Introduction
Gastric cancer is a malignant tumor that has a
marked impact on human health: There were 989,600 novel cases of
gastric cancer in 2012 globally and 738,000 mortalities owing to
gastric cancer, ranking fourth in cancer incidence and second in
mortality rate (1). China is a
country with high incidence of gastric cancer, with 42% of patients
with gastric cancer worldwide being in China (2). The third nationwide retrospective sample
survey revealed that gastric cancer is the third most common cause
of cancer-associated mortality; since the census is not yet
universal, ~30% of patients with stomach cancer are at locally
advanced stage, 30% are subject to distant metastasis at the time
of diagnosis, and the remaining 40% are resectable, among which 60%
may experience relapse or metastasis. Therefore, ~84% of patients
with gastric cancer eventually become advanced (3).
Following research into human cancer, an increasing
number of drugs are being developed to target tumor characteristics
and are being applied clinically as molecularly targeted therapies
(4). In advanced gastric cancer,
there has been progress in the research and application of
molecular targeted drugs (5).
Subsequently, clinical studies on targeted drugs alone, including
anti-human epidermal growth factor-2, -epidermal growth factor
receptor, -MET and -phosphoinositide 3-kinase (PI3K)/protein kinase
B (AKT)/mechanistic target of rapamycin signaling pathways as well
as anti-angiogenesis, or in combination with chemotherapy for
advanced gastric cancer have been reported (5,6).
Gastric cancer is a common cancer of the digestive
system, of which the prognosis is poor when metastasis occurs in
patients (7). Gastric cancer is
associated with the inactivation of tumor suppressor genes
(7). Recent studies have demonstrated
that the Wnt signaling pathway is abnormally activated in certain
types of cancer (7,8). Glycogen synthase kinase-3β (GSK3β) is an
important molecule involved in the Wnt signaling pathway, which is
a serine/threonine kinase involved in the regulation of microtubule
dynamics, proliferation, apoptosis, angiogenesis and cell motility,
as well as other functions (9). GSK3β
acts on substrates including oncogenic transcription factors and
oncoproteins, to participate in the occurrence of certain tumors,
and, for example, the inactivation of GSK3β phosphorylation results
in the inhibition of GSK3β activity and the expression induces
epithelial-mesenchymal transition, participating in tumor invasion
and metastasis (9).
Resibufogenin is a commonly used natural medicinal
ingredient, extracted from the dried secretions of the Asiatic toad
Bufo gargarizans, which is involved in a variety of
biological activities, including analgesia, anti-inflammation,
anesthesia, as well as anticancer, anti-radiation, cardiac
protection, etc (10). Therefore, it
has important clinical value of medical treatment. It has been
demonstrated that the traditional Chinese medicine Venenum Bufonis
exhibits pharmacological activity in the clinical treatment of
malignant tumors, prompting it to be of interest for further
research (11). According to the
anticancer activity of Venenum Bufonis, a variety of active
ingredients have been isolated (12).
As one of the active ingredients in Venenum Bufonis, resibufogenin,
the natural medicine monomeric molecule, is hypothesized to induce
the apoptosis of and inhibit the proliferation of tumor cells
(13). In the present study, the
anticancer effect of resibufogenin induced in gastric carcinoma
cells and potential underlying molecular mechanisms were
investigated.
Materials and methods
Cell culture
Human gastric carcinoma MGC-803 cells were
maintained and cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 2 mmol/l glutamine, 100 U/ml penicillin and 100
µg/ml streptomycin, and cultured at 37°C in a humidified atmosphere
containing 5% CO2. Resibufogenin was purchased from
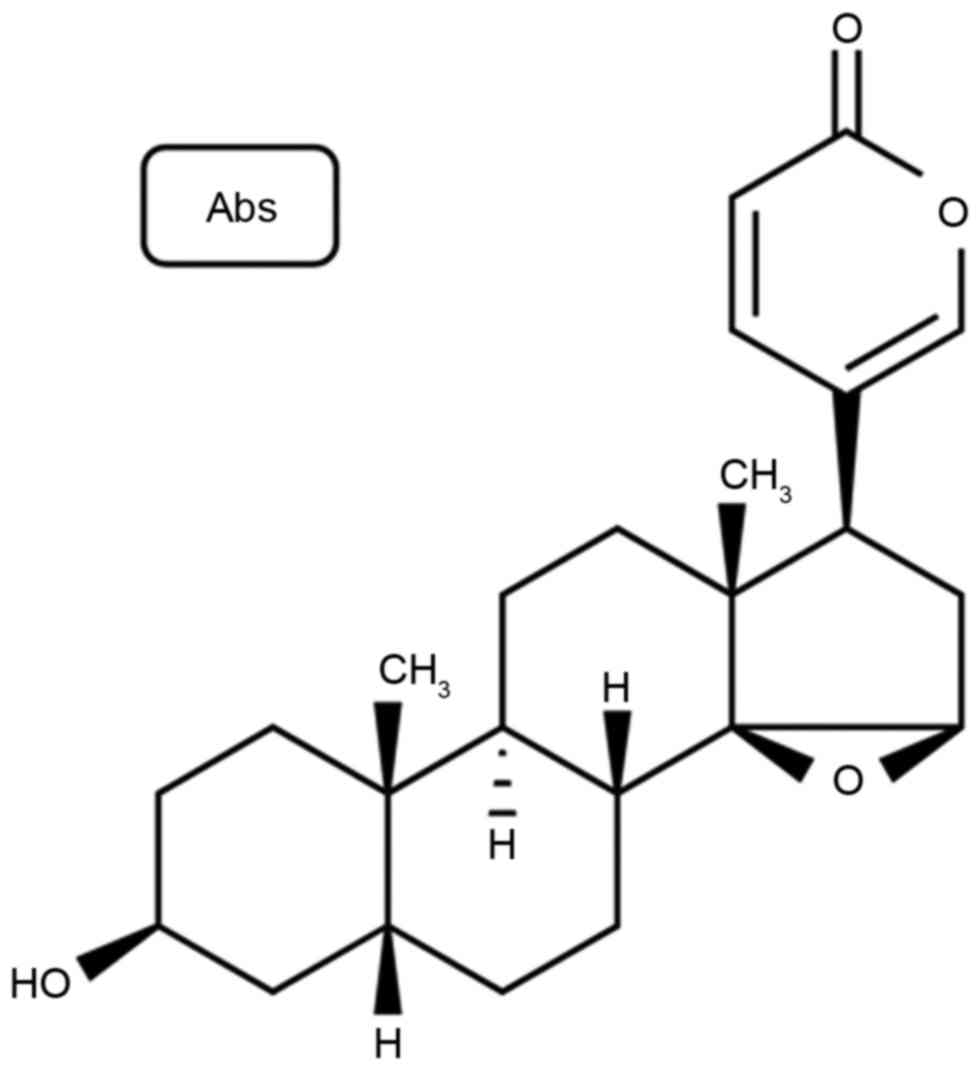
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany), and its structural
formula is presented in Fig. 1.
