Introduction
Gastric cancer is the fifth most prevalent
malignancy and the third leading cause of cancer-related death
worldwide, and 70% of cases occur in east Asia (1,2), with
China alone accounting for 42% of all newly diagnosed cases
(3). However, the incidence of
gastric cancer has decreased in most industrialized countries over
the past three decades. The complete surgical removal of the tumor
is an effective treatment for early gastric cancer, but at the time
of diagnosis, the majority of gastric cancer patients are at an
advanced stage of disease. The treatment of gastric cancer remains
a challenge, and despite advances in surgical techniques,
chemotherapy, and radiotherapy, the survival rate of gastric cancer
patients remains unsatisfactory. The 5-year survival rate for
advanced gastric cancer ranges from 25 to 35% (4,5), and this
low rate is largely attributable to postoperative metastasis
(6). There is still a large
discrepancy between the success of treatment in mainland China and
in Japan or Korea. Improvements in the early diagnosis and
treatment of gastric cancer may continue to be the most effective
strategy for improving patient survival. To improve the diagnosis
and treatment of gastric cancer, an effective therapeutic target
for this disease is required.
The mitochondrion is an organelle that is in a
continuous state of dynamic fission-fusion equilibrium (7), which is important for maintaining normal
cellular functions. If the equilibrium of the mitochondrion is
disturbed, its energy-generating function is damaged and its DNA
mutates. Mitochondrial dysfunction is closely related to tumor
occurrence. The characteristics of tumor cells, such as their
unlimited growth, resistance to apoptosis, evasion of immunological
surveillance, invasion, and distant metastasis, are all related to
mitochondrial dysfunction. Therefore, researchers have proposed new
strategies that target the mitochondrion (8–10). A
comprehensive understanding of the effects of mitochondria in the
occurrence and development of tumors could provide theoretical
guidance and new strategies for tumor therapies that target this
organelle. Dynamin-1-like protein (DNM1L) belongs to the dynein GTP
enzyme superfamily. DNM1L contains a unique proline-rich structural
domain and three functional domains. The N-terminal domain has GTP
enzyme activity; a dynein-like domain occurs in the middle region;
and the C-terminal domain exerts a dynein-isogeny GTP enzyme effect
(11,12). DNM1L is a key regulatory factor
mediating the dynamic fission-fusion equilibrium of the
mitochondrion (13). It is usually
found in cytosol, but after it is spliced, DNM1L is recruited by
FIS1 in the mitochondrial outer membrane, and translocates from the
cytosol to the mitochondrion, where it forms an oligomer. DNM1L
then assumes a cyclical structure around the mitochondrion at the
fission site, and ruptures the organelle by hydrolyzing GTP and
extruding the mitochondrial membrane (14).
In this study, we determined the expression of DNM1L
in patients with gastric cancer to gain a better understanding of
DNM1L as a prognostic marker of this disease and the relationship
between its expression and the clinicopathological features of the
disease. We also examined the effects of DNM1L on the
proliferation, apoptosis, and invasion capacity of gastric cancer
MKN-45 cells. Our findings provide novel insights into the role of
DNM1L in gastric cancer.
Materials and methods
Cells
Human gastric cancer cells SGC-7901 and AGS cells,
and human embryonic kidney (293) cells were purchased from Shanghai
Institutes for Biological Sciences (shanghai, China), and human
gastric cancer MKN-45 cells were from the China Center for Type
Culture Collection (Wuhan, China). All these cells were cultured in
RPMI-1640 medium (Gibco BRL, Paisley, Scotland, UK) supplemented
with 10% fetal calf serum (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany).
Reagents
SYBR Master Mix was purchased from Takara Bio, Inc.
(Otsu, Japan). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) kit was
obtained from Gen-View Scientific, Inc. (El Monte, FL, USA). The
anti-DNM1L antibody (ab56788) was from Abcam (Cambridge, MA,
USA).
Patients and preparation of tissue
samples
In this study, we used a study cohort of 150
patients who underwent radical gastrotomy for gastric
adenocarcinoma between January 2005 and December 2009 at The Second
Affiliated Hospital of Wenzhou Medical University (Wenzhou, China)
and were followed-up by December 2015. No patient was treated with
radiotherapy or chemotherapy before surgery. The clinical
information, including age, sex, tumor size, histological
differentiation, recurrence, and metastasis, was obtained from the
patients' medical records. The pathological TNM status was assessed
according to the criteria of the TNM Classification of Malignant
Tumors, American Joint Committee on Cancer (edition 7, 2010). This
study was approved by the Ethics Committee of The Second Affiliated
Hospital of Wenzhou Medical University, and the need for informed
consent was waived. However, written informed consent for radical
gastrotomy and the use of data for research purposes were obtained
from patient prior to treatment.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was prepared with TRIzol Reagent (Pufei,
Shanghai, China) and reverse transcribed with M-MLV reverse
transcriptase (Promega Corporation, Madison, WI, USA). qPCR was
performed with the SYBR Green Real-Time PCR Master Mix on the ABI
Prism 7500 Sequence Detection System (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The primers used to
amplify the DNM1L and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) transcripts in the SYBR-Green assay were: DNM1L-F,
5′-GGTGAACCCGTGGATGATAAA-3′ and DNM1L-R,
5′-CCTCAGGCACAAATAAAGCAG-3′, which generated a 265-bp product; and
GAPDH-F, 5′-TGACTTCAACAGCGACACCCA-3′ and GAPDH-R,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The data were analyzed with the ABI
7500 System SDS software. The DNM1L mRNA expression levels were
standardized to those of GAPDH mRNA by calculating ΔCq=Cq
(DNM1L)-Cq (GAPDH). All experiments were performed in triplicate
and repeated three times.
