Introduction
As the fourth most common type of malignant tumor in
females, cervical cancer causes an unacceptably high mortality rate
worldwide (1). Cervical cancer may be
divided into two major subgroups, including cervical squamous cell
carcinoma and cervical adenocarcinoma (2). As the dominant cervical cancer type,
cervical squamous cell carcinoma accounts for 80–90% of all
cervical cancer cases (3). Human
papillomaviruses (HPV) infection has been demonstrated to be
closely associated with the occurrence and development of cervical
cancer (3,4), and associations between certain HPV
genotypes and the incidence of cervical cancer are well-established
(5). With the development of HPV
infection screening programs and a continually increasing HPV
vaccination rate, the incidence of cervical squamous cell carcinoma
has been significantly reduced (3–5). However,
the incidence of cervical adenocarcinoma has been demonstrated to
have increased from 5 to 24% in the previous 30 years (6,7), due to
cervical cancer also being caused by factors other than HPV
infection, and the prognosis of HPV-negative cervical cancer is
usually poor (7). Therefore, the
development of novel prevention and treatment modalities for
cervical cancer are required.
The development of cervical cancer is a complex
process with various internal and external factors involved
(8). A recent study suggested that
Erb-b2 receptor tyrosine kinase 3 (ERBB3) is likely to be involved
in the development of cervical cancer (8). However, the functionality of ERBB3 in
the pathogenesis of this disease remains unclear. A previous study
indicated that ERBB3 may promote the migration and invasion of
breast cancer cells and increased resistance of cancer cells to
targeted therapy (9). In contrast,
degradation of ERBB3 mediated by E3 ubiquitin-protein ligase NRDP1
(NRDP1) was demonstrated to inhibit the migration and invasion of
human glioma cells (10). In light of
the data from previous studies, it is reasonable to hypothesize
that ERBB3 may also participate in the progression of cervical
cancer by regulating the migration and invasion of cancer
cells.
In the present study, the expression levels of ERBB3
in tumor and normal tissues of patients with cervical squamous cell
carcinoma and cervical adenocarcinoma and in different cervical
lines with or without HPV infection were measured by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
compared. Additionally, the effects of ERBB3 on cancer cell
proliferation, migration and invasion were also investigated.
Materials and methods
Patients and tissue collection
A total of 25 females with cervical squamous cell
carcinoma and 25 females with cervical adenocarcinoma who were
treated at the Luodian Hospital (Shanghai, China) from July 2014 to
July 2016 were enrolled in the present study. All patients were
diagnosed by pathological and imaging examinations. All included
patients were diagnosed with cervical cancer and were being treated
for the first time. Patients with other types of malignancies (such
as lung, liver, gastric cancers), other severe diseases (such as
cardiovascular disease) and other cervical diseases (such as
cervicitis and cervical ectropion) were excluded. The age of the
patients with cervical squamous cell carcinoma ranged from 26 to 71
years, with an average age of 55±7.7 years. The age of the patients
with cervical adenocarcinoma ranged from 25 to 80 years, with an
average age of 57±9.9 years. All patients were treated with
surgical resection, and tumor and normal tissues ≥5 cm around the
tumor were collected during surgery. All patients provided written
informed consent. The present study was approved by the ethics
committee of the Luodian Hospital (Shanghai, China).
Cell lines and cell culture
Human cervical squamous cell carcinoma SiHa (HPV
positive) and C33A (HPV negative) cell lines, and human normal
cervical Ect1/E6E7 (HPV positive) and HCvEpC (HPV negative) cell
lines were purchased from American Type Culture Collection (ATCC;
Manassas, VA, USA). All cells were cultured in ATCC-formulated
Eagle's Minimum Essential Medium (cat no. 30–2003; ATCC) containing
10% fetal bovine serum (Thermo Fisher Scientific) in an incubator
(37°C, 5% CO2). Cells were harvested during the
logarithmic growth phase for subsequent experiments.
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used to extract
total RNA from the tumor tissues, adjacent healthy tissues and
in vitro cultured cells of SiHa, C33A, Ect1/E6E7 and HCvEpC
cell lines. Tumor and normal tissues were ground in liquid nitrogen
prior to the addition of TRIzol® reagent. Following
this, cDNA was then synthesized using SuperScript III Reverse
Transcriptase (Thermo Fisher Scientific, Inc.) with total RNA as
the template. SYBR® Green Real-Time PCR Master Mixes
(Thermo Fisher Scientific, Inc.) and cDNA were then used to prepare
the PCR reaction system. ERBB3 primers (cat. no. qHsaCIP0031829)
were purchased from Bio-Rad Laboratories, Inc. (Hercules, CA, USA).
The primers of the endogenous control β-actin were: Forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
The PCR was conducted on a CFX96 Touch™ Real-Time PCR Detection
System (Bio-Rad Laboratories, Inc.). PCR thermocycler conditions
were: 95°C for 45 sec, followed by 40 cycles of 95°C for 10 sec and
60°C for 45 sec, and the final extension step at 72°C for 5 min.
mRNA levels were quantified using the 2−ΔΔCq method
(11), and the relative expression
level of each gene was normalized to the endogenous control
β-actin. This experiment was repeated 3 times.