Cell viability assay
The effects of resibufogenin on the proliferation of
MGC-803 cells were determined using an MTT assay. MGC-803 cells
were seeded in 96-well plates (1×104 cells/well) and
then treated with 0, 1, 2, 4 and 8 µM resibufogenin for 12, 24 and
48 h at 37°C. MTT was then added to the cells for another 4 h.
Culture medium was removed and MTT formazan crystals were dissolved
with dimethylsulfoxide. The viability of MGC-803 cells was
determined at 490 nm using a microplate reader.
Determination of apoptosis using
annexin V staining
The effects of resibufogenin on the apoptosis of
MGC-803 cells were determined with annexin V staining. MGC-803
cells were seeded in 6-well plates (1×106 cells/well)
and then treated with 0, 2, 4 and 8 µM resibufogenin for 48 h at
37°C. Cells were resuspended in binding buffer (BD Biosciences,
Franklin Lakes, NJ, USA) and stained with 5 µl annexin
V-fluorescein isothiocyanate in darkness for 30 min at room
temperature. Subsequently, 1 µl propidium iodide was added to 100
µl samples from each of the cell suspensions in darkness for 10 min
at room temperature. The stained cells were measured using flow
cytometry on a FACSCalibur instrument (v6, BD Biosciences).
Determining caspase-3 and caspase-8
activity using caspase activity kits
The effects of resibufogenin on caspase-3 and
caspase-8 activity in MGC-803 cells were determined using caspase
activity kits. MGC-803 cells were seeded in 96-well plates
(1×104 cells/well) and treated with 0, 2, 4 and 8 µM
resibufogenin for 48 h. N-acetyl
(Ac)-Asp-Glu-Val-Asp-p-nitroanilide (pNA; C1116; Caspase 3 Activity
Assay kit, Beyotime Institute of Biotechnology) and
Ac-Ile-Glu-Thr-Asp-pNA (Caspase 8 Activity Assay kit, cat. no.
C1152; Beyotime Institute of Biotechnology) were added to the cells
and incubated for 1 h at 37°C. Caspase-3 and caspase-8 activity
were measured at 490 nm using a microplate reader.
Western blot analysis
Cells were lysed in buffer radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) for 30 min at 4°C. Protein content was measured using
bicinchoninic acid (Beyotime Institute of Biotechnology). Proteins
(50 µg) in resulting cell lysates were separated by SDS-PAGE (8–10%
gel) and electrotransferred onto polyvinylidene difluoride
membranes. Following blocking with 5% nonfat dry milk in TBS
containing 0.1% Tween-20 for 1 h at 37°C, membranes were incubated
with anti-Bax (cat. no. 5023, 1:2,000, Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-Bcl-2 (cat. no. 3498, 1:2,000, Cell
Signaling Technology, Inc.), anti-cyclin D1 (cat. no. 2978,
1:2,000, Cell Signaling Technology, Inc.), anti-cyclin E (cat. no.
4132, 1:2,000, Cell Signaling Technology, Inc.), anti-AKT (cat. no.
4685, 1:2,000, Cell Signaling Technology, Inc.), anti-p-AKT (cat.
no. 4060, 1:2,000, Cell Signaling Technology, Inc.), anti-p-GSK3β
(cat. no. 12456, 1:2,000, Cell Signaling Technology, Inc.),
anti-β-catenin (cat. no. 8480, 1:2,000, Cell Signaling Technology,
Inc.) and anti-GAPDH (cat. no. 5174, 1:5,000, Cell Signaling
Technology, Inc.) antibodies at 4°C overnight. Following washing
with TBS containing 0.1% Tween-20, membranes were incubated with
horseradish peroxidase-conjugated anti-rabbit immunoglobulin G
secondary antibody (cat. no. A0545; 1:40,000; Sigma-Aldrich; Merck
KGaA) for 1 h at 37°C and subsequently visualized with an enhanced
chemiluminescence detection system (ECL2 Western Blotting
substrate; Pierce; Thermo Fisher Scientific, Inc.).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis of the data was performed using
analysis of variance and Tukey's post-hoc test for comparison
between treatments and controls. P<0.05 was considered to
indicate a statistically significant difference.