Lentiviral short hairpin RNA (shRNA)
vector construction and transfection
A DNM1L-directed shRNA (sh-DNM1L:
5′-gcTACTTTACTCCAACTTATT-3′) was designed based on the DNM1L
gene sequence (accession no. NM_005690) and synthesized as follows:
forward The oligonucleotides
5′-CcgggcTACTTTACTCCAACTTATTCTCGAGAATAAGTTGGAGTAAAGTAGCTTTTTg-3′
and reverse 5′-aat tca aaa agc
TACTTTACTCCAACTTATTCTCGAGAATAAGTTGGAGTAAAGTAGC-3′ were annealed and
inserted downstream from the U6 promoter in the lentiviral vector
GV248 (Jikai, Shanghai, China). A lentivirus carrying an
shRNA-targeting nonsilencing sequence (5′-TTCTCCGAACGTGTCACGT-3′)
was used as the control (sh-Ctrl). The lentiviruses were generated
by transfecting 293 cells with the GV248-sh-DNM1L plasmid, together
with pHelper 1.0 and pHelper 2.0, with polyethylenimine
(Sigma-Aldrich; Merck KGaA). For cell infection, the viral
supernatants were supplemented with Polybrene (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and incubated with the cells
for 8 h. HepG2 cells were transfected with the lentiviral
particles, and selected with puromycin (2 µg/ml) for 7 days. The
cells stably expressing the shRNAs were confirmed with RT-qPCR.
Cell proliferation assay
Cells in the logarithmic growth phase were collected
and digested with trypsin (Gibco; Thermo Fisher Scientific, Inc.).
The cell suspension was prepared in culture medium at a density of
2×103 cells/ml (100 µl) in a 96-well plate, and the
cells were incubated at 37°C under 5% CO2. Cell
proliferation was determined on days 1, 2, 3, 4, and 5 after
seeding, with the MTT assay kit (Gen-View Scientific, Inc.),
according to the manufacturer's instructions. Optical densities
(ODs) were measured at 490 nm with a microplate reader (Infinite
M1000; Tecan Group Ltd., Männedorf, Switzerland). Two independent
experiments were performed.
Cell invasion assay
Culture medium (500 µl) without fetal bovine serum
was added to the upper and lower chambers of a Transwell apparatus
and incubated at 37°C for 2 h to form a Matrigel matrix hydrate.
After the Matrigel matrix was hydrated, the chambers were
transferred into new wells. A cell invasion assay was performed in
a 24-well Transwell chamber with 8 µm pores (Corning Incorporated,
Corning, NY, USA), according to the manufacturer's instructions.
Cells were suspended in serum-free medium and seeded into Transwell
inserts coated with growth-factor-reduced Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA). The bottom wells were filled with
complete medium. After 24 h, Giemsa stain (Sigma-Aldrich; Merck
KGaA) was added to the cells, and each chamber was photographed
under an inverted fluorescence microscope (Olympus Corporation,
Tokyo, Japan) for analysis. Nine random microscope fields
(magnification, ×200) were counted for each chamber and three
independent experiments were performed.
Cell apoptosis assay
Cells were collected, seeded in 96-well plates, and
allowed to grow to 80% confluence. The cells were then transferred
to medium without serum or growth factors for 5 days. The apoptosis
assay was performed with the Annexin V Apoptosis Detection kit APC
(eBioscience; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The data were analyzed with the CellQuest
software. Three independent experiments were performed.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue sections (4
µm) were dewaxed, rehydrated in xylene and then in a 100-70%
alcohol gradient, and washed in water. Antigen retrieval was
performed with Antigen Unmasking Solution (Vectorlabs, Burlingame,
CA, USA), according to manufacturer's instructions. The sections
were blocked with 1% bovine serum albumin for 30 min at 24°C, and
were then incubated with a primary rabbit polyclonal anti-DNM1L
antibody (diluted 1:100; Abcam, Cambridge, UK) for 45 min at 24°C,
followed by a secondary goat anti-rabbit immunoglobulin G (IgG)
antibody for 30 min at 24°C. The slides were washed three times
with PBS. Positive staining was visualized with a DAB kit (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
for 5 min and then counterstained in hematoxylin. PBS was used
instead of the primary antibody for the negative control.
Immunostaining was blindly and semiquantitatively evaluated by two
observers with no knowledge of the clinical data of the patients.
