Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies globally. In particular, HCC is more frequent
in developing countries like China compared with in developed
countries like the United States of America, as viral hepatitis
infections [hepatitis B virus (HBV) and hepatitis C virus (HCV)]
are more common in developing countries (1). Concurrently, the frequency of HBV is
higher compared with HCV infection in China (2). Untreated viral hepatitis infection often
leads to chronic liver disease and cirrhosis; in these conditions,
almost one-third of patients will ultimately develop HCC over a
period of several years (3). In
addition, HCC is most common in men; the primary reason for this
may be differences in lifestyle, including alcohol consumption or
smoking habits (4). Each year,
~500,000 incident cases were diagnosed as HCC globally (4). The treatment options for early-stage HCC
include surgical resection and liver transplantation (5). Although early diagnostic markers have
been developed in previous decades, the long-term survival of
patients with HCC remains poor (6).
Therefore, identification of valuable diagnostic markers and novel
therapeutic strategies are a major challenge in HCC.
Prostate and breast cancer overexpressed 1
(PBOV1), also termed UROC28 or UC28, is a human
protein-coding gene with a 2,501-bp single-exon mRNA and an open
reading frame encoding a protein of 135 amino acids (7). The gene was first characterized by An
et al (7) in 2000 as being
overexpressed at the protein and mRNA levels in prostate, breast
and bladder cancer tissues, as well as in the glandular epithelium
(7–10). An additional study confirmed that the
expression of PBOV1 was increased in prostate cancer compared with
adjacent benign epithelium, and PBOV1 overexpression promoted cell
proliferation and colony formation ability, and tumorigenic ability
in vitro (11). Notably, PBOV1
is poorly conserved in mammalian evolution and is expressed in
multiple types of human tumors, but only rarely in normal tissue
samples (12). It was also identified
that the expression of PBOV1 in prostate cancer cells is
upregulated by dihydrotestosterone (12). In addition, PBOV1 transcription in
breast cancer cells was demonstrated to be downregulated by
estradiol in a dose-dependent manner (13), and the mRNA and protein expression of
PBOV1 was identified to be upregulated in ovarian cancer cell lines
(14). However, there were negative
associations between high PBOV1 expression and ascending
histological grade and late TNM stage (14). It was also suggested that patients
with high PBOV1 expression experience longer overall survival
times; and, therefore, it was hypothesized that PBOV1 may serve as
a tumor-suppressor gene in ovarian cancer, which is different from
previous data regarding prostate, breast and bladder cancer
(14). Therefore, additional studies
are required to understand the role of PBOV1 in the development of
cancer.
Considering the poor prognosis of HCC, and that the
role of PBOV1 in cancer remains incompletely characterized, the
present study aimed to investigate the expression pattern of PBOV1
in HCC and its association with clinicopathological features for
the first time. The effectiveness of PBOV1 as an independent
prognostic factor was assessed using multivariate analysis.
Materials and methods
Patients and frozen tissue
samples
A total of 109 patients (54 females and 55 males;
age range, 41–75 years; mean age, 57) with HCC who received
treatment between March 2006 and February 2010 at Zhongnan Hospital
of Wuhan University were enrolled. Written informed consent was
obtained from each patient. A total of 2 patients exhibited IVa
stage disease, and the maximum tumor size of these patients was
<13 cm. The tumor stages of all the enrolled patients were
classified according to the 7th Tumor-Node-Metastasis (TNM)
classification system of the International Union Against Cancer
(15). The patients who did not
receive percutaneous ablation, radiotherapy or chemoembolization
prior to surgery were selected and enrolled. The study was approved
and monitored by the Ethics Committee of Zhongnan Hospital of Wuhan
University (Hubei, China), and conformed to the guidelines of the
Declaration of Helsinki.
Paired tumor and non-tumor liver tissue samples were
obtained from all patients immediately following liver resection
(16), and snap frozen at −80°C until
use. The tissues were collected from the patients during surgery,
and the normal tissues were obtained from the patients at an ~5-cm
margin from the tumor tissues. The pathological diagnosis was
confirmed in all cases by the Department of Pathology, Zhongnan
Hospital of Wuhan University. Clinicopathological features of the
patients were recorded in the follow-up survey. In addition, the
associations between PBOV1 expression and clinicopathological
features were analyzed using a χ2 test, and the results
are summarized in Table I.
 | Table I.Associations between PBOV1 expression
with the clinicopathological features of hepatocellular
carcinoma. |
Table I.
