue specimens were obtained with
permission from the Medical Ethics Committee of The Third
Affiliated Hospital of Qiqihar Medical University. The median age
of all patients was 66.57 years (range, 43–78 years). None of the
patients received chemotherapy, radiotherapy or immunotherapy
before surgery. The 14 tumor samples were staged according to the
2002 tumor node metastasis (TNM) classification with 0 as
non-invasive (pTa), 7 as invasive pT1 and 8 as invasive pT2.
Cell Counting Kit-8 (CCK-8) assay
NCI-H1299 cells were planted in 96-well plates with
a density of 1×104 per well and cultured to 80%
confluence, followed by incubating with various concentrations of
TBMS1 for indicated time, with five replicates for each testing
point including the negative control, positive control and blank
wells. Thereafter, the cell viability was measured by Enhanced Cell
Counting Kit-8 (Beyotime Institute of Biotechnology, Haimen, China)
completely following the manufacturer's directions (14–16).
Optical density (OD) values were evaluated at 450 nm by a
microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Hoechst staining
NCI-H1299 cells were inoculated onto coverslips at a
density of 5 ×104 per well in 12-well plates. When
reached to 80% confluences, the suspension was decanted and cells
were treated with 10 µM TBMS1 for 48 h. Hoechst staining assay was
performed with the Hoechst Staining kit (Beyotime Institute of
Biotechnology) following the manufacturer instructions. Briefly,
the cells on coverslips were fixed for 20 min using indicated
stationary liquid and stained at room temperature for 5 min using a
total of 0.5 ml Hoechst 33258 solution with dropwise addition. Then
the coverslips were mounted inversely onto slides with indicated
anti-fluorescein quencher and observed under a fluorescence
microscope (Olympus Corporation, Tokyo, Japan).
Flow cytometric analysis of cell
apoptosis
According to the instructions of the Annexin
V-FITC/PI apoptosis detection kit (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China), the collected 5×105 cells were
resuspended in 500 µl binding buffer, mixed sequentially with 5 µl
Annexin V-FITC and 5 µl PI and incubated for 15 min in the dark at
room temperature. Cell apoptosis was assessed immediately with flow
cytometry and analyzed with CellQuest software (BD Biosciences,
Franklin Lakes, NJ, USA).
Wound healing assay
Cells were inoculated in 6-well plates until 80%
confluence. A wound was gently created on each cell monolayer by a
200 µl pipette tip and rinsed with a FBS-free RPMI-1640 medium to
remove detached cells. Then the cells were grown in FBS free
PMI-1640 medium supplemented with or without drug treatment for 48
h, and migrating cells were imaged under an inverted microscope.
The migration rate was the ratio of the migrated distance to the
initial distance.
Transwell invasion assay
The matrigel-based invasion assay was carried out
using a 6-well transwell system (Corning Incorporated, Corning, NY,
USA) with a matrigel (BD Biosciences) pre-coated polycarbonate
membrane at the upper chamber. The collected cells were resuspended
in FBS-free RPMI-1640 medium with or without indicated drug and
plated in the upper chamber with a density of 2×104 per
well. 700 µl RPMI-1640 containing 10% FBS was added into the lower
chamber. After 48 h of incubation, the non-invading cells on the
upper surface of the membrane were removed with cotton swabs. The
invading cells at the undersurface of the membrane were fixed with
4% paraformaldehyde for 20 min and stained by crystal violet for
5–10 min. The invading cells in each group were calculated in five
randomly selected fields under an inverted microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-PCR)
Total RNA from tissue samples or NCI-H1299 cells was
extracted by an RNA extraction kit (Tiangen Biotech Co., Ltd.,
Beijing, China) according to the manufacturer instructions and was
reverse-transcribed into cDNA. 2 µl cDNA was amplified with 10 µl
Bestar® SybrGreen qPCR masterMix (DBI®
Bioscience, Ludwigshafen, Germany), 1 µl primers and 7 µl
ddH2O in an Mx3000P (Agilent Technologies, Inc., Santa
Clara, CA, USA) with the following cycling profile: Initial
denaturation at 95°C for 2 min, 40 cycles consisting of 94°C for 20
sec, 58°C for 20 sec, and 72°C for 20 sec. Primer sequences were:
miR-126-5p, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCGCGTA-3′
(sense) and 5′-ACACTCCAGCTGGGCATTATTACTTTTGGTA-3′ (antisense);
miR-29, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTAACCG-3′ (sense)
and 5′-ACACTCCAGCTGGGTAGCACCATCTGAAATC-3′ (antisense); miR-128,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCTCAG-3′ (sense) and
5′-ACACTCCAGCTGGGCGGGGCCGTAGCACTGT-3′ (antisense); miR-206,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCACAC-3′ (sense) and
5′-ACACTCCAGCTGGGTGGAATGTAAGGAAGTG-3′ (antisense); VEGFR-A,
5′-AGGGCAGAATCATCACGAAGT-3′ (sense) and 5′-AGGGTCTCGATTGGATGGCA-3′
(antisense); VEGFR-2, 5′-ATAGAAGGTGCCCAGGAAAAG-3′ (sense) and
5′-GTCTTCAGTTCCCCTCCATTG-3′ (antisense); MEK1,
5′-GGGCTTCTATGGTGCGTTCTA-3′ (sense) and 5′-CCCACGGGAGTTGACTAGGAT-3′
(antisense); ERK, 5′-TCTGGAGCAGTATTACGACCC-3′ (sense) and
5′-CTGGCTGGAATCTAGCAGTCT-3′ (antisense); β-actin
5′-ATCGTGCGTGACATTAAGGAGAAG-3′ (sense) and
5′-AGGAAGGAAGGCTGGAAGAGTG-3′ (antisense); U6,
5′-CTCGCTTCGGCAGCACA-3′ (sense) and 5′-AACGCTTCACGAATTTGCGT-3′
(antisense). Relative expression was obtained by 2−ΔΔCT
method. β-actin and U6 were served as internal controls for cells
and tissues respectively.