DNM1L staining was scored for the percentage of positive cells and
the intensity of staining in the cytoplasm. The scoring system for
intensity was: 0, no staining; 1, weak staining; 2, moderate
staining; and 3, strong staining. The scoring system for the
percentage of stained tumor cells was: 0, < 5% stained cells; 1,
5–25% stained cells; 2, 26–50% stained cells; and 3, >51%
stained cells. A final score was the sum of the staining intensity
and the percentage of stained cells: 0–1, negative (−), 2–3, weakly
positive (+); 4–6 positive (++); >6, strongly positive (+++). A
score of 0–1 was considered DNM1L negative and a score ≥was
considered DNM1L positive.
Statistical analysis
The SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA) was used for all statistical analyses. Measurement data were
expressed as means ± standard errors of the means (SEM). Multigroup
comparisons of the means were performed using one-way analysis of
variance with post hoc Student-Newman-Keuls test. Comparisons of
count data were made with a χ2 test or Fisher's exact
test. The Kaplan-Meier statistic was used to analyze survival, and
the differences among groups were analyzed with the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
DNM1L expression in gastric
adenocarcinoma cells
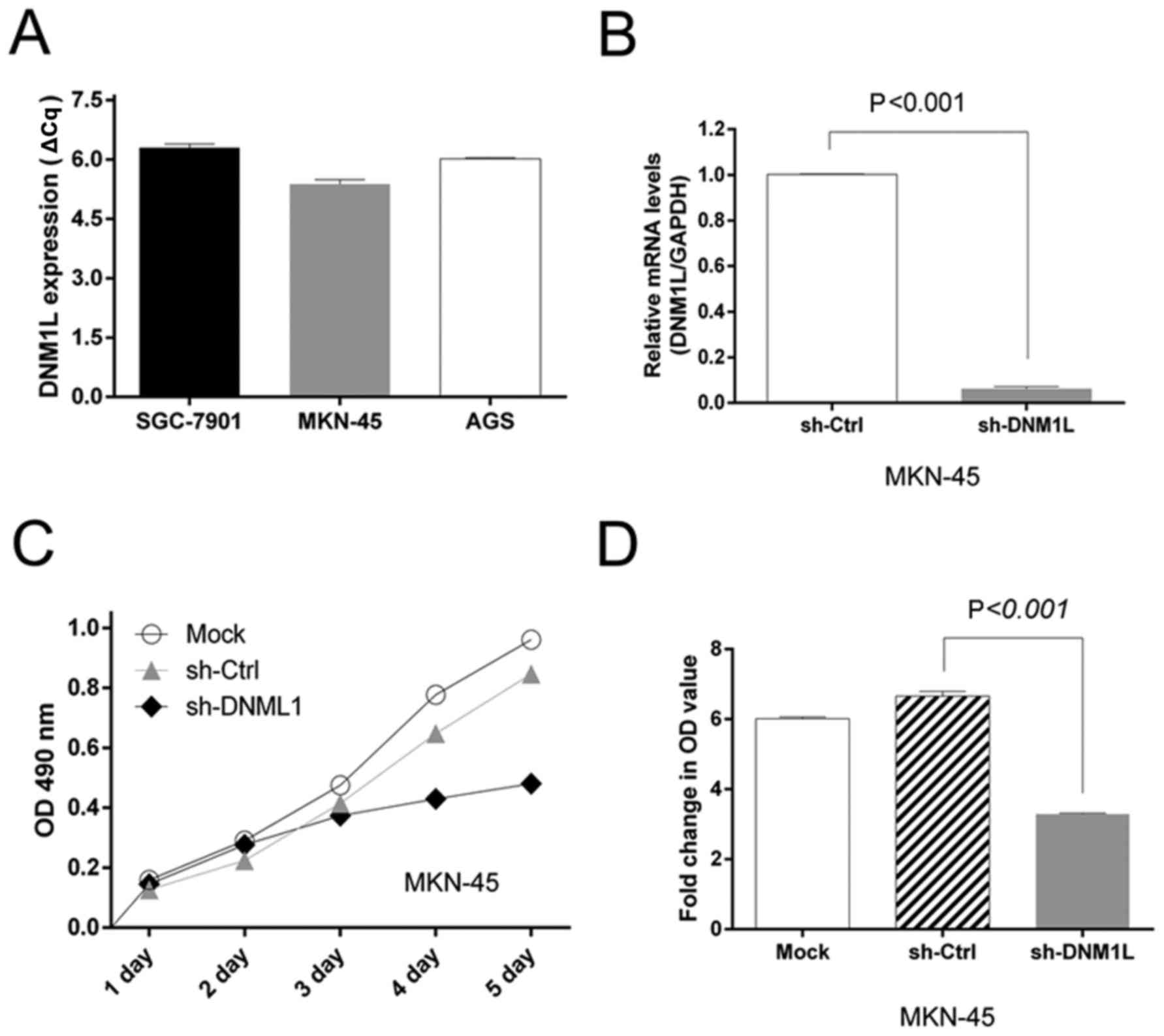
We screened a panel of three gastric cancer cell
lines (AGS, SGC-1901, and MKN-45) for DNM1L mRNA expression. High
levels of DNM1L mRNA were observed in all three cell lines. The
MKN-45 cells had the highest DNM1L mRNA expression levels and were
selected for subsequent experiments (Fig.
