Introduction
In 2012 the World Health Organization (WHO)
estimated that cardiovascular disease accounted for 30% of the
total number of mortalities per year and is the leading cause of
mortality worldwide (1). WHO predicts
that cardiovascular mortality will rapidly increase up to 2030
(1,2).
Vascular endothelial cells possess numerous biological functions,
including the regulation of vascular tone, secretion of vasoactive
substances and anti-platelet aggregation, serving an important role
in maintaining the function of the cardiovascular system. A
previous study demonstrated that the incidence of vascular disease
in patients with impaired endothelial function was significantly
increased compared with that in patients with normal endothelial
function (3). Endothelial cell injury
induced by oxidative stress is an important cause of vascular
endothelial dysfunction and serves a crucial function in the
occurrence of cardiovascular diseases (4). Vascular endothelial cell apoptosis is
the primary form of oxidative stress injury and a prelude to the
occurrence and evolution of multiple types of cardiovascular
diseases (5). Wild type P53 is bound
to four specific sites in the form of tetramer, which is associated
with cell stress response, cell cycle and cell apoptosis (6).
The S100 protein, whose molecular weight is 11–12
kDa, belongs to the family of calcium binding proteins. It is
highly homologous with calmodulin (7,8). The S100
protein family serves an important function in cell proliferation,
differentiation, muscle contraction, gene expression and cell
apoptosis (9,10). Dysfunction of S100 protein may cause
different diseases. S100 calcium-binding protein A4 (S100A4) is a
member of the S100 family and is associated with the occurrence,
development and prognosis of tumors (11). Studies have indicated that the S100A4
protein not only is expressed in the cardiovascular endothelium,
but also functions in promoting angiogenesis (12,13).
Previous studies have indicated that S100A4 binds
the C terminal region of the P53 protein, which affects the
subcellular localization and transcriptional activity of P53
(14). This combination affects
apoptosis. The aim of the present study was to investigate whether
the S100A4 protein exhibits a protective effect on oxidative stress
injury of endothelial cells and to elucidate its mechanism. By
establishing an oxidative stress damage model in vascular
endothelial cells, the effect of S100A4 protein on oxidative stress
injury of vascular endothelial cells was observed. The effect of
S100A4 protein on cardiovascular endothelial cell apoptosis through
the P53 pathway was also examined. Based on these experiments, the
present study preliminarily clarified the regulatory mechanism of
S100A4 protein on the apoptosis of vascular endothelial cells.
Materials and methods
Antibodies and reagents
The following antibodies were used: P53 (cat. no.
10442-1-AP; ProteinTech Group, Inc. (Chicago, IL, USA), Caspase 3
(cat. no. ab13847; Abcam, Cambridge, MA, USA), p17-specific
antibody (cat. no. 25546-1-AP; Proteintech Group, Inc.), S100A4
(ab41532; Abcam) and β-actin (cat. no. 60008-1-Ig; Proteintech
Group, Inc.). S100A4 (cat. no. ab41532) was purchased from Abcam.
The following reagents were used: Rat Lactic Dehydrogenase (LDH;
cat. no. E-0672; Meilian Shengwu, Shanghai, China), nitrous oxide
(NO; cat. no. KGE001; R&D Systems, Inc. Minneapolis, MN, USA),
Malondialdehyde (MDA; cat. no. ab118970; Abcam), superoxide
dismutase (SOD; cat. no. DYC3419-2; R&D Systems). Rat
recombinant S100A4 protein was expressed and purified in the
Performance Medicine Laboratory of Tianjin Institute of Health and
Environmental Medicine (Tianjin, China). Cell Death Detection kit
(cat. no. G3250, Promega) was from Promega Corporation (Madison,
WI, USA). Hydrogels incubated with PBS alone were used as negative
controls. All the experiments were repeated at least three times.
The microscopy (OLMPUS CK40, Olympus corporation, Tokyo, Japan) was
preserved in cell culture room and utilized to observe endothelial
cells without fixing embedding and staining at 25°C. The
magnification used was ×100.
Cell culture and treatment
Rat aortic endothelial cells (RAECs) were isolated
and cultured as described previously, with minor modifications
(15). The thoracic aorta were
excised from 3 male Wistar rats (150–180 g) after they were
sacrificed humanely by injecting with 40 mg anesthetic (chloral
hydrate) per 100 g of body weight and immediately placed in cold
PBS containing 100 U/ml penicillin and 100 mg/ml streptomycin. The
aorta was cut into 1 mm-wide rings subsequent to the removal of the
periadventitial fat. Following transfer to a T-25 cm2
flasks (NalgeNunc International, Penfield, NY, USA), the rings were
cultured in Medium 199 (cat. no. 11150059, Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 20% fetal bovine
serum (FBS; cat. no. 10099141, Gibco; Thermo Fisher Scientific,
Inc.), 2.5 ng/ml basic fibroblast growth factors, 100 U/ml
penicillin and 100 mg/ml streptomycin. The aorta rings were placed
at 37°C in a humidified atmosphere with 5% CO2 for 72–80
h without movement. All pieces of aorta rings were removed when
cells migrated. In experiments, M199 medium supplemented with 1%
bovine serum albumin (cat. no. B2064; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used. All experiments were performed with
RAECs up to 4 passages. Male Wistar rats were gained from the
Experimental Animal Room of the Institute of Health and
Environmental Medicine (Tianjin, China). The rats were maintained
under standard conditions (ambient temperature 21–23°C; with a 12 h
dark-light cycle) with ad libitum access to food and tap water.
This study was approved by the committee of the Institute of Health
and Environmental Medicine, Tianjin, China.
The cells were divided into the following groups: i)
A normal control group incubated in Medium 199 (NC); ii) a
H2O2 injury group incubated in Medium 199
containing 100 µM H2O2 (HI); and two
S100A4-treated groups incubated with iii) 50 µM or iv) 100 µM
S100A4 in Medium 199 containing 100 µMH2O2
[(HI+S100A4 (50 µM)/HI+S100A4 (100 µM)]. In the MTT and ELISA
analysis, two groups were added to detect whether S100A4 had
damaged the cells. They were NC+S100A4 (50 µM) and NC+S100A4 (100
µM) groups.
Cell proliferation assay
Cell proliferation was determined using an MTT
assay. A total of ~3×103 endothelial cells were plated
into each well of a 96-well plate. Following overnight incubation
at 37°C, the cells were treated with 100 µM
H2O2, H2O2+S100A4
(50/100 µM) for 48 h. Then the medium was removed and MTT (20 µl of
5 mg/ml) was added to each well and incubated at 37°C for 4 h.