Western blot analysis
Total proteins in NCI-H1299 cells were lysed by
NP-40 lysate (Beyotime Institute of Biotechnology) containing 1%
phenylmethanesulfonyl fluoride (PMSF). The protein concentration
was determined using a bicinchoninic acid (BCA) protein assay kit
(Beyotime Institute of Biotechnology). 20 µg proteins in each
sample was loaded and separated by SDS-polyacrylamide gel
electrophoresis (PAGE) and transferred onto polyvinylidene fluoride
(PVDF) membranes (EMD Millipore, Billerica, MA, USA). The blots
were then blocked with 5% non-fat milk overnight at 4°C and
incubated at 4°C overnight with the specific primary antibodies as
follows: anti-VEGFR-2, anti-ERK1/2, anti-p-ERK1
(pT202/pY204)+p-ERK2 (pT185/pY187) (all 1:1,500 diluted; Abcam,
Cambridge, MA, USA); anti-MEK1, anti-p-MEK1 (pS298) (both 1:1,000
diluted; Abcam). After washed with TBST for three times, the
membranes were incubated with a secondary goat anti-rabbit IgG-HRP
antibody (1:20,000 diluted; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) at 37°C for 40 min. An enhanced
chemiluminescence (ECL; EMD Millipore) detection method was
employed to visualize the target bands, and relative protein
intensities were analyzed by Gel-Pro-Analyzer software (Media
Cybernetics, Rockville, MD, USA). GAPDH was used as an internal
control.
Immunofluorescence assay
NCI-H1299 cells were grown on coverslips with
appropriate treatment and fixed with 4% formaldehyde for 15 min
followed by permeabilized with 0.1% Triton X-100 (Ameresco, Inc.,
Framingham, MA, USA) for 30 min. After washed three times with PBS,
the coverslips were blocked with goat serum (Solarbio, Beijing,
China) for 1 h at room temperate. Subsequently, cells were stained
with primary antibodies against VEGF-A (1:200 diluted; Abcam) at
4°C overnight followed by incubated with Cy3-labeled goat
anti-rabbit IgG secondary antibody (1:200; Beyotime Institute of
Biotechnology) for 1 h at room temperature. Unbound antibodies at
each step were washed three times by PBS. Thereafter, cells were
stained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min and
finally rinsed with PBS. The coverslips were mounted inversely onto
slides with neutral gum and observed under a fluorescence
microscope.
Statistical analysis
Statistical analysis was carried out by GraphPad
Prism v5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
All values except for the clinical data are reported as mean ±
standard deviation (SD). Differences between groups in RT-PCR
detection of clinical data were calculated with unpaired Student's
t-test. Other differences comparison between groups were analyzed
with one-way analysis of variance followed by Tukey's Bonferroni
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
TBMS1 suppresses proliferation and
enhances apoptosis of NCI-H1299 cells
To explore the effect of TBMS1 on the survival of
NCI-H1299 cells, we conducted CCK-8, Hoechst staining and flow
cytometry to detect the proliferation and apoptosis of NCI-H1299