Results
Resibufogenin inhibits the growth of
gastric carcinoma cells
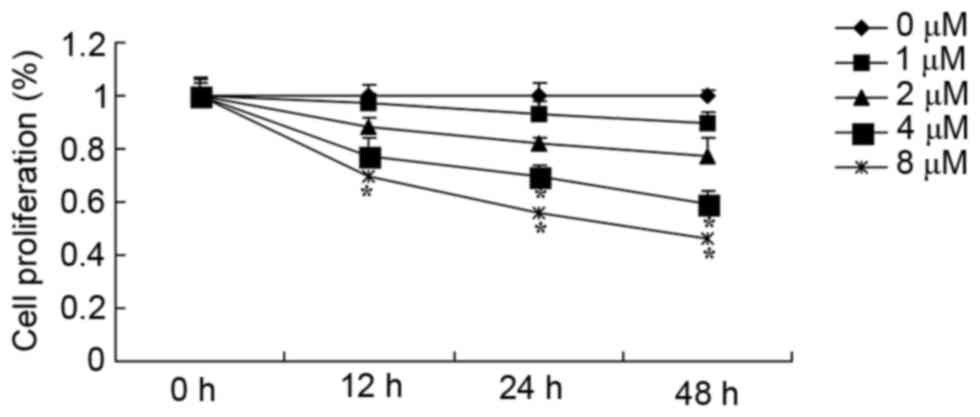
Results presented in Fig.
2 reveal that resibufogenin inhibited cell proliferation of
MGC-803 cell in a time- and dose-dependent manner. Following
resibufogenin treatment for 24 and 48 h, 4 and 8 µM resibufogenin
effectively inhibited the viability of MGC-803 cells; treatment
with 8 µM resibufogenin for 12 h effectively inhibited the
viability of MGC-803 cells. Resibufogenin groups were compared with
the untreated control.
Resibufogenin induces apoptosis of
gastric carcinoma cells
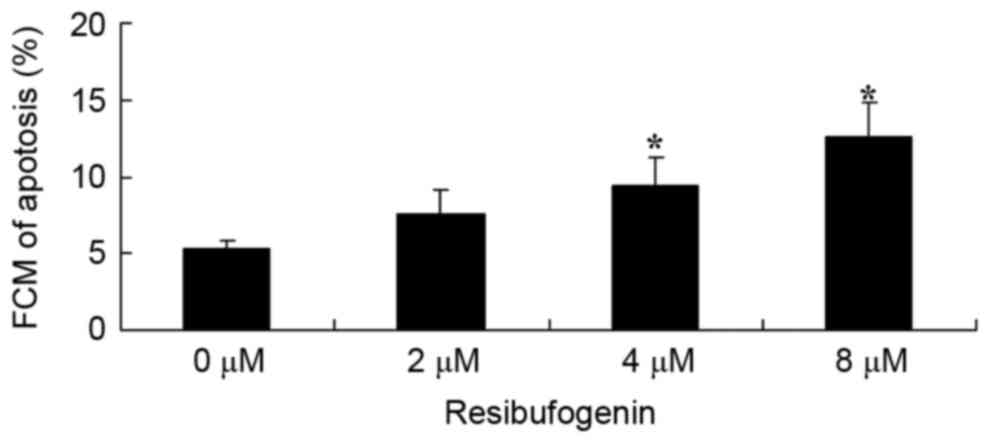
To investigate the anticancer effect of
resibufogenin on the apoptosis of gastric carcinoma cells, the
apoptotic rate was determined using flow cytometry following
annexin V staining. Fig. 3 revealed
that 4 and 8 µM resibufogenin significantly induced apoptosis of
MGC-803 cells at 48 h, compared with the untreated control.
Resibufogenin induces caspase-3 and
caspase-8 activity of gastric carcinoma cells
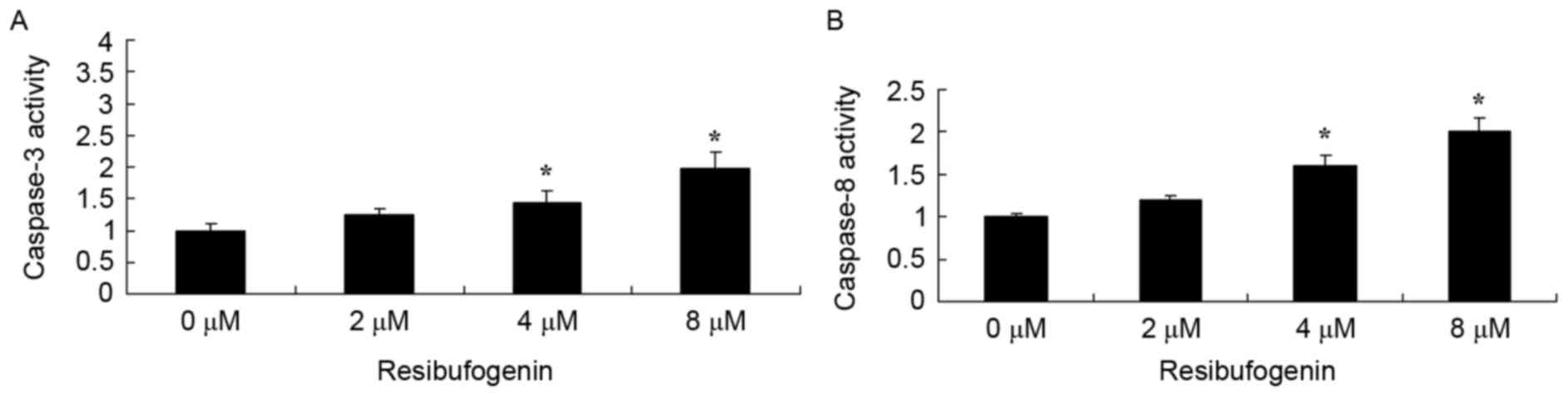
The molecular mechanism of apoptosis in the
anticancer effect of resibufogenin was investigated in MGC-803
cells. Caspase-3 and caspase-8 activity of MGC-803 cell were
determined using commercial kits. Fig.
