Introduction
Breast cancer is one of the most common carcinoma in
females worldwide, accounting for approximately 1,384,155 new
cases, and 459,000 deaths annually (1). Chemotherapy is considered one of the
most effective treatment for patients with breast cancer and can
improve overall survival in patients (2). Nevertheless, due to chemotherapy
resistance and the lack of effective predictors, the clinical
efficacy of chemotherapy is limited. Disease relapse may occur in
months or years, due to chemotherapeutic resistance acquired during
treatment (3,4). There are two types of chemotherapy
resistance, the first is intrinsic resistance which is
predominantly related to the heterogeneity of tumor cells due to
tumor stem cells (5,6), and the other is acquired resistance,
which occurs over the course of treatment (7).
Previous studies have focused on the tumor itself,
while ignoring the effects of tumor surroundings on tumor growth
(4). Recently, emerging evidence has
indicated that as the predominant components of stroma cells in the
tumor environment, carcinoma associated fibroblasts (CAFs) have
been reported to be crucial in the progression and chemotherapy
effect of breast cancer (8–11). Dangi-Garimella et al (12), reported that CAFs promote gemcitabine
resistance in pancreatic cancer through MT1 and matrix
metalloproteinase (MMP) in CAFs mediated expression of HMGA2 by
secreted Collagen I. Additionally, some researchers have
demonstrated that inhibition of the p53 response in CAFs can
improve the efficacy of anticancer treatment by increasing the
anti-angiogenic effects of chemotherapy and radiotherapy in mice
(13). Given the relationship between
CAFs and chemotherapy resistance, we hypothesized that CAFs may be
impaired, and the expression of secreted factors involved in
chemotherapy resistance may be altered, following chemotherapeutic
treatment. However, which factors have changed and whether these
changes will influence the chemotherapeutic effect of Taxotere on
breast cancer cells remain unclear.
Our previous microarray analysis showed that MMP-1
in CAFs under co-culture conditions is upregulated before and after
Taxotere treatment (14). However,
whether the overexpressed MMP-1 and its target protein Collagen IV
could affect the chemotherapeutic effect of Taxotere on mammary
tumor cells and its specific mechanism were not in-depth
investigate at that time. MMP-1 in tumor cells can promote growth,
invasion and metastasis of tumors, and is closely related to the
prognosis (15–17). However, MMP-1 in CAFs has not been
reported that involved in the chemotherapy of tumor with Taxotere
yet, which secreted from CAFs served as ECM proteins.
Based on our previous work, we hypothesized that
highly expressed MMP-1 in CAFs is a key gene that regulates the
chemotherapeutic effect of Taxotere on tumor cells. The aim of the
present study was to further investigate the function and molecular
mechanism of CAFs in protecting breast cancer cells against
chemotherapeutic treatment and possibly providing novel predictors
for chemotherapeutic efficiency and feasible for targeted
therapy.
Materials and methods
Ethics statement
The present study was performed with the approval of
the Institutional Review Board and Human Ethics Committee of Xuanwu
Hospital of Capital Medicine University, China, and was carried out
in accordance with The Declaration of Helsinki of the World Medical
Association. Written informed consent was obtained from all
patients prior to surgery to collect the samples for research
purposes. And these patients did not receive any form of
chemotherapy, endocrine therapy or radiotherapy treatments prior to
their surgery.
Breast cancer cell line and cell
culture of CAFs
The tumor removal was performed by Professor Kang at
Xuanwu Hospital, Beijing, China. Three breast cancer tissue samples
were obtained between January and February 2016, then were
immediately placed in DMEM (SH30022.01; HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS, 10099-141; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and antibiotics and incubated in a vacuum cup filled with
ice (only tissues more than those needed for clinical diagnoses
were harvested for the present study). And all samples'
histopathological diagnoses were determined as triple negative
breast cancer by pathologist. Tissues were minced into pieces,
washed with PBS three times and digested for 8–12 h at 37°C in
prepared reagent containing 0.1% collagenase type I and 0.1%
hyaluronidase (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
digested pellet was resuspended in fresh DMEM containing 10% FBS
(and all steps were performed under sterilized condition) (14,18).
The human breast cancer cell line MDA-MB-231 was
obtained from the Laboratory of Xuanwu Hospital, Capital Medical
University and was cultured in DMEM supplemented with 10% FBS at
37°C in a humidified atmosphere of 5% CO2 according to a
standard procedure. Cell counting was performed with a VWR
hemocytometer (Hausser Scientific Company, Horsham, PA, USA).
Reagents
GM6001, a specialized MMP inhibitor (Abcam,
Cambridge, MA, USA) was prepared from lyophilized powder and the
final solvent concentration in the medium was 2 µM. The lyophilized
powder of type IV collagen (Sigma-Aldrich; Merck KGaA) was
reconstituted in sterile PBS, and the final solvent concentration
in the medium was 20 µg/ml at 4°C. MMP-1 (anti-rabbit monoclonal
antibody; Abcam), Collagen IV (Col IV;, anti-rabbit polyclonal
antibody; Abcam) and GAPDH antibodies were purchased from Abcam,
and secondary antibodies (anti-rabbit IgG-HRP; Abcam) were
purchased from Santa Cruz Biotechnology, Inc., Dallas, TX, USA. The
final solvent concentration of Taxotere (Sanofi, Shanghai, China)
in the medium was 20 ng/ml based on the IC50 value from our
previous experiment (14).