Plates were agitated at low speed (16.77 × g) 37°C for 10 min and
the purple-colored precipitates of formazan were dissolved in 150
µl dimethyl sulfoxide. Absorbance was measured at 490 nm using an
ELISA plate reader. The reduction in viability of in
H2O2-treated endothelial cells was expressed
as a percentage compared with non-H2O2
treated control cells. Control cells were considered 100%
viable.
ELISA assay
The levels of LDH (Rat LDH ELISA kit; cat. no.
E-0672; Meilian Shengwu), NO (Total Nitric Oxide and
Nitrate/Nitrite Parameter assay kit; cat. no. KGE001; R&D
Systems), MDA (MDA assay kit; cat. no. ab118970; Abcam), and SOD
(Human/Mouse/Rat Total SOD2/Mn-SOD DuoSet IC ELISA kit; cat. no.
DYC3419-2; R&D Systems) from endothelial cell medium were
quantified by ELISA assay according to the manufacturer's
protocol.
Assessment of apoptosis by TUNEL
staining
Cells apoptosis was determined by double-labeling
TUNEL immunofluorescence staining. A total of 2×104
RAECs with Medium 199 (cat. no. 11150059; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS were seeded into each
well of a 48-well plate. Following the aforementioned experimental
treatments, cells were fixed in 4% paraformaldehyde for 30 min at
room temperature. Subsequently, the cells were incubated with 3%
H2O2 in methanol for 10 min at room
temperature to block endogenous peroxidase activity, and were then
incubated with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min
at 4°C. After washing in PBS, the cells were incubated with the
TUNEL reaction mixture containing 5 µl enzyme solution and 45 µl
fluorochrome-labeled solution at 37°C for 60 min in the dark. For
counterstaining, the cells were incubated with DAPI for 15 min at
room temperature. The cells were observed using a fluorescence
microscope, in which 10 fields were randomly selected. The TUNEL+
and DAPI+ nuclei in the cells were counted manually. The percentage
of apoptotic cells was calculated as the ratio of the number of
TUNEL-positive cells to the total number of cells, which were
counted using a fluorescence microscope.
Western blot analysis
The 4 groups of cells (described above) were
harvested. The cells samples were homogenized in a
Radio-Immunoprecipitation Assay (RIPA) lysis buffer [50 mM Tris (pH
7.4), 150 mM NaCl, 1% TritonX-100, 1% sodium deoxycholate, 0.1%
SDS]. Protein concentrations were determined with a BCA assay kit
(Pierce; Thermo Fisher Scientific, Inc.). The proteins (10 µl in
each line) were separated using a 10% gel with SDS-PAGE and
transferred onto nitrocellulose membranes. Membranes were blocked
with TBS-Tween-20 (0.05%) containing 5% non-fat milk for 2 h at
room temperature and incubated with the following primary
antibodies, anti-β-actin (1:1,000, 60008-1-Ig, Proteintech Group,
Inc.), anti-P53 (1:500, 10442-1-AP, Proteintech Group, Inc.),
anti-cleaved-caspase-3 (1:1,000, 25546-1-AP, Proteintech Group,
Inc.), anti-S100A4 (1:250, ab41532, Abcam) overnight at 4°C. The
membranes were then incubated with secondary antibody, goat
anti-rabbit antibodies (1:5,000, ZB-2301, ZSGB-Bio) conjugated with
horseradish peroxidase for 1 h at room temperature (25°C). A
SuperSignal™-enhanced chemiluminescent substrate (Pierce; Thermo
Fisher Scientific, Inc.) was applied to the probed membrane for 3
min at room temperature. The blots were quantified via densitometry
using ImageJ software (version 1.37; National Institutes of Health,
Bethesda, MA, USA).
Immunoprecipitation (IP)
Endothelial cells were seeded in 10-cm dishes,
followed by stimulation (37°C) with or without 100 µM
H2O2 for 48 h. Subsequently, the HepG2 cell
(Tianjin Saierbio Biotechnology Co., Ltd., Tianjin, China) lysates
were prepared with 1% Tris-Triton cell lysis buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA) pre-mixed with 1 mM
phenylmethanesulfonyl fluoride on ice for 30 min and centrifuged
(4°C) at 12,000 × g for 30 min. The supernatants were incubated
(4°C) overnight with 30 µl Dynabeads protein A or protein G (Thermo
Fisher Scientific, Inc.) pre-coated with anti-S100A4 (1:250, Abcam)
antibodies. The immunocomplexes were subjected to western blot
analysis. The normal corresponding immunoglobulin G control was
assayed simultaneously.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using a Student's t-test for
paired samples or a single-factor analysis of variance with
Student-Newman-Keuls post-hoc test as appropriate by SPSS software
(version 20.0; IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
S100A4 may inhibit the cell
morphological changes induced by H2O2
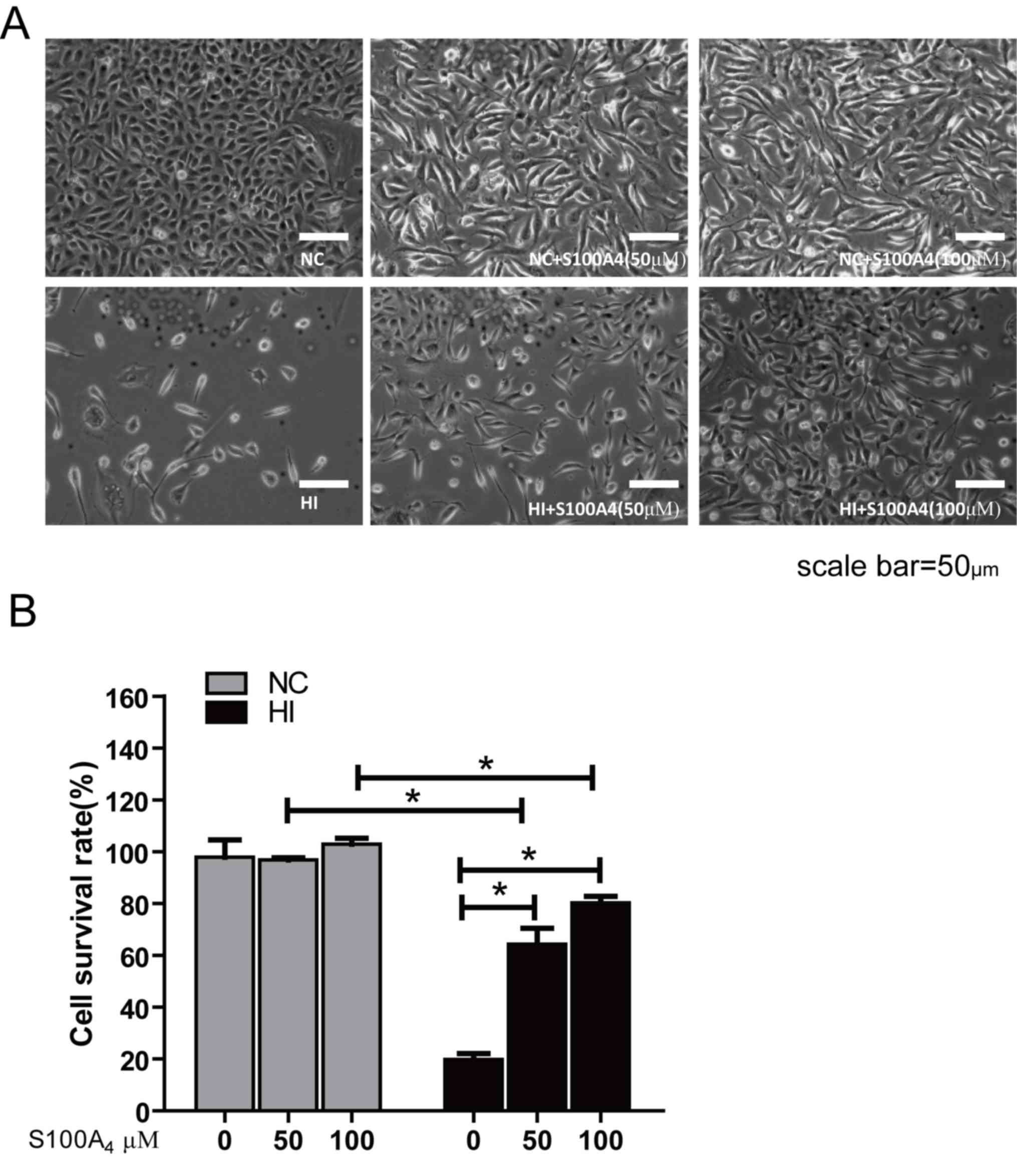
Visible under a phase contrast microscope, in the NC
group the endothelial cells were polygonal and exhibited a clear
structure, uniform size and single paving stone-like close spacing.