4 indicated that 4 and 8 µM resibufogenin significantly
increased caspase-3 and caspase-8 activity of MGC-803 cell at 48 h,
compared with the untreated control.
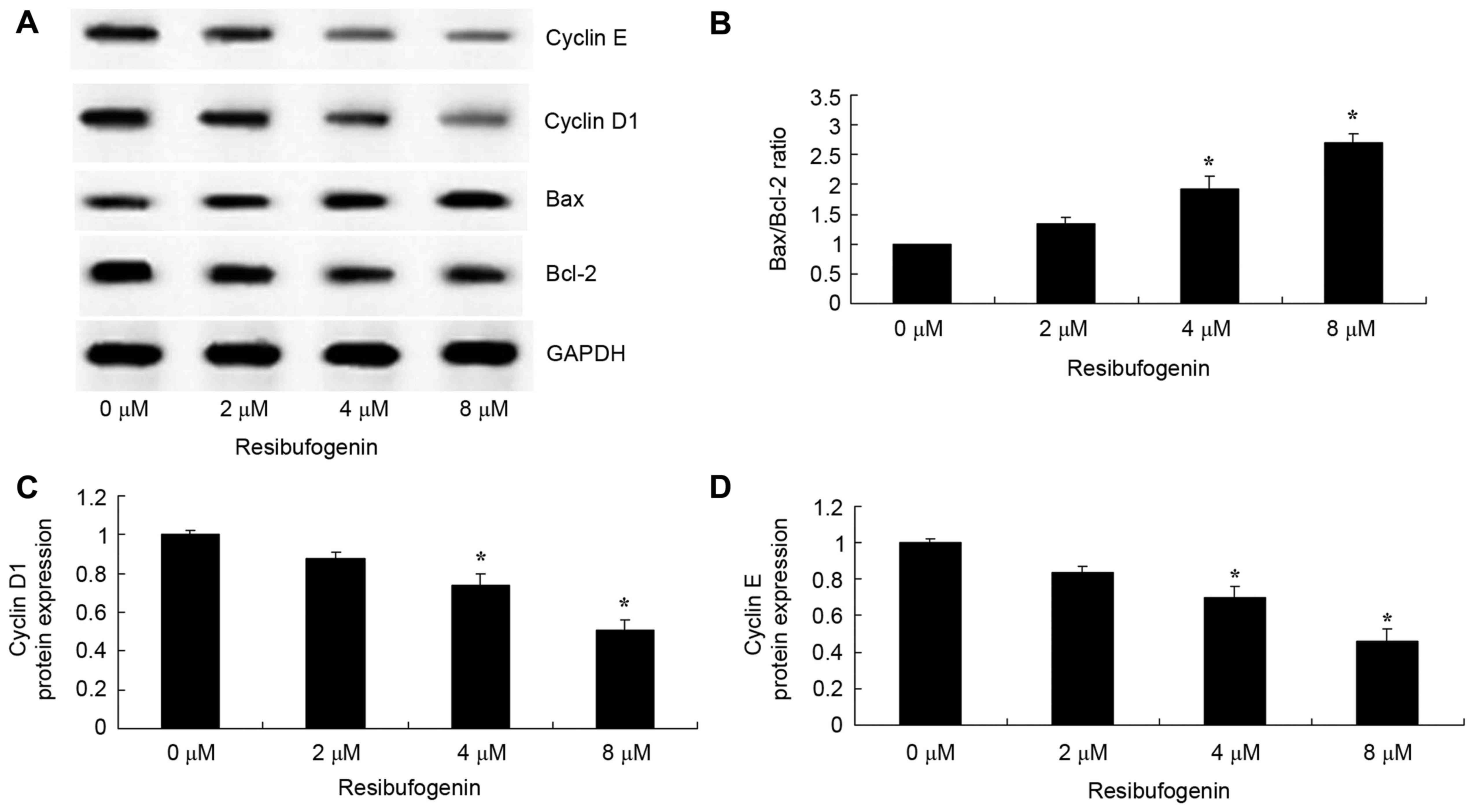
Resibufogenin increases Bax/Bcl-2, and
inhibited cyclin D1 and cyclin E protein expression in gastric
carcinoma cells
The mechanism of apoptosis in the anticancer effect
of resibufogenin was investigated in MGC-803 cells. Bax and Bcl-2
protein expression were measured using western blot analysis. The
results demonstrated that Bax protein expression was significantly
induced and Bcl-2 protein expression was significantly suppressed
by treatment with 4 and 8 µM resibufogenin at 48 h, compared with
the untreated control (Fig. 5A and
B). The results from western blot analysis revealed that cyclin
D1 and cyclin E protein expression was significantly suppressed
following treatment with 4 and 8 µM resibufogenin at 48 h, compared
with the untreated control (Fig. 5A, C
and D).
