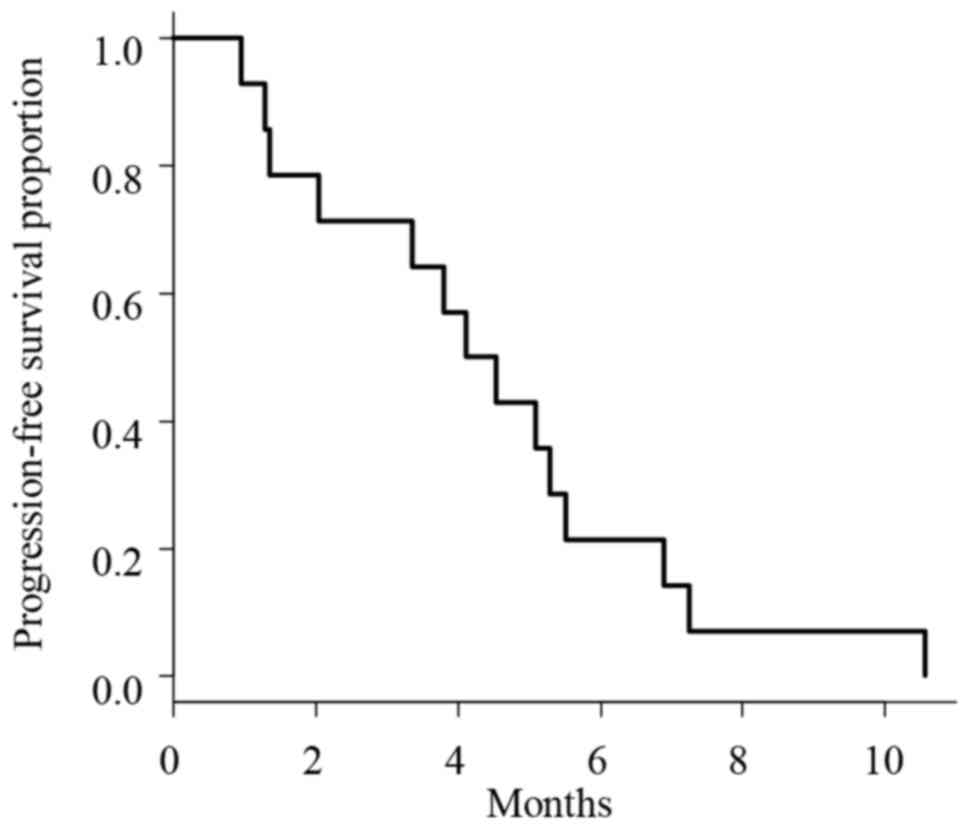
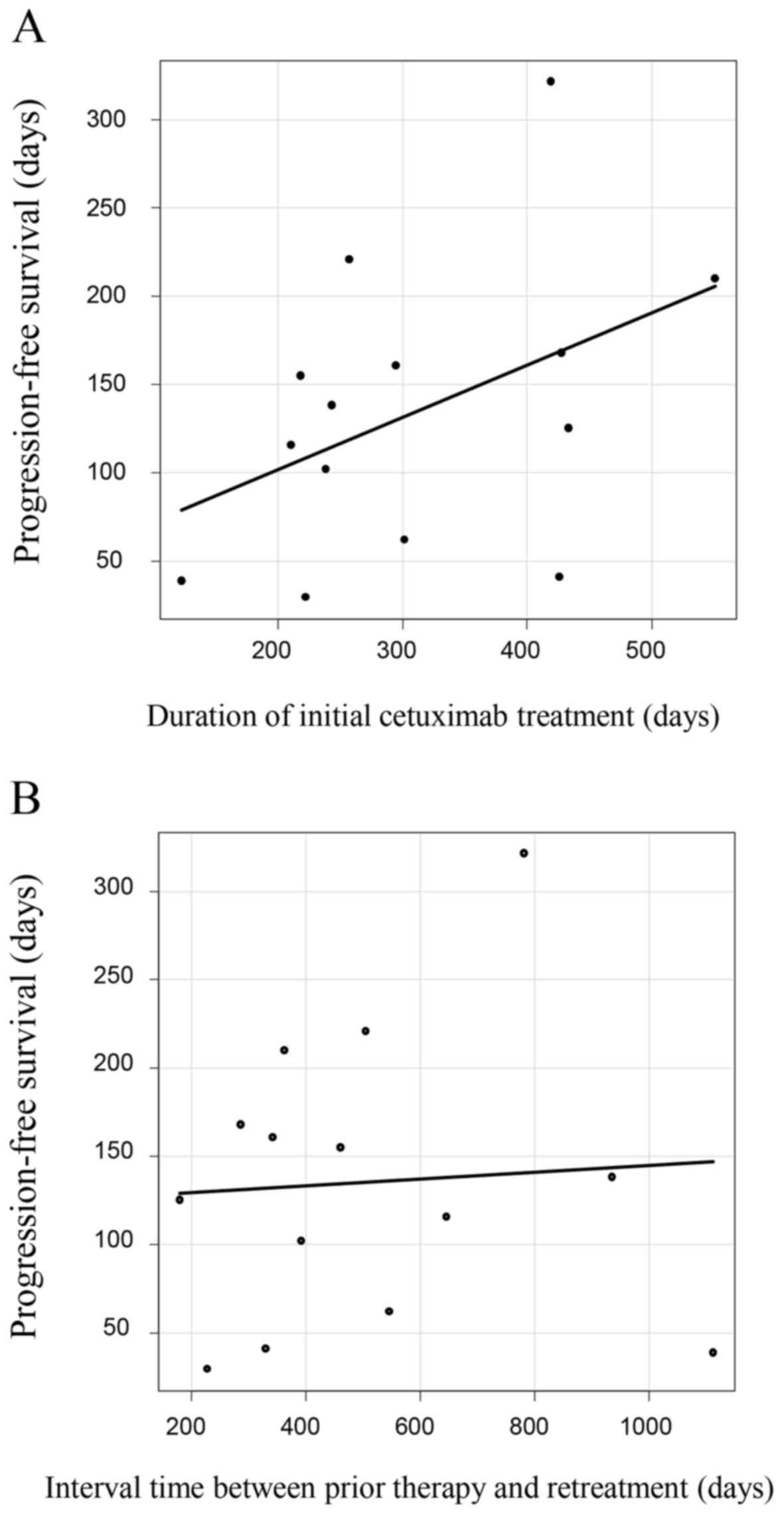