cells with TBMS1 incubation for different h. We found that cell
viability exhibited dose-dependent inhibitions after TBMS1
administration for 48 h, and the inhibitory effect was sharply
increased at 10 µM TBMS1 treatment, so we chose 10 µM TBMS1 for
subsequent experiments (P<0.001; Fig.
1A). Moreover, the proliferation of NCI-H1299 cells was
inhibited significantly since 10 µM TBMS1 treating for 24 h, and
the growth inhibitory rate elevated to the peak between the points
of 24 and 48 h treatment (P<0.001; Fig. 1B), the inhibition effect at 72 h was
not as prominent as at 48 h (P<0.01). Hence, we chose 48 h
treatment time for subsequent experiments. As shown in Fig. 1C, cells were regular with uniform
chromatin in control, but nuclear condensation, fragmentation and
apoptotic bodies were appeared in a portion of NCI-H1299 cells
administrated with TBMS1 or 5-FU. From flow cytometry assay, the
total apoptosis rate was stimulated by 3.78 folds in TBMS1-treated
cells compare with control (P<0.001; Fig. 1D). The above results demonstrated that
TBMS1 suppresses proliferation and enhances apoptosis of NCI-H1299
cells.
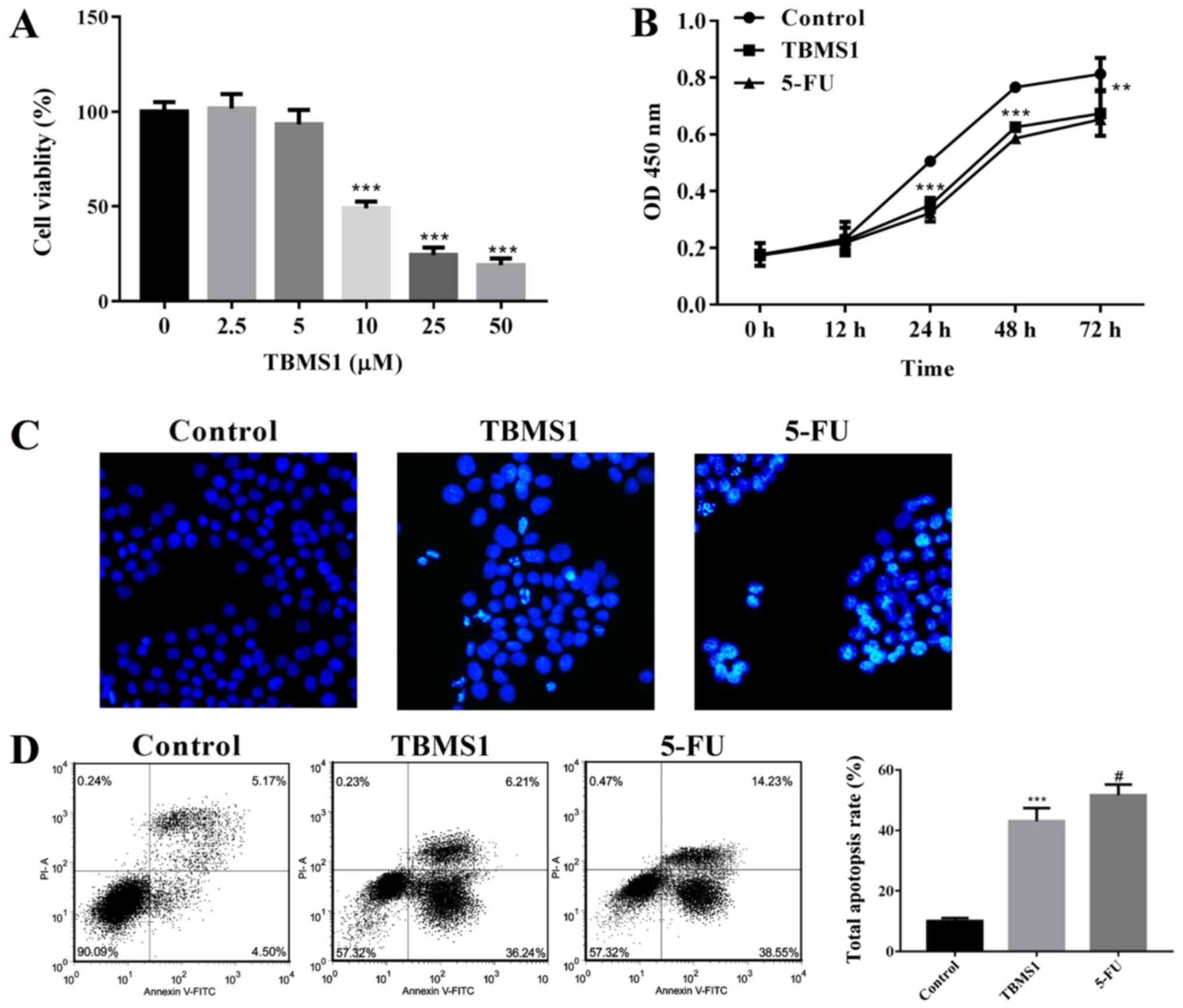 | Figure 1.TBMS1 suppresses proliferation and
enhances apoptosis of NCI-H1299 cells (A) NCI-H1299 Cells were
seeded in 96-well plates and exposed to increased concentrations of
TBMS1 (0, 2.5, 5, 10, 25, 50 µM) for 48 h, followed by CCK-8 assay.
(B) cells were seeded in 96-well plates and subjected to 10 µmol/l
TBMS1 at indicated intervals from 0 up to 72 h, followed by CCK-8
assay. (C) Cells were planted on coverslips and incubated with 10
µmol/l TBMS1 for 48 h, Hoechst staining was employed to detect cell
apoptosis. Representative examples of images are shown. Apoptotic
cells appeared to be bright white. Magnification, ×200. (D) Cell
apoptosis was examined by Annexin V-FITC and propidium iodide
stained flow cytometry. Cells are characterized as healthy cells
(bottom left quadrant), early apoptotic cells (bottom right
quadrant), necrotic cells (top left quadrant) and late apoptotic
cells (top right quadrant), and the total apoptosis rate is
calculated on the right. Above experiments were performed for three
times. Non-TBMS1 treated control was used as negative control, and
5-FU treated cells was served as positive control. Data are
expressed as mean ± SD. Compared with non-TBMS1 treated control,
**P<0.01, and ***P<0.001; compared with 5-FU treated cells,
#P<0.05. TBMS1, Tubeimoside-1. |
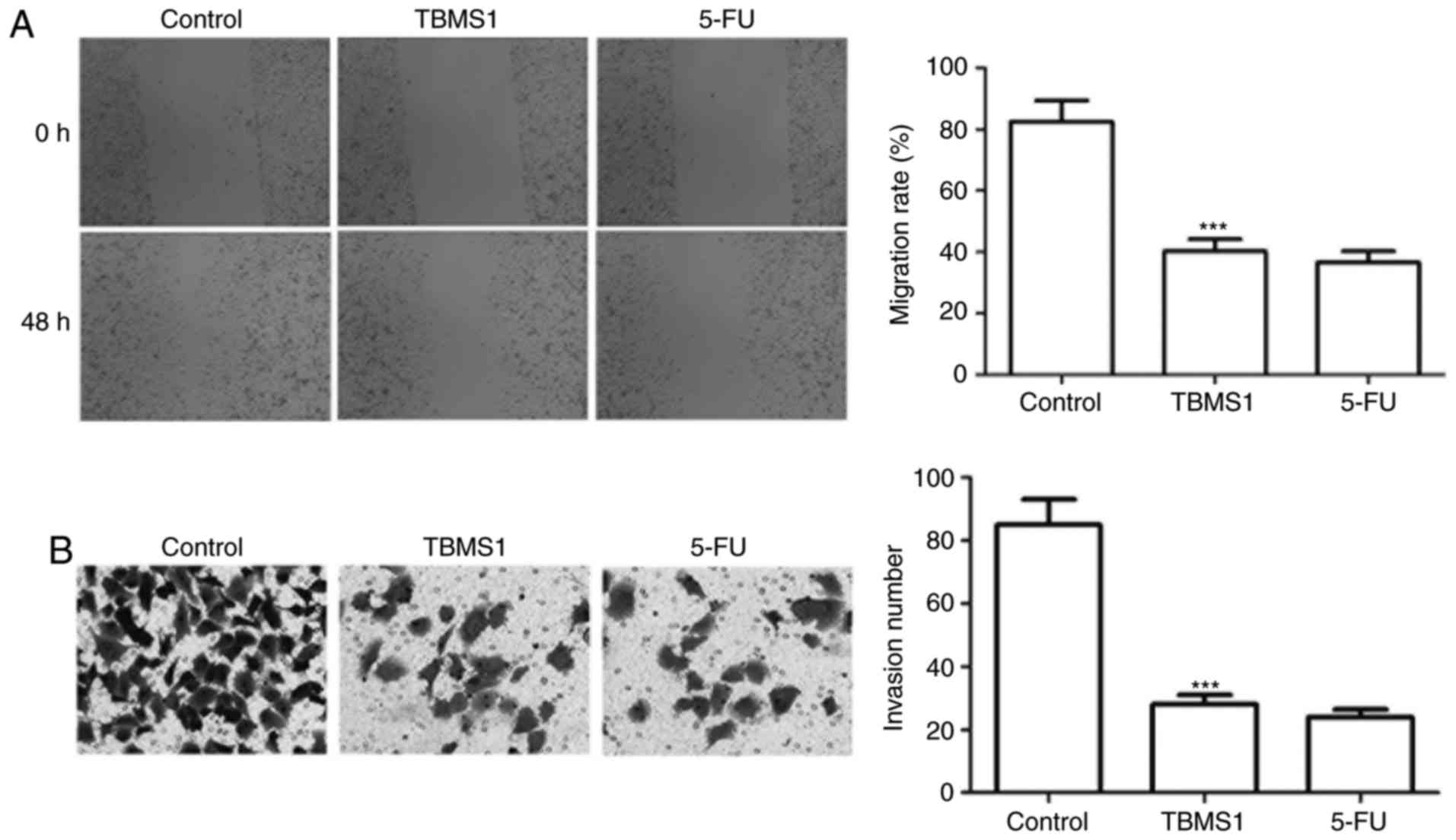
TBMS1 inhibits the migration and
invasion of NCI-H1299 cells
Wound healing and Transwell invasion assay were
employed to address the effect of TBMS1 on the migration and
invasion of NCI-H1299 cells. The migration rate of NCI-H1299 cells
with TBMS1 treatment was declined by 2.05 folds than control
(P<0.001; Fig. 2A), and the
numbers of invading cells was 28.80±3.40, which was remarkably
decreased compared with control cells (85.76±8.23) (P<0.001;
Fig. 2B), indicating that TBMS1
inhibits the migration and invasion of NCI-H1299 cells.