1A). We engineered MKN-45-based cells with lentiviral
transfection to express DNM1L shRNA. The level of DNM1L mRNA in the
lenti-sh-DNM1L-transfected MKN-45 cells was examined with qPCR
(Fig. 1B).
DNM1L accelerates MKN-45 cell
proliferation
An MTT assay was used to assess cell viability as a
marker of cell proliferation. MTT detected the proliferation of
MKN-45 cells transfected with the sh-DNM1L lentiviral vector
(sh-DNM1L) or the control vector (sh-Ctrl). The depletion of DNM1L
significantly impaired the proliferation of MKN-45 cells (Fig. 1C). Moreover, the MTT OD value on day
5, normalized to that on day 1, was lower in the DNM1L-knockdown
cells than in MKN-45 cells transfected with the control vector
(Fig. 1D). This suggests that the
depletion of DNM1L by shRNA in MKN-45 cells caused a dramatic
reduction in cell proliferation.
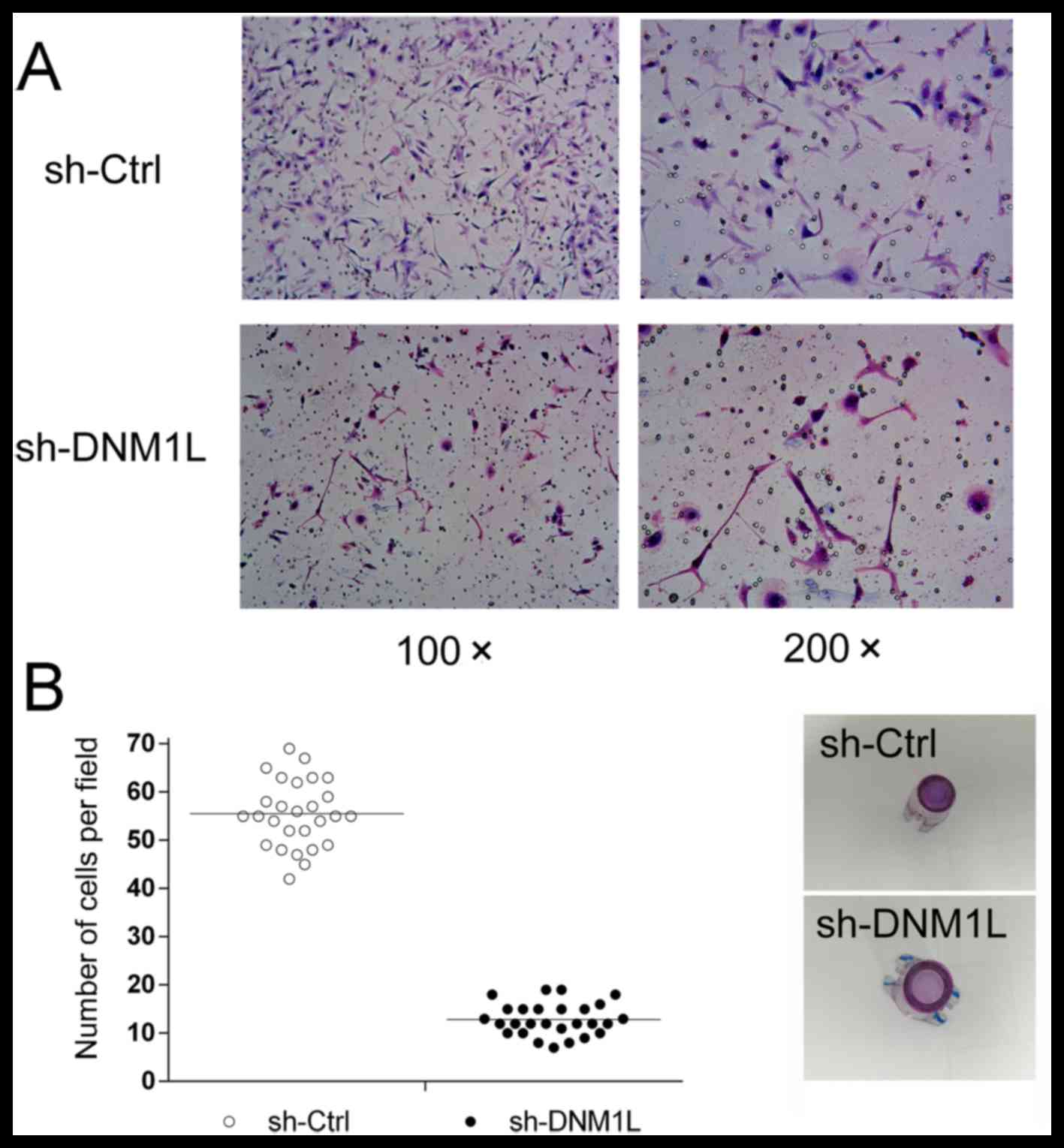
DNM1L promotes the invasion of MKN-45
cells
A Transwell invasion assay was used to explore the
effect of DNM1L on the invasion of MKN-45 cells. The number of
DNM1L-knockdown cells in the high-power lens of each field of view
was much lower than the number of MKN-45 cells transfected with the
control vector (Fig. 2), suggesting
that DNM1L promotes the invasion of MKN-45 cells.