The cell morphology of the NC+S100A4 groups was almost identical to
that of the NC group. In the HI group, the endothelial cells
exhibited shrinkage, roundness and clearance. There were vacuoles
and nuclear chromatin condensation and aggregation in the cytoplasm
of the cell. Cells demonstrated a disordered arrangement, losing
the typical single layer paving stone-like arrangement. In the
HI+S100A4 groups, cell morphology and structure were predominantly
clear (Fig. 1A). The arrangement and
morphology of the cells was not markedly changed compared with the
NC group.
The MTT assay easily and effectively evaluates the
activity of cells. The cell survival rate of the oxidative stress
injury group was significantly decreased compared with that of the
NC group (P<0.05), suggesting that H2O2
may cause endothelial cell death. There were no significant
differences in cell survival rate between the NC, NC+S100A4 (50 µM)
and NC+S100A4 (100 µM) groups following administration of S100A4
protein, which indicated that exogenous S100A4 protein demonstrated
no significant effect on endothelial cells. In addition, the cell
survival rate of the 50 and 100 µM HI+S100A4 groups was
significantly increased compared with that in the HI group, but
remained significantly decreased compared with that of the NC group
(P<0.05) and the cell survival rate of HI+S100A4 (100 µM) group
was increased compared with that of the HI+S100A4 (50 µM) group
(Fig. 1B). This indicated that S100A4
protein may improve the survival rate of these cells.
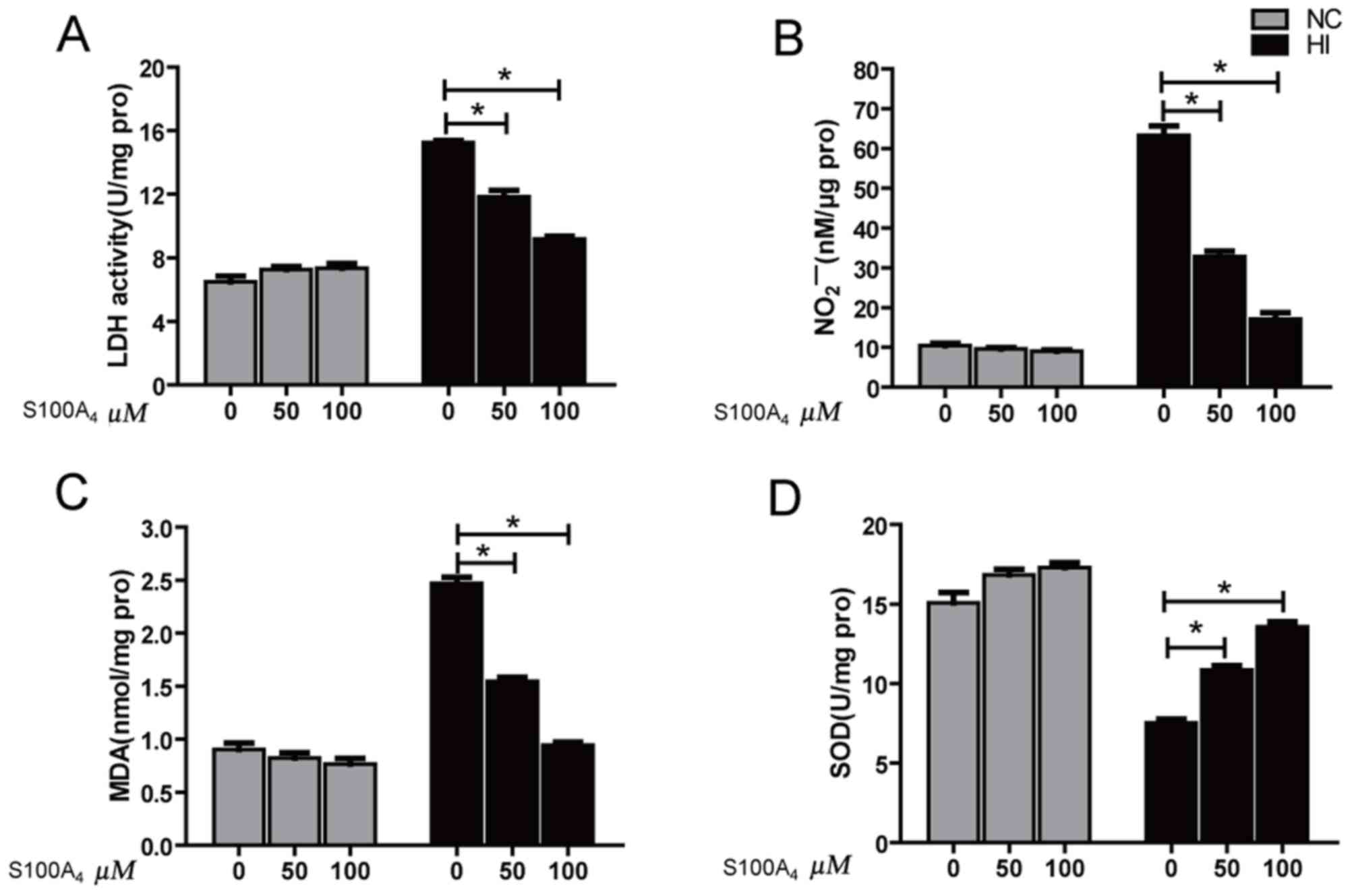
Effect of S100A4 protein on LDH, NO,
MDA and SOD in cultured endothelial cells induced by oxidative
stress
The results indicated that the LDH activity and MDA
content of HI group were significantly increased compared with
those of the NC group (P<0.01), while NO content and SOD
activity were significantly decreased compared with those of the NC
group (P<0.01), suggesting that H2O2
oxidatively damaged these cells. However, the LDH values of the 50
and 100 µM HI+S100A4 groups were significantly decreased compared
with that of the HI group, but remained significantly increased
compared with that of the NC group (P<0.05). The LDH value of
the HI+S100A4 (100 µM) group was decreased compared with that of
the HI+S100A4 (50 µM) group (P<0.05; Fig. 2A). The NO content of 50 and 100 µM
HI+S100A4 groups was significantly increased compared with that of
the HI group, but remained significantly decreased compared with
that of the NC group (P<0.05; Fig.
