Introduction
Thyroid cancer is the most common tumor of the
endocrine system, and its incidence rate has remarkably increased
over the past several decades (1).
Thyroid cancer can be classified into four types depending on
pathological type: Papillary, follicular, medullary and anaplastic
thyroid cancer. Papillary thyroid cancer (PTC) is the most common
type of thyroid malignant tumor that accounts for approximately 90%
of all thyroid cancer (2). In most
cases, patients with PTC have an excellent prognosis after they
undergo surgical resection combined with radioiodine and
levothyroxine treatment. However, 10–15% of patients suffering from
PTC with relapses and distant metastases frequently elicit a poor
response to standard treatments and achieve poor clinical outcomes
(3). Therefore, molecular mechanisms
underlying the formation and progression of PTC must be elucidated
to improve its diagnosis, therapy and prevention.
MicroRNAs (miRNAs) are a group of small non-coding
RNAs that regulate gene expression through translation repression
or mRNA degradation by binding to the 3′-untranslated region of a
target mRNA (4). miRNAs are involved
in the regulation of cell survival, proliferation and migration by
mediating the expression of their target genes (5). Alterations in miRNA expression are
likely implicated in PTC development and progression. miR-329
located on 14q32.31 participates in the progression of several
cancers (6–13). However, the expression levels,
biological functions and associated molecular mechanism of miR-329
in PTC have yet to be elucidated.
In the present study, we measured miR-329 expression
in PTC tissues and cell lines. We also investigated the regulatory
roles of miR-329 in PTC cells. Moreover, we explored the underlying
molecular mechanism of its actions in PTC cells.
Materials and methods
Tissue sample collection
Paired PTC and adjacent non-tumor tissues were
collected from 20 patients who underwent surgical resection at
Linyi Central Hospital. All patients had not received chemotherapy
or radiotherapy before surgery. Tissue samples were collected
during surgery, frozen in liquid nitrogen, and then stored until
total RNAs were extracted. Informed consent was obtained from each
patient, and the study protocol and consent procedures were
approved by the ethics committee of Linyi Central Hospital (Linyi,
China).
Cell culture and cell
transfection
Human PTC cell lines (TPC-1 and BCPAP) and human
immortalized follicular cell line (Nthy-ori3-1) were obtained from
the ATCC (Manassas, VA, USA). BCPAP originally classified as a
thyroid gland papillary cancer but is now considered to be a poorly
differentiated thyroid gland cancer (14). In the present study, BCPAP acts as
poorly differentiated thyroid cancer cell line to further confirm
the experiments results of TPC-1 and not affect the outcomes of
this study. The cell lines were authenticated using short-tandem
repeat profiling with BMR Genomics. The cells were cultured in DMEM
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences)
in a humidified atmosphere with 5% CO2 and humidified
sphere of 95% at 37°C. miR-329 mimic and negative control (miR-NC)
were purchased from GE Healthcare Life Sciences. A lentiviral
packaging kit was purchased from Shanghai GeneChem (Shanghai,
China). Lentiviruses carrying miR-329 or miR-NC were packaged in
HEK293T cells and harvested from the culture supernatant in
accordance with the kit manufacturer's instructions. Stable cell
lines were established by selecting infected TPC-1 and BCPAP cells
with puromycin (HyClone; GE Healthcare Life Sciences). WNT1
overexpression plasmid was achieved using pcDNA3.1/WNT1
transfection. siRNA targeting human Wnt1 was obtained from Santa
Cruz Biotechnology, Inc., (sc-36839; Dallas, TX, USA). siWnt1 (5
nmol) was transfected into the cells according to the
manufacturer's protocol. Transient transfection was performed with
a Lipofectamine 2000 Reagent in accordance with the manufacturer's
instruction.
Colony formation assay
Cell proliferation was analyzed by using the plate
colony formation assay. A total of 400 cells from each group were
seeded in a new six-well plate and cultured for approximately 10
days until colony formation was observed. The colonies were fixed
with methanol and stained with 1% crystal violet (Beyotime
Institute of Biotechnology, Haimen, China). Images of the colonies
were obtained, and the colonies with more than 50 cells were
counted under a microscope (Olympus Corporation, Tokyo, Japan).
CCK-8 assay
The viability of TPC-1 and BCPAP cells was
determined by using the CCK-8 assay. TPC-1 and BCPAP cells stably
transfected with miR-329 mimic or miR-NC were seeded in 96-well
plates at a density of 1×103 cells per well and then
cultured for 24, 48, and 72 h before performing the CCK-8 assay.
After 4 h of incubation with CCK-8 at 37°C, the absorbance (OD
value) at a wavelength of 450 nm was detected and used for
calculating cell viability.
Cell migration and invasion
assays
Cell migration and invasion assays were analyzed by
using Transwell chamber in accordance with the manufacturer's
instruction. For the invasion assay, the upper sides of the filters
were coated with 50 µl of Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA). TPC-1 and BCPAP cells stably transfected with miR-329
mimic or miR-NC were plated at a density of 5×104 cells
per well in the upper chamber without serum. The lower chamber was
filled with 600 µl of the DMEM medium with 10% FBS to act as the
nutritional attraction. After incubation for 8 h (migration) and 24
h (invasion), the cells on the upper membrane surface were removed,
whereas the invasive cells attached to the lower surface of the
membrane insert were fixed with 70% methanol for 30 min and stained
with 0.1% crystal violet for 10 min. Then, the cells were counted
in five randomly selected fields per well under a light microscope
(Olympus Corporation).