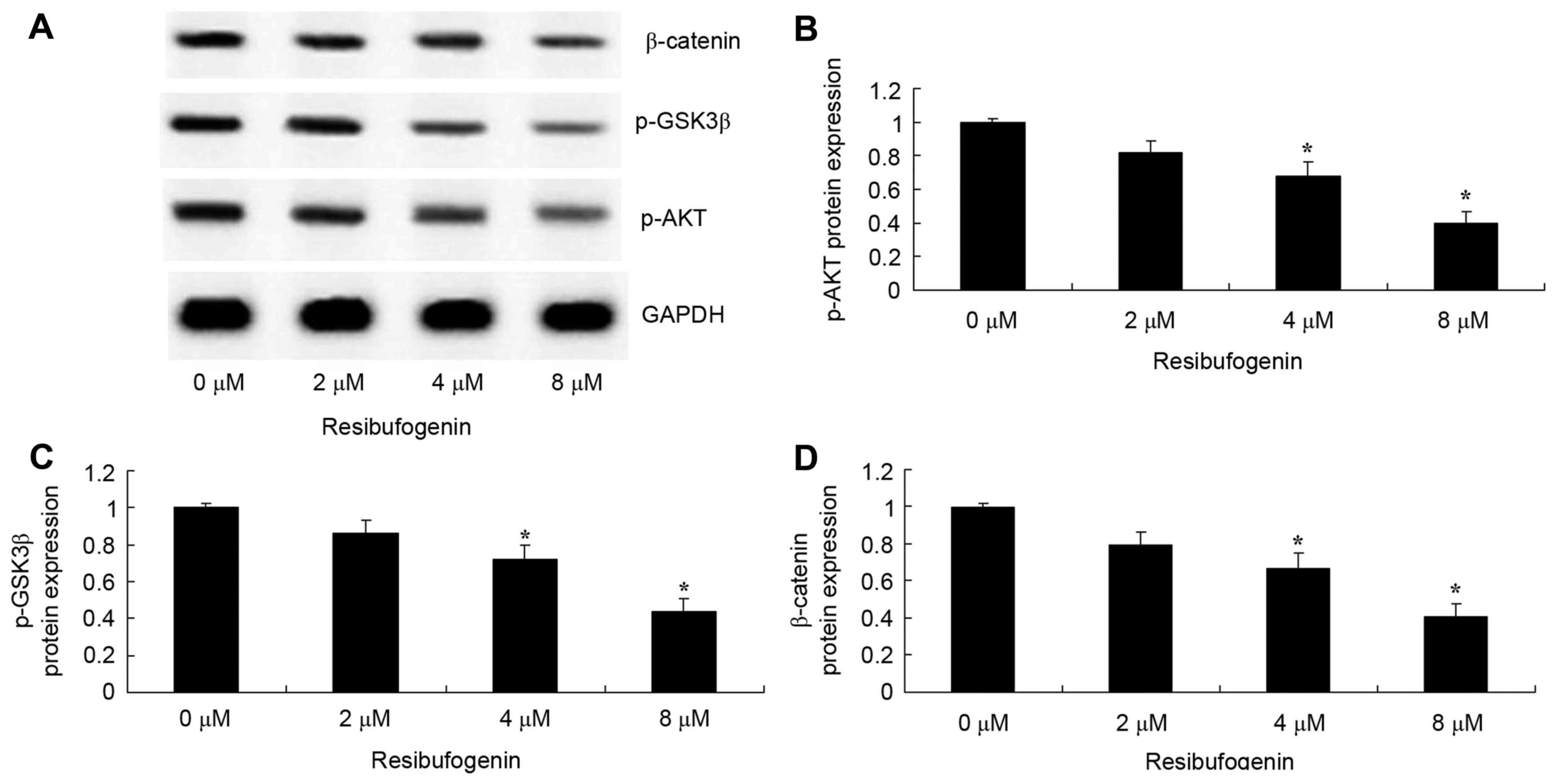
Resibufogenin suppresses AKT/p-AKT,
p-GSK3β and β-catenin protein expression in gastric carcinoma
cells
AKT/p-AKT protein expression of gastric carcinoma
cell was investigated using western blot analysis. Resibufogenin
treatment at 4 and 8 µM significantly decreased p-AKT, p-GSK3β and
β-catenin protein expression in MGC-803 cell at 48 h, compared with
the untreated control, respectively (Fig.
6).
Discussion
Gastric cancer is the most common gastrointestinal
malignant tumor with the most associated mortalities owing to its
strong local invasiveness and easy metastasis (14). The pathogenesis of gastric cancer is
complex as a result of multiple factors, multiple genes and
multiple stages, and in which the cell signal transduction pathway
serves an important function in its occurrence and development
(15). The results of the present
study demonstrated for the first time, to the best of our
knowledge, that resibufogenin, a component of the traditional
Chinese medicine Venenum Bufonis, inhibited viability and induced
apoptosis in MGC-803 cells. Ichikawa et al (16) demonstrated that resibufogenin induces
apoptosis of human malignant tumor cells.