2B). The content of NO in the HI+S100A4 (100 µM) group was
increased compared with that in the HI+S100A4 (50 µM) group
(P<0.05; Fig. 2B). The MDA content
results demonstrated that different doses of S100A4 protein may
significantly reverse the increase in MDA levels induced by
H2O2 injury (P<0.05; Fig. 2C). The SOD activities in the 50 and
100 µM HI+S100A4 groups were significantly increased, compared with
the HI group (P<0.05; Fig. 2D).
The experimental results indicated that S100A4 protein prevented
oxidative damage.
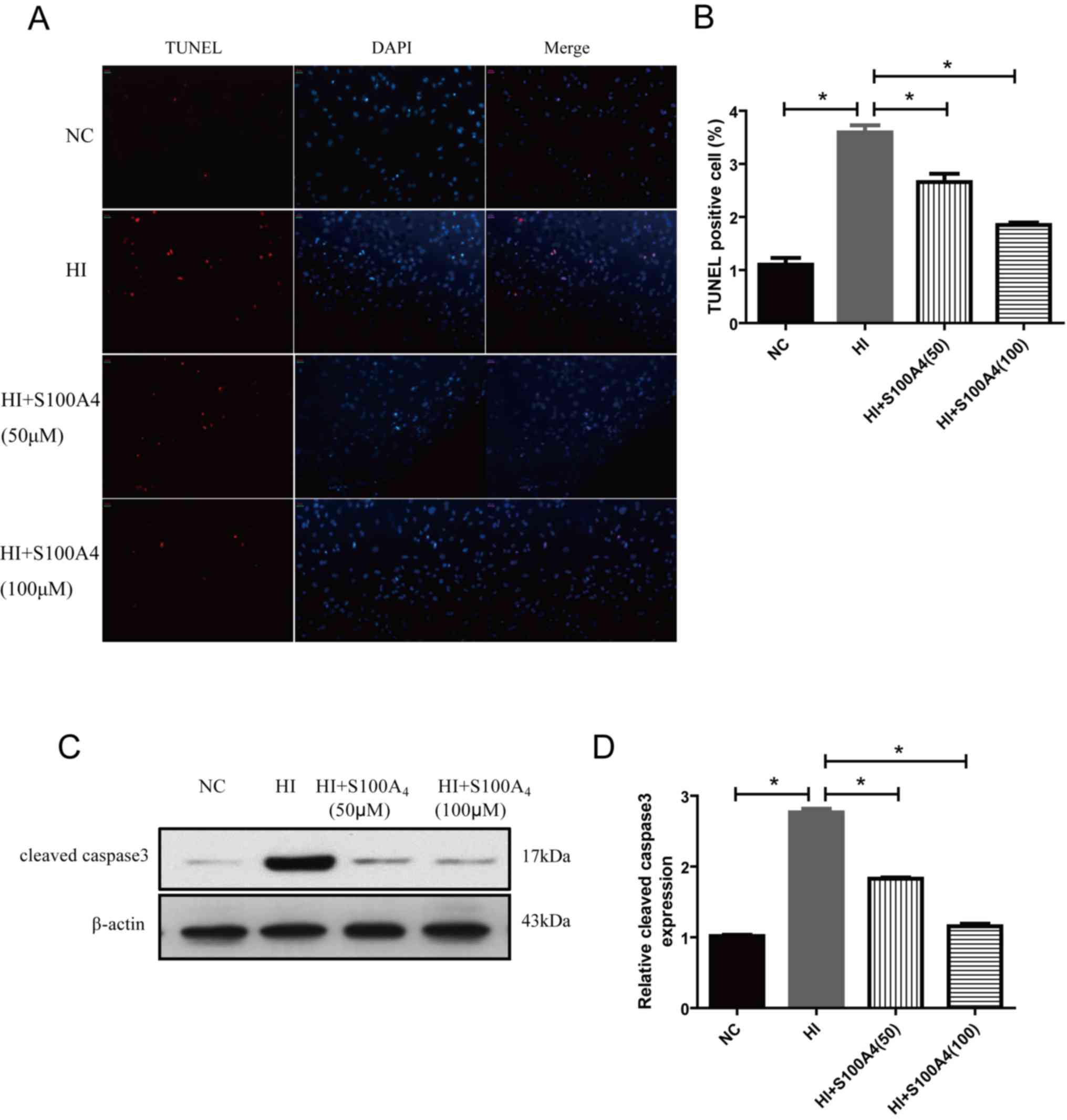
Effect of S100A4 protein on the rate
of endothelial cell apoptosis induced by oxidative stress
The results demonstrated that the endothelial cell
apoptosis rate of the oxidative stress injury group was
significantly increased compared with that of the control group
(P<0.01), suggesting that H2O2 may induce
endothelial cell injury and cell apoptosis. The endothelial cell
apoptosis rate in the HI+S100A4 (50 µM) and HI+S100A4 (100 µM)
group was significantly decreased compared with that in the HI
group (P<0.05; Fig. 3A and B).
Since mitochondrial function is associated with
caspase activity (16), the function
of caspase 3 was assessed. The expression of cleaved (active)
caspase 3 expression was demonstrated using western blot analysis.
In Fig. 3, western blot analysis of
cleaved caspase 3 (Fig. 3C and D) was
performed following H2O2 administration in
ECs and revealed significant caspase 3 activity compared with NC
(P<0.05). However, S100A4 significantly inhibited cleaved active
caspase 3 activity. The results indicated that S100A4 protein
exhibited prevented oxidative damage, inhibiting apoptosis.