Immunohistochemistry (IHC)
IHC staining for α-smooth muscle actin (α-SMA),
multi-cytokeratin (CK) and Vimentin (all purchased from ZSGB-Bio,
Beijing, China) was performed. CAFs were seeded in chamber slides
for 24 h and fixed in cold acetone for 10 min. After antigen
retrieval and blocking of endogenous peroxidase in 3% hydrogen
peroxide, the CAFs were incubated with primary antibodies at 4°C in
a moist chamber overnight (PBS was used as a control). Specific
signals were visualized by incubation with a peroxidase-coupled
secondary antibody for 10 min, followed by incubation with 3,
3′-diaminobenzidine (DAB). Counterstaining was performed with
hematoxylin and 0.1% hydrochloric acid (HCL) for 5 min, and the
slides were cover slipped.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-PCR was performed to confirm differential gene
expression in cultured CAFs before and after Taxotere treatment (20
ng/ml for 24 h), using a Bio-Rad IQ5 Real-Time PCR System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). RNA was isolated from
fibroblasts using TRIzol reagent (Thermo Fisher Scientific, Inc.).
cDNA was synthesized using 1 µg total RNA, oligo (dT), and
SuperscriptTM III reverse transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
primers for the candidate genes (COL4A1, COL4A2, COL4A3, COL4A4,
COL4A5, COL4A6, MMP-1) were designed with Primer Express Software
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Predicted PCR
product sequences were verified using BLAST for recognition of
target and non-target sequences: Human COL4A1, Forward
5′-CTCCACGAGGAGCACAGC-3′ and Revese 5′-CCTTTTGTCCCTTCACTCCA-3′;
Human COL4A2, Forward 5′-GCCAGTGCTACCCTGAGAAA-3′ and Revese
5′-CGGGGAATCCTTGTAATCCT-3′; Human COL4A3, Forward
5′-CAGGTGCTCCTGCTGCC-3′ and Revese 5′-GCACTGGCCTTTGTCTTTACA-3′;
Human COL4A4, Forward 5′-TGTGTTCCTGAAAAGGGGTC-3′ and Revese
5′-CCTTTCTCTCCTGAAAGCCC-3′; Human COL4A5, Forward
5′-TACTGGCCCTGAGTCTTTGG-3′ and Revese 5′-TTTCCCCTTTTATGCCACTG′;
Human COL4A6, Forward 5′-CTGCTCCTGGTTACGTTGTG-3′andRevese
5′-GGAAAACACTGACAGCTCCC′; Human MMP-1, Forward
5′-TTCGGGGAGAAGTGATGTTC-3′ and Revese 5′-TTGTGGCCAGAAAACAGAAA-3′;
Conditions for the Real-time PCR reactions were as follows: 10 min
at 95°C followed by 40 cycles of 15 sec at 95°C; 15 sec, 58°C and
35 sec, 72°C. The mRNA expression level was determined using the
2−∆∆Cq method, in which relative quantification of mRNA
expression level was calculated using β-actin as the internal
reference.
Western blot analysis
The cell culture, drug treatment and group
classification protocols were the same as those for the RT-PCR
analyses. Cells were lysed using RIPA buffer with protease and
phosphatase inhibitors. Following SDS-PAGE analyses, proteins were
transferred to nitrocellulose membranes, blocked and incubated with
primary antibodies. Secondary antibodies were detected with
streptavidin-horseradish peroxidase (HRP). Chemi-luminescent
detection was achieved using Western Lightning ECL reagent (Thermo
Fisher Scientific, Inc.). The target bands of the gel were
semi-quantified by densitometric analysis using an image software
program.
Proliferation assay (CCK-8)
Cell proliferation was detected by the Cell Counting
Kit-8 (CCK-8) assay. The harvested MDA-MB-231 cells were diluted
with DMEM at a concentration of 5×104 cells/insert,
while CAFs were diluted to a concentration of 2×104
cells/insert. For the proliferation assay, cells were divided into
four groups: Control group presented mono-culture MDA-MB-231, CO
group presented MDA-MB-231 co-cultured with CAFs, CO+GM6001 group
presented CO group with GM6001 treatment, CO+Col IV group presented
CO group with Collagen IV treatment. Then, MDA-MB-231 cells were
added to each lower chamber, and CAFs were added to the upper
chamber. The 0.4 µm pore transwell inserts (Costar, USA) were used
for this assay. After incubation for 24 h, all four groups were
treated with Taxotere. Then, the cells were cultured for 24, 48 or
72 h, the old medium was discarded, and 10 µl CCK-8 (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) in 100 µl culture
medium was added to each well and incubated for another 3 h. The
absorbance was measured at wavelength of 450 nm.
Flow cytometry (FCM)
For analysis of apoptosis, cell culture, drug
treatment and the group classification methods were performed as
described above. After being resuspended in 500 µl binding buffer,
the MDA-MB-231 cells were stained with 5 µl Annexin-V-FITC and 1 µl
PI (Alexa Fluor 488 Annexin V/Dead Cell Apoptosis kit; Invitrogen;
Thermo Fisher Scientific, Inc.), in the dark at room temperature
for 15 min. Finally, cell apoptosis was measured by a FACSAria flow
cytometer (Cytoflex; Beckman Coulter, Inc., Shanghai, China).
Invasion assay
Invasion assays were performed using 8 µm pore
transwell inserts (Costar). The harvested MDA-MB-231 cells were
used at a concentration of 2×104 cells/insert, while
CAFs were used at a concentration of 1×104 cells/insert.
Matrigel (356237; BD Biosciences, Franklin Lakes, NJ, USA) was
equilibrated with serum-free DMEM at a 1:3 ratios on ice, and 50
µl/cm2 matrigel was added to each filter. The group
classification, cell culture and drug treatment protocols were the
same as those for the proliferation assay. The MDA-MB-231 cells
were added to the upper chamber, and CAFs were added to the lower
chamber. At the end of the incubation period, the cells on the
upper filters were removed with a cotton swab, and the filters were
fixed in 4% formaldehyde and stained with 1% crystal violet. The
number of cells that invaded into the lower surface of the membrane
were counted from 5 randomized fields at 100 times magnifications
using an inverted microscope (Olympus IX70; Olympus Corporation,
Tokyo, Japan). The assay was performed twice, each time in
triplicate.