RNA extraction and quantitative real
time PCR (qPCR)
Total RNAs (inclusive of miRNAs) were extracted from
cells or xenograft tissues using Trizol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) in accordance with the
manufacturer's protocol. To measure the expression levels of
miR-329, qPCR assay was performed, and RNU48 served as the internal
control. To analyze the mRNA levels of WNT1, total RNAs were
reversely transcribed by oligodT primer using PrimeScript RT
Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). GAPDH
served as the endogenous control. The relative expression levels of
miR-329 and WNT1 were calculated as the inverse log of ΔΔCT and
normalized to the reference. The primers used for amplification
were as follows: miR-329 Forward 5′-GGGAACACACCTGGTTAAC-3′, Reverse
5′-CAGTGCGTGTCGTGGAGT-3′ RNU48 Forward
5′-TGATGATGACCCCAGGTAACTCTGAGTG-3′, Reverse
5′-GTCAGAGCGCTGCGGTGATGGCATCAGC-3′ GAPDH Forward
5′-GACCTGACCTGCCGTCTAG-3′ Reverse 5′-ACTCCTGCTTGCTGATCCAC-3′ WNT1
Forward 5′-AGCCCTAGCTGCCAACAGTA-3′ Reverse
5′-GGAATTGCCATTTGCACTCT-3′
Western blot analysis
Equal amounts of the protein from lysates of PTC
cells were subjected to 10% SDS-PAGE and then transferred onto PVDF
membranes. The immunoreactive bands were first incubated with the
primary antibodies, including WNT1 (1:500) and GAPDH (1:1,000; both
from Santa Cruz Biotechnology, Inc.) antibodies. The intensity of
protein bands was detected by Image-Pro Plus v.6.0 software
(National Institutes of Health, Bethesda, MD, USA). GAPDH served as
the loading control.
Dual luciferase reporter assay
The wild-type (WT) and mutated putative (mut)
miR-329 target sequences in the WNT1 3′UTR were amplified from
human WNT1 cDNA by PCR and then cloned into the SacI and HindIII
sites of the pmiRNA-report firefly luciferase vector (GeneChem).
TPC-1 and BCPAP cells were seeded in a 24-well plate and
co-transfected with the WT or MUT reporter plasmid, a Renilla
luciferase plasmid, and miR-329 mimic or miR-NC. Luciferase
activities were measured 24 h after transfection using a dual
luciferase assay kit (Promega Corporation, Madison, WI, USA).
Firefly luciferase activity was normalized to its corresponding
Renilla luciferase activity.
Tumourigenesis assay in nude mice
All experimental procedures involving animals were
approved by the Ethics Committee of Linyi Central Hospital
(Shandong, China). 5-week-old BALB⁄c athymic nude mice (n=12,
female, weight range; 20–22 g) (Jilin University, Changchun, China)
and maintained in a SPF environment with constant humidity (45–50%)
and constant temperature (25–27°C) under a 12 h light/dark cycle
with free access to food and water. BCPAP cells stably transformed
with miR-329 or miR-NC were inoculated subcutaneously into the
right flanks of nude mice. Each experimental group included six
mice. Tumor growth was measured every 7 days from injection using a
digital caliper, and the tumor volume was calculated by the
following formula: tumor volume=(length × width2)/2.
After 42 d, the mice were sacrificed, and xenografts were
harvested.
Statistical analysis
Statistical analyses were performed using SPSS
v.13.0 software (SPSS, Inc., Chicago, IL, USA). Differences between
two groups were assessed using Student's t-test (two-tailed). Data
of more than two groups were analyzed using one way ANOVA with post
hoc test by Tukey's test. Correlations between WNT1 and miR-329
were analyzed using Spearman rank correlation. Each experiment was
performed at least three times. P<0.05 was considered to
indicate a statistically significant difference and results are
represented as means ± standard deviations (SD).
Results
miR-329 is downregulated in PTC
tissues and cell lines
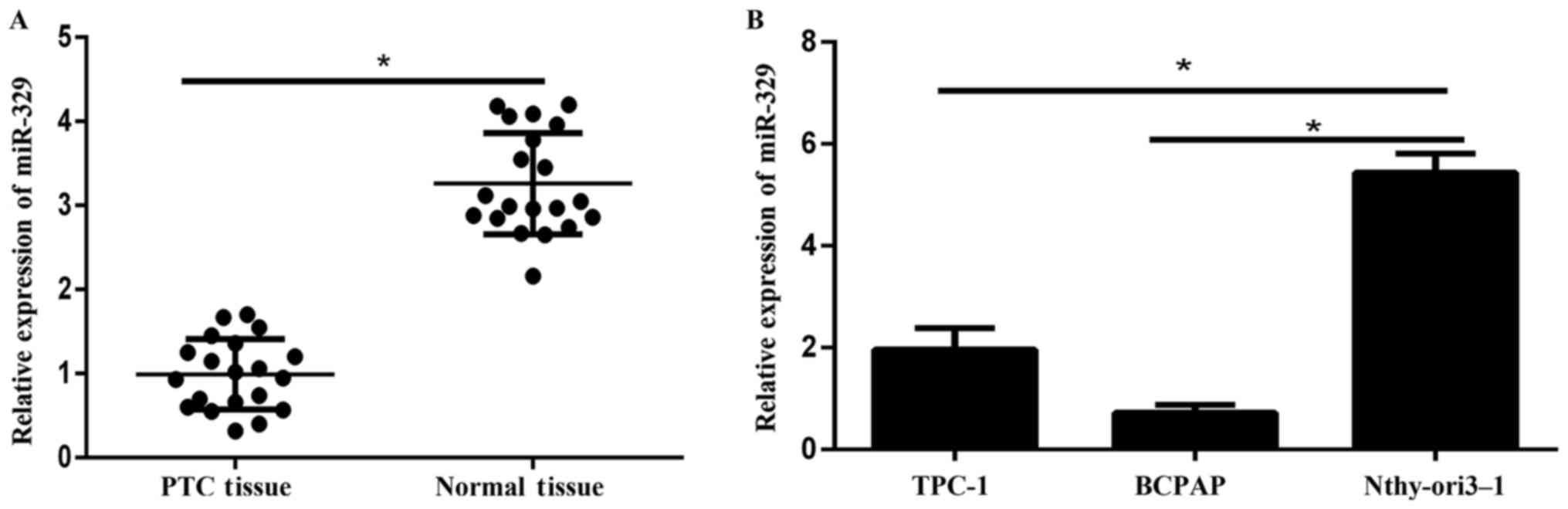
The expression levels of miR-329 in 20 paired
samples (PTC specimens and corresponding adjacent non-tumor
tissues) were detected via qPCR. Results showed that miR-329
expression was remarkably lower in tumor tissues compared to
adjacent normal tissues (Fig. 1A). In
addition, the expression of miR-329 in PTC cell lines (TPC-1,
BCPAP) was significantly reduced compared to that in the human
immortal follicular thyroid cell Nthy-ori3-1 (Fig. 1B).