Establishment of ERBB3 small
interfering (si)RNA silencing cell lines
ErbB-3 siRNA (h) sc-35327 and control siRNA-A sc-370
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). Transfection (Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to transfect 10 nM siRNA into
5×105 cells. Cells were cultured for another 48 h before
subsequent experiments. Cells without transfection were used as
control, and cells transfected with 10 nM control siRNA-A was used
as negative control.
Western blot analysis
Total protein was extracted from cells of SiHa,
C33A, Ect1/E6E7and HCvEpC cell lines using a RIPA solution (Thermo
Fisher Scientific, Inc.). The BCA method was used for protein
determination. A total of 30 µg protein from each sample was
subjected to electrophoresis using 10% SDS-PAGE gel, followed by
transfer to a polyvinylidene fluoride membrane. Following washing
with TBST, membranes were incubated with 5% skimmed milk at room
temperature for 2 h. Following washing with TBST, primary
antibodies including rabbit anti-CFTR antibody (1:1,000; cat. no.
ab5470), rabbit anti-MTK1 antibody (1:2,000; cat. no. ab186125),
and rabbit anti-β-actin antibody (1:1,000; cat. no. ab8226; all
Abcam, Cambridge, UK) overnight at 4°C. Following washing with
TBST, membranes were incubated with anti-rabbit IgG-HRP secondary
antibody (1:1,000; cat. no. MBS435036; MyBioSource, Inc., San
Diego, CA, USA) at room temperature for 2 h. Signals were detected
following the addition of ECL detection reagent (Sigma-Aldrich:
Merck KGaA, Darmstadt, Germany). Image J V1.6 software (National
Institutes of Health, Bethesda, MD, USA) was then used to normalize
the relative expression level of each protein to endogenous control
β-actin. This experiment was repeated 3 times.
Cell migration and invasion assay
The cell migratory ability was detected by Transwell
cell migration assay (BD Biosciences, Franklin Lakes, NJ, USA).
Briefly, 5×104 cells of SiHa and C33A cell lines in