The caspase family has >10 members which are
among the most important factors in the apoptotic process (17). The members of the caspase family serve
distinct functions in the apoptotic pathway, in which caspase-2,
−8, −9 and −10 are primarily involved in the initiation of
apoptosis (17). Caspase-3, −6 and −7
may be combined with a variety of substrates for hydrolysis,
leading to chromatin condensation and nuclear disintegration, and
are therefore apoptosis executioner proteins (18). Currently, there are two pathways to
trigger apoptosis, namely the extrinsic pathway and the intrinsic
pathway. The intrinsic pathway is closely associated with the
development of tumor cells (18). The
mitochondria undergo a series of changes in the intrinsic pathway,
including the change in mitochondrial membrane permeability, the
damage to the energy metabolic pathway, the release of cytochrome
c, the activation of caspase family, which cause the cascade
reaction mediated by caspase-9 and other key enzymes downstream. In
addition, the mitochondria may promote apoptosis by generating free
radicals, including reactive oxygen species (13). In the present study, resibufogenin was
identified to induce caspase-3 and caspase-8 activity in MGC-803
cells. This suggests that caspase activity is associated with the
anticancer effect of resibufogenin in gastric carcinoma.
As aforementioned, caspase-3 induces apoptosis. In
addition, growth factors may also mediate mitochondrial apoptotic
pathway through the PI3K/AKT signaling pathway (19) in which growth factors identify and
bind with its receptor, to activate PI3K (20). The activated PI3K activates AKT
(20), which is an important factor
in the regulation of Bcl-2-associated agonist of cell death (BAD)
protein, an important member of the Bcl-2 family, involved in
mitochondrial apoptosis (21). In
addition, protein kinase C inhibits BAD, thus serving an important
regulatory function in apoptosis (22). The results of the present study
suggest that resibufogenin treatment significantly decreases p-AKT
protein expression in MGC-803 cells and that the growth suppression
and cell death induced by resibufogenin may occur despite the
decrease in p-AKT expression.
Following various types of cell stress signal, the
pro-apoptotic proteins in the Bcl-2 gene family are activated and
interact with anti-apoptotic proteins to inactivate the cell
(8). The anti-apoptotic and
pro-apoptotic members in the Bcl-2 family proteins possess
conserved α-helix and homologous domains (23). When the mitochondria initiate
apoptosis, the Bax protein family members are located downstream of
the apoptotic signaling pathways, and Bcl homology (BH)3
domain-specific proteins act upstream of the signaling pathway
(24). Anti-apoptotic members of
Bcl-2 family, such as Bcl-2 and Bcl-XL, contain homologous
sequences BH1-BH4 (23). The
interactions between anti- and pro-apoptotic proteins undermine the
stability of the mitochondrial membrane, causing the release of
apoptotic factors from the mitochondria into the cytoplasm
(24). In the present study, it was
revealed that resibufogenin significantly induced Bax/Bcl-2 protein
expression in MGC-803 cells. Therefore, the effects of
resibufogenin on Bax/Bcl-2 may be useful to the regulation of the
anticancer effect on gastric carcinoma.
The Bcl-2 signaling pathway is also known as the
mitochondrial apoptosis pathway. Bcl-2 is widely present in normal
tissue and embryonic tissue cells, including nerve cells, skin
cells, and embryonic kidney cells and cartilage. The Bcl-2 family
serves an important function in apoptosis. Bcl-2 itself is able to
block or delay the apoptosis induced by a variety of
chemotherapeutic drugs by blocking the apoptosis signal
transduction system. Wang et al (25) reported that resibufogenin inhibited
cell proliferation by inducing the apoptosis of HepG2 cells by
regulation of the Bax/Bcl-2 ratio. Therefore, Bcl-2 gene is also a
survival gene.
A previous study demonstrated that the classical Wnt
signaling pathway serves an important regulatory function in the
cell proliferation, differentiation and migration, which are
associated with a number of tumors with high incidence, such as
gastric cancer (7). GSK3β is a type
of multifunctional serine/threonine protein kinase (7). A previous study identified that GSK3β is
one of the major rate-limiting enzymes involved in glucose
metabolism, which is able to phosphorylate glycogen synthase and
inhibit glycogen synthesis (26). A
subsequent study demonstrated that GSK3β participates in important
physiological processes including cell differentiation,
proliferation and apoptosis, in addition to glucose metabolism
(26). As an important molecule of
multimeric protein complex adenomatous polyposis coli
(APC)/GSK3β/β-catenin in the classical Wnt signaling pathway, it
serves a key function to phosphorylate serine/threonine residues at
the N-terminus of β-catenin, to regulate β-catenin by forming a
degradation complex with axin and APC (27). It has been reported that GSK3β is
overexpressed in a variety of tumor tissues, including kidney,
colon and gastric cancer (26). In
the present study, resibufogenin significantly suppressed p-GSK3β
protein expression of MGC-803 cells. Ichikawa et al
(16) demonstrated that resibufogenin
induces apoptosis of human malignant tumor cells through the
degradation of cyclin D1 caused by the activation of GSK-3β. The
results of the present study suggest that GSK3β expression may be
involved in overcoming the anticancer effect of resibufogenin on
gastric carcinoma cells.