Statistical analysis
All statistical analyses were performed using SPSS
v.22.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism software
(GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as
the mean ± standard deviation for three independent experiments.
One-way ANOVA with Bonferroni post hoc analysis was used for
multiple group parametric comparisons of proliferation, invasion
and apoptosis assays; Student's t-test for two groups parametric
comparisons of RT-qPCR. P<0.05 was considered to indicate a
statistically significant difference and is indicated by an
asterisk.
Results
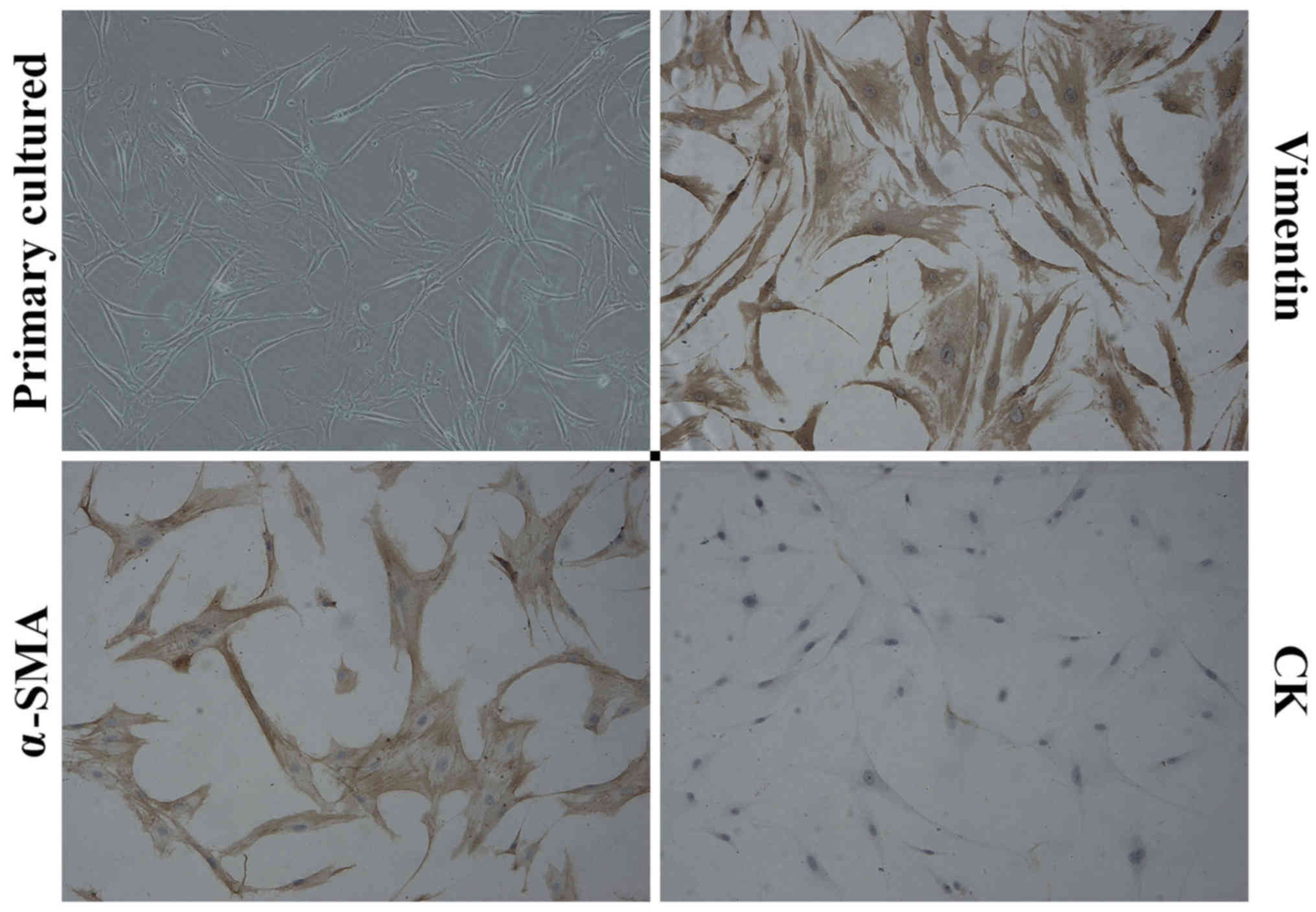
Characterization of primary cultured
CAFs
Primary cells were cultured from the surgically
resected breast cancer tumors, which were confirmed as triple
negative breast cancer by pathologist. The obtained primary cells
showed stable characteristic and the fourth or fifth passage cells
were used for our experiment. The cultured cells had a flat spindle
shape, abundant cytoplasm and an ovoid nuclear morphology.
Immunostaining showed that the primary cultured CAFs expressed
α-SMA and Vimentin, which are CAF-specific biomarkers, but not CK,
an epithelial cell biomarker (Fig.
1).