serum-free RPMI-1640 medium (Thermo Fisher Scientific, Inc.) were
transferred to the upper chamber, while RPMI-1640 medium
supplemented with 20% fetal calf serum (Sigma-Aldrich: Merck KGaA)
was used to fill the lower chamber. The cells were incubated for 24
h at 37°C, and stained with 0.5% crystal violet (Sigma-Aldrich:
Merck KGaA) at room temperature for 20 min. Stained cells were
counted under an optical microscope (magnification, ×20; Olympus
Corporation, Tokyo, Japan). The same method was used to perform the
invasion assay, with the exception that the upper chamber was
pre-coated with Matrigel® (EMD Millipore, Billerica, MA,
USA) at room temperature for 2 h prior to experimentation. Cells
transfected with control siRNA-A sc-370 were used as the negative
control. Cells without any transfection were used as a control.
This experiment was repeated 3 times. Cells were counted under a
light microscope (Olympus, Japan), and cell numbers were normalized
to that of control group which was set to 100.
Cell proliferation assay
The cell proliferation assay was performed using a
CCK-8 kit (Sigma-Aldrich: Merck KGaA) according to manufacturer's
protocol. A total of 100 µl cell suspension containing
5×103 cells of SiHa and C33A cell lines were added into
each well of 96-well plates, and CCK-8 solution (10 µl) was added
into each well and were incubated for 12, 24, 48, 72 or 96 h at
37°C. Following incubation at 37°C for an additional 4 h,
absorbance at 450 nm was measured using a microplate reader
(Bio-Rad Laboratories, Inc.). Cells transfected with control
siRNA-A sc-370 were used as the negative control. Cells without any
transfection were used as a control. This experiment was repeated 3
times.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
all statistical analyses. Data were expressed as mean ± standard
deviation. Comparisons of data between two groups were performed
using unpaired Student's t-test. Comparisons of data among multiple
groups were performed using one-way analysis of variance followed