Associations between PBOV1 expression
with the clinicopathological features of hepatocellular
carcinoma.
|
|
| PBOV1 expression,
n |
|
|---|
|
|
|
|
|
|---|
| Characteristic | N | High | Low | P-value |
|---|
| Sex |
|
|
|
|
| Male | 55 | 39 | 16 | NS |
|
Female | 54 | 34 | 20 |
|
| Age, years |
|
|
|
|
| ≥50 | 62 | 38 | 24 | NS |
|
<50 | 47 | 35 | 12 |
|
| Maximal tumor size,
cm |
|
|
|
|
| ≥5 | 67 | 50 | 17 | 0.032 |
|
<5 | 42 | 23 | 19 |
|
| Serum AFP level,
ng/ml |
|
|
|
|
| ≥400 | 61 | 44 | 17 | NS |
|
<400 | 48 | 29 | 19 |
|
| HBsAg |
|
|
|
|
|
Positive | 50 | 34 | 16 | NS |
|
Negative | 59 | 39 | 20 |
|
| Tumor metastasis |
|
|
|
|
| No | 58 | 43 | 15 | 0.035 |
| Yes | 51 | 30 | 21 |
|
| TNM stage |
|
|
|
|
| I–II | 49 | 27 | 22 | 0.017 |
|
III–IV | 60 | 46 | 14 |
|
Cell lines and transfection
Two HCC cell lines (HCCLM3 and HEP3B) and one normal
liver cell line (L02) were obtained from the American Type Culture
Collection (Manassas, VA, USA). These cell lines were cultured in
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.). All
cells were maintained at 37°C in a humidified atmosphere containing
5% CO2 and 95% air.
PBOV1 short hairpin RNAs (shRNAs) were designed
according to a previous study, and synthesized by Shanghai GeneChem
Co., Ltd. (Shanghai, China) (10).
The shRNA sequences were as follows: shRNA 1,
5′-CCAGCCAAGTAACTGAACCAT; and shRNA 2, GCAGACACACTTGACCATGAA-3′.
HCCLM3 and HEP3B cells were transfected with 4 µg PBOV1 shRNA using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 24 h, as described previously (17).
RNA extraction, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted from all cultured cell lines
and the 109 tissue samples using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Purified RNA was additionally treated with
RNase-free DNase I (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany).
The first-strand cDNA was synthesized using a
RevertAid First Strand cDNA Synthesis kit (cat. no. K1621;
Fermentas; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA),
including oligo(dT) primers. The whole experimental procedure was
performed following the manufacturer's protocol. The resulting cDNA
was stored at −20°C until use.
RT-qPCR was performed using an ABI Prism 7500
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and SYBR-Green I (Invitrogen; Thermo Fisher
Scientific, Inc.). PCR primers targeting the PBOV1 coding sequence
were designed based on GenBank cDNA AF189270 (https://www.ncbi.nlm.nih.gov/gene/?term=AF189270).
PBOV1-specific primers used were as follows: Forward,
5′-AAGGAACCAGAAATATGAGG-3′ and reverse, 5′-TTTGGATAAGTAGAGAAGAC-3′.
The amplification was performed in the following conditions: 1 min
at 95°C, then 35 cycles of 30 sec at 95°C, 30 sec at 58°C and 40
sec at 72°C, and a final elongation step at 72°C for 5 min. The
housekeeping gene GAPDH was used as an internal control to
normalize the gene expression data. The GAPDH-specific primers used
were as follows: Forward, 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and
reverse, 5′-CATGTGGGCCATGAGGTCCACCAC-3′. The same PCR conditions
were used. The expression level of PBOV1 was calculated as
2−[(Cq of PBOV1)-(Cq of GAPDH)], where Cq represents the
quantification cycle value for each transcript (18). Experiments were repeated in triplicate
to ensure accuracy. The levels of PBOV1 were used to classify these
patients into high or low PBOV1 expression group (threshold value,
1.05).
Western blot assay
Total proteins were extracted from 109 fresh tissue
pairs and the cultured cells using a Total Protein Extraction kit
(cat. no. KGP2100) with protease inhibitor, phosphatase inhibitor,
and PMSF (Nanjing Keygen Biotech, Co., Ltd., Nanjing, China),
according to the manufacturer's protocol. The protein concentration
was measured using a Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). Equal amounts (50 µg) of each protein sample
were loaded and separated via 10% SDS-PAGE. Then, the samples were
transferred onto a PVDF membrane, and the membrane was blocked with
5% fat-free milk at room temperature for 2 h. The membrane was
probed with an anti-PBOV1 antibody (1:1,000 dilution; cat. no.
ab70018) and an anti-GAPDH antibody (1:1,000 dilution; cat. no.
ab181602; both Abcam, Cambridge, MA, USA) at 4°C overnight.