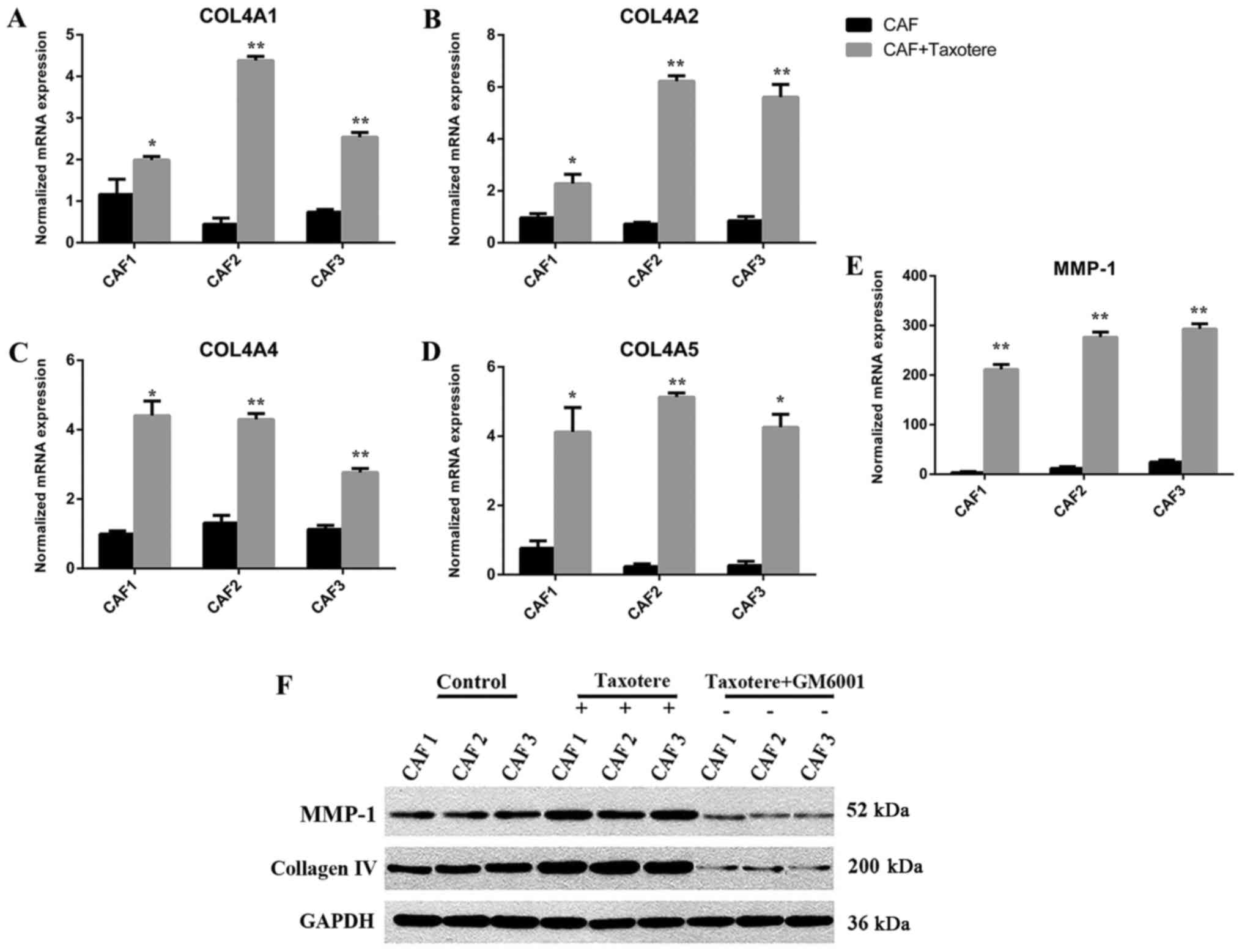
Chemotherapy induced MMP-1 and
collagen IV expression in CAFs
After Taxotere treatment, the gene expression levels
of MMP-1 and synthesis of Collagen IV (COL4A1, COL4A2, COL4A4,
COL4A5, P<0.05) were highly upregulated in CAFs as shown by
RT-PCR, MMP-1 in particular showed significantly increased
expression (P<0.01; Fig. 2A-E),
while there were no statistical significant in COL4A3 (CAF1,
P=0.026; CAF2, P=0.114; CAF3, P=0.083), COL4A6 (CAF1, P=0.02; CAF2,
P=0.18, CAF3, P=0.846). Western blotting was performed to assess
the protein levels of MMP-1 and Collagen IV. The results showed