by Least Significant Difference post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of ERBB3 mRNA in tumor and
normal tissues
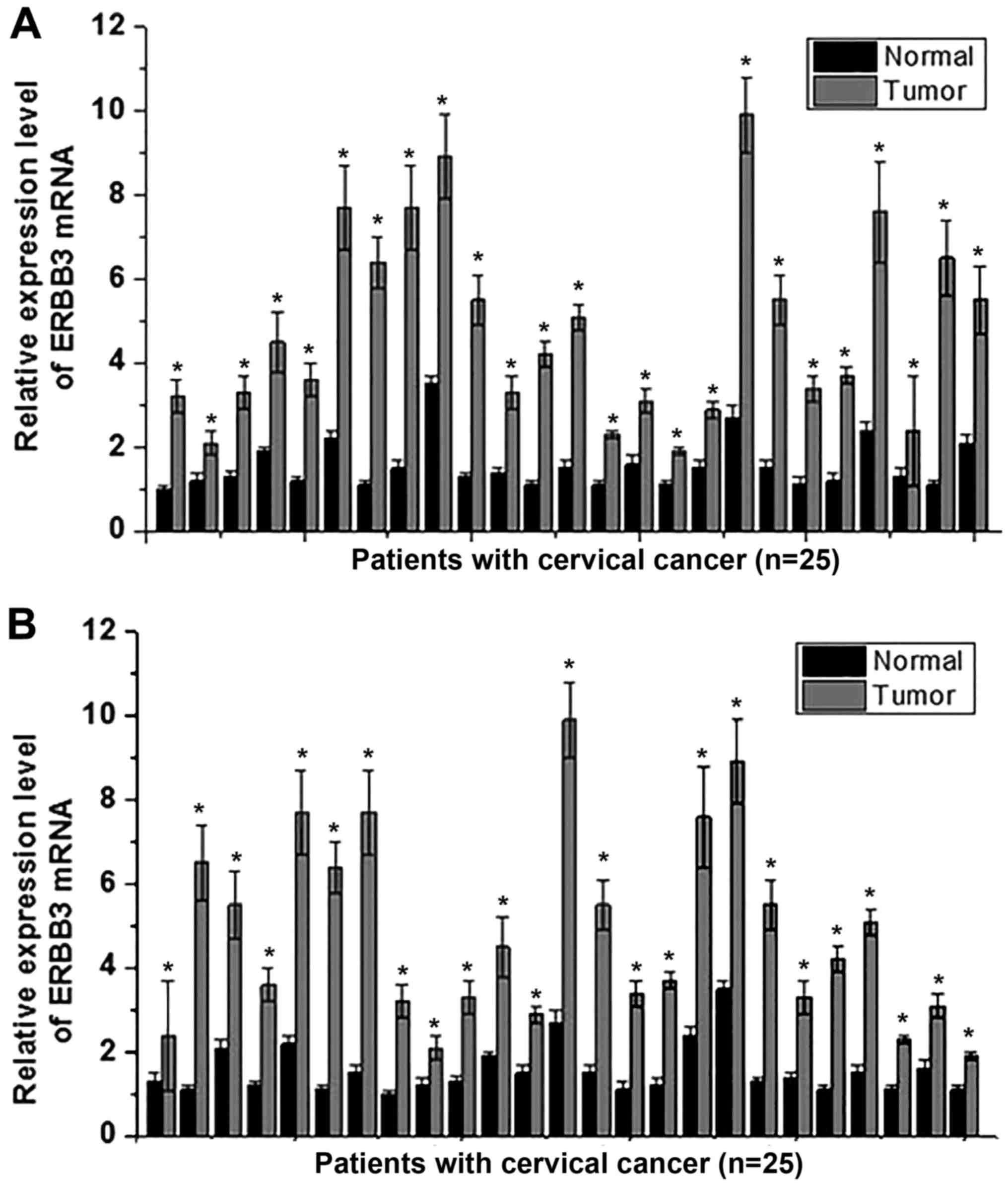
Expression levels of ERBB3 mRNA in tumor and normal
tissues in 25 patients with cervical squamous cell carcinoma and 25
patients with cervical adenocarcinoma were detected by RT-qPCR. The
results indicated that the expression level of ERBB3 mRNA was
significantly higher in tumor tissue compared with normal tissue
(P<0.05) in all 25 patients with cervical squamous cell
carcinoma (Fig. 1A) and in all 25
patients with cervical adenocarcinoma (Fig. 1B). These data suggests that the
downregulation of ERBB3 is likely to be involved in the
pathogenesis of cervical squamous cell carcinoma and cervical
adenocarcinoma.
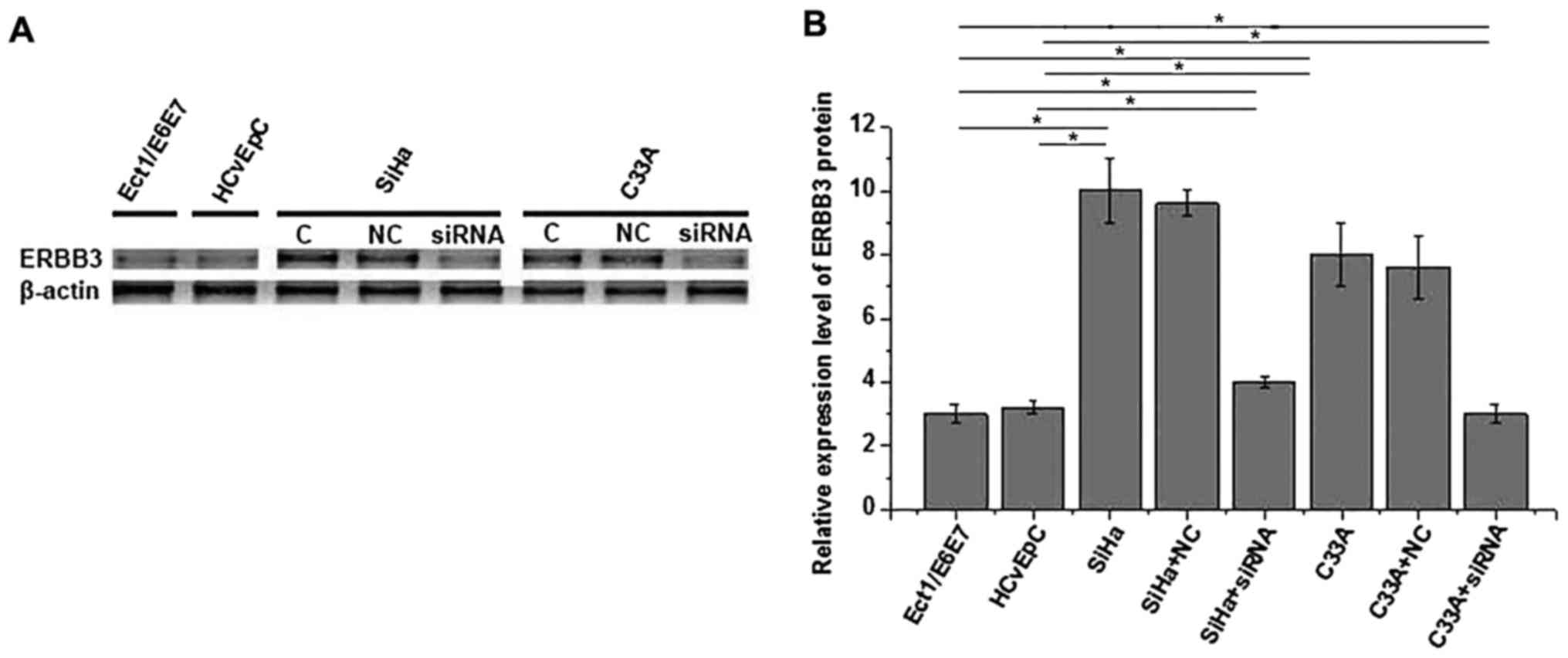
Expression of ERBB3 protein in
different cell lines
In the present study, human cervical squamous cell
carcinoma SiHa (HPV positive) and C33A (HPV negative) cell lines
and human normal cervical Ect1/E6E7 (HPV positive) and HCvEpC (HPV
negative) cell lines were used. As demonstrated in Fig. 2., no significant difference in the
expression level of ERBB3 protein was identified between the
Ect1/E6E7 and HCvEpC cells, or between the SiHa and C33A cells,
indicating that HPV infection has no significant effect on ERBB3
expression in cervical squamous cell carcinoma and normal cervical
cell lines. The expression level of ERBB3 was demonstrated to be
significantly increased in the SiHa and C33A cells compared with in
the Ect1/E6E7 and HCvEpC cells, indicating an increased expression
level in cervical squamous cell carcinoma. In addition, the
expression level of ERBB3 was significantly decreased following
siRNA silencing, indicating that the ERBB3-silenced cell lines were
established successfully.
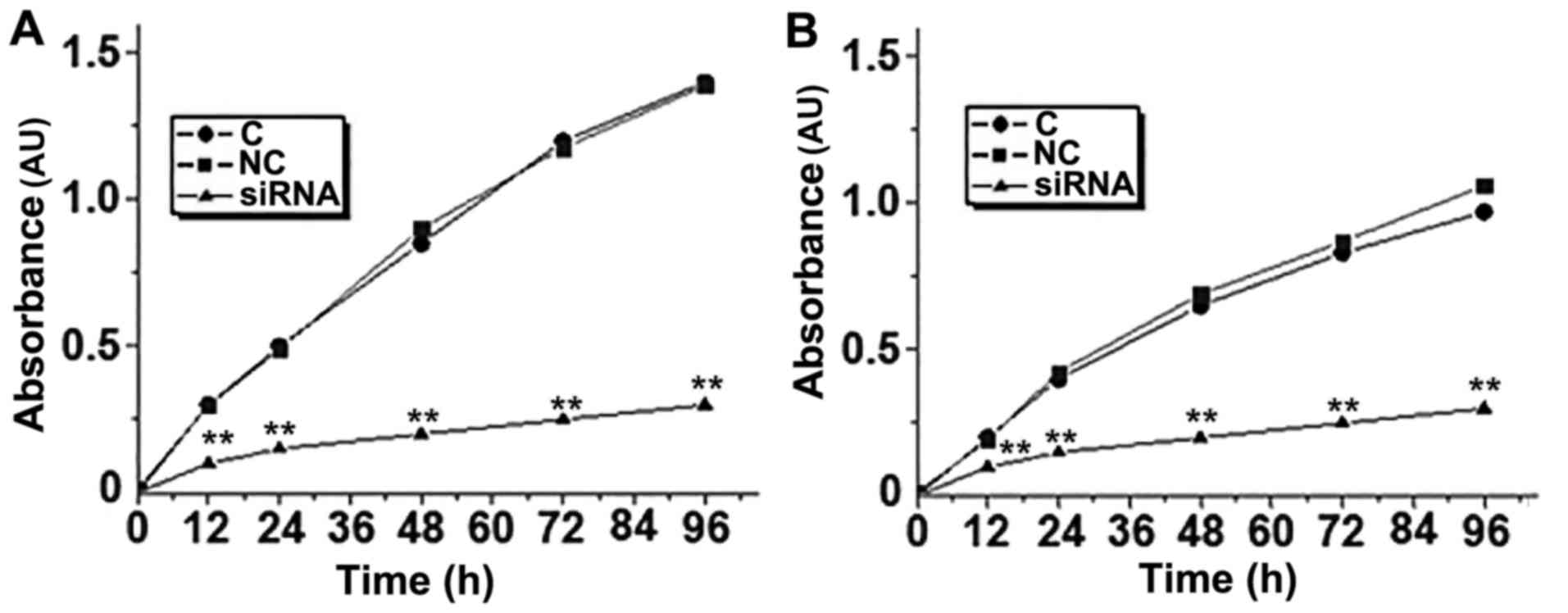
Effects of ERBB3 siRNA silencing on
the proliferation of SiHa and C33A cells
As demonstrated in Fig.