Expression of PBOV1 was determined with a horseradish
peroxidase-conjugated anti-rabbit IgG (1:3,000 dilution; cat. no.
ab6721; Abcam) at room temperature for 2 h. The signals were
developed using SuperSignal West Pico PLUS (Pierce; Thermo Fisher
Scientific, Inc.) and quantified using Bio-Rad Gel Doc EZ system
with the Image Lab 3.0 software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), according to the manufacturer's protocol
(19). Experiments were repeated in
triplicate to ensure accuracy.
MTT assay
The effect of PBOV1 on cell proliferation was
assessed with an MTT assay (Sigma-Aldrich; Merck KGaA), as
described previously (20). Cells
were seeded into 96-well culture plates at a density of
1×104/well. At various time points (12, 24, 36 and 48
h), 10 µl (50 µg) MTT was added to each well and incubated for 4 h
at 37°C. Next, the medium was removed, and 150 µl dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA) was added to dissolve the
resultant formazan crystals. Absorbance was measured using a
microplate reader at 450 nm. All experiments were performed in
triplicate.
Statistical analysis
All statistical analyses were performed using the
SPSS 10.0 statistical software package (SPSS, Inc., Chicago, IL,
USA). The data was presented as mean ± standard deviation. The
associations between PBOV1 expression and clinicopathological
characteristics were analyzed using a χ2 test. The
survival probability was calculated using the Kaplan-Meier method,
and the statistical differences were examined using a log-rank
test. Multivariate analysis was performed to assess the variables
deemed significant in univariate analyses, each performed using a
Cox proportional hazards regression model. Differences between two
groups were calculated using a Student's t-test. One-way analysis
of variance and Tukey's post-hoc test were performed to analyze the
statistical differences between ≥3 groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