that protein levels in CAFs were significantly different before and
after chemotherapy (P=0.00; Fig. 2F).
The variations in gene and protein expression of MMP-1 and Collagen
IV were consistent.
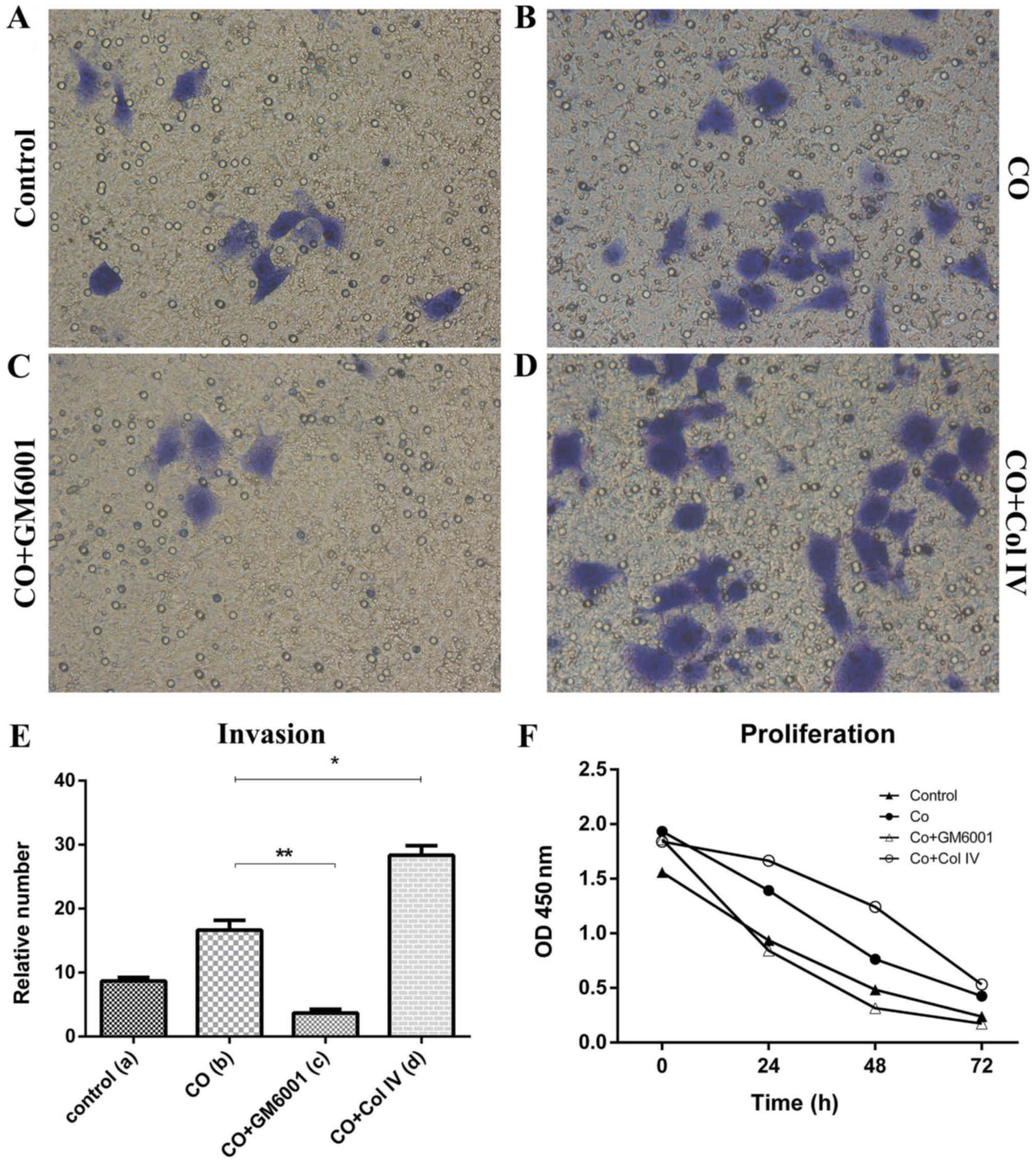
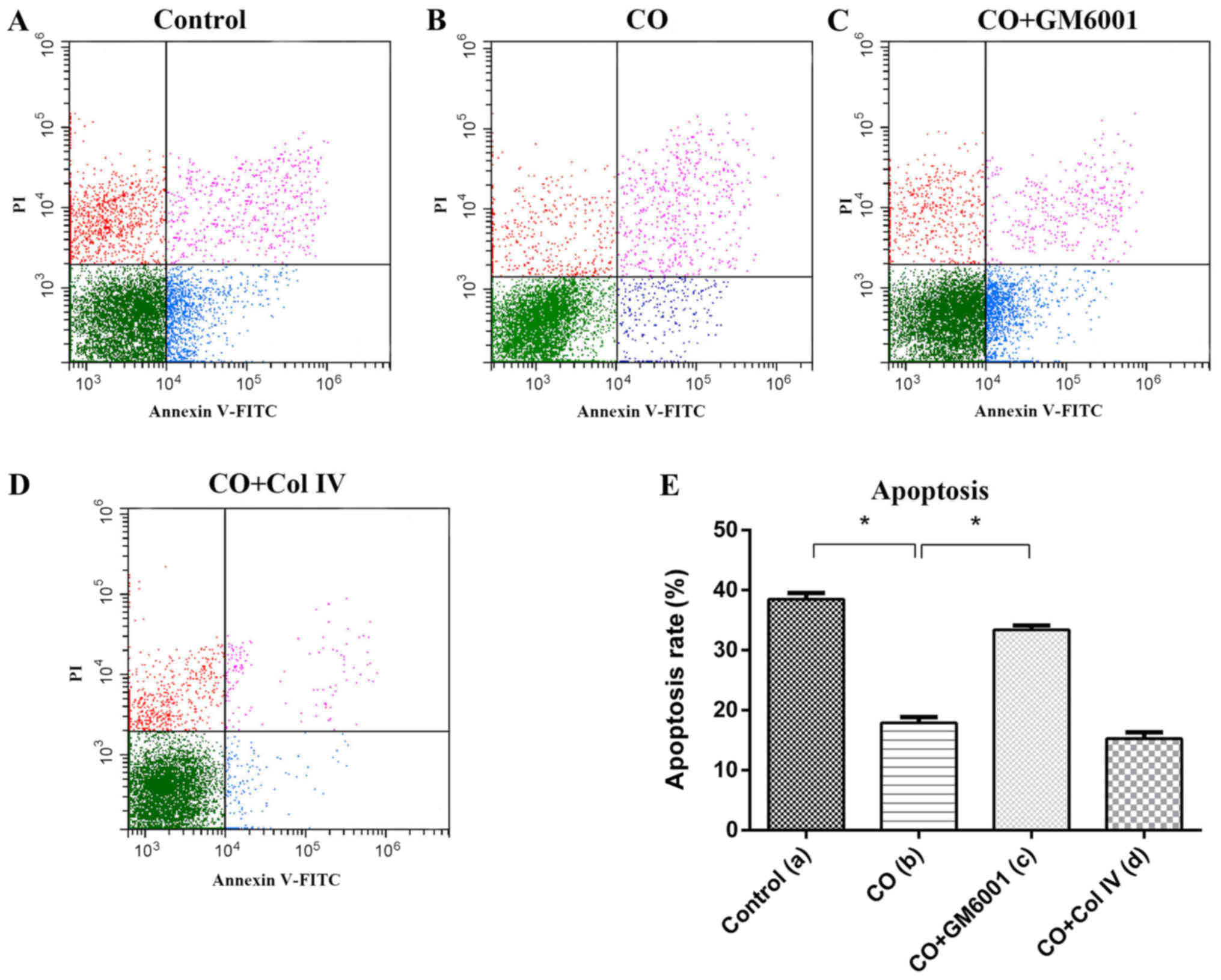
CAFs promoted breast cancer cells
resistance to chemotherapeutic effects
After Taxotere treatment, CO group displayed
increased proliferation (24 h, P=0.01, 48 h, P=0.036) and invasion
(P=0.00; Fig. 3E and F), but
decreased apoptosis was decreased dramatically (P=0.002), compared
with those of Control group (Fig.