Overexpression of miR-329 reduced the
cell proliferation in PTC cells
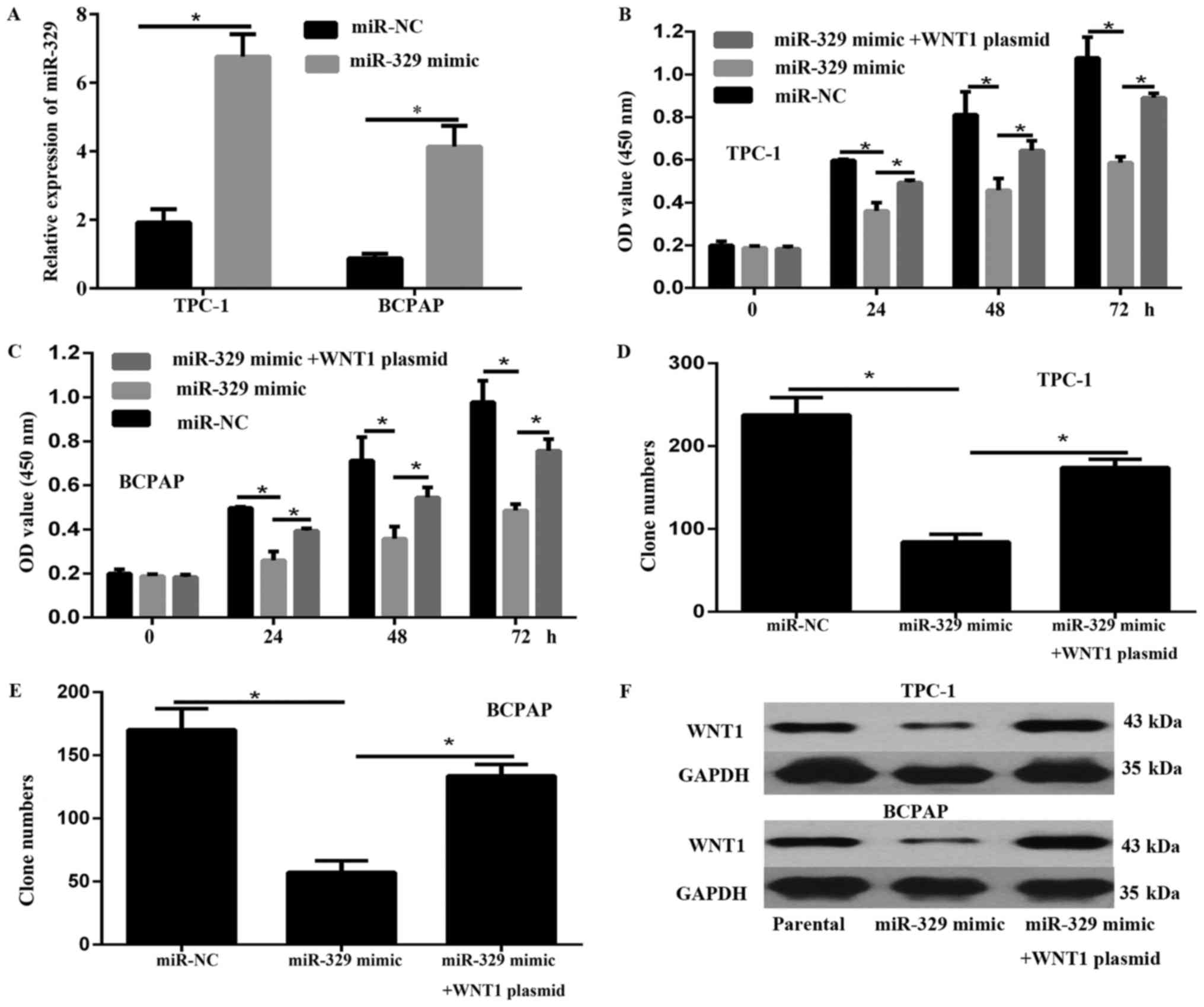
The overexpression of miR-329 was achieved by
transfection with miR-329 mimic in TPC-1 and BCPAP, as verified
using qPCR assays (Fig. 2A). As
exhibited by CCK-8 assays, the cell viability of TPC-1 and BCPAP
cells was significantly inhibited after miR-329 overexpression
compared to that of cells transfected with miR-NC (Fig. 2B and C). In addition, results of the
colony formation assay illustrated that the proliferation of
miR-329 overexpression cells was significantly decreased relative
to that of cells transfected with miR-NC (Fig. 2D and E). Furthermore, the results of
the western blot revealed that miR-329 overexpression significantly
decreased the expression of the WNT1 oncogene (Fig. 2F).