PBOV1 is upregulated in HCC cell lines
and tissues
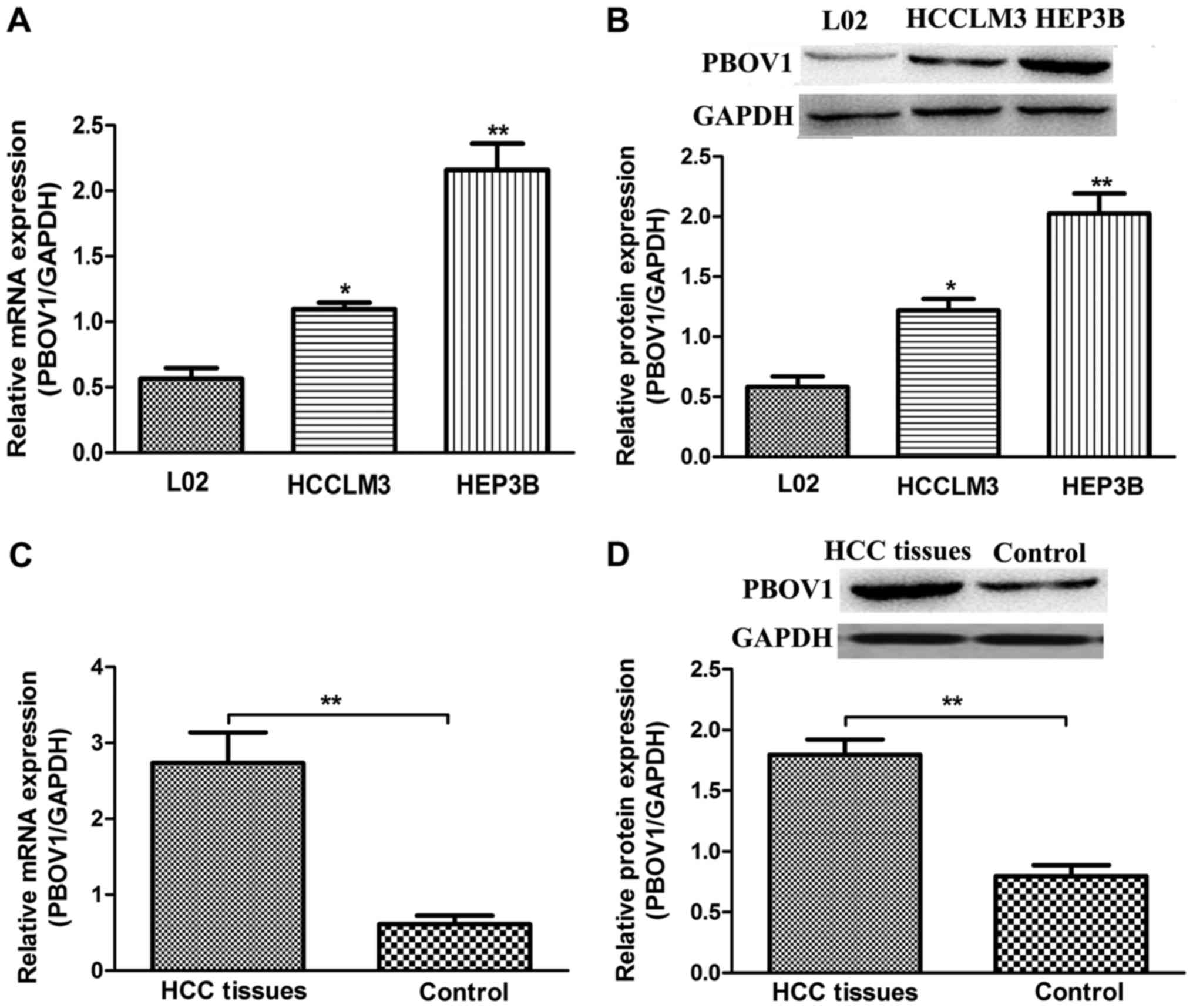
The mRNA and protein levels of PBOV1 were examined
in L02, HCCLM3 and HEP3B cell lines using RT-qPCR and western blot
analysis, respectively. The data indicated that the PBOV1
expression levels (mRNA and protein) in the HCC cell lines were
significantly increased compared with in the normal liver L02 cell
line (P<0.05; Fig. 1A and B). Of
the HCC cell lines investigated the HEP3B cell line exhibited the
highest mRNA and protein PBOV1 expression levels. Collectively,
these results suggest that PBOV1 is upregulated in HCC cell
lines.
To investigate whether PBOV1 may be involved in the
progression of HCC, the mRNA and protein expression levels of PBOV1
in all the collected HCC tissues and their paired adjacent
noncancerous tissues were determined. It was observed that the
expression of PBOV1 (mRNA and protein) was significantly
upregulated in the HCC tissues when compared with in the matched
noncancerous tissues (P<0.01; Fig. 1C
and D). Among the 109 patients, 73 patients (66.97%) exhibited
higher PBOV1 expression levels in tumor tissues compared with in
the matched noncancerous tissues, and, thus, these 73 patients were
assigned to the high PBOV1 expression group. The remaining 36
patients who exhibited lower PBOV1 expression levels were assigned
to the low PBOV1 expression group. These data demonstrate the
upregulated status of PBOV1 in HCC; and it was hypothesized that
PBOV1 may serve an important role in the progression of HCC.
PBOV1 promotes proliferation of HCC
cell lines
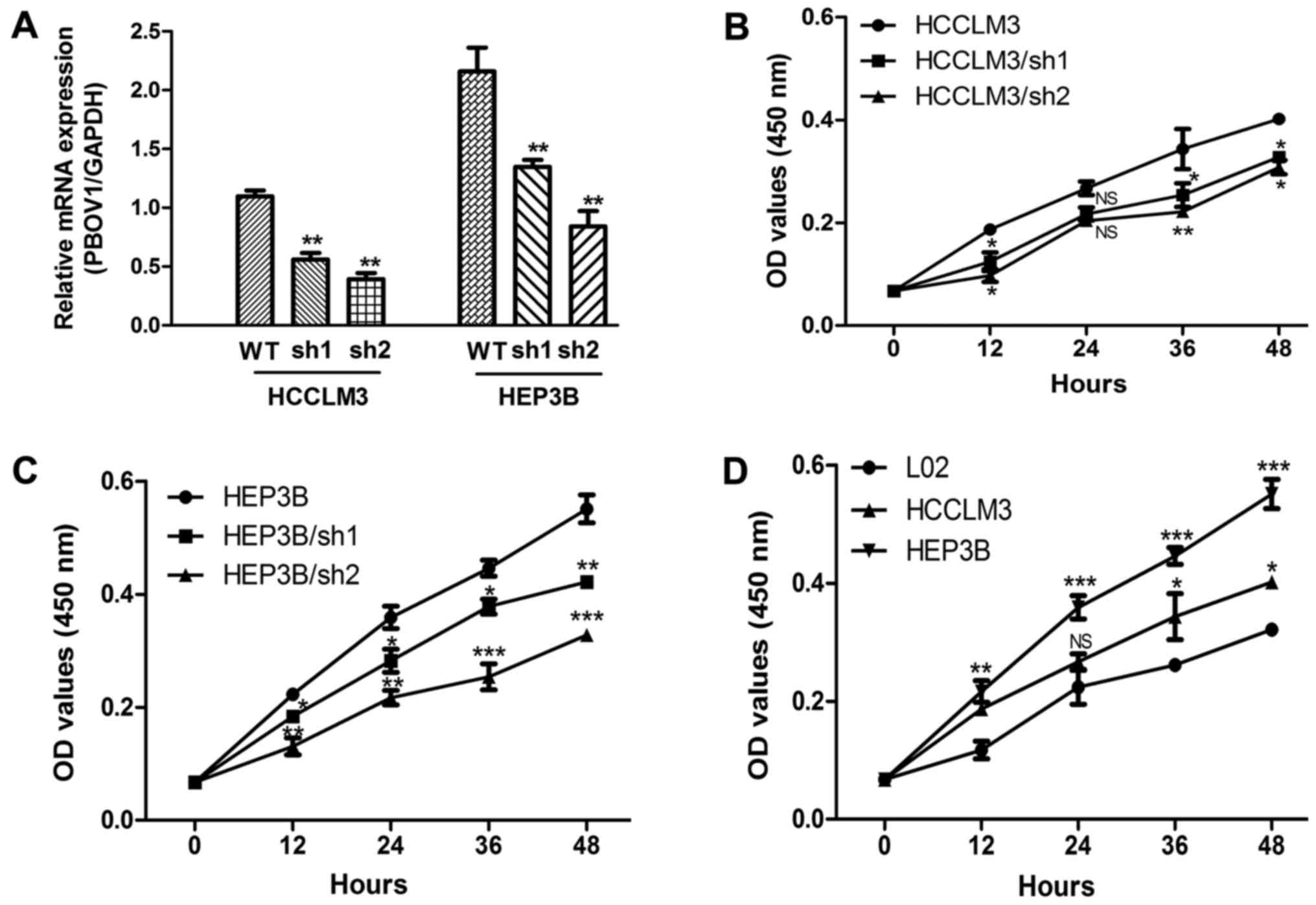
In order to assess the oncogenic activity of PBOV1
in HCC, a stable PBOV1-knockdown HCC cell line was established
using specific shRNA. The PBOV1 mRNA expression level of these
transfected cell lines was assessed by RT-qPCR. As expected, the
transfection of the PBOV1-shRNA into the HCC cell lines resulted in