4E). Thus, CAFs could protect breast cancer cells against the
effects of chemotherapy.
GM6001 increased chemosensitivity of
breast cancer cells
To further investigate the role of MMP-1 in CAFs on
breast cancer chemotherapy, we used GM6001, an inhibitor of MMP-1,
to decrease the expression of MMP-1 in CAFs. After GM6001 was added
to the Taxotere-treated CAFs (CO+GM6001), MMP-1 in CAFs protein
expression was substantially decreased, compared to that of CO
group as shown by western blot analysis. Additionally, MDA-MB-231
cell proliferation (24 h, P=0.00, 48 h, P=0.02) and invasion
(P=0.00) showed significant decreased (Fig. 3E and F), but apoptosis was
significantly increased (P=0.013) between CO+GM6001 and CO group
(Fig. 4E). Thus, we concluded MMP-1
plays an important role in CAF induced protecion of breast cancer
cells against chemotherapy (Taxotere). Upregulated expression of
MMP-1 in CAFs increased the chemotherapy resistance, while
decreased MMP-1 expression in CAFs promoted chemosensitivity of
breast cancer cells.
Collagen IV promoted breast cancer
cells resistance to chemotherapy
To evaluate the effect of Collagen IV secreted from
CAFs on MDA-MB-231 cells chemotherapeutic effect, we treated
co-cultured cells with Collagen IV after chemotherapy (CO+Col IV).
The proliferation (24 h, P=0.035; 48 h, P=0.01) and invasion assays
(P=0.00) showed that there were significant differences, compared
to that of the CO group (Fig. 3E and
F). The proliferation and invasion of MDA-MB-231 cells were
strongly enhanced by addition of Collagen IV after chemotherapy.
These results indicated that Collagen IV could promote resistance
of breast cancer cells to Taxotere. However, the apoptosis assay
indicated that there was no significant difference between CO and
CO+Col IV group after chemotherapy (P=0.487; Fig. 4E).
Discussion
Breast cancer is a complex disease, involving tumor
cells themselves and the tumor microenvironment, which includes
multiple components of the extracellular matrix (ECM), such as
collagen, as well as cellular components, such as fibroblasts
(19,20). Many investigations have shown that
CAFs play an important role in tumor initiation, progression,
apoptosis and chemotherapy resistance (19,21,22).
Overall, CAFs are induced to adapt to the drugs used for treatment,
which is consistent with the hypothesis that CAFs co-evolve along
with the tumor cells (23). Moreover,
the resistance of breast cancer to a comprehensive range of
chemotherapeutic drugs and the lack of useful predictive markers of
drug response are ongoing problems (24,25). As
the majority of researchers have focused on endo-crinotherapy and
CAFs after treatment, conversely, few studies have examined CAF
induced chemotherapy resistance (26–28). And
the molecular mechanism underlying CAF-mediated chemotherapy
resistance is still ambiguous.
The aim of the present study was to further
investigate the function and molecular mechanism of CAFs in
protecting breast cancer cells against chemotherapeutic treatment.
Moreover, based on our previous work, we hypothesized that highly
expressed MMP-1 is a key gene that regulates the chemotherapeutic
effect of Taxotere on breast tumor cells.
To verify this hypothesis, CAFs were first isolated
from primary invasive ductal human breast tumors following surgical
resection and were used with MDA-MB-231 cells for co-culture to
simulate the tumor growth microenvironment. After addition of
Taxotere, high expression in MMP-1 gene and protein levels were
observed by RT-PCR and Western blot analyses, respectively. After
Taxotere treatment, CO group displayed increased proliferation and
invasion, but the apoptosis was decreased dramatically, compared
with those of Control group. Hence, we concluded that up-regulated
MMP-1 in CAFs under co-cultured conditions decreased the
therapeutic efficacy of Taxotere on breast cancer cells. These
assays also indicated that chemosensitivity was significantly
increased when MMP-1 expression was inhibited by GM6001. These
results are consistent with the hypothesis. MMP-1 is predominantly
produced by CAFs, and it could be increased following stimulation
(15). MMPs play a crucial role in
proliferation, invasion, metastasis and apoptosis of tumor cells
(15–17). Faller WJ founded that MMPs have a
significant effect on tumor resistance to gemcitabine (29). These research results were consistent
with our data. Thus, based on our data, as the main component of
ECM, MMP-1 was hypothesized to directly affect tumor cells and
increase the ECM abundance by regulating the synthesis of collagen
and reducing blood flow to limit the transport of drugs.