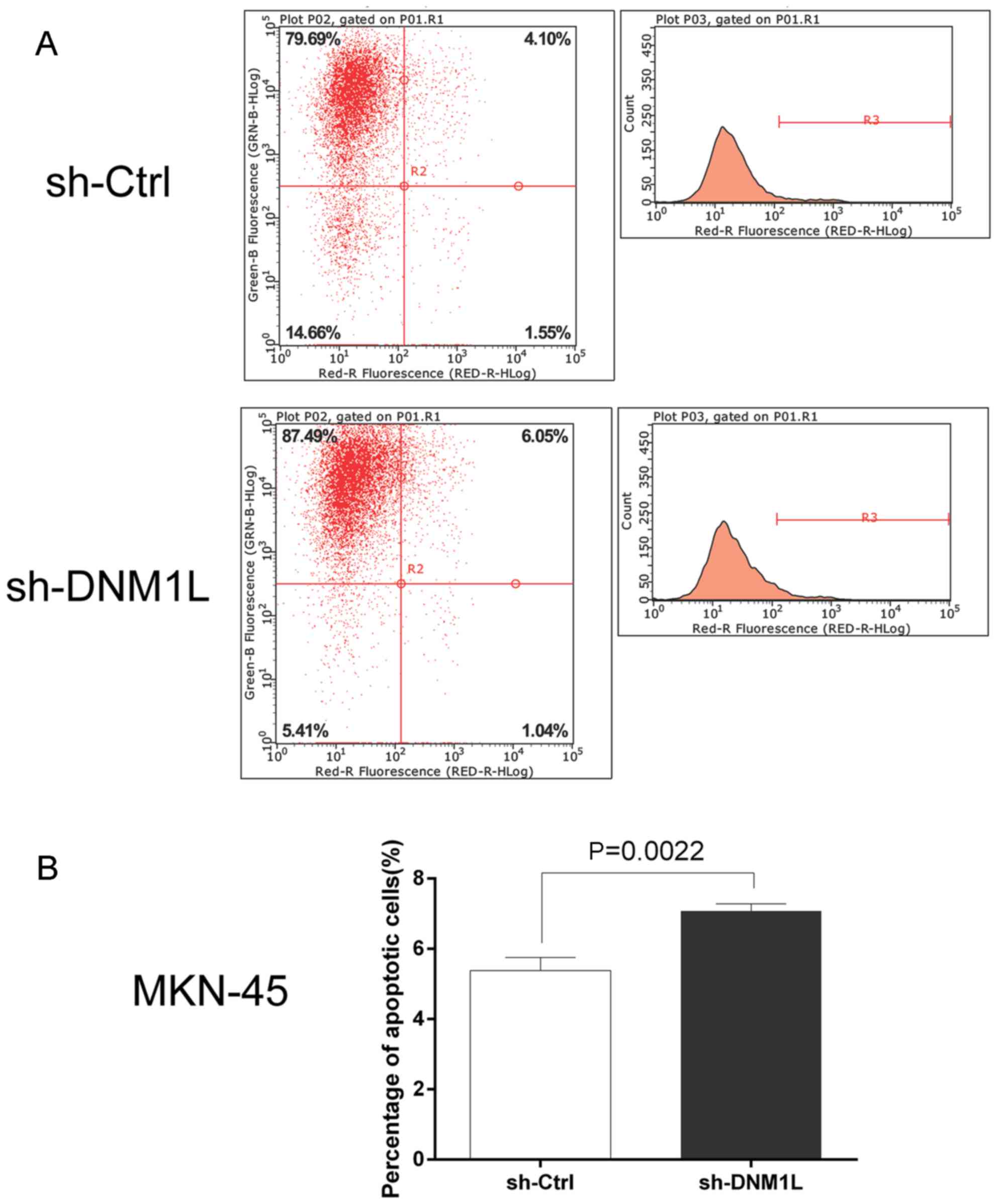
DNM1L inhibits the apoptosis of MKN-45
cells
The Annexin V Apoptosis Detection kit APC was used
to investigate the effect of DNM1L on cell apoptosis. Flow
cytometry showed that the apoptosis rate was higher in the
DNM1L-knockdown cells than in the MKN-45 cells transfected with the
control vector (Fig. 3). This
suggests that DNM1L plays an important role in resistance to
apoptosis.