significantly decreased PBOV1 mRNA expression levels when compared
with the cell lines without shRNA transfection (all P<0.01;
Fig. 2A). Furthermore, the
proliferation rates of these cell lines were measured using an MTT
assay. The suppression of PBOV1 expression significantly inhibited
the proliferation of the HCCLM3 cell line in all the time points
selected except in 24 h (P<0.05; Fig.
2B). However, the cell proliferation rate of HEP3B was
inhibited by shRNAs in all the time points selected (all P<0.05;
Fig. 2C) The proliferation rates of
HCC and normal liver cell lines were also compared. The results in
Fig. 2D indicated that the cell
proliferation rates of the HEP3B cell line were significant
increased compared with the normal liver cell line in all the time
points selected (P<0.05). Additionally, the cell proliferation
rates of HCCLM3 cell line was higher than normal liver cell line in
all the time points selected except in 24 h (P<0.05; Fig. 2D). Taken together, these results
suggest the PBOV1 expression is an important regulator of HCC cell
proliferation.
Association between PBOV1 expression
and clinicopathological features
In order to analyze the associations between PBOV1
expression and the clinicopathological characteristics of patients
with HCC, the 109 patients were divided into two groups according
to the PBOV1 expression level. The clinicopathological features
were collected from all 109 patients and summarized in Table I. A χ2 test was conducted
to evaluate the association between PBOV1 expression and all the
clinicopathological features. As indicated in Table I, PBOV1 expression was significantly
associated with maximal tumor size (P=0.032), tumor metastasis
(P=0.035), and tumor stage (P=0.017), while no significant
associations between PBOV1 expression and other variables, such as
age, sex, hepatitis B surface antigen (HBsAg) and serum AFP, were
observed (all P>0.05).