miR-329 inhibited the migration and
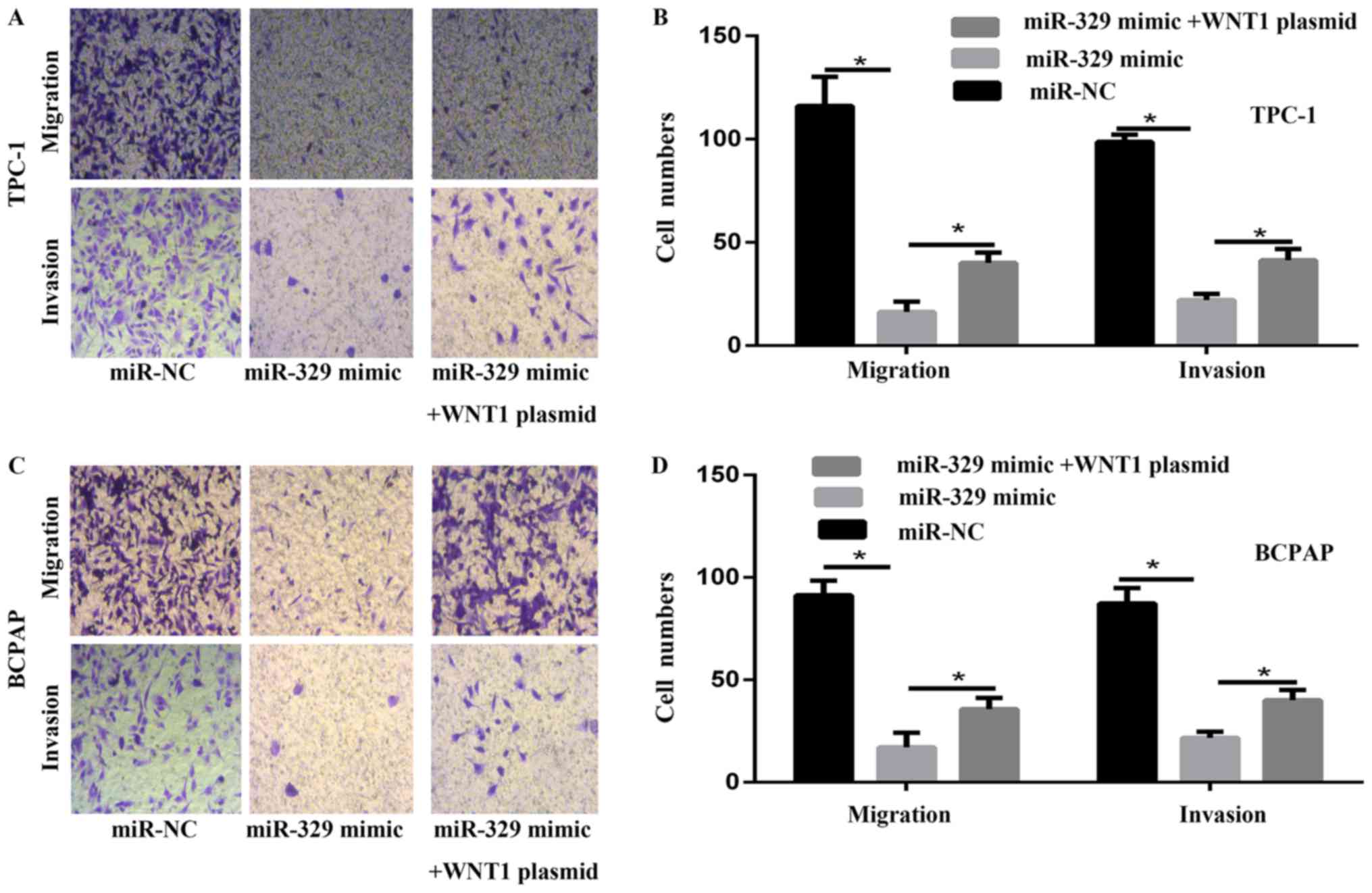
invasion capability of PTC cells in vitro
To evaluate the migration and invasion potential of
PTC cells transfected with miR-329 mimic, trasnswell assay was
performed in vitro. The overexpression of miR-329
significantly decreased the number of PTC cells capable of
migration and invasion (Fig. 3A-D).
These results suggested that miR-329 reduced the migration and
invasion of PTC cells in vitro.