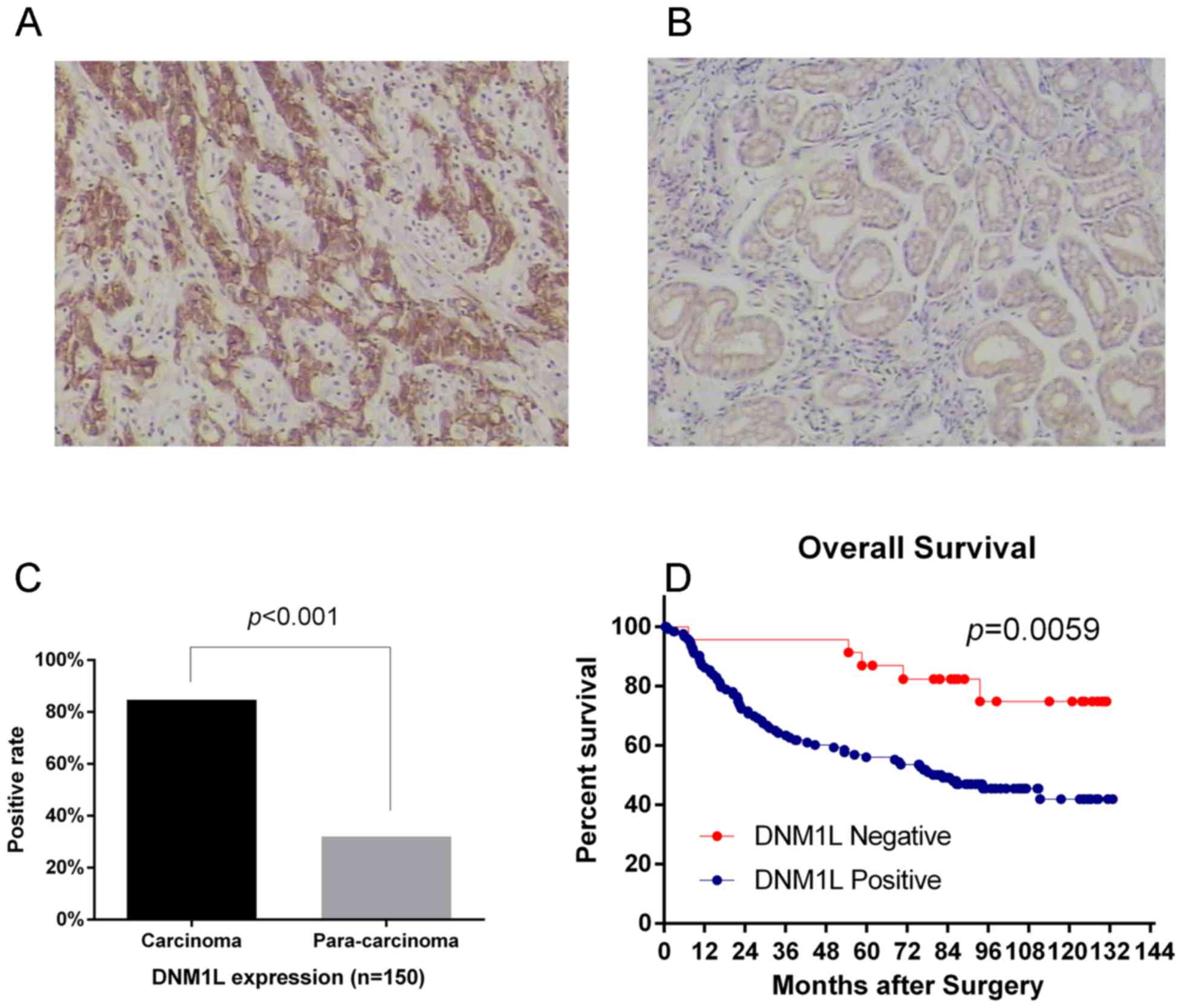
Increased DNM1L expression is
associated with a poor prognosis in gastric adenocarcinoma
patients
We used immunohistochemistry to analyze the
expression of the DNM1L protein in 150 primary gastric
adenocarcinomas and the corresponding pericarcinoma tissues.
Immunostaining revealed that DNM1L expression was significantly
higher in the carcinoma specimens (84.67%) than that in the
pericarcinoma specimens (32%) (Fig.
4A-C). We also analyzed the associations between DNM1L
expression and variables such as age, sex, tumor diameter, tumor
grade, depth of invasion, TNM stage, and lymph-node metastasis.
DNM1L expression associated significantly with the depth of
invasion, lymph-node metastasis, and TNM stage, but not with the
other factors tested (Table I).
DNM1L-positive patients had a poorer survival outcome than
DNM1L-negative patients [78 months (mean), 95% confidence interval
(CI): 68.6–87.4 vs. 113.5 months, 95% CI: 99.4–127.7] (Fig. 4D).
 | Table I.Association of DNM1L expression with
clinicopathological factors. |
Table I.