As a cell cycle regulation factor, cyclin D1 is an
important target gene of the classical Wnt signaling pathway
(28). A previous study demonstrated
that cyclin D1 is overexpressed in gastric cancer; following the
introduction of an antisense oligonucleotide probe to the gastric
cancer cells of nude mice, cancer cell growth was markedly
controlled following cyclin D1 inhibition and eventual loss of
tumorigenicity (28). Cyclin D1
expression and GSK3β expression are negatively associated. The Wnt
signaling pathway is activated abnormally to promote the occurrence
and metastasis of gastric cancer, with decreased expression of
GSK3β in gastric tissue, thus contributing to the depolymerization
of the multiprotein degradation complex APC/axin/GSK-3β/β-catenin,
resulting in the accumulation and activation of β-catenin in the
nucleus, thereby activating its target gene, cyclin D1, downstream
(29). Cyclin D1 is useful for
monitoring the progress of gastric cancer clinically (30). In the present study, resibufogenin
suppression of cyclin D1 and cyclin E protein expression in gastric
carcinoma cells was investigated. resibufogenin was demonstrated to
significantly suppress β-catenin protein expression in MGC-803
cells. Ichikawa et al (16)
demonstrated that resibufogenin induces apoptosis of human
malignant tumor cells through the degradation of cyclin D1 caused
by the activation of GSK3β. The results of the present study
suggested that resibufogenin may suppress the cyclin D1- and cyclin
E-independent pathway. Taken together, the results of the present
study suggested that there is crosstalk between cyclin D1/E and
β-catenin signaling pathways, affected by resibufogenin treatment
on gastric carcinoma cells.
In conclusion, the results of the present study
demonstrate for the first time, to the best of our knowledge, that
resibufogenin effectively inhibits cell viability, induces
apoptosis, and induces caspase-3 and caspase-8 activity in MGC-803
cells by suppressing the PI3K/AKT/GSK3β signaling pathway.
Therefore, resibufogenin may be a possible treatment for gastric
carcinoma.
References
|
1
|
Kawanaka M, Watari J, Kamiya N, Yamasaki
T, Kondo T, Toyoshima F, Ikehara H, Tomita T, Oshima T, Fukui H, et
al: Effects of Helicobacter pylori eradication on the development
of metachronous gastric cancer after endoscopic treatment: Analysis
of molecular alterations by a randomised controlled trial. Br J
Cancer. 114:21–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Cheng L, Xie Z and Li Z: Impact of
preoperative oral liquid carbohydrate on postoperative insulin
resistance in gastric cancer patients and its associated study.
Zhonghua Wei Chang Wai Ke Za Zhi. 18:1256–1260. 2015.(In Chinese).
PubMed/NCBI
|
|
3
|
Park SR, Hong YS, Lim HS, Seong MW, Kong
SY, Kim SY, Park YI and Jung KH: Phase I clinical and
pharmacokinetic/pharmacogenetic study of a triplet regimen of
S-1/irinotecan/oxaliplatin in patients with metastatic colorectal
or gastric cancer. Cancer Chemother Pharmacol. 72:953–964. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li NA, Wang W, Xu B and Gong H: miR-196b
regulates gastric cancer cell proliferation and invasion via
PI3K/AKT/mTOR signaling pathway. Oncol Lett. 11:1745–1749. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan CX, Zhou ZW, Yang YX, He ZX, Zhang X,
Wang D, Yang T, Pan SY, Chen XW and Zhou SF: Danusertib, a potent
pan-Aurora kinase and ABL kinase inhibitor, induces cell cycle
arrest and programmed cell death and inhibits epithelial to
mesenchymal transition involving the PI3K/Akt/mTOR-mediated
signaling pathway in human gastric cancer AGS and NCI-N78 cells.
Drug Des Devel Ther. 9:1293–1318. 2015.PubMed/NCBI
|
|
6
|
Riquelme I, Tapia O, Espinoza JA, Leal P,
Buchegger K, Sandoval A, Bizama C, Araya JC, Peek RM and Roa JC:
The gene expression status of the PI3K/AKT/mTOR pathway in gastric
cancer tissues and cell lines. Pathol Oncol Res. 22:797–805. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang B, Yang Y, Shi X, Liao W, Chen M,
Cheng AS, Yan H, Fang C, Zhang S, Xu G, et al: Proton pump
inhibitor pantoprazole abrogates adriamycin-resistant gastric
cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin
signaling and epithelial-mesenchymal transition. Cancer Lett.
356:704–712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo H, Cui H, Peng X, Fang J, Zuo Z and
Deng J, Wang X, Wu B, Chen K and Deng J: Modulation of the PI3K/Akt
pathway and Bcl-2 family proteins involved in chicken's tubular
apoptosis induced by nickel chloride (NiCl2). Int J Mol
Sci. 16:22989–23011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang X, Zheng D, Hu P, Zeng Z, Li M,
Tucker L, Monahan R, Resnick MB, Liu M and Ramratnam B: Glycogen
synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the
β-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids
Res. 42:2988–2998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chu Q, Xu H, Gao M, Guan X, Liu H, Deng S,
Huo X, Liu K, Tian Y and Ma X: Liver-targeting Resibufogenin-loaded
poly(lactic-co-glycolic acid)-D-α-tocopheryl polyethylene glycol
1000 succinate nanoparticles for liver cancer therapy. Int J
Nanomedicine. 11:449–463. 2016.PubMed/NCBI
|
|
11
|
Xin XL, Sun JH, Wang XB, Xi RG, Wang G,
Lan R, Su DH, Li H, Huo XK and Wang C: Microbial transformation of
resibufogenin by Curvularia lunata AS 3.4381. J Asian Nat Prod Res.