3., the proliferative abilities of the SiHa and C33A cells were
decreased significantly following ERBB3 siRNA silencing. These
results suggest that ERBB3 expression is important for the
proliferation of human cervical squamous cell carcinoma cells.
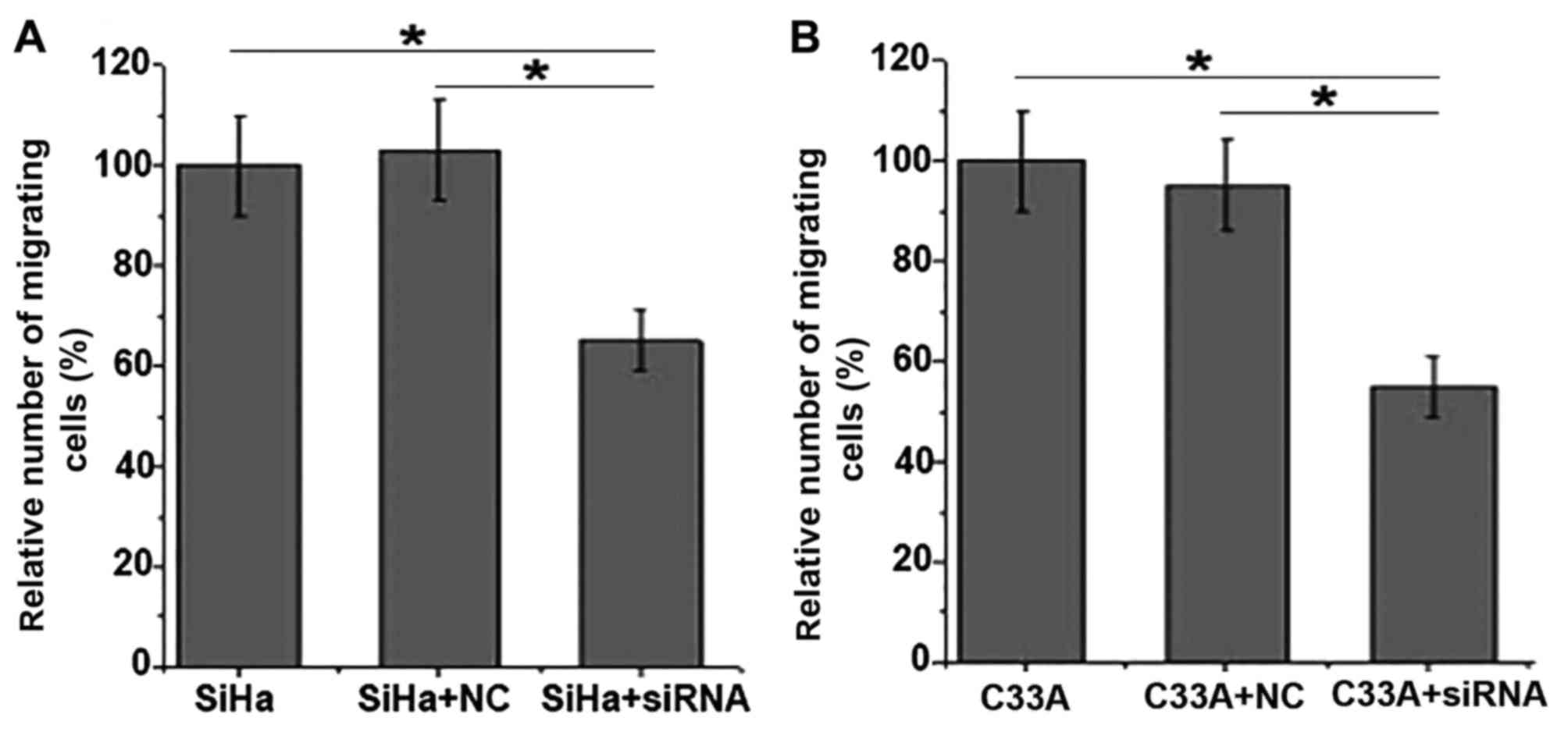
Effects of ERBB3 siRNA silencing on
the migration of SiHa and C33A cells
As indicated in Fig.
4, the results of the Transwell migration assay demonstrated
that the migratory abilities of the SiHa and C33A cells were
significantly decreased following EBRR3 siRNA silencing. These
results suggest that EBRRS expression is important for the
migration of SiHa and C33A cells.