TBMS1 increases the expression of
miR-126-5p
According to Ricciuti et al (17), we chose miR-29, miR-206, miR-126-5p
and miR-128, which are all downregulated in NSCLC and play roles in
inhibiting cell growth, migration and invasion, to compare their
expressions with or without TBMS1 administration in NSCLC tissues
and NCI-H1299 cells. First, we collected 14 cases of pathological
specimens from patients with NSCLC and 14 cases of normal adjacent
tissues for RT-PCR detection. We found that the expression level of
miR-29, miR-206, miR-126-5p and miR-128 were all notably reduced in
NSCLC tissues, while the downregulated expressions of miR-29 and
miR-126-5p were particularly significant (P<0.05; Fig. 3A-D). Next, although miR-29 and
miR-126-5p were both apparently upregulated in NCI-H1299 cells upon
TBMS1 treatment for 48 h compared with control, TBMS1 treatment,
the increased level of miR-126-5p in NCI-H1299 cells following
TBMS1 treatment was much higher than 5-FU treatment (P<0.001;
Fig. 3E-H). So we selected miR-126-5p
for subsequent exprements. These results demonstrated that TBMS1
could increase the expression of miR-126-5p in NCI-H1299 cells.
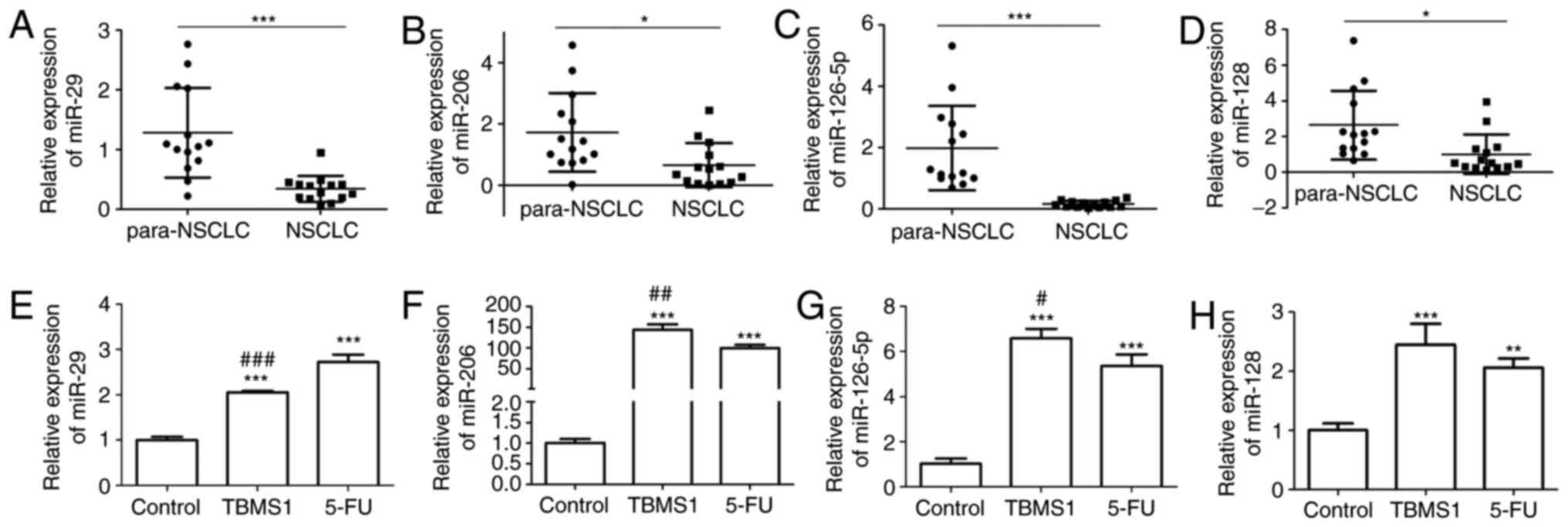 | Figure 3.TBMS1 increases the expression of