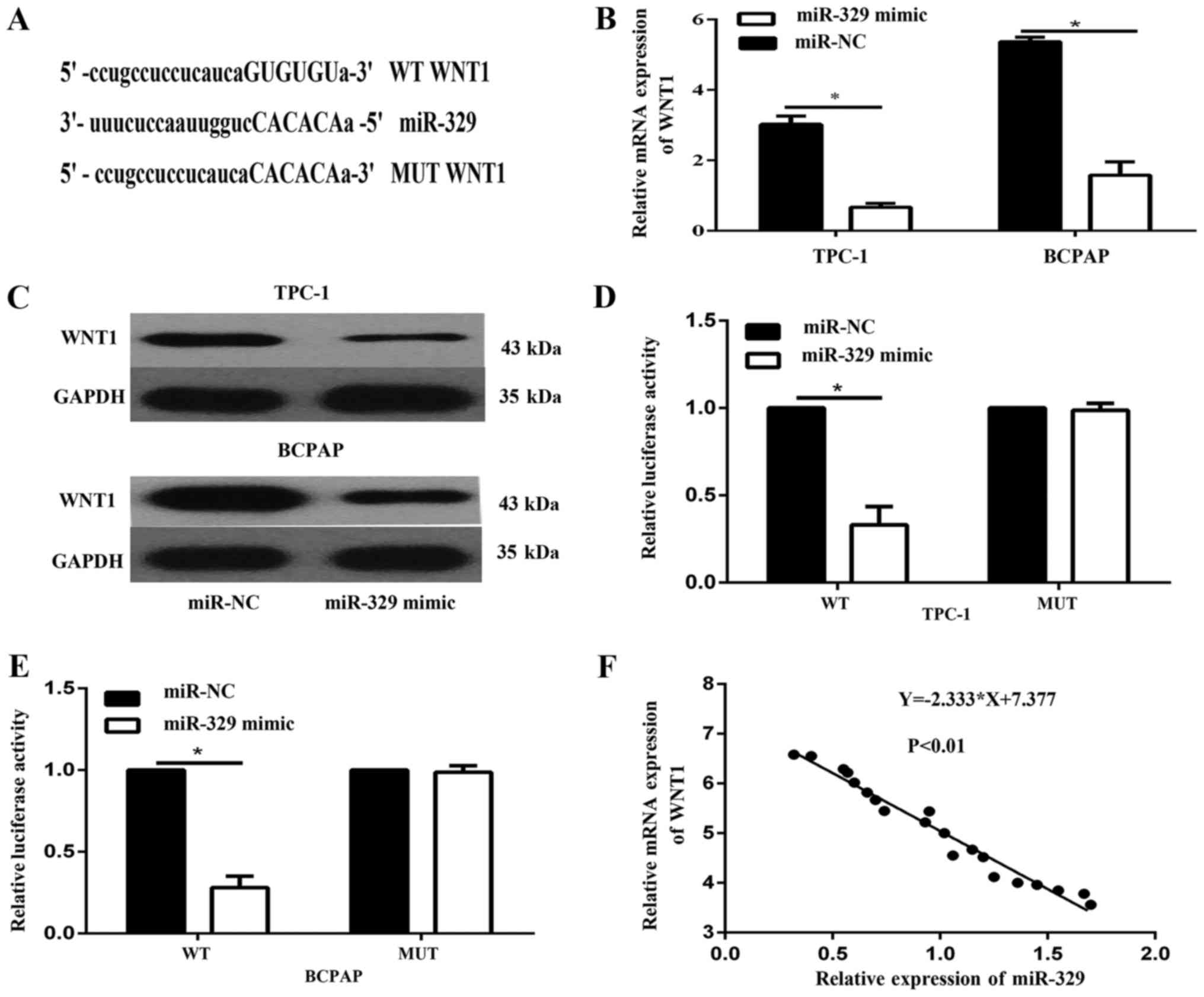
WNT1 was a direct target gene of
miR-329
We identified WNT1 mRNA as one of the putative
miR-329 targets by using the microrna.org
and Targetscan 7.2 (http://www.targetscan.org/vert72/) (Fig. 4A). To demonstrate whether miR-329
affects WNT1 expression in PTC, we examined the expression of WNT1
in TPC-1 and BCPAP cells transfected with miR-329 mimic. The data
showed that levels of WNT1 mRNA (Fig.
4B) and protein (Fig. 4C) were
both decreased in TPC-1 and BCPAP cell transfected with miR-329
mimic. To further verify that WNT1 mRNA is a direct target of
miR-329, luciferase reporter assays was analyzed. The results
illustrated that luciferase activity was remarkedly reduced by
miR-329 transduction in TPC-1 and BCPAP cells expressing a reporter
driven by the WT WNT1 3′-UTR, but not the mutated WNT1 3′-UTR
(Fig. 4D and E). Furthermore, we
showed that the mRNA expression of miR-329 was reversely correlated
with WNT1 in PTC tissues (Fig.
4F).
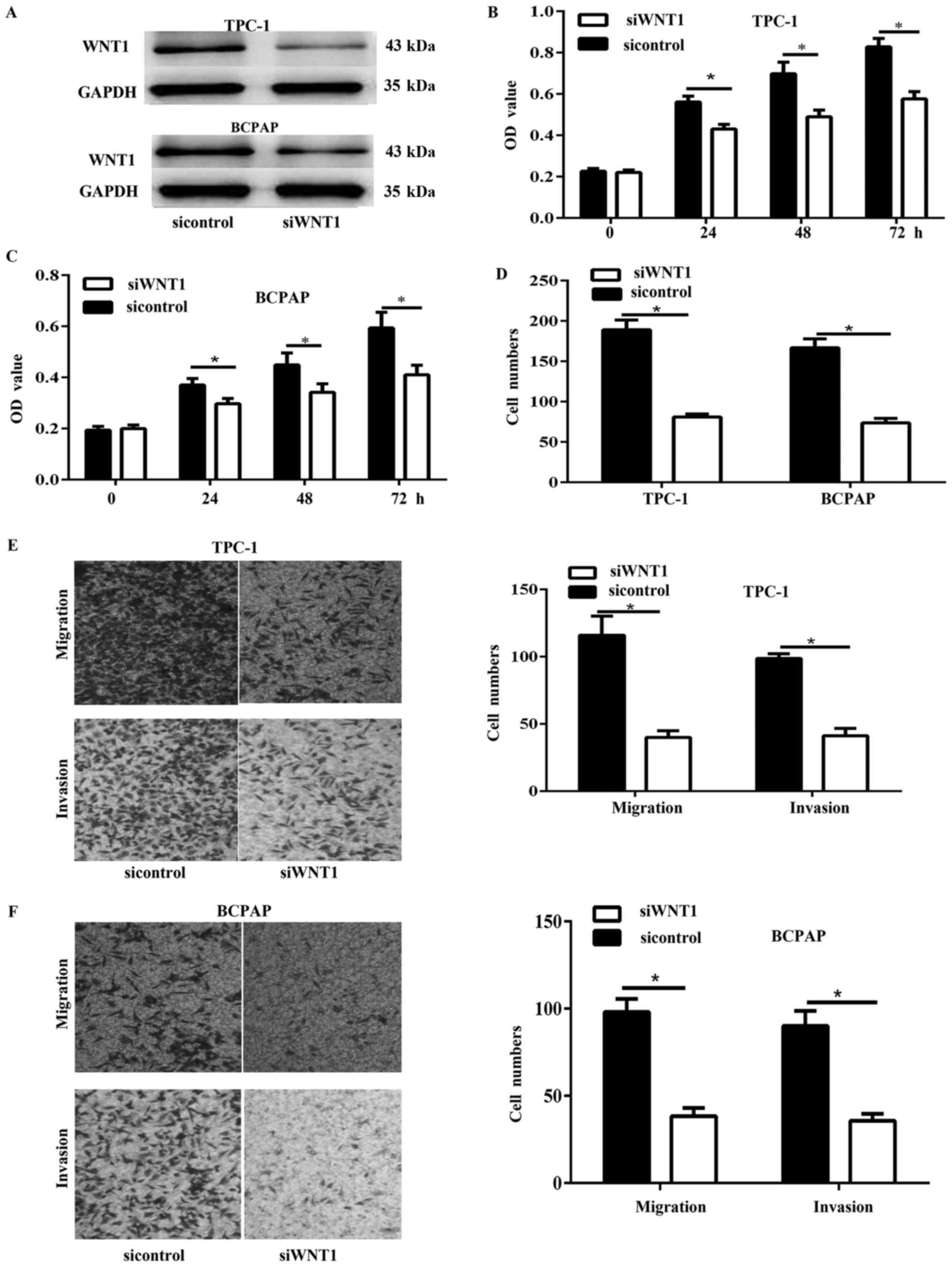
WNT1 silencing inhibited PTC cell
viability, proliferation, migration and invasion in vitro
To further determine whether WNT1 play a critical
role in PTC progression, we performed in vitro function loss
analyses by WNT1 silencing with siWNT1. Results of western blot