Prognostic value of high PBOV1
expression in HCC
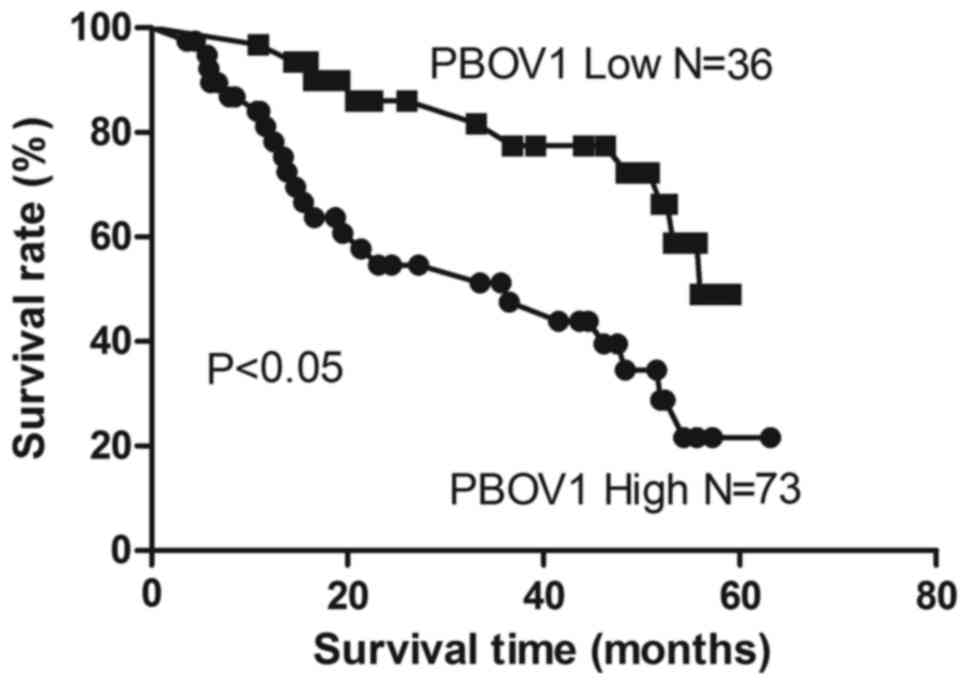
The association between PBOV1 expression and the
survival of patients with HCC following surgery was investigated
using the Kaplan-Meier analysis and log-rank test. As demonstrated
in Fig. 3, patients with high PBOV1
expression levels exhibited a significantly shorter survival time
following surgery compared with patients with low PBOV1 expression
levels (P<0.05). Univariate analysis revealed that among the
collected clinicopathological features, maximal tumor size, tumor
metastasis, tumor stage and PBOV1 expression were significantly
associated with the overall survival of HCC patients (all
P<0.05; Table II), while the
other features were not statistically significant prognosticators
(all P>0.05; Table II).
Multivariate analysis using the Cox proportional hazards model for
all variables identified that PBOV1 expression was a significant
independent predictor of poor prognosis in patients with HCC in
addition to maximal tumor size, tumor metastasis and tumor stage
(all P<0.05; Table II).
 | Table II.Univariate and multivariate analyses
of overall survival. |
Table II.
Univariate and multivariate analyses
of overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| PBOV1 | 2.416 | 1.176–4.962 | 0.016 | 2.142 | 1.146–4.004 | 0.010a |
| Age | 1.604 | 0.869–2.960 | 0.131 | 1.714 | 0.888–3.310 | 0.108 |
| Sex | 1.666 | 0.897–3.095 | 0.106 | 1.613 | 0.828–3.140 | 0.160 |
| Maximal tumor
size | 2.262 | 1.112–4.599 | 0.024 | 2.253 | 1.165–4.358 | 0.015a |
| Serum AFP
level | 1.950 | 0.960–3.961 | 0.065 | 1.824 | 0.952–3.494 | 0.070 |
| HBsAg | 1.838 | 0.975–3.462 | 0.059 | 1.746 | 0.918–3.320 | 0.089 |
| Tumor
metastasis | 2.083 | 1.036–4.188 | 0.039 | 2.140 | 1.097–4.176 | 0.026a |
| TNM stage | 2.029 | 1.030–3.996 | 0.040 | 1.912 | 1.012–3.612 | 0.046a |
Discussion
Previous data have demonstrated that one prominent
feature of various tumor types is the abundant upregulation of
various transcripts, a number of which have an uncharacterized
function (21–23). The PBOV1 is gene encoding a
protein of 135 amino acids in length, located at chromosome 6q23-24
region (7). PBOV1 has been
identified to be overexpressed in a number of types of human
cancer, including ovarian (14),
breast (13), endometrial and
prostate cancer (11). Previous
studies have demonstrated that overexpression of PBOV1 promoted
cell proliferation, cell cycle progression and tumorigenicity in
vitro, whereas the knockdown of PBOV1 reduced these effects
(11,14). However, whether or not PBOV1 has
similar expression pattern in HCC as these aforementioned cancer
types remains unknown.