16:290–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mou LY, Xin XL, Chen L, Dong PP, Lan R, Su
DH, Huang J, Wang JH and Zhan LB: Biotransformation of
resibufogenin by Actinomucor elegans. J Asian Nat Prod Res.
16:623–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Fu XQ, Zhou W, Yu HG, Yu JP and Luo
HS: LY294002 potentiates the anti-cancer effect of oxaliplatin for
gastric cancer via death receptor pathway. World J Gastroenterol.
17:181–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao J, Chen Y, Li W, Gong J, Zhou Z, Deng
Y, Wang L, Ren D, Wang J, Peng J and Lan P: Dose-dense biweekly
docetaxel combined with 5-fluorouracil as first-line treatment in
advanced gastric cancer: A phase II trial. Med Oncol. 32:3342015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi D, Bao YS and Liu YP:
Individualization of metal stents for management of gastric outlet
obstruction caused by distal stomach cancer: A prospective study.
Gastrointest Endosc. 78:277–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ichikawa M, Sowa Y, Iizumi Y, Aono Y and
Sakai T: Resibufogenin induces G1-phase arrest through the
proteasomal degradation of cyclin D1 in human malignant tumor
cells. PLoS One. 10:e01298512015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dolatkhah H, Movahedian A, Somi MH, Aghaei
M, Samadi N, Mirza-Aghazade A and Esfahani A: Effect of PUFAs oral
administration on the amount of apoptotic caspases enzymes in
gastric cancer patients undergoing chemotherapy. Anticancer Agents
Med Chem. 17:93–101. 2017.PubMed/NCBI
|
|
18
|
Frejlich E, Rudno-Rudzińska J, Janiszewski
K, Salomon L, Kotulski K, Pelzer O, Grzebieniak Z, Tarnawa R and
Kielan W: Caspases and their role in gastric cancer. Adv Clin Exp
Med. 22:593–602. 2013.PubMed/NCBI
|
|
19
|
Su CC and Chiu TL: Tanshinone IIA
decreases the protein expression of EGFR, and IGFR blocking the
PI3K/Akt/mTOR pathway in gastric carcinoma AGS cells both in vitro
and in vivo. Oncol Rep. 36:1173–1179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tapia O, Riquelme I, Leal P, Sandoval A,
Aedo S, Weber H, Letelier P, Bellolio E, Villaseca M, Garcia P and
Roa JC: The PI3K/AKT/mTOR pathway is activated in gastric cancer
with potential prognostic and predictive significance. Virchows
Arch. 465:25–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu Z, Yang J, Wei Y and Li J: Effects of
piceatannol and pterostilbene against β-amyloid-induced apoptosis
on the PI3K/Akt/Bad signaling pathway in PC12 cells. Food Funct.
7:1014–1023. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xing X, Zhang L, Wen X, Wang X, Cheng X,
Du H, Hu Y, Li L, Dong B, Li Z and Ji J: PP242 suppresses cell
proliferation, metastasis, and angiogenesis of gastric cancer
through inhibition of the PI3K/AKT/mTOR pathway. Anticancer Drugs.
25:1129–1140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Liu Z, Cao BB, Qiu YH and Peng YP:
TGF-β1 neuroprotection via inhibition of microglial activation in a
rat model of parkinson's disease. J Neuroimmune Pharmacol.
12:433–446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanninen KM and White AR: Type-I
interferons in Parkinson's disease: Innate inflammatory response
drives fate of neurons in model of degenerative brain disorder: An
editorial comment on ‘Type-I interferons mediate the
neuroinflammatory response and neurotoxicity induced by rotenone’.
J Neurochem. 141:9–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang DL, Qi FH, Xu HL, Inagaki Y, Orihara
Y, Sekimizu K, Kokudo N, Wang FS and Tang W: Apoptosis-inducing
activity of compounds screened and characterized from cinobufacini
by bioassay-guided isolation. Mol Med Rep. 3:717–722.
2010.PubMed/NCBI
|
|
26
|
Wang L, Yin J, Wang X, Shao M, Duan F, Wu
W, Peng P, Jin J, Tang Y, Ruan Y, et al: C-type lectin-like
receptor 2 suppresses AKT signaling and invasive activities of
gastric cancer cells by blocking expression of phosphoinositide
3-kinase subunits. Gastroenterology. 150:1183–1195.e16. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cho YJ, Kim JH, Yoon J, Cho SJ, Ko YS,
Park JW, Lee HS, Lee HE, Kim WH and Lee BL: Constitutive activation
of glycogen synthase kinase-3beta correlates with better prognosis
and cyclin-dependent kinase inhibitors in human gastric cancer. BMC
Gastroenterol. 10:912010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Cui J, Yu Q, Wu X, Pan A and Li L:
Evaluation of CCND1 amplification and CyclinD1 expression: Diffuse
and strong staining of CyclinD1 could have same predictive roles as
CCND1 amplification in ER positive breast cancers. Am J Transl Res.
8:142–153. 2016.PubMed/NCBI
|
|
29
|
Seiler R, Thalmann GN, Rotzer D, Perren A
and Fleischmann A: CCND1/CyclinD1 status in metastasizing bladder
cancer: A prognosticator and predictor of chemotherapeutic
response. Mod Pathol. 27:87–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Neal JT, Peterson TS, Kent ML and
Guillemin K: H. pylori virulence factor CagA increases intestinal
cell proliferation by Wnt pathway activation in a transgenic
zebrafish model. Dis Model Mech. 6:802–810. 2013. View Article : Google Scholar : PubMed/NCBI
|