miR-126-5p RT-PCR analysis of miR-29 (A), miR-206 (B), miR-126-5p
(C) and miR-128 (D) expression levels in NSCLC tissues and
paraneoplastic tissues. U6 was served as an internal control,
compared with para-NSCLC, *P<0.05 and ***P<0.001. RT-PCR
analysis of miR-29 (E), miR-206 (F), miR-126-5p (G) and miR-128 (H)
expression levels in NCI-H1299 cells with or without indicated drug
incubation for 48 h, which data are presented as mean ± SD, and
β-actin was used as an internal control, compared with control,
**P<0.01, ***P<0.001; compared with 5-FU,
#P<0.05, ##P<0.01 and
###P<0.001. The above experiments were repeated three
times. TBMS1, Tubeimoside-1; NSCLC, non-small cell lung cancer. |
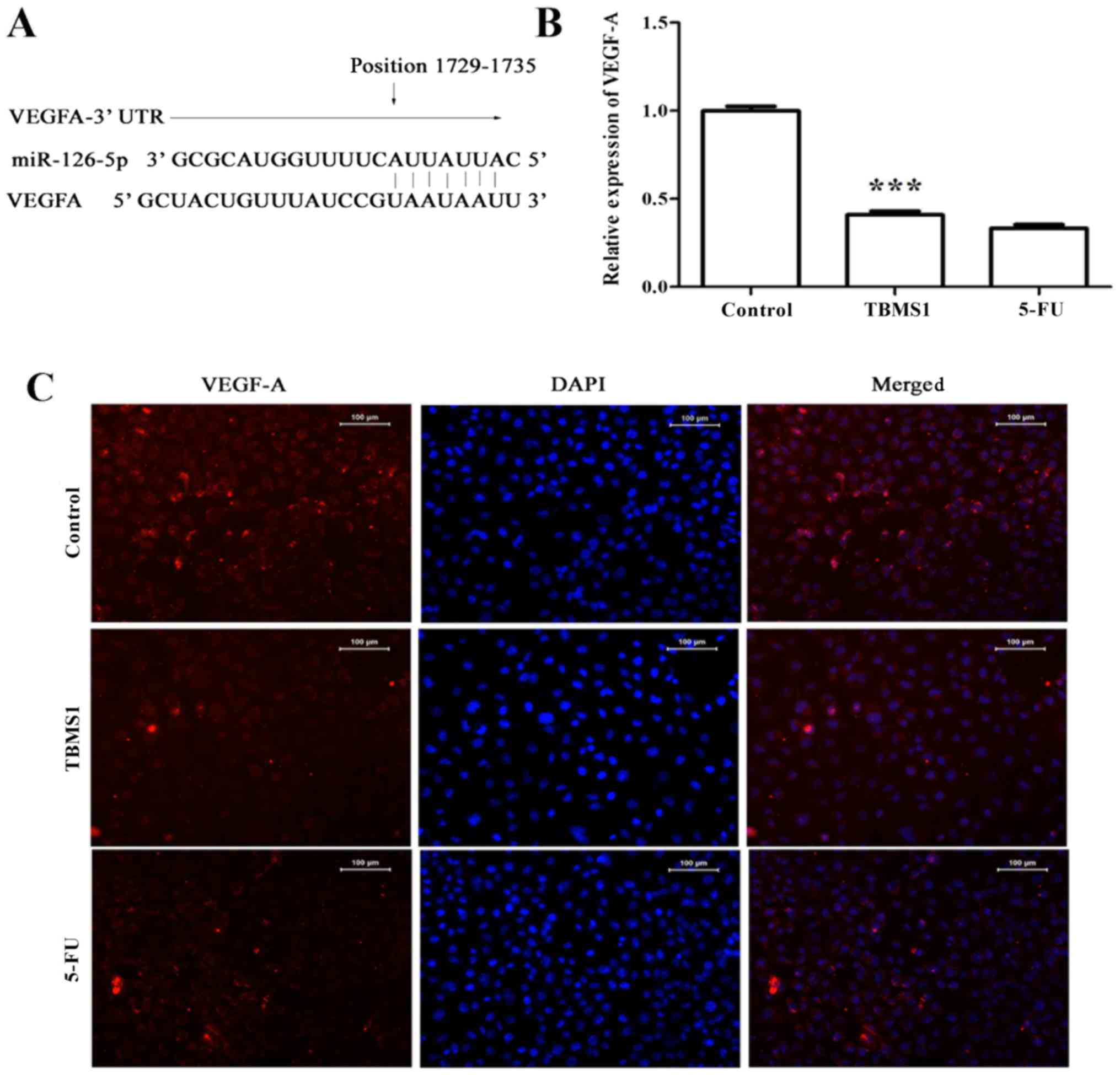
TBMS1 downregulates the expression of
miR-126-5p-targeted VEGF-A
It is a well establish fact that VEGF-A is one of
the target genes of miR-126-5p (Fig.
4A), so we carried out RT-PCR and immunofluorescence assay to
evaluate the expression of VEGF-A in TBMS1-treated NCI-H1299 cells.
Compared with non-TBMS1 treated control cells, the expression of
VEGF-A was decreased significantly at both mRNA and protein levels
(Fig. 4B and C), suggesting that
TBMS1 induced overexpressing miR-126-5p directly downregulates
VEGF-A level in NCI-H1299 cells.