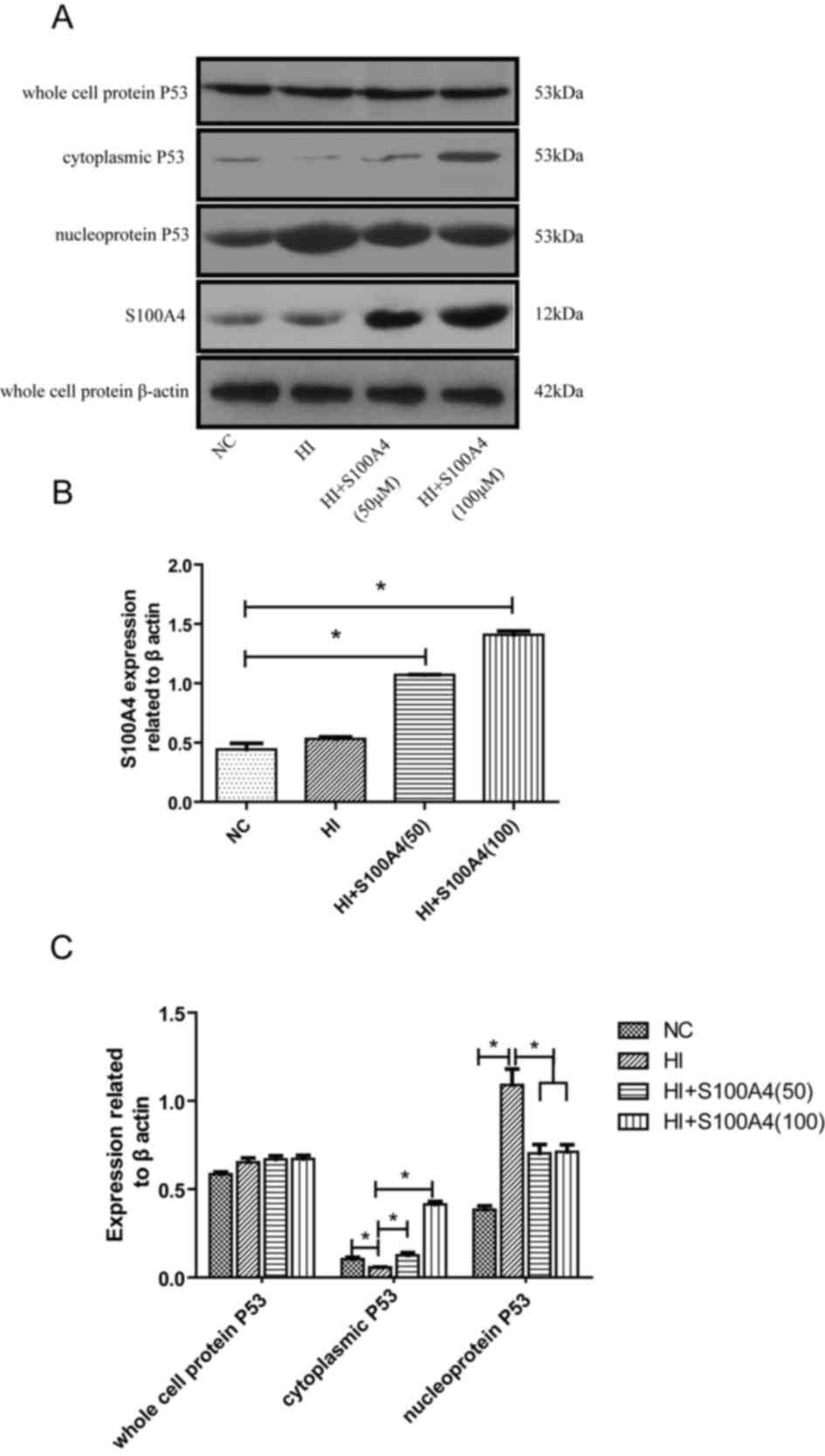
Determination of total protein,
cytoplasmic protein and nuclear protein of endothelial cell protein
by western blot analysis
In Fig. 4, following
S100A4 intervention, the level of intracellular S100A4 protein was
significantly increased in the S100A4 groups (P<0.05). Although
there was no significant change in the expression of P53 in the
cell, the distribution of P53 protein in the cell was altered; the
effect of S100A4 protein on P53 was dose-dependent. As the dose of
S100A4 increased, the aggregation of P53 in the cytoplasm increased
and the corresponding distribution in the nucleus decreased. This
suggests that S100A4 protein may inhibit P53 movement from the
cytoplasm into the nucleus. Therefore, we hypothesize that the
function of S100A4 protein in endothelial cell apoptosis is
inhibited in the oxidative stress damage model of endothelial cells
and that this effect is mediated by P53.