showed that the expression of WNT1 was significantly decreased in
TPC-1 and BCPAP cells (Fig. 5A). Cell
viability was detected via CCK-8 assay. The results showed that
knockdown of WNT1 in PTC cells significantly inhibited cell
viability (Fig. 5B and C).
Furthermore, the colony formation assay showed that the
proliferation of WNT1 silencing cells was significantly inhibited
(Fig. 5D). In addition, the transwell
migration and invasion assay showed that the WNT1 silencing
significantly decreased the migration and invasion capability of
TPC-1 and BCPAP cells (Fig. 5E and
F).
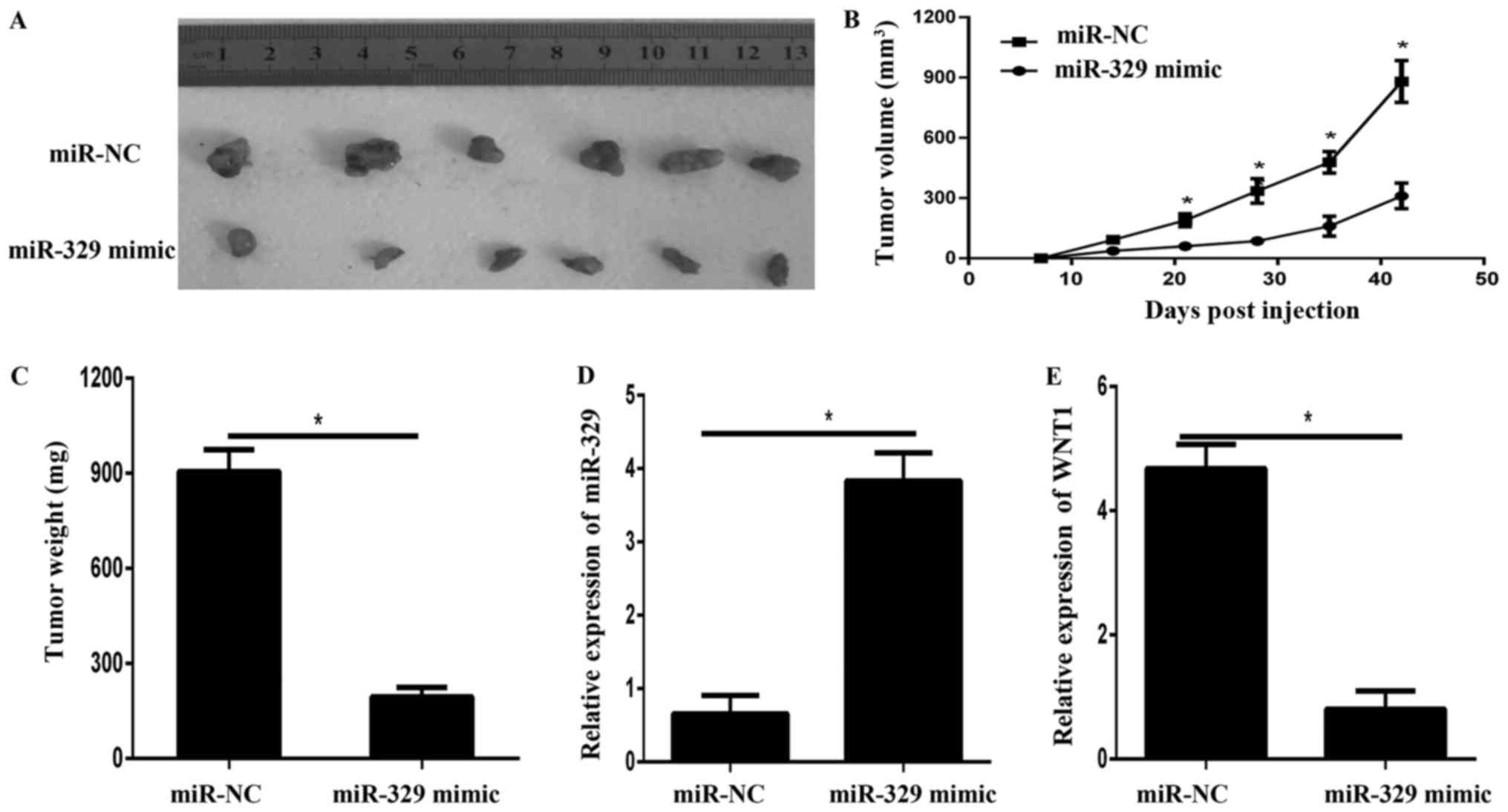
miR-329 suppressed tumor growth in
vivo
To evaluate the effects of miR-329 on tumor
progression in vivo, we extended our investigation by
subcutaneous implantation of stably overexpressing miR-329 BCPAP
cells in nude mice. Tumor volume were examined every 7 d for 42 d,
at which point the tumors were removed for photograph (Fig. 6A) and analysis. As shown in Fig. 6B and C, the results showed that the
volumes and weight of xenografts derived from cells with miR-329
mimic were significantly smaller than that of miR-NC group. In
addition, the qPCR results showed that the expression of miR-329
was significantly enhanced in xenografts from miR-329
mimic-transfected cells (Fig. 6D).