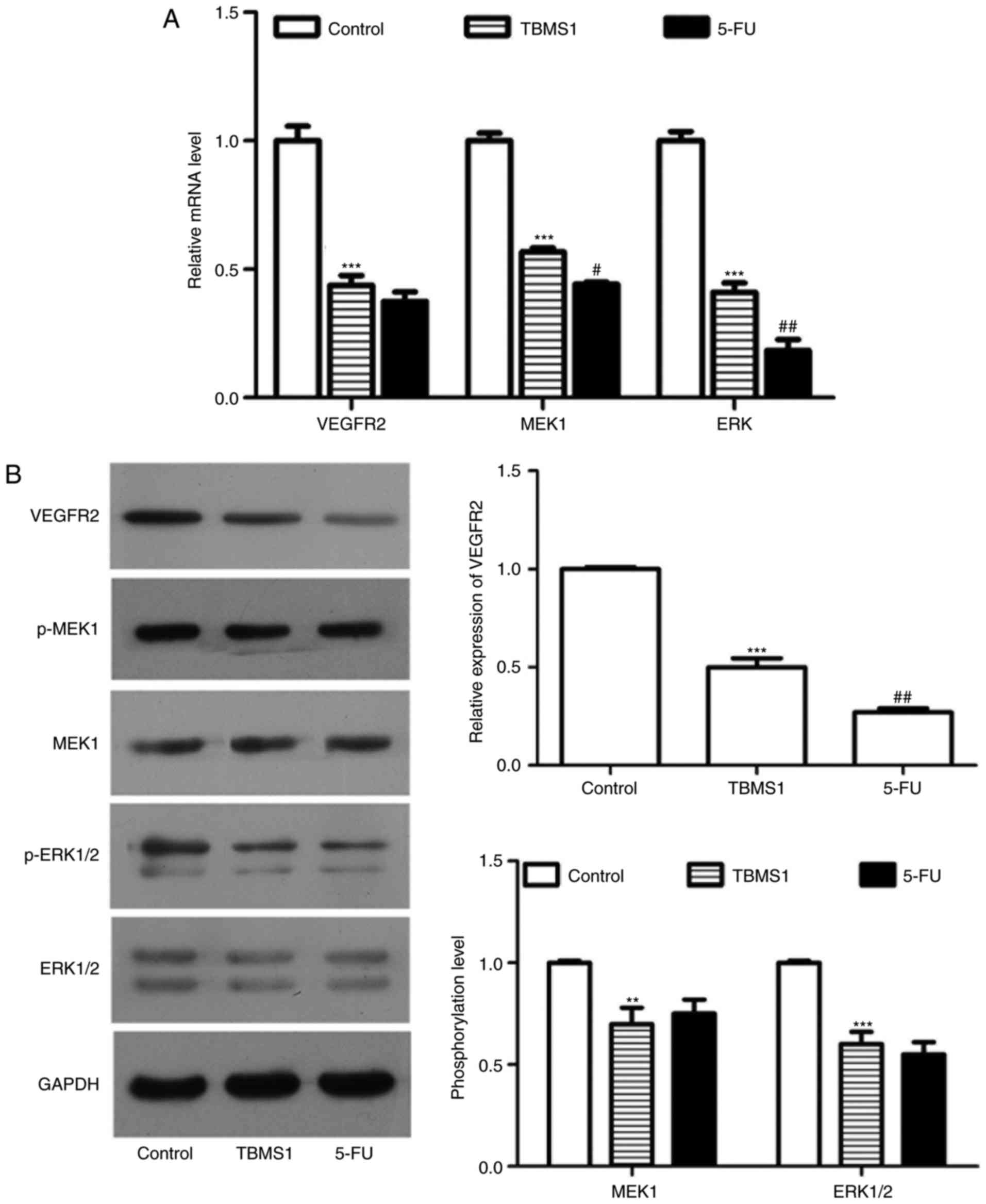
TBMS1 inactivates VEGFR2 mediated ERK
pathway
To identify the alteration of VEGFR-2 mediated ERK
pathway, we performed RT-PCR and western blot to detect the
expression or phosphorylation of VEGFR-2, MEK1 and ERK1/2. The mRNA
and protein levels of VGFR-2 in NCI-H1299 cells incubated with
TBMS1 for 48 h were 2.28-fold and 2.0-fold lower than those in the
respective control cells (P<0.001; Fig. 5A and B). Moreover, the expression of
MEK1 and ERK were both sharply reduced at mRNA levels in comparison
with control (P<0.001; Fig. 5A).
At the same time, the phosphorylation level of MEK1 and ERK1/2 were
both decreased significantly (P<0.001; Fig. 5B). Taken together, TBMS1 inactivates
VEGFR2 mediated ERK pathway in NCI-H1299 cells.
Discussion
Previous researches revealed the cytostatic and
pro-apoptotic effects of TBMS1 in multiple cancer cells, including
lung cancer (8,9). But less is known about the internal
event of NSCLC cells during the anti-tumor effects of TBMS1. Our
study confirmed that TBMS1 suppressed the proliferation, migration
and invasion, boosted the apoptosis of NCI-H1299 cells through
overexpressing miR-126-5p, subsequently resulted in the inhibition
of VEGF-A/VEGFR2/ERK pathway.
Hao et al (8)
and Lin et al (9), both
identified that TBMS1 could inhibit growth and induce apoptosis in
lung cancer cells, which was basically consistent with our study
using NCI-H1299 cell line. NCI-H1299 cell line originates from a
lymph node metastasis in patients with NSCLC and accepted early
radiotherapy. The most obvious feature of NCI-H1299 is its absence
of pro-apoptotic p53 expression. Singla et al (18) and Wu et al (19) have demonstrated the possibility of
metastasis induced by NCI-H1299 cells in mice model. In this study,
we showed that TBMS1 blocked the migration and invasion of
NCI-H1299 cells significantly, first indicating the anti-metastatic
effect of TBMS1 in NSCLC cells.
The pre-miR-126, located in the epidermal growth
factor-like domain 7 (EGFL7) gene, produces two mature miRNA
chains, miR-126-3p (referring to the 3′ part of the miR-126
transcript) and miR-126-5p (referring to the 5′ part of the miR-126
transcript, also called miR-126*) (20). Shibayama et al (21), proved that upregulation of miR-126-5p
was associated with drug resistance to cytarabine and poor
prognosis in acute myeloid leukemia (AML) patients. Further studies
reported that miR-126-5p acted as a tumor suppressor in many tumors
such as prostate cancer, melanoma and breast cancer, as evidenced
by repressing the proliferation and invasion of cancer cells
(22–26). Clinical researches revealed that
miR-126-5p was notably downregulated in lung cancer patients
(26). Similarly, miR-126-5p was
remarkably downregulated in the tissues we recruited from NSCLC
patients compared with control, but the expression of miR-126-5p
was raised much higher after TBMS1 administration in NCI-H1299
cells, suggesting that TBMS1 inhibited the proliferation and
metastasis through increasing the expression miR-126-5p in NSCLC
cells.
Vascular endothelial growth factor-A (VEGF-A), an
important member of VEGF family, is upregulated in multiple
malignant tumors, such as cancers of breast, lung, brain, pancreas,
ovarian, kidney and bladder, which presents highly correlation with
staging, pathological grading and a poor prognosis (27–32).
Besides its essential role in regulating physiologic and pathologic
angiogenesis, VEGF-A also triggers the growth, survival, and
migration of cancer cells (33).