Thus, we continue to study the change of MMP-1
targeted protein: Collagen. And the results showed that Collagen IV
was upregulated in CAFs after chemotherapy and enhanced breast
cancer cell resistance to chemotherapeutic effects, Collagen IV
expression significantly decreased, along with MMP-1 expression,
after GM6001 was added. Proliferation and invasion assays showed
that addition of exogenous Collagen IV weakened the
chemotherapeutic effect of Taxotere on breast tumor cells. Collagen
IV is a member of super-collagen family of proteins, predominantly
secreted by stromal cells and plays an important role in tumor
progression and drug effects and is consist of 3 peptide chains,
which encoding genes including COL4A1-COL4A6 (30–33). So,
we verified the 6 encoding genes; while, only COL4A1, COL4A2,
COL4A4, COL4A5 had statistically significance. It was reported
Collagen IV was highly expressed in ovarian cancer after
gemcitabine treatment and promoted the acquisition of ovarian cells
resistance to gemcitabine (33). The
results indicated that Collagen IV, which is secreted by CAFs and
is a component of the ECM, may induce cell adhesion-mediated drug
resistance by interacting with integrin receptors of cancer cells
and then reducing the chemotherapy effects. Interestingly, our
results showed that Collagen IV protein expression significantly
decreased after treatment with GM6001, which is consistent with the
alteration in MMP-1. This phenomenon contrasts with the finding
that MMPs degraded Collagen. We inferred that the degradation and
synthesis of the Collagen protein are in a dynamic equilibrium, and
under certain conditions or stimuli, the synthesis of Collagen
protein is greater than the degradation by MMPs. It was also
demonstrated that MMP-1 could directly affect tumor cells and be
treated as not only a kind of ECM proteolytic enzymes, but also
should be taken as an enzyme that involved in the signaling between
cells and cells, cells and stoma, even as a protein equipped signal
potential amplification ability. TGF-β is involved in the classical
pathway of collagen secretion and could be activated with latent
TGF-β (LTBP-1) by MMPs (34,35). Bates et al (36) founded that the collagen levels
significantly decreased after TGF-β neutralizing antibody was added
to block the TGF-β pathway. Hence, combining these findings with
our study results, it was suggested TGF-β pathway maybe the
reactive regulator between MMP-1 and Collagen IV: After Taxotere
treatment, CAF secreted MMP-1 synergized with Collagen VI to
decrease the chemotherapeutic effect of Taxotere on breast cancer
cells by the TGF-β pathway.
There are some limitations in our study. Collagen IV
was added exogenously, and no negative intervetions were taken to
decrease Collagen IV expression. Thus, Collagen IV can not be
identified as the key gene to regulate the effet of Taxotere on
tumor cells. In addition, the relationship between MMP-1 and
Collagen IV should be further verified by TGF-β pathway study.
What'more, the present study was focused on triple-negative breast
cancer. What's the influence of Taxotere on hormone receptors of
breast cancer needed to be discussed in the future. However, MMP-1
synergized with Collagen IV in CAFs should be confirmed as the key
regulator that regulates the chemotherapeutic effect of Taxotere on
tumor cells by in vitro experiments in the present
study.
In summary, we cultured CAFs of primary breast
cancer samples, and provided new evidence showing that CAFs induced
breast cancer cell resistance against the chemotherapeutic effect
of Taxotere and elucidated the underlying molecular mechanism. The
observation that high expression of MMP-1 synergy with Collagen IV
in CAFs plays an important role in reducing the efficacy of
Taxotere in breast cancer cells and maybe react via the TGF-β
pathway. This provides a theoretical basis for the chemotherapeutic
effect of CAFs on breast tumor cells and a novel approach to
enhance the chemosensitivity of tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172517), the
Specialized Research Fund for the Doctoral Program of Higher
Education of China (grant no. 20111107110001), the cancer control
program of Beijing Breast Disease Society (grant no. 2025-8-8) and
the Beijing Municipal Health System Academic Leaders of High-Level
Health Personnel Program (grant no. 2011-2-28).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HK designed the experiments, provided critical
reagents and experimental expertise and supervised the study. QC
designed and performed the experiments, generated the figures and
wrote the manuscript. BW and KL collected the tumor tissues. HS, YZ
and TH assisted with some of the experiments.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board and Human Ethics Committee of Xuanwu Hospital of Capital
Medicine University. Written informed consent was obtained from all
patients prior to their inclusion within the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tao Z, Shi A, Lu C, Song T, Zhang Z and
Zhao J: Breast cancer: Epidemiology and etiology. Cell Biochem
Biophys. 72:333–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carrara GF, Scapulatempo-Neto C,
Abrahão-Machado LF, Brentani MM, Nunes JS, Folgueira MA and Vieira
RA: Breast-conserving surgery in locally advanced breast cancer
submitted to neoadjuvant chemotherapy. Safety and effectiveness
based on ipsilateral breast tumor recurrence and long-term
follow-up. Clinics (Sao Paulo). 72:134–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tafe LJ: Molecular mechanisms of therapy
resistance in solid tumors: Chasing ‘moving’ targets. Virchows
Arch. 471:155–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: An evolving concept. Nat Rev Cancer. 12:133–143.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niero EL, Rocha-Sales B, Lauand C, Cortez
BA, de Souza MM, Rezende-Teixeira P, Urabayashi MS, Martens AA,
Neves JH and Machado-Santelli GM: The multiple facets of drug
resistance: One history, different approaches. J Exp Clin Cancer
Res. 33:372014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo H, Tu G, Liu Z and Liu M:
Cancer-associated fibroblasts: A multifaceted driver of breast
cancer progression. Cancer Lett. 361:155–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tao L, Huang G, Song H, Chen Y and Chen L:
Cancer associated fibroblasts: An essential role in the tumor
microenvironment. Oncol Lett. 14:2611–2620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ashida S, Kawada C and Inoue K: Stromal
regulation of prostate cancer cell growth by mevalonate pathway
enzymes HMGCS1 and HMGCR. Oncol Lett. 14:6533–6542. 2017.PubMed/NCBI
|
|
11
|
Du H and Che G: Genetic alterations and
epigenetic alterations of cancer-associated fibroblasts. Oncol
Lett. 13:3–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dangi-Garimella S, Krantz SB, Barron MR,
Shields MA, Heiferman MJ, Grippo PJ, Bentrem DJ and Munshi HG:
Three-dimensional collagen I promotes gemcitabine resistance in
pancreatic cancer through MT1-MMP-mediated expression of HMGA2.