S100A4 interacts with P53 in the
nucleus
S100 family proteins have no known enzymatic
activity; therefore, it may be hypothesized that S100 proteins
function through interaction with other proteins to regulate the
function of interacting proteins. Previous studies have focused on
non-muscle myosin IIA and P53 as potential S100A4-interacting
proteins. Therefore, the present study investigated the potential
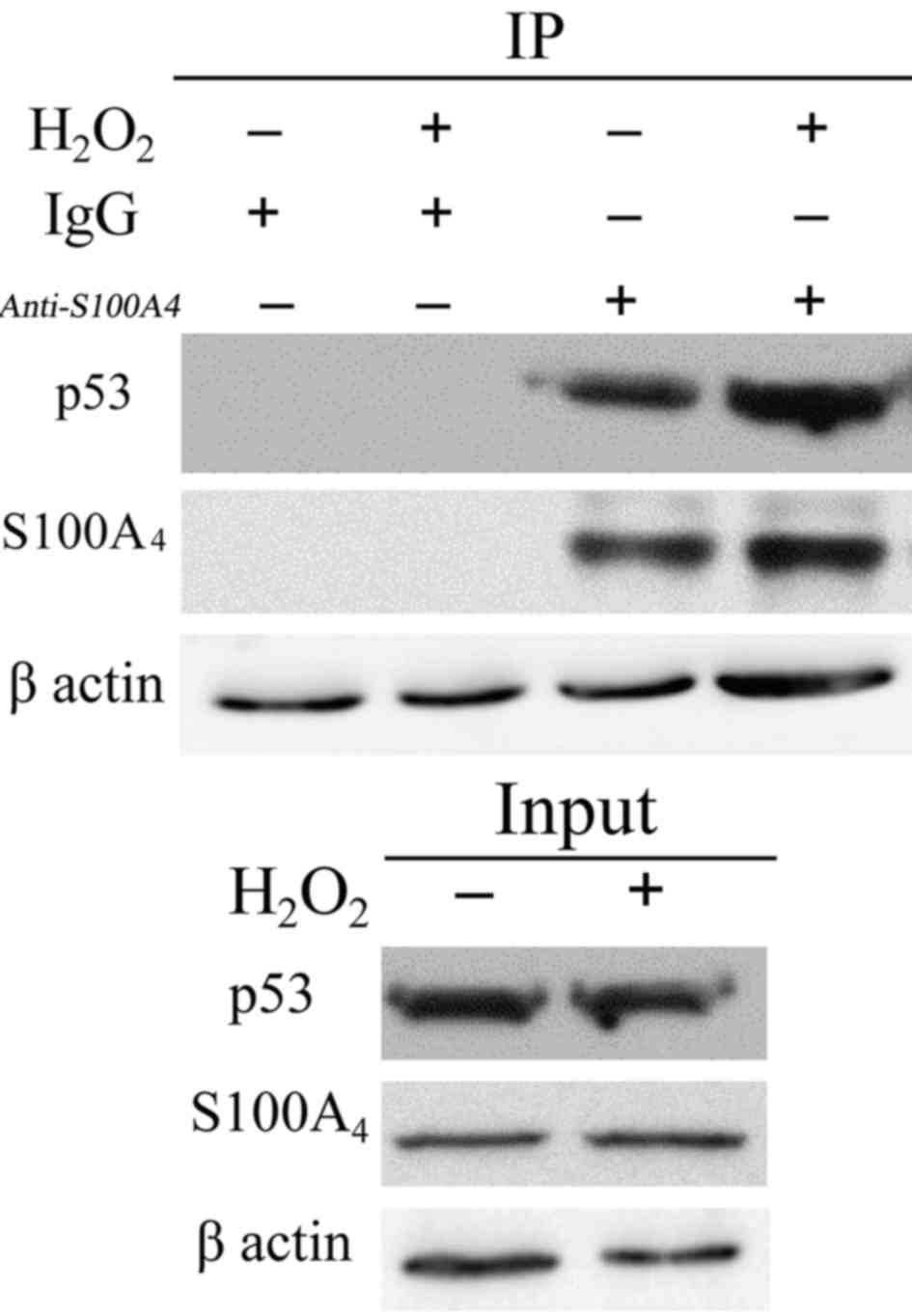
interaction between S100A4 and P53. IP of endogenous S100A4 in
endothelial cells resulted in the co-precipitation of endogenous
P53 in untreated cells (Fig. 5).
Following H2O2 treatment, the total cell P53
did not change significantly. However, P53 in the nucleus was
significantly increased. P53 binding to S100A4 was also
significantly increased in the nucleus (P<0.05). Therefore, we
hypothesized that S100A4 may combine with P53 in the nucleus to
increase the degradation of P53, thereby inhibiting the apoptosis
of cells.
Discussion
The vascular endothelium is composed of a monolayer
of vascular endothelial cells, which is a barrier between
circulating blood and vascular smooth muscle. The vascular
endothelium participates in the contraction and relaxation of blood
vessels, the formation of blood vessels, thrombosis and the
inflammatory response (17,18). Oxidative stress is the key factor that
causes changes in endothelial function and cell damage (19,20).
Oxygen free radicals, collectively known as reactive oxygen species
(ROS), are one of the primary factors that cause oxidative damage
of endothelial cells (18,19). Free radical reactions cause cell
membrane lipid peroxidation, and then alterations to the cell
transport and enzyme functions (19,20).
Oxidative stress is associated with the pathological process of the
endothelial cells, including increasing vascular endothelial cell
permeability, affecting cell proliferation, increasing leukocyte
infiltration and interfering with the signal transduction in the
cell, which causes cell death and apoptosis (21). It serves a key function in the
occurrence and development of vascular diseases (22). Therefore, it is important to prevent
the occurrence of cardiovascular disease through the protection of
vascular endothelial cells from oxidative damage.
The S100 protein family is one of the largest
families of calcium-binding proteins. Including the S100A4 protein,
at present, there are 21 members of the S100 family (23). Previous studies have demonstrated
abnormal expression of S100 in numerous diseases, including
psoriasis, Alzheimer's disease, cystic fibrosis, cardiomyopathy,
muscle atrophy (spinal cord) lateral sclerosis and cancer (24,25). The
S100A4 protein belongs to the S100 protein family and is composed
of 101 amino acids with a molecular weight of 11.5 kD (23). Studies have confirmed that the S100A4
protein may promote the invasion and metastasis of tumors and
regulate the function of the cell, including cell growth and cell
signal transduction (26,27). Multiple studies demonstrate that the
S100A4 protein is associated with vascular regeneration and
extracellular matrix remodeling and may even be used as an
independent vascular-stimulating factor (13,28).
Schmidt-Hansen et al (29)
identified that intracellular S100A4 stimulates endothelial cells
to produce matrix metalloproteinases, which promotes the remodeling
of the extracellular matrix and the degradation of matrix
remodeling is a necessary step in angiogenesis. An additional study
indicated that the release of S100A4 from the cornea of the
implanted rat cornea may induce novel blood vessel formation
(30). Furthermore, the interaction
of S100A4 protein and membrane protein may promote the activation
of plasminogen activator, which is induced by the activation of
plasminogen activator (31).
To assess the function of S100A4 protein on
endothelial cell damage induced by oxidative stress and the
potential mechanism, the present study selected 100 µM
H2O2-induced injury for 12 h to establish a
model of endothelial cell oxidative stress injury (32). The results indicated that the
oxidative stress caused the loss of the typical morphology of the
endothelial cells. The cells exhibited shrinkage and roundness, the
gap increased and vacuoles in the cytoplasm of cells were not
arranged in order. The activity of LDH was increased, the content
of NO was decreased and the content of MDA was increased, while the
activity of SOD was decreased. The survival rate of endothelial
cells was decreased and the apoptosis rate was increased. The
experiment suggested that oxidative stress resulted in the injury
of endothelial cells. S100A4 prevented oxidative stress to protect
endothelial cells.