Effects of ERBB3 siRNA silencing on
the invasion of SiHa and C33A cells
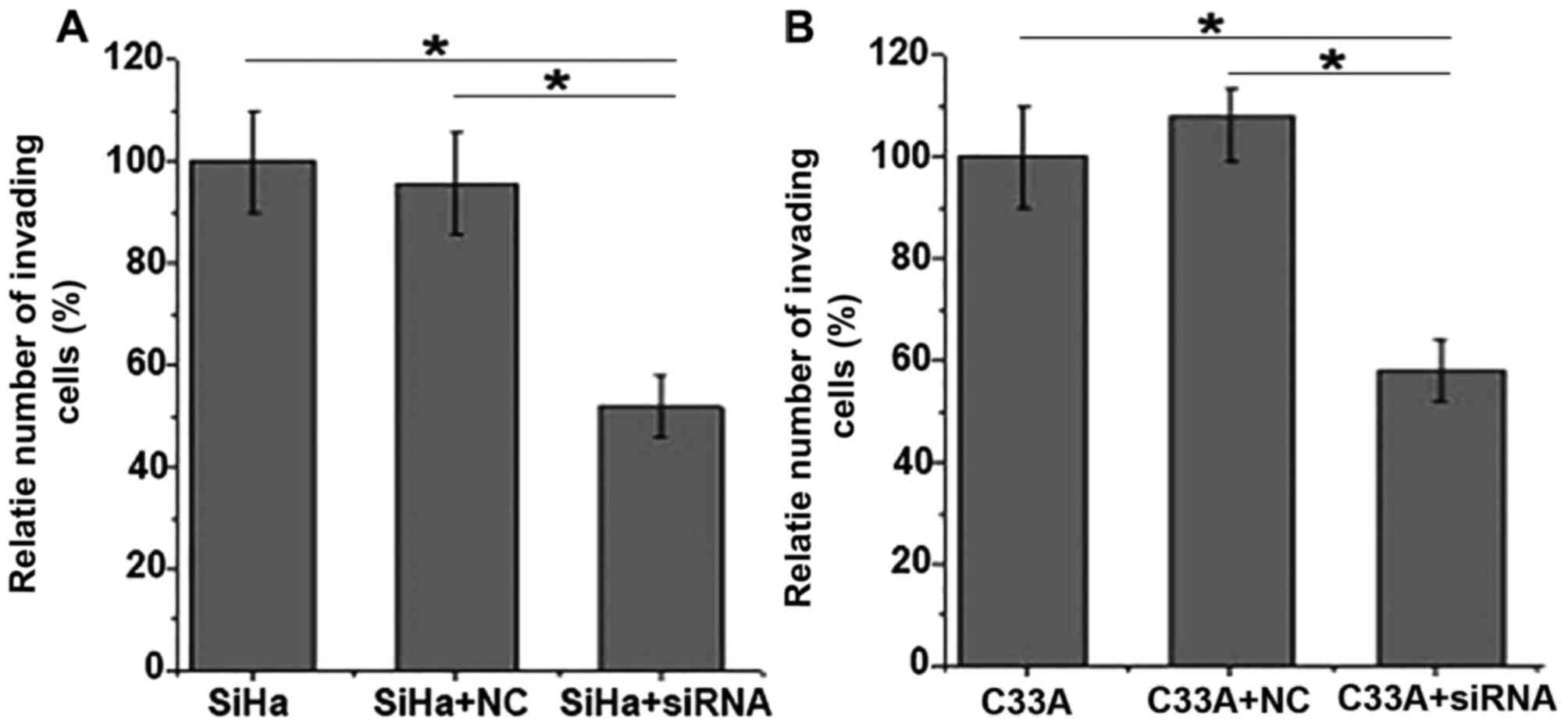
As demonstrated in Fig.
5, the results of the invasion assay demonstrated that the
invasive abilities of the SiHa and C33A cells were significantly
decreased following EBRR3 siRNA silencing. These results suggest
that the EBRRS expression is important for the invasion of SiHa and
C33A cells.
Effects of ERBB3 siRNA silencing on
expression of MTK-1 protein in SiHa and C33A cells
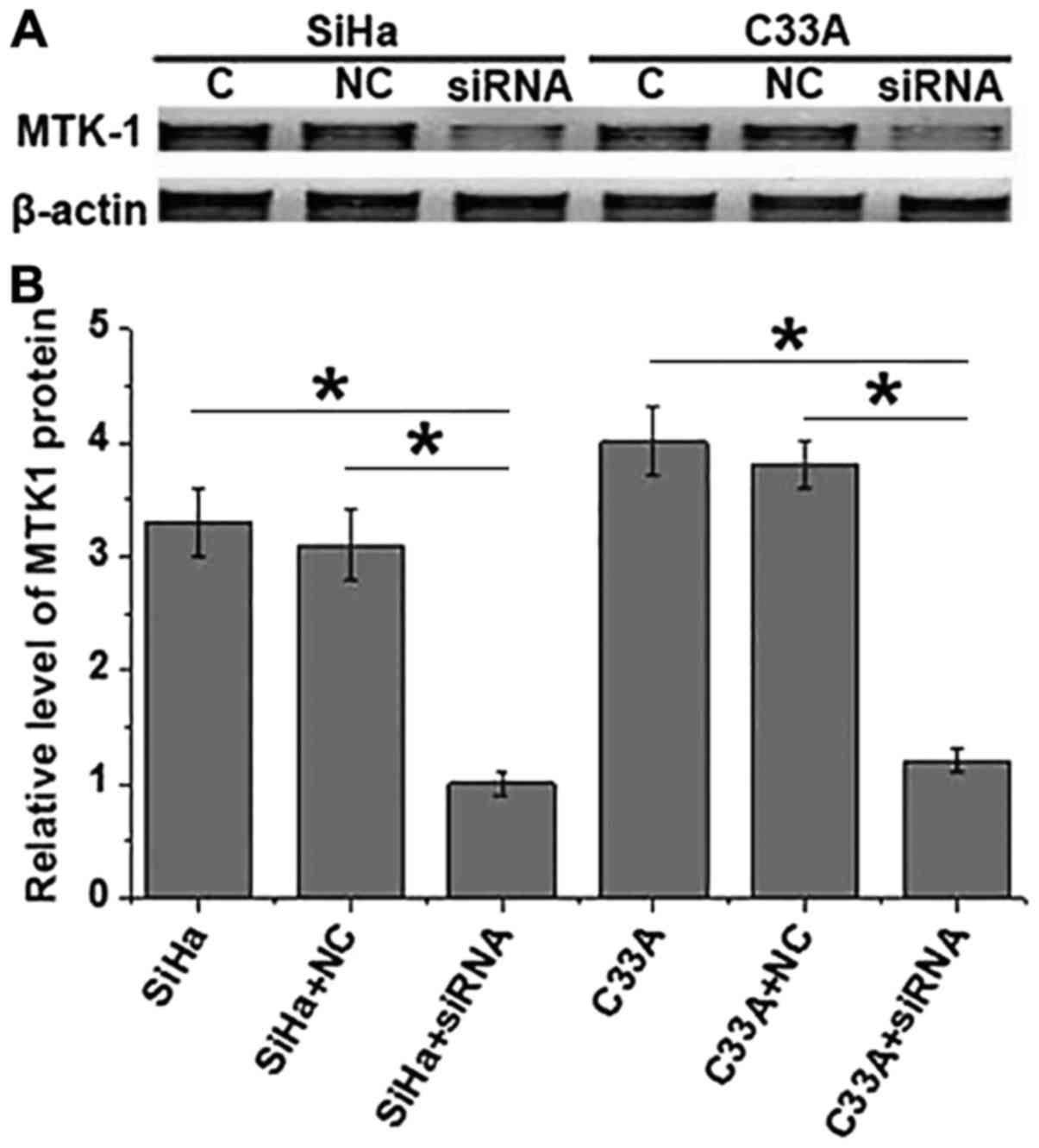
As indicated in Fig.
6, compared with SiHa and C33A cells without ERBB3 siRNA
silencing, the expression level of the MTK-1 protein was
significantly decreased in the SiHa and C33A cells with ERBB3 siRNA
silencing. These results suggest that the expression level of the
MTK-1 protein was decreased with the decreased expression level of
ERBB3.
Discussion
As a membrane-bound protein, the functions of ERBB3
have been demonstrated to be closely associated with the occurrence
and development of various human diseases, including different
types of cancer (12,13). In the study of breast cancer, Yan
et al (12) suggested that
ERBB3 served a role as an oncogene to promote the progression of
breast cancer, while the miR-143/145 cluster was revealed to
suppress the cell proliferation and invasion of breast cancer cells
(12). The phosphorylation of ERBB3
was also demonstrated to be closely associated with the activation
of the phosphoinositide 3-kinase/protein kinase B signaling pathway
in the progression of a variety of types of cancer (13). In addition to its role in the
pathogenesis of tumors, ERBB3 also serves a role in the formation
of drug resistance developed during long-term treatment (14). Consequently, ERBB3-targeted therapy
has been widely used in the treatment of cancer (15). A recent study has demonstrated that