Prior studies proved that VEGF-A is one of the target genes of
miR-126-5p (34). Tang et al
(35), pointed out that decreased
expression of miR-126-5p upregulated VEGF-A and contributed to
lipopolysaccharide-induced acute lung injury. Liu et al
(34), demonstrated that
downregulated VEGF by miR-126 could inhibit the proliferation of
lung cancer cells. In this study, we showed the significantly
reduced mRNA and protein VEGF-A levels in TBMS1 treated NCI-H1299
cells, indicating that the increased miR-126-5p expression targets
VEGF-A, which may be associated with the anti-tumor effect of
TBMS1.
VEGF-A mediates its activity mainly via 2 receptor
tyrosine kinases (RTKs): VEGF receptor 1 (VEGFR-1) and VEGF
receptor 2 (VEGFR-2). VEGFR-2 is the most biologically responsible
for cell proliferation and migration (36). Extracellular signal regulated kinase
(ERK)1/2 pathway belongs to MAPKs pathway that are highly conserved
three stage kinase cascade amplification pathway models from yeast
to mammals. ERK1/2 pathway is generally described as
Ras-Raf-MEK-ERK1/2 model. Activated ERKs translocates into nucleus
and stimulates multiple transcription factors to promote the
expression of related genes in mitotic G0/G1 phase (37). Santos et al (38), found a strong pro-apoptotic effect of
the intracellular VEGFR-2 inhibitor through inhibiting ERK1/2
pathway. Furthermore, overexpression of VEGF-A augmented cell
migration and invasion through VEGF-A/VEGFR2/MEK/ERK1/2 signaling
(39). Our experiment showed the
significant downregulation of VEGFR-2 and an apparently inhibitory
of the activation of ERK1/2 pathway upon TBMS1 administration. We
therefore speculate that TBMS1 suppresses the proliferation,
migration and invasion and promotes the apoptosis of NCI-H1299
cells through overexpressing miR-126-5p, which ultimately reduces
VEGF-A/VEGFR-2/ERK1/2 pathway.
In summary, our present study revealed the
cytostatic and anti-metastatic effects of TBMS1, which may induced
by TBMS1 increased miR-126-5p expression and subsequent inactivated
VEGF-A/VEGFR-2/ERK1/2 pathway in NCI-H1299 cells. Our data
preliminarily identified the significant roles of TBMS1 and the
potential mechanism in vitro. TBMS1 may become a promising
candidate for NSCLC therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The authors declare that all available data is
presented in this submitted article.
Authors' contributions
HBS, HXB and JS conceived and designed the study.
HBS, HXB, XYS, HYD, YFJ, HJM, WL, GHL and RZG performed the
experiments. HBS and JS wrote the paper. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
All tissue specimens were obtained with permission
from the Medical Ethics Committee of The Third Affiliated Hospital
of Qiqihar Medical University. All participants have read and
signed the written informed consent.
Consent for publication
All participants have read and signed the written
informed consent for the publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
National Lung Screening Trial Research
Team, . Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD,
Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM and Sicks JD:
Reduced lung-cancer mortality with low-dose computed tomographic
screening. N Engl J Med. 365:395–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minguet J, Smith KH and Bramlage P:
Targeted therapies for treatment of non-small cell lung
cancer-Recent advances and future perspectives. Int J Cancer.
138:2549–2561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Villaruz LC and Socinski MA: Is there a
role of nab-paclitaxel in the treatment of advanced non-small cell
lung cancer? The data suggest yes. Eur J Cancer. 56:162–171. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA A Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
5
|
Yin Y, Chen W, Tang C, Ding H, Jang J,
Weng M, Cai Y and Zou G: NF-kB, JNK and p53 pathways are involved
in tubeimoside-1-induced apoptosis in HepG2 cells with oxidative
stress and G (2)/M cell cycle arrest. Food Chem Toxicol.
49:3046–3054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu Y, Korbel C, Scheuer C, Nenicu A,
Menger MD and Laschke MW: Tubeimoside-1 suppresses tumor
angiogenesis by stimulation of proteasomal VEGFR2 and Tie2
degradation in a non-small cell lung cancer xenograft model.
Oncotarget. 7:5258–5272. 2016.PubMed/NCBI
|
|
7
|
Bian Q, Liu P, Gu J and Song B:
Tubeimoside-1 inhibits the growth and invasion of colorectal cancer
cells through the Wnt/β-catenin signaling pathway. Int J Clin.
8:12517–12524. 2015.
|
|
8
|
Hao W, Wang S and Zhou Z: Tubeimoside-1
(TBMS1) inhibits lung cancer cell growth and induces cells
apoptosis through activation of MAPK-JNK pathway. Int J Clin Exp
Pathol. 8:12075–12083. 2015.PubMed/NCBI
|
|
9
|
Lin Y, Xie G, Xia J, Su D, Liu J, Jiang F
and Xu Y: TBMS1 exerts its cytotoxicity in NCI-H460 lung cancer
cells through nucleolar stress-induced p53/MDM2-dependent
mechanism, a quantitative proteomics study. Biochim Biophys Acta.
1864:204–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia G, Wang Q, Wang R, Deng D, Xue L, Shao
N, Zhang Y, Xia X, Zhi F and Yang Y: Tubeimoside-1 induces glioma
apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome
C/Caspase-3 pathway. Onco Targets Ther. 8:303–311. 2015.PubMed/NCBI
|
|
11
|
Zhang Y, Xu XM, Zhang M, Qu D, Niu HY, Bai
X, Kan L and He P: Effects of tubeimoside-1 on the proliferation
and apoptosis of BGC823 gastric cancer cells in vitro. Oncol Lett.