Therefore, it is required to examine the expression
level of PBOV1 in HCC, in order to additionally investigate whether
or not PBOV1 participates in the progression of HCC. The expression
of PBOV1 in one normal liver cell line and two HCC cell lines were
examined, and the results demonstrated that PBOV1 was significantly
upregulated in the HCC cell lines, compared with in the normal cell
line. Considering this, a total of 109 paired HCC tumor tissues and
adjacent noncancerous tissues were collected. It was identified
that, in comparison with the adjacent noncancerous in tissues,
PBOV1 mRNA and protein levels were upregulated in HCC tumor
tissues. These data in HCC were the same as the results from the
analyses in ovarian, breast, endometrial and prostate cancer, and
suggest that PBOV1 may serve a role in the tumorigenesis of HCC.
However, how PBOV1 regulates HCC progression requires additional
exploration.
Using PBOV1-specific shRNA, a stable PBOV1-knockdown
HCC cell line was created. The PBOV1 mRNA expression data revealed
the successful PBOV1 knockdown in the selected HCC cell lines.
Then, the cell proliferation rate analysis results indicated that
the introduction of PBOV1 shRNA significantly downregulated the
cell proliferation rates. Concurrently, the normal liver cell line,
with the lowest PBOV1 expression among all the cell lines used,
also exhibited the lowest cell proliferation rate. Therefore, the
results of the present study suggested that PBOV1 overexpression
may promote HCC cancer cell proliferation and described a potential
mechanism of how PBOV1 promotes tumorigenesis in HCC; however, the
detailed mechanism requires additional study. A previous study
investigating the effect of PBOV1 on prostate cells revealed that
the overexpression of PBOV1 suppressed cell cycle inhibitors
cyclin-dependent kinase inhibitor 1 and cyclin-dependent kinase
inhibitor 1B, increased retinoblastoma protein phosphorylation
levels and cyclin D1 expression and suggested that PBOV1
contributed to G1/S transition (11), which may also be a mechanism for the
proliferation-stimulatory effect of PBOV1.
The 109 archived paraffin-embedded HCC tumor tissues
were additionally divided into two groups according to the PBOV1
expression level, and the association of PBOV1 expression with the
clinicopathological features was analyzed. Notably, it was
identified that the expression of PBOV1 in HCC was closely
associated with well-known tumor malignancy indicators, including
tumor size, tumor metastasis and tumor stage. Concurrently, whether
or not the HBV infection was associated with PBOV1 expression, as
HBV infection is a major cause of HCC, was analyzed. However, it
was demonstrated that the PBOV1 expression was not associated with
HBsAg, and therefore it was hypothesized that there was no
association between HBV infection and PBOV1 expression level. Then,
the association between PBOV1 expression and survival duration was
assessed using a Kaplan-Meier analysis. The log-rank test indicated
that PBOV1 expression level was inversely associated with overall
survival of the patients with HCC. Next, the multivariate Cox
proportional hazards model suggested that PBOV1 expression was an
independent predictor for the overall survival duration of patients
with HCC.
In conclusion, PBOV1 overexpression is a common
feature in patients with HCC. Additionally, the present study
provides the clinical evidence that PBOV1 is an independent
prognostic factor for the outcome of patients with HCC. However,
the present study had certain limitations, including the fact that
the associations between HCV infection, degree of hepatitis, liver
cirrhosis and the expression of PBOV1 were not analyzed.
Independent validation of these clinical data and additional
investigation of the detailed effects of PBOV1 on cell behaviors
are required. Nevertheless, the present study provides a basis for
the development of a novel diagnostic and prognostic biomarker for
HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China-Xinjiang Joint Fund (grant no.
U1403222).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CBX, ZBZ, SJY, YFW and QFY conceived and designed
the experiments, performed the experiments, and analyzed the data.
CBX and QFY wrote the paper.
Ethics approval and consent to
participate
The study was approved and monitored by the Ethics
Committee of Zhongnan Hospital of Wuhan University, and conformed
to the ethical guidelines of the Helsinki Declaration. Written
informed consent was obtained from all the enrolled patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cui Y and Jia J: Update on epidemiology of
hepatitis B and C in China. J Gastroenterol Hepatol. 28 Suppl
1:S7–S10. 2013. View Article : Google Scholar
|
|
3
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: Incidence and risk
factors. Gastroenterology. 127 5 Suppl 1:S35–S50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Block TM, Mehta AS, Fimmel CJ and Jordan
R: Molecular viral oncology of hepatocellular carcinoma. Oncogene.