ERBB3 is likely to be involved in the occurrence of cervical cancer
(8). However, the functionality of
ERBB3 in cervical cancer remains unknown. The expression of ERBB3
is usually altered in tumor tissue, and it has been indicated that
all ERBB family members including ERBB3 were highly expressed in
ovarian carcinoma tissues, and that the increased expression level
of ERBB3 may be used as an indicator in the pathological evaluation
of this disease (16). In the present
study, the expression level of ERBB3 was identified to be
significantly increased in cervical cancer tissue compared with
normal tissue in patients with cervical squamous cell carcinoma and
cervical adenocarcinoma. Furthermore, the expression level of ERBB3
was also significantly increased in cervical cancer cell lines,
when compared with normal cervical cell lines. These data suggest
that it is highly probable that the downregulation of ERBB3
expression is involved in the pathogenesis of cervical squamous
cell carcinoma and cervical adenocarcinoma.
HPV infection is the primary contributor to the
incidence of cervical cancer (3,4). It is
well-known that 15 out of 100 known HPV genotypes cause cervical
cancer, and HPV 16 and 18 are responsible for ~70% of all cervical
cancer cases (17). Although HPV
infection serves an essential role in the development of the
majority of cervical cancers, HPV infection itself is not enough to
trigger the onset of cervical cancer tumor, in which the
involvement of multiple host factors, including genetic factors and
environmental factors, is required (18). In the present study, no significant
difference in expression level of ERBB3 was identified between the
normal cervical cells with and without HPV infection and between
cervical cancer cells with and without HPV infection. These data
suggests that ERBB3 is not likely to be associated the HPV
infection-dependent tumorigenesis of cervical cancer.