However, the mRNA expression of WNT1 was remarkedly decreased in
the xenografts tissues derived from the cells transfected with
miR-329 mimic (Fig. 6E).
Overexpression of WNT1 overcame the
miR-329-mediated inhibition of cell progression
We investigated whether WNT1 overexprssion rescued
the progression inhibition effect of miR-329 mimic in PTC cells.
The expression of WNT1 was analyzed by western blot (Fig. 2F). Results of CCK-8 (Fig. 2B and C) and colony formation assay
(Fig. 2D and E) showed that WNT1
overexprssion reversed the inhibition of cell viability and
proliferation. Results of transwell migration and invasion assay
showed that WNT1 overexpression significantly reversed the
inhibition of cell migration and invasion via regulating WNT1 in
PTC cell (Fig. 3A-D).
Discussion
In the present study, we showed that miR-329 was
downregulated in human PTC tissues compared with that in adjacent
normal tissues, and its levels were lower in the PTC cell lines
TPC1 and BCPAP than in the normal cell line. Further studies
illustrated that miR-329 overexpression suppressed PTC cell
viability, proliferation, migration and invasion in TPC1 and BCPAP
cells. The present study also demonstrated that miR-329 directly
targeted WNT1 to inhibit the biological behaviour of PTC cells.
Spearman's correlation analysis revealed that the expression of
miR-329 was inversely correlated with mRNA expression of WNT1 in
PTC tissues. miR-329 also suppressed tumor growth in nude mice by
inhibiting WNT1. These findings illustrated that miR-329 inhibited
the development and progression of PTC.
Previous studies reported that miR-329 expression is
decreased in several different types of cancers (6–13). Wang
et al (6), reported that
miR-329 was downregulated in pancreatic cancer and involved in the
inhibition of pancreatic cancer progression by regulating the
GRB2/pERK pathway (6). miR-329 might
partially inhibit neuroblastoma progression by targeting KDM1A
(7) and play an important role in
lung cancer progression through the inhibition of cell
proliferation, migration, invasion and apoptosis by targeting
oncogenic MET (9). In gastric cancer
progression, miR-329 possibly prevented cell proliferation,
migration and invasion by targeting TIAM1 (10). However, expression of miR-329 in oral
squamous cell carcinoma specimens was increased compared with that
in matched tissue ulcerative colitis, suggesting its function as an
oncogene (11). Therefore, miR-329
performed dual functions: tumor suppressor or oncogene dependent on
specific cancer types. In the present study, results showed that
expression of miR-329 was downregulated in PTC tissues and cell
lines, suggesting the tumor suppressive role of miR-329 in PTC
progression. To further illustrate the function of miR-329 in PTC
development, we conducted cell transfection and found that miR-329
overexpression significantly reduced the viability, proliferation
and migration and invasion of PTC cells. These results illustrated
that miR-329 repressed tumour progression in PTC carcinogenesis,
and further demonstrated that the same gene might serve as oncogene
or tumor suppressor depending on the type of tissue and the context
in which they were expressed.
Many miR-329 targets have been identified in various
types of cancer. In the present study, WNT1 was determined as a
target gene of miR-329 in PTC. miR-329 could also regulate the
viability, proliferation and metastasis of PTC cells by targeting
WNT1. WNT1, the first member of the 19 known members of the human
Wnt family, has been shown to promote cancer progression because it
triggers cell proliferation and metastasis (15,16). WNT1
binds to specific Frizzled surface receptors of cells to activate
different signalling pathways, resulting in the accumulation and
nuclear localisation of the downstream molecule β-catenin protein
(17–19). In this study, an important molecular
association between miR-329 and WNT1 was demonstrated. Dual
luciferase reporter assays illustrated that WNT1 was one of the
direct target genes of miR-329. Furthermore, the ectopic expression
of WNT1 remarkably enhanced the viability, proliferation and
migration and invasion of PTC cells reduced by miR-329 mimic,
suggested that WNT1 might be one of the functional target genes of
miR-329. In addition, the mRNA expression of WNT1 was inversely
correlated with miR-329 levels in PTC tissues. At last, the
knockdown of WNT1 repressed PTC cell viability, proliferation and
migration and invasion in vitro. These results implied WNT1
is a functional target gene of miR-329 in PTC.