The important mechanism of oxygen free
radical-induced cell and tissue damage is to induce peroxidation of
the polyunsaturated fatty acids in the biological membrane,
consequently producing lipid peroxide. Lipid peroxidation may be
associated with the occurrence of a chain reaction of
polyunsaturated fatty acids (28). As
one of the unsaturated fatty acids, linoleic acid can induce both
NF-κB and AP-1 transcriptional activation (33). Lipid free radicals and their
degradation products (MDA) are formed, causing membrane fluidity,
permeability and integrity damage and then the cell membrane is
destroyed (34,35). ROS mainly includes oxygen ions and
peroxides. As a superoxide scavenging enzyme, active SOD is able to
neutralize O2− and presents antioxidative
effect (36). With the aid of SOD,
superoxide radical anion or hyperoxide in biological tissues can be
changed into hydrogen peroxide (HO) and singlet oxygen
(1O2). HO may be changed into water by
catalase or glutathione peroxidase (36). Therefore, the content of MDA may
reflect the degree of lipid peroxidation and indirectly reflect the
degree of cell damage. SOD activity indirectly reflects the ability
of the body to clear oxygen free radicals. Oxidative stress may
increase the secretion of endothelin-1 and diminish the
bioavailability of nitric oxide (NO) (3,37). These
vasoactive molecules promote the contraction of blood vessels and
then initiate a series of post-injury reactions, resulting in the
occurrence of cardiovascular disease (38,39).
Oxidative stress may lead to endothelial dysfunction
(40,41), resulting in decreased NO levels and
increased LDH release (42). The
results indicated that H2O2 may increase the
content of MDA, the activity of LDH was increased and that the
activity of SOD was decreased, suggesting that the endothelial
cells were damaged by lipid peroxidation. S100A4 protein
intervention may be effective against oxygen free radical
damage.
Therefore, we hypothesized that the protective
mechanism of S100A4 on the endothelial cells may be associated with
the protection of cell mitochondria, increased cell activity.
Endothelial cell activity is key to the maintenance
of endothelial function. If the apoptosis rate of endothelial cells
exceeds the normal level, it will affect the integrity of the blood
vessels and induce the injury of endothelial cells. The P53 gene is
an important anti-cancer gene. A previous study demonstrated that
wild type P53 may induce the apoptosis of leukocytes (43). Three members of the S100 protein
family, namely S100B, S100A2 and S100A4, physiologically interact
with P53 in a calcium dependent manner. Phosphorylation and
acetylation of P53 may be affected by the interaction of S100
protein, which results in the regulation of the subcellular
localization and transcriptional activity of P53 (44). Previously, studies have focused on the
role of the P53 gene in cardiovascular diseases and have indicated
that P53 serves an important function in the oxidative stress of
blood vessels as an important type of transcription protein
(45,46). The results of the present study
suggested that oxidative stress causes an increased rate of
endothelial cell apoptosis and P53 may be the target protein of
S100A4. Subsequent to the cells being administered S100A4 protein,
the total level of P53 protein was not increased but the
distribution of P53 protein in the cells was changed, primarily
with an increase in the aggregation of P53 in the cytoplasm and a
decrease in the distribution of P53 in the nucleus. As the dose of
S100A4 increased, the aggregation of P53 in the cytoplasm increased
and the corresponding distribution in the nucleus decreased. This
suggested that S100A4 protein may inhibit P53 movement from the
cytoplasm into the nucleus.
To conclude, exogenous S100A4 protein may reverse
oxidative stress damage, improve the survival rate of cells and
inhibit the apoptosis of endothelial cells. The protective
mechanism of S100A4 protein on the apoptosis of endothelial cells
may be mediated by inhibiting P53. This provides a reference for
the additional exploration of the application of S100A4 protein in
endothelial cell damage induced by oxidative stress.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81373108 and
30971421).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TW and BS developed the project. XM, XG and ZZ
performed experiments and wrote the manuscript. XZ, LW and MY
analyzed the data. KW and HR helped with computational analysis.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the committee of the
Institute of Health and Environmental Medicine, Tianjin, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shanthi M, Tim A, Douglas B, Francesco B,
Jeremy L, Cecile M, Vladimir P, Leanne R, Vera Da Costa E Silva and
Gretchen S: Global status report on concommunicable diseases
2014[R]. Geneva: World Health Organization 9; 2014
|
|
2
|
World Health Organization, . Fact Sheet
no. 317. Geneva: WHO; 2011
|
|
3
|
Crimi E, Ignarro LJ and Napoli C:
Microcirculation and oxidative stress. Free Radi Res. 41:1364–1375.
2007. View Article : Google Scholar
|
|
4
|
Loscalzo J: Redox dysregulation in
vascular pathobiology. Free Radic Biol Med. 75 Suppl 1:S22014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marchenko ND and Moll UM: Mitochondrial
death functions of p53. Mol Cell Oncol. 1:e9559952014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tarabykina S, Griffiths TR, Tulchinsky E,
Mellon JK, Bronstein IB and Kriajevska M: Metastasis-associated
protein S100A4: Spotlight on its role in cell migration. Curr
Cancer Drug Targets. 7:217–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moroz OV, Antson AA, Murshudov GN,
Maitland NJ, Dodson GG, Wilson KS, Skibshøj I, Lukanidin EM and
Bronstein IB: The three-dimensional structure of human S100A12.
Acta Crystallogr D Biol Crystallogr. 57:20–29. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ridinger K, Schäfer BW, Durussel I, Cox JA
and Heizmann CW: S100A13 biochemical characterization and
subcellular localization in different cell lines. J Biol Chem.
275:8686–8694. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazzuccheli L: Protein S100A4: Too long
overlooked by pathologists? Am J Pathol. 160:7–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donato R: S100: A multigenic family of
calcium-modulated proteins of the EF-hand type with intracellular
and extracelluar functions roles. Int J Biochem Cell Biol.
33:637–668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garrett SC, Varney KM, Weber DJ and
Bresnick AR: S100A4, a mediator of metastasis. J Biol Chem.
281:677–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ambartsumian N, Grigorian M and Lukanidin
E: Genetically modified mouse models to study the role of
metasasis-promoting S100A4(mts1) protein in metastatic mammary
cancer. J Dairy Res. 72:27–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt-Hansen B, Klingelhöfer J,
Grum-Schwensen B, Christensen A, Andresen S, Kruse C, Hansen T,
Ambartsumian N, Lukanidin E and Grigorian M: Functional
significance of metastasis-including S100A4(Mts1) in tumor-stroma
interplay. J Biol Chem. 279:24498–24504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orre LM, Panizza E, Kaminskyy VO, Vernet
E, Gräslund T, Zhivotovsky B and Lehtiö J: S100A4 interacts with
p53 in the nucleus and promotes p53 degradation. Oncogene.