Association of DNM1L expression with
clinicopathological factors.
|
|
| DNM1L expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
parameters | No. | Negative | Positive | Chi-square | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
<60 | 63 | 12 | 51 | 1.154 | 0.283 |
| ≥60 | 87 | 11 | 76 |
|
|
| Sex |
|
|
|
|
|
| Male | 105 | 17 | 88 | 0.198 | 0.656 |
|
Female | 45 | 6 | 39 |
|
|
| Tumor diameter
(cm) |
|
|
|
|
|
|
<5.0 | 99 | 17 | 82 | 0.758 | 0.384 |
| ≥5.0 | 51 | 6 | 45 |
|
|
| Tumor grade |
|
|
|
|
|
| Well | 25 | 6 | 19 | 2.284 | 0.319 |
|
Moderately | 70 | 11 | 59 |
|
|
|
Poorly | 55 | 6 | 49 |
|
|
| Depth of
invasion |
|
|
|
|
|
|
T1+T2 | 56 | 13 | 43 | 4.257 | 0.039 |
|
T3+T4 | 94 | 10 | 84 |
|
|
| TNM-stage |
|
|
|
|
|
| I+II | 70 | 16 | 54 | 5.723 | 0.017 |
|
III+IV | 80 | 7 | 73 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
Negative | 60 | 16 | 44 | 9.894 | 0.002 |
|
Positive | 90 | 7 | 83 |
|
|
Discussion
Mitochondria are highly dynamic organelles in living
cells, and display continuous movement, fusion, and fission,
forming the mitochondrial reticulum. The correct regulation of
mitochondrial fission and fusion is essential for cellular
homoeostasis. The balance between mitochondrial fission and fusion
is controlled by a small cohort of mediators (OPA1, MFN1, and MFN2
for fusion and DNM1L for fission) (15). The organelle is closely associated
with metabolism, development, and the death of cells (16). DNM1L is the key regulator mediating
mitochondrial fission and fusion (16–18). It is
primarily cytoplasmic, with a smaller fraction localizing to the
outer mitochondrial membrane. The translocation of DNM1L from the
cytoplasm to the mitochondrion appears to play a critical role in
the regulation of mitochondrial fission (19). DNM1L has been implicated in
neurodegenerative diseases (20,21) and
tumorigenesis of various kinds in humans. It is expressed at high
levels in HCT116 and SW480 human colon cancer cells; the
overexpression of DNM1L promotes the proliferation of colon cancer
cells, and the downregulation of DNM1L reduces the proliferation
and increases the apoptosis of these cells in vitro
(22). Both immunohistochemistry and
in vitro protein analyses showed that DNM1L expression is
upregulated in oncocytic thyroid tumors, and the overexpression of
DNM1L is associated with malignant oncocytic thyroid tumors. The
inhibition of DNM1L activity also affects the migration and
invasion capacities of thyroid cancer cells (23).
However, its role in gastric carcinoma remains
unclear. Therefore, in this study, we investigated the expression
and biological functions of DNM1L in gastric adenocarcinoma
specimens and MKN-45 cells. Consistent with previous studies, we
found that DNM1L was markedly upregulated in human gastric
adenocarcinoma specimens relative to pericarcinoma specimens. DNM1L
was also highly expressed in gastric carcinoma cell lines. DNM1L
expression was associated with the depth of invasion, lymph-node
metastasis, and TNM stage. Increased DNM1L expression was
associated with a poor prognosis in gastric adenocarcinoma
patients. Based on these data, DNM1L could be an attractive target
for cancer therapy and warrants further exploration.
To study the effects of DNM1L knockdown on gastric
carcinoma cells, a lentivirus-mediated shRNA approach was used to
establish a cell line containing DNM1L-shRNA. An MTT assay, flow
cytometry, and a Transwell assay were used to investigate the
effects of DNM1L knock-down on cell proliferation, apoptosis, and
migration/invasion, respectively. Our data revealed dramatically
reduced MKN-45 cell proliferation and the induction of apoptosis
after the transfection of an shRNA targeting DNM1L. We also found
that the downregulation of DNM1L in MKN-45 cells reduced their
invasive and migratory capacities. These results suggest that DNM1L
is directly involved in the occurrence and invasiveness of gastric
cancer.
In summary, our findings demonstrate, for the first
time, that DNM1L expression is upregulated in gastric
adenocarcinoma. It also correlates with lymphatic metastasis,
infiltration depth, and the TNM stage, and could be a useful
prognostic indicator of the survival of patients with gastric
adenocarcinoma. The knockdown of DNM1L with shRNA potently reduced
the proliferation and invasion of cells and induced their apoptosis
in vitro. In future studies, we will increase the number of
gastric cancer patients and include other pathological types of
gastric cancer, besides adenocarcinoma, to investigate the role of
DNM1L in gastric cancer. More gastric cancer cell lines should also
be examined to determine the effects of DNM1L on cell
proliferation, invasion, and other biological properties, both
in vitro and in vivo.
Acknowledgements
The authors would like to thank Ms. Li-Wei Xie
(Department of Pathology, The Second Affiliated Hospital and
Children's Hospital of Wenzhou Medical University, Wenzhou, China)
for her technical assistance in performing the immunohistochemical
studies.
Funding
This study was supported by the Projects of Medical
and Health Technology in Zhejiang Province (no. 2017KY453) and the
Zhejiang Provincial Natural Science Foundation (no.