5:801–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weng XY, Ma RD and Yu LJ: Apoptosis of
human nasopharyngeal carcinoma CNE-2Z cells induced by tubeimoside
I. Ai Zheng. 22:806–811. 2003.PubMed/NCBI
|
|
13
|
Peng Y, Zhong Y and Li G: Tubeimoside-1
suppresses breast cancer metastasis through downregulation of CXCR4
chemokine receptor expression. BMB Rep. 49:502–507. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ni N, Zhang D, Xie Q, Chen J, Wang Z, Deng
Y, Wen X, Zhu M, Ji J, Fan X, et al: Effects of let-7b and TLX on
the proliferation and differentiation of retinal progenitor cells
in vitro. Sci Rep. 4:66712014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu M, Sun L, Zhou J, Zhao Y and Deng X:
Dihydroartemi-sinin-induced apoptosis is associated with inhibition
of sarco/endoplasmic reticulum calcium ATPase activity in
colorectal cancer. Cell Biochem Biophys. 73:137–145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shan S, Lv Q, Zhao Y, Liu C, Sun Y, Xi K,
Xiao J and Li C: Wnt/β-catenin pathway is required for epithelial
to mesenchymal transition in CXCL12 over expressed breast cancer
cells. Int J Clin Exp Pathol. 8:12357–12367. 2015.PubMed/NCBI
|
|
17
|
Ricciuti B, Mecca C, Crino L, Baglivo S,
Cenci M and Metro G: Non-coding RNAs in lung cancer. Oncoscience.
1:674–705. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singla AK, Downey CM, Bebb GD and Jirik
FR: Characterization of a murine model of metastatic human
non-small cell lung cancer and effect of CXCR4 inhibition on the
growth of metastases. Oncoscience. 2:263–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu W, Bi C, Credille KM, Manro JR, Peek
VL, Donoho GP, Yan L, Wijsman JA, Yan SB and Walgren RA: Inhibition
of tumor growth and metastasis in non-small cell lung cancer by
LY2801653, an inhibitor of several oncokinases, including MET. Clin
Cancer Res. 19:5699–5710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meister J and Schmidt MH: miR-126 and
miR-126*: New players in cancer. Sci World J. 10:2090–2100. 2010.
View Article : Google Scholar
|
|
21
|
Shibayama Y, Kondo T, Ohya H, Fujisawa S,
Teshima T and Iseki K: Upregulation of microRNA-126-5p is
associated with drug resistance to cytarabine and poor prognosis in
AML patients. Oncol Rep. 33:2176–2182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Musiyenko A, Bitko V and Barik S: Ectopic
expression of miR-126*, an intronic product of the vascular
endothelial EGF-like 7 gene, regulates prostein translation and
invasiveness of prostate cancer LNCaP cells. J Mol Med (Berl).
86:313–322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Felli N, Felicetti F, Lustri AM, Errico
MC, Bottero L, Cannistraci A, De Feo A, Petrini M, Pedini F,
Biffoni M, et al: miR-126&126* restored expressions play a
tumor suppressor role by directly regulating ADAM9 and MMP7 in
melanoma. PLoS One. 8:e568242013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y,
Li C, Chong M, Ibrahim T, Mercatali L, et al: miR-126 and miR-126*
repress recruitment of mesenchymal stem cells and inflammatory
monocytes to inhibit breast cancer metastasis. Nat Cell Biol.
15:284–294. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sanfiorenzo C, Ilie MI, Belaid A, Barlési
F, Mouroux J, Marquette CH, Brest P and Hofman P: Two panels of
plasma microRNAs as non-invasive biomarkers for prediction of
recurrence in resectable NSCLC. PLoS One. 8:e545962013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vosa U, Vooder T, Kolde R, Vilo J,
Metspalu A and Annilo T: Meta-analysis of microRNA expression in
lung cancer. Int J Cancer. 132:2884–2893. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshiji H, Gomez DE, Shibuya M and
Thorgeirsson UP: Expression of vascular endothelial growth factor,
its receptor and other angiogenic factors in human breast cancer.
Cancer Res. 56:2013–2016. 1996.PubMed/NCBI
|
|
28
|
Volm M, Koomägi R and Mattern J:
Prognostic value of vascular endothelial growth factor and its
receptor Flt-1 in squamous cell lung cancer. Int J Cancer.
74:64–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hatva E, Kaipainen A, Mentula P,
Jääskeläinen J, Paetau A, Haltia M and Alitalo K: Expression of
endothelial cell-specific receptor tyrosine kinases and growth
factors in human brain tumors. Ame J Pathol. 146:368–378. 1995.
|
|
30
|
Ellis LM, Takahashi Y, Fenoglio CJ, Cleary
KR, Bucana CD and Evans DB: Vessel counts and vascular endothelial
growth factor expression in pancreatic adenocarcinoma. Eur J
Cancer. 34:337–340. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boocock CA, Charnock-Jones DS, Sharkey AM,
McLaren J, Barker PJ, Wright KA, Twentyman PR and Smith SK:
Expression of vascular endothelial growth factor and its receptors
flt and KDR in ovarian carcinoma. J Natil Cancer Inst. 87:506–516.
1995. View Article : Google Scholar
|
|
32
|
Brown LF, Berse B, Jackman RW, Tognazzi K,
Manseau EJ, Dvorak HF and Senger DR: Increased expression of
vascular permeability factor (vascular endothelial growth factor)
and its receptors in kidney and bladder carcinomas. Am J Pathol.
143:1255–1262. 1993.PubMed/NCBI
|
|
33
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu B, Peng XC, Zheng XL, Wang J and Qin
YW: MiR-126 restoration down-regulate VEGF and inhibit the growth
of lung cancer cell lines in vitro and in vivo. Lung Cancer.
66:169–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang R, Pei L, Bai T and Wang J:
Down-regulation of microRNA-126-5p contributes to overexpression of
VEGFA in lipopolysaccharide-induced acute lung injury. Biotechnol
Lett. 38:1277–1284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsumoto K and Ema M: Roles of VEGF-A
signalling in development, regeneration and tumours. J Biochem.
156:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Santos SC and Dias S: Internal and
external autocrine VEGF/KDR loops regulate survival of subsets of
acute leukemia through distinct signaling pathways. Blood.
103:3883–3889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tian Y, Xie Q, Tian Y, Liu Y, Huang Z, Fan
C, Hou B, Sun D, Yao K and Chen T: Radioactive 125I seed
inhibits the cell growth, migration and invasion of nasopharyngeal
carcinoma by triggering DNA damage and inactivating VEGF-A/ERK
signaling. PLoS One. 8:e740382013. View Article : Google Scholar : PubMed/NCBI
|