32:5531–5540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang XH, Chen SF, Jin HM and Hu RM:
Differential analyses of angiogenesis and expression of growth
factors in micro- and macrovascular endothelial cells of type 2
diabetic rats. Life Sci. 84:240–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hao J, Shen W, Tian C, Liu Z, Ren J, Luo
C, Long J, Sharman E and Liu J: Mitochondrial nutrients improve
immune dysfunction in the type 2 diabetic Goto-Kakizaki rats. J
Cell Mol Med. 13:701–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chesterman CN: Vascular endothelium,
haemostasis and thrombosis. Blood Rev. 2:88–94. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang X, Yang X, Peng Y and Lin J:
Protective effects of lycopene against
H2O2-induced oxidative injury and apoptosis
in human endothelial cells. Cardiovasc Drugs Ther. 23:439–448.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kadl A and Leitinger N: The role of
endothelial cells in the resolution of acute inflammation. Antioxid
Redox Signal. 7:1744–1754. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pober JS and Sessa WC: Evolving functions
of endothelial cells in inflammation. Nat Rev Immunol. 7:803–815.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramagopal UA, Dulyaninova NG, Varney KM,
Wilder PT, Nallamsetty S, Brenowitz M, Weber DJ, Almo SC and
Bresnick AR: Structure of the S100A4/myosin-IIA complex. BMC Struct
Biol. 20:13–31. 2013.
|
|
22
|
Toya SP and Malik AB: Role of endothelial
injury in disease mechanisms and contribution of progenitor cells
in mediating endothelial repair. Immunobiolog. 217:569–580. 2012.
View Article : Google Scholar
|
|
23
|
Boye K and Maelandsmo GM: S100A4 and
metastasis: A small actor playing many roles. Am J Pathol.
176:528–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marenholz I, Heizmann CW and Fritz G: S100
proteins in mouse and man: From evolution to function and pathology
(including an update of the nomenclature). Biochem Biophys Res
Commun. 322:1111–1122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Santamaria-Kisiel L, Rintala-Dempsey AC
and Shaw GS: Calcium-dependent and -independent interactions of the
S100 protein family. Biochem. 396:201–214. 2006. View Article : Google Scholar
|
|
26
|
Schneider M, Kostin S, Strøm CC, Aplin M,
Lyngbaek S, Theilade J, Grigorian M, Andersen CB, Lukanidin E,
Hansen Lerche J and Sheikh SP: S100A4 is regulated in injured
myocardium and promotes growth and survival of cardiac myocytes.
Cardiovasc Res. 75:40–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chaabane C, Otsuka F, Virmani R and
Bochaton-Piallat ML: Biological responses in stented arteries.
Cardiovasc Res. 99:353–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nowak JZ: Oxidative stress,
polyunsaturated fatty acids-derived oxidation products and
bisretinoids as potential inducers of CNS diseases: Focus on
age-related macular degeneration. Pharmacol Rep. 65:288–304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmidt-Hansen B, Ornås D, Grigorian M,
Klingelhöfer J, Tulchinsky E, Lukanidin E and Ambartsumian N:
Extracellular S100A4(mts1) stimulates invasive growth of mouse
endothelial cells and modulates MMP-13 matrix metalloproteinase
activity. Oncogene. 23:5487–5495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ambartsumian N, Klingelhöfer J, Grigorian
M, Christensen C, Kriajevska M, Tulchinsky E, Georgiev G, Berezin
V, Bock E, Rygaard J, et al: The metastasis associated Mts1(S100A4)
protein could act as an angiogenic factor. Oncogene. 20:4685–4695.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Semov A, Moreno MJ, Onichtchenko A,
Abulrob A, Ball M, Ekiel I, Pietrzynski G, Stanimirovic D and
Alakhov V: Metastasis-associated protein S100A4 induces
angiogenesis through interaction with Annexin II and accelerated
plasmin formation. J Biol Chem. 280:20833–20841. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sakamoto T, Repasky WT, Uchida K, Hirata A
and Hirata F: Modulation of cell death pathways to apoptosis and
necrosis of H2O2-treated rat thymocytes by
lipocortin I. Biochem Biophys Res Comunun. 220:643–647. 1996.
View Article : Google Scholar
|
|
33
|
Toborek M, Lee YW, Garrido R, Kaiser S and
Hennig B: Unsaturated fatty acids selectively induce an
inflammatory environment in human endothelial cells. Am J Clin
Nutr. 75:119–125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mazière C, Conte MA, Degonville J, Ali D
and Mazière JC: Cellular enrichment with polyunsaturated fatty
acids induces an oxidative stress and activates the transcription
factors AP1 and NFkappaB. Biochem Biophys Res Commun. 265:116–122.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alexander-North LS, North JA, Kiminyo KP,
Buettner GR and Spector AA: Polyunsaturated fatty acids increase
lipid radical formation induced by oxidant stress in endothelial
cells. J Lipid Res. 35:1773–1785. 1994.PubMed/NCBI
|
|
36
|
Nowak JZ: Oxidative stress,
polyunsaturated fatty acids-derived oxidation products and
bisretinoids as potential inducers of CNS diseases: Focus on
age-related macular degeneration. Pharmacol Rep. 65:288–304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takeda N and Manabe I: Cellular interplay
between cardiomyocytes and nonmyocytes in cardiac remodeling. Int J
Inflam. 2011:5352412011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Raij L: Hypertension, endothelium, and
cardiovascular risk factors. Am J Med. 90 Supple 2A:13S–18S. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smith EJ and Rothman MT: Antiproliferative
coatings for the treatment of coronary heart disease: What are the
targets and which are the tools? J Interv Cardiol. 16:475–483.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Onat D, Brillon D, Colombo PC and Schmidt
AM: Human vascular endothelial cells: A model system for studying
vascular inflammation in diabetes and atherosclerosis. Curr Diab
Rep. 11:193–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Parnell E, Smith BO, Palmer TM, Terrin A,
Zaccolo M and Yarwood SJ: Regulation of the inflammatory response
of vascular endothelial cells by EPAC1. Br J Pharmacol.
166:434–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma S, Yao S, Tian H, Jiao P, Yang N, Zhu P
and Qin S: Pigment epithelium-derived factor alleviates endothelial
injury by inhibiting Wnt/β-catenin pathway. Lipids Health Dis.
16:312017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yonish-Rouach E, Grunwald D, Wilder S,
Kimchi A, May E, Lawrence JJ, May P and Oren M: p53-mediated cell
death: Relationship to cell cycle control. Mol Cell Biol.
13:1415–1423. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mueller A, Schäfer BW, Ferrai S, Weibel M,
Makek M, Höchli M and Heizmann CW: The calcium-binding protein
S100A2 interact with p53 and modulates its transcriptional
activity. J Biol Chem. 280:29186–29193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma JQ, Ding J, Zhang L and Liu CM: Ursolic
acid protects mouse liver against CCl4-induced oxidative stress and
inflammation by the MAPK/NF-κB pathway. Environ Toxicol Pharmacol.
37:975–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tilstra JS, Robinson AR, Wang J, Gregg SQ,
Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, et al:
NF-κB inhibition delays DNA damage-induced senescence and aging in
mice. J Clin Invest. 122:2601–2612. 2012. View Article : Google Scholar : PubMed/NCBI
|