Cancer Res. 71:1019–1028. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burdelya LG, Komarova EA, Hill JE, Browder
T, Tararova ND, Mavrakis L, DiCorleto PE, Folkman J and Gudkov AV:
Inhibition of p53 response in tumor stroma improves efficacy of
anticancer treatment by increasing antiangiogenic effects of
chemotherapy and radiotherapy in mice. Cancer Res. 66:9356–9361.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rong G, Kang H, Wang Y, Hai T and Sun H:
Candidate markers that associate with chemotherapy resistance in
breast cancer through the study on Taxotere-induced damage to tumor
microenvironment and gene expression profiling of
carcinoma-associated fibroblasts (CAFs). PLoS One. 8:e709602013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roberti MP, Arriaga JM, Bianchini M,
Quintá HR, Bravo AI, Levy EM, Mordoh J and Barrio MM: Protein
expression changes during human triple negative breast cancer cell
line progression to lymph node metastasis in a xenografted model in
nude mice. Cancer Biol Ther. 13:1123–1140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chabottaux V and Noel A: Breast cancer
progression: Insights into multifaceted matrix metalloproteinases.
Clin Exp Metastasis. 24:647–656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li K, Kang H, Wang Y, Hai T, Rong G and
Sun H: Letrozole-induced functional changes in carcinoma-associated
fibroblasts and their influence on breast cancer cell biology. Med
Oncol. 33:642016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aboussekhra A: Role of cancer-associated
fibroblasts in breast cancer development and prognosis. Int J Dev
Biol. 55:841–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sambi M, Haq S, Samuel V, Qorri B, Haxho
F, Hill K, Harless W and Szewczuk MR: Alternative therapies for
metastatic breast cancer: Multimodal approach targeting tumor cell
heterogeneity. Breast Cancer (Dove Med Press). 9:85–93.
2017.PubMed/NCBI
|
|
21
|
Kubo N, Araki K, Kuwano H and Shirabe K:
Cancer-associated fibroblasts in hepatocellular carcinoma. World J
Gastroenterol. 22:6841–6850. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Madar S, Goldstein I and Rotter V: ‘Cancer
associated fibroblasts’-more than meets the eye. Trends Mol Med.
19:447–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Junttila MR and de Sauvage FJ: Influence
of tumour micro-environment heterogeneity on therapeutic response.
Nature. 501:346–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perez EA: Impact, mechanisms, and novel
chemotherapy strategies for overcoming resistance to anthracyclines
and taxanes in metastatic breast cancer. Breast Cancer Res Treat.
114:195–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dittmer J and Leyh B: The impact of tumor
stroma on drug response in breast cancer. Semin Cancer Biol.
31:3–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shekhar MP, Santner S, Carolin KA and Tait
L: Direct involvement of breast tumor fibroblasts in the modulation
of tamoxifen sensitivity. Am J Pathol. 170:1546–1560. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Campisi J, Higano C, Beer TM,
Porter P, Coleman I, True L and Nelson PS: Treatment-induced damage
to the tumor microenvironment promotes prostate cancer therapy
resistance through WNT16B. Nat Med. 18:1359–1368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanker AB, Estrada MV, Bianchini G, Moore
PD, Zhao J, Cheng F, Koch JP, Gianni L, Tyson DR, Sánchez V, et al:
Extracellular matrix/integrin signaling promotes resistance to
combined inhibition of HER2 and PI3K in HER2+ breast
cancer. Cancer Res. 77:3280–3292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Faller WJ, Rafferty M, Hegarty S, Gremel
G, Ryan D, Fraga MF, Esteller M, Dervan PA and Gallagher WM:
Metallothionein 1E is methylated in malignant melanoma and
increases sensitivity to cisplatin-induced apoptosis. Melanoma Res.
20:392–400. 2010.PubMed/NCBI
|
|
30
|
Iyengar P, Combs TP, Shah SJ, Gouon-Evans
V, Pollard JW, Albanese C, Flanagan L, Tenniswood MP, Guha C,
Lisanti MP, et al: Adipocyte-secreted factors synergistically
promote mammary tumorigenesis through induction of anti-apoptotic
transcriptional programs and proto-oncogene stabilization.
Oncogene. 22:6408–6423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Irwin WA, Bergamin N, Sabatelli P,
Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M,
Volpin D, Bressan GM, et al: Mitochondrial dysfunction and
apoptosis in myopathic mice with collagen VI deficiency. Nat Genet.
35:367–371. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tokes AM, Szasz AM, Farkas A, Toth AI,
Dank M, Harsanyi L, Molnar BA, Molnar IA, Laszlo Z, Rusz Z and
Kulka J: Stromal matrix protein expression following preoperative
systemic therapy in breast cancer. Clin Cancer Res. 15:731–739.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sherman-Baust CA, Weeraratna AT, Rangel
LB, Pizer ES, Cho KR, Schwartz DR, Shock T and Morin PJ: Remodeling
of the extracellular matrix through overexpression of collagen VI
contributes to cisplatin resistance in ovarian cancer cells. Cancer
Cell. 3:377–386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tatti O, Vehvilainen P, Lehti K and
Keski-Oja J: MT1-MMP releases latent TGF-beta1 from endothelial
cell extracellular matrix via proteolytic processing of LTBP-1. Exp
Cell Res. 314:2501–2514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Z, Wang S, Wu M, Zeng W, Wang X and
Dong Z: TGFβ1 and HGF protein secretion by esophageal squamous
epithelial cells and stromal fibroblasts in oesophageal
carcinogenesis. Oncol Lett. 6:401–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bates AL, Pickup MW, Hallett MA, et al:
Stromal matrix metalloproteinase 2 regulates collagen expression
and promotes the outgrowth of experimental metastases. J Pathol.
2015.235(5): 773–783. View Article : Google Scholar : PubMed/NCBI
|