ERBB3 may participate in the development of
different types of cancer by regulating the migration and invasion
of tumor cells (12,13). A recent study has indicated that ERBB3
may promote the development of breast cancer by increasing the
migratory and invasive abilities of breast cancer cells (9), while the increased degradation of ERBB3
mediated by NRDP1 was identified to reduce the migratory and
invasive abilities of human glioma cells (10). In the present study, ERBB3 siRNA
silencing was identified to significantly reduce the proliferative,
migratory and invasive abilities of cervical cancer cells,
indicating that ERBB3 expression is important for the migration and
invasion of cervical cancer cells. A previous study demonstrated
that ERBB3 may interact with MTK1 to regulate cell migration and
extracellular acidification (19). In
the present study, the expression level of MTK1 protein was
identified to be significantly decreased in cervical cancer cells
with ERBB3 siRNA silencing compared with the cells without ERBB3
siRNA silencing, indicating an interaction between ERBB3 and MTK1
in cervical cancer cells.
In conclusion, the expression level of ERBB3 was
significantly increased in cervical cancer tissue compared with
normal tissue in patients with cervical squamous cell carcinoma and
cervical adenocarcinoma, and ERBB3 expression was not altered by
HPV infection. ERBB3 expression is important for the proliferation,
migration and invasion of cervical cancer cells. The function of
ERBB3 in the development of cervical cancer is likely to be
achieved through the interaction with MTK1. Future studies with a
greater number of patients are required to further confirm the
conclusions drawn in the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JD and LM designed the experiments, JD and SZ
performed the experiments, LW and MY analyzed the data and JD wrote
the manuscript. All authors read the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Luodian Hospital and written informed consent was
obtained from all patients.
Consent for publication
All patients provided written informed consent for
publication.
Competing interests
The authors declare no that there are competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang PM, Chou CJ, Tseng SH and Hung CF:
Bioinformatics and in vitro experimental analyses identify
the selective therapeutic potential of interferon gamma and
apigenin against cervical squamous cell carcinoma and
adenocarcinoma. Oncotarget. 8:46145–46162. 2017.PubMed/NCBI
|
|
3
|
zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiffman M, Castle PE, Jeronimo J,
Rodriguez AC and Wacholder S: Human papillomavirus and cervical
cancer. Lancet. 370:890–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hildesheim A, Gonzalez P, Kreimer AR,
Wacholder S, Schussler J, Rodriguez AC, Porras C, Schiffman M,
Sidawy M, Schiller JT, et al: Impact of human papillomavirus (HPV)
16 and 18 vaccination on prevalent infections and rates of cervical
lesions after excisional treatment. Am J Obstet Gynecol.
215:212.e1–212.e15. 2016. View Article : Google Scholar
|
|
7
|
Galic V, Herzog TJ, Lewin SN, Neugut AI,
Burke WM, Lu YS, Hershman DL and Wright JD: Prognostic significance
of adenocarcinoma histology in women with cervical cancer. Gynecol
Oncol. 125:287–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cancer Genome Atlas Research Network:
Integrated genomic and molecular characterization of cervical
cancer. Nature. 543:378–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
del Pilar Camacho-Leal M, Sciortino M and
Cabodi S: ErbB2 receptor in breast cancer: Implications in cancer
cell migration, invasion and resistance to targeted therapy. Breast
Cancer Biol Med. 2017. View
Article : Google Scholar
|
|
10
|
Shi H, Gong H, Cao K, Zou S, Zhu B, Bao H,
Wu Y, Gao Y, Tang Y and Yu R: Nrdp1-mediated ErbB3 degradation
inhibits glioma cell migration and invasion by reducing cytoplasmic
localization of p27(Kip1). J Neurooncol. 124:357–364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan X, Chen X, Liang H, Deng T, Chen W,
Zhang S, Liu M, Gao X, Liu Y, Zhao C, et al: miR-143 and miR-145
synergistically regulate ERBB3 to suppress cell proliferation and
invasion in breast cancer. Mol Cancer. 13:2202014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michael N, Hopkins M and Jura N: Mechanism
of PI3K activation by the HER3/ErbB3 receptor. 2016.
|
|
14
|
Lyu H, Huang J, Edgerton SM, Thor AD and
Liu B: Abstract B20: Role of ErbB3 in tumorigenesis and drug
resistance in ErbB2-driven breast cancer. 2015. View Article : Google Scholar
|
|
15
|
Ma J, Lyu H, Huang J and Liu B: Targeting
of erbB3 receptor to overcome resistance in cancer treatment. Mol
Cancer. 13:1052014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davies S, Holmes A, Lomo L, Steinkamp MP,
Kang H, Muller CY and Wilson BS: High incidence of ErbB3, ErbB4 and
MET expression In ovarian cancer. Int J Gynecol Pathol. 33:4022014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Castellsagué X: Natural history and
epidemiology of HPV infection and cervical cancer. Gynecol Oncol.
110:S4–S7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schiffman M and Wentzensen N: Human
papillomavirus infection and the multistage carcinogenesis of
cervical cancer. Cancer Epidemiol Biomarkers Prev. 22:553–560.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sollome JJ, Thavathiru E, Camenisch TD and
Vaillancourt RR: HER2/HER3 regulates extracellular acidification
and cell migration through MTK1 (MEKK4). Cell Signal. 26:70–82.
2014. View Article : Google Scholar : PubMed/NCBI
|