22:5093–5107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
An G, Ng AY, Meka CS, Luo G, Bright SP,
Cazares L, Wright GL Jr and Veltri RW: Cloning and characterization
of UROC28, a novel gene overexpressed in prostate, breast, and
bladder cancers. Cancer Res. 60:7014–7020. 2000.PubMed/NCBI
|
|
8
|
Loizidou MA, Cariolou MA, Neuhausen SL,
Newbold RF, Bashiardes E, Marcou Y, Michael T, Daniel M, Kakouri E,
Papadopoulos P, et al: Genetic variation in genes interacting with
BRCA1/2 and risk of breast cancer in the Cypriot population. Breast
Cancer Res Treat. 121:147–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Samusik N, Krukovskaya L, Meln I, Shilov E
and Kozlov AP: PBOV1 is a human de novo gene with tumor-specific
expression that is associated with a positive clinical outcome of
cancer. PLoS One. 8:e561622013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doak SH, Jenkins SA, Hurle RA, Varma M,
Hawizy A, Kynaston HG and Parry JM: Bone morphogenic factor gene
dosage abnormalities in prostatic intraepithelial neoplasia and
prostate cancer. Cancer Genet Cytogenet. 176:161–165. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan T, Wu R, Liu B, Wen H, Tu Z, Guo J,
Yang J and Shen G: PBOV1 promotes prostate cancer proliferation by
promoting G1/S transition. Onco Targets Ther. 9:787–795. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krukovskaia LL, Samusik ND, Shilov ES,
Polev DE and Kozlov AP: Tumor-specific expression of PBOV1, a new
gene in evolution. Vopr Onkol. 56:327–332. 2010.(In Russian).
PubMed/NCBI
|
|
13
|
Kamagata C, Tsuji N, Kondoh K, Sasaki M,
Kobayashi D, Yagihashi A and Watanabe N: Enhanced expression of the
UROC28 gene in human breast cancer: Relationship to ERBB2 gene
expression. Anticancer Res. 22:4087–4091. 2002.PubMed/NCBI
|
|
14
|
Wang L, Niu CH, Wu S, Wu HM, Ouyang F, He
M and He SY: PBOV1 correlates with progression of ovarian cancer
and inhibits proliferation of ovarian cancer cells. Oncol Rep.
35:488–496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual. 7th ed.
Springer; New York: pp. 191–200. 2010
|
|
16
|
Yamashita Y, Taketomi A, Shirabe K,
Aishima S, Tsuijita E, Morita K, Kayashima H and Maehara Y:
Outcomes of hepatic resection for huge hepatocellular carcinoma
(≥10 cm in diameter). J Surg Oncol. 104:292–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hahn WC, Dessain SK, Brooks MW, King JE,
Elenbaas B, Sabatini DM, DeCaprio JA and Weinberg RA: Enumeration
of the simian virus 40 early region elements necessary for human
cell transformation. Mol Cell Biol. 22:2111–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Zhang N, Song LB, Liao WT, Jiang LL,
Gong LY, Wu J, Yuan J, Zhang HZ, Zeng MS and Li M: Astrocyte
elevated gene-1 is a novel prognostic marker for breast cancer
progression and overall patient survival. Clin Cancer Res.
14:3319–3326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song L, Wang L, Li Y, Xiong H, Wu J, Li J
and Li M: Sam68 up-regulation correlates with, and its
down-regulation inhibits, proliferation and tumourigenicity of
breast cancer cells. J Pathol. 222:227–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma W, Yu Q, Jiang J, Du X, Huang L, Zhao L
and Zhou QI: miR-517a is an independent prognostic marker and
contributes to cell migration and invasion in human colorectal
cancer. Oncol Lett. 11:2583–2589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang G, Huang J, Zhu H, Ju S, Wang H and
Wang X: Overexpression of GRO-β is associated with an unfavorable
outcome in colorectal cancer. Oncol Lett. 11:2391–2397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang B, Tang F, Wang Z, Qi G, Liang X, Li
B, Yuan S, Liu J, Yu S and He S: Overexpression of CTNND1 in
hepatocellular carcinoma promotes carcinous characters through
activation of Wnt/β-catenin signaling. J Exp Clin Cancer Res.
35:822016. View Article : Google Scholar : PubMed/NCBI
|