In summary, the present study is the first to
provide evidence that miR-329 is downregulated in thyroid cancer
tissues and cell lines. miR-329 also functions as a tumor
suppressor of PTC growth by targeting WNT1. This newly identified
miR-329/WNT1 link provides new insight into the mechanisms
underlying PTC development, and suggests that targeting the
miR-329/WNT1 axis may represent a promising therapeutic strategy
for PTC treatment. Nevertheless, further studies are needed to
determine the exact mechanism of decreased miR-329 expression
during the progression of PTC and to further explore other possible
targets of miR-329 in PTC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LW and DM conceived and designed the experiments. DM
wrote and revised the manuscript. FP, XM and KW conducted the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Linyi Central Hospital. All patients provided written
informed consent.
Patient consent for publication
All patients provided written informed consent for
the publication of their data and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liebner DA and Shah MH: Thyroid cancer:
Pathogenesis and targeted therapy. Ther Adv Endocrinol Metab.
2:173–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gonzalez-Gonzalez R, Bologna-Molina R,
Carreon-Burciaga RG, Gómezpalacio-Gastelum M, Molina-Frechero N and
Salazar-Rodriguez S: Papillary thyroid carcinoma: Differential
diagnosis and prognostic values of its different variants: Review
of the literature. ISRN Oncol. 2011:9159252011.PubMed/NCBI
|
|
3
|
Shi X, Liu R, Basolo F, Giannini R, Shen
X, Teng D, Guan H, Shan Z1, Teng W, Musholt TJ, et al: Differential
clinicopathological risk and prognosis of major papillary thyroid
cancer variants. J Clin Endocrinol Metab. 101:264–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orang Valinezhad A, Safaralizadeh R and
Kazemzadeh-Bavili M: Mechanisms of miRNA-mediated gene regulation
from common downregulation to mRNA-specific upregulation. Int J
Genomics. 2014:9706072014.PubMed/NCBI
|
|
5
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Lu X, Zhang T, Wen C, Shi M, Tang
X, Chen H, Peng C, Li H, Fang Y, et al: mir-329 restricts tumor
growth by targeting grb2 in pancreatic cancer. Oncotarget.
7:21441–21453. 2016.PubMed/NCBI
|
|
7
|
Yang H, Li Q, Zhao W, Yuan D, Zhao H and
Zhou Y: miR-329 suppresses the growth and motility of neuroblastoma
by targeting KDM1A. FEBS Lett. 588:192–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang P, Luo Y, Duan H, Xing S, Zhang J, Lu
D, Feng J, Yang D, Song L and Yan X: MicroRNA 329 suppresses
angiogenesis by targeting CD146. Mol Cell Biol. 33:3689–3699. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun CC, Li SJ, Zhang F, Pan JY, Wang L,
Yang CL, Xi YY and de Li J: Hsa-miR-329 exerts tumor suppressor
function through down-regulation of MET in non-small cell lung
cancer. Oncotarget. 7:21510–21526. 2016.PubMed/NCBI
|
|
10
|
Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J
and Feng F: By downregulating TIAM1 expression, microRNA-329
suppresses gastric cancer invasion and growth. Oncotarget.
6:17559–17569. 2015.PubMed/NCBI
|
|
11
|
Shiah SG, Hsiao JR, Chang WM, Chen YW, Jin
YT, Wong TY, Huang JS, Tsai ST, Hsu YM and Chou ST: Downregulated
miR329 and miR410 promote the proliferation and invasion of oral
squamous cell carcinoma by targeting Wnt-7b. Cancer Res.
74:7560–7572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao B, Tan L, He B, Liu Z and Xu R:
miRNA-329 targeting E2F1 inhibits cell proliferation in glioma
cells. J Transl Med. 11:1722013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Liang J, Zhang Z, Lou H, Zhao L, Xu
Y and Ou R: MicroRNA-329-3p targets MAPK1 to suppress cell
proliferation, migration and invasion in cervical cancer. Oncol
Rep. 37:2743–2750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saiselet M, Floor S, Tarabichi M, Dom
Hébrant A, van Staveren WC and Maenhaut C: Thyroid cancer cell
lines: An overview. Front Endocrinol (Lausanne).
3:1332012.PubMed/NCBI
|
|
15
|
Benad P, Rauner M, Rachner TD and Hofbauer
LC: The anti-progestin RU-486 inhibits viability of MCF-7 breast
cancer cells by suppressing WNT1. Cancer letters. 312:101–108.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stanczak A, Stec R, Bodnar L, Olszewski W,
Cichowicz M, Kozlowski W, Szczylik C, Pietrucha T, Wieczorek M and
Lamparska-Przybysz M: Prognostic significance of Wnt-1, β-catenin
and E-cadherin expression in advanced colorectal carcinoma. Pathol
Oncol Res. 17:955–963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wieczorek M, Paczkowska A, Guzenda P,
Majorek M, Bednarek AK and Lamparska-Przybysz M: Silencing of Wnt-1
by siRNA induces apoptosis of MCF-7 human breast cancer cells.
Cancer Biol Ther. 7:268–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang JM, Huang FC, Kuo MH, Wang ZF, Tseng
TY, Chang LC, Yen SJ, Chang TC and Lin JJ: Inhibition of cancer
cell migration and invasion through suppressing the Wnt1-mediating
signal pathway by G-quadruplex structure stabilizers. J Biol Chem.
289:14612–14623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sasaya K, Sudo H, Maeda G, Kawashiri S and
Imai K: Concomitant loss of p120-catenin and β-catenin membrane
expression and oral carcinoma progression with E-cadherin
reduction. PLoS One. 8:e697772013. View Article : Google Scholar : PubMed/NCBI
|