LY18H160048).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and XY are researchers working in cancer biology
and carried out the study. WZ and LZ undertook the statistical
analysis. YZ along with DL designed the work and interpreted the
results. YZ contributed to the writing of the manuscript. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Second Affiliated Hospital of Wenzhou Medical University, and
the need for informed consent was waived. However, written informed
consent for radical gastrotomy and the use of data for research
purposes were obtained from patient prior to treatment.
Patient consent for publication
As the approval for the use of data with written
informed consent was obtained from patients and all identifying
information was removed, the consent for publication was waived for
this retrospective observational study.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei
WQ, Qiao YL and Inoue M: Comparative epidemiology of gastric cancer
between Japan and China. World J Gastroenterol. 17:4421–4428. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waddell T, Chau I, Cunningham D, Gonzalez
D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G,
Wadsley J, et al: Epirubicin, oxaliplatin and capecitabine with or
without panitumumab for patients with previously untreated advanced
oesophagogastric cancer (REAL3): A randomised, open-label phase 3
trial. Lancet Oncol. 14:481–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smalley SR, Benedetti JK, Haller DG,
Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson
JA, Jessup JM, et al: Updated analysis of SWOG-directed intergroup
study 0116: A phase III trial of adjuvant radiochemotherapy versus
observation after curative gastric cancer resection. J Clin Oncol.
30:2327–2333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coburn NG, Lourenco LG, Rossi SE, Gunraj
N, Mahar AL, Helyer LK, Law C, Rabeneck L and Paszat L: Management
of gastric cancer in Ontario. J Surg Oncol. 102:54–63. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roy M, Reddy PH, Iijima M and Sesaki H:
Mitochondrial division and fusion in metabolism. Curr Opin Cell
Biol. 33:111–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boland ML, Chourasia AH and Macleod KF:
Mitochondrial dysfunction in cancer. Front Oncol. 3:2922013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ubah OC and Wallace HM: Cancer therapy:
Targeting mitochondria and other sub-cellular organelles. Curr
Pharm Des. 20:201–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bossy-Wetzel E, Barsoum MJ, Godzik A,
Schwarzenbacher R and Lipton SA: Mitochondrial fission in
apoptosis, neurodegeneration and aging. Curr Opin Cell Biol.
15:706–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imai Y and Lu B: Mitochondrial dynamics
and mitophagy in Parkinson's disease: Disordered cellular power
plant becomes a big deal in a major movement disorder. Curr Opin
Neurobiol. 21:935–941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang CR and Blackstone C: Dynamic
regulation of mitochondrial fission through modification of the
dynamin-related protein Drp1. Ann N Y Acad Sci. 1201:34–39. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cronin-Furman EN, Borland MK, Bergquist
KE, Bennett JP Jr and Trimmer PA: Mitochondrial quality, dynamics
and functional capacity in Parkinson's disease cybrid cell lines
selected for Lewy body expression. Mol Neurodegener. 8:62013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Archer SL: Mitochondrial
dynamics-mitochondrial fission and fusion in human diseases. N Engl
J Med. 369:2236–2251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mishra P and Chan DC: Mitochondrial
dynamics and inheritance during cell division, development and
disease. Nat Rev Mol Cell Biol. 15:634–646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frank S, Gaume B, Bergmann-Leitner ES,
Leitner WW, Robert EG, Catez F, Smith CL and Youle RJ: The role of
dynamin-related protein 1, a mediator of mitochondrial fission, in
apoptosis. Dev Cell. 1:515–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tailor D, Hahm ER, Kale RK, Singh SV and
Singh RP: Sodium butyrate induces DRP1-mediated mitochondrial
fusion and apoptosis in human colorectal cancer cells.
Mitochondrion. 16:55–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park SJ, Park YJ, Shin JH, Kim ES, Hwang
JJ, Jin DH, Kim JC and Cho DH: A receptor tyrosine kinase
inhibitor, Tyrphostin A9 induces cancer cell death through Drp1
dependent mitochondria fragmentation. Biochem Biophys Res Commun.
408:465–470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DuBoff B, Gotz J and Feany MB: Tau
promotes neurodegeneration via DRP1 mislocalization in vivo.
Neuron. 75:618–632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan J, Liu XH, Han MZ, Wang YM, Sun XL, Yu
N, Li T, Su B and Chen ZY: Blockage of GSK3beta-mediated Drp1
phosphorylation provides neuroprotection in neuronal and mouse
models of Alzheimer's disease. Neurobiol Aging. 36:211–227. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue-Yamauchi A and Oda H: Depletion of
mitochondrial fission factor DRP1 causes increased apoptosis in
human colon cancer cells. Biochem Biophys Res Commun. 421:81–85.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferreira-da-Silva A, Valacca C, Rios E,
Populo H, Soares P, Sobrinho-Simões M, Scorrano L, Maximo V and
Campello S: Mitochondrial dynamics protein Drp1 is overexpressed in
oncocytic thyroid tumors and regulates cancer cell migration. PLoS
One. 10:e01223082015. View Article : Google Scholar : PubMed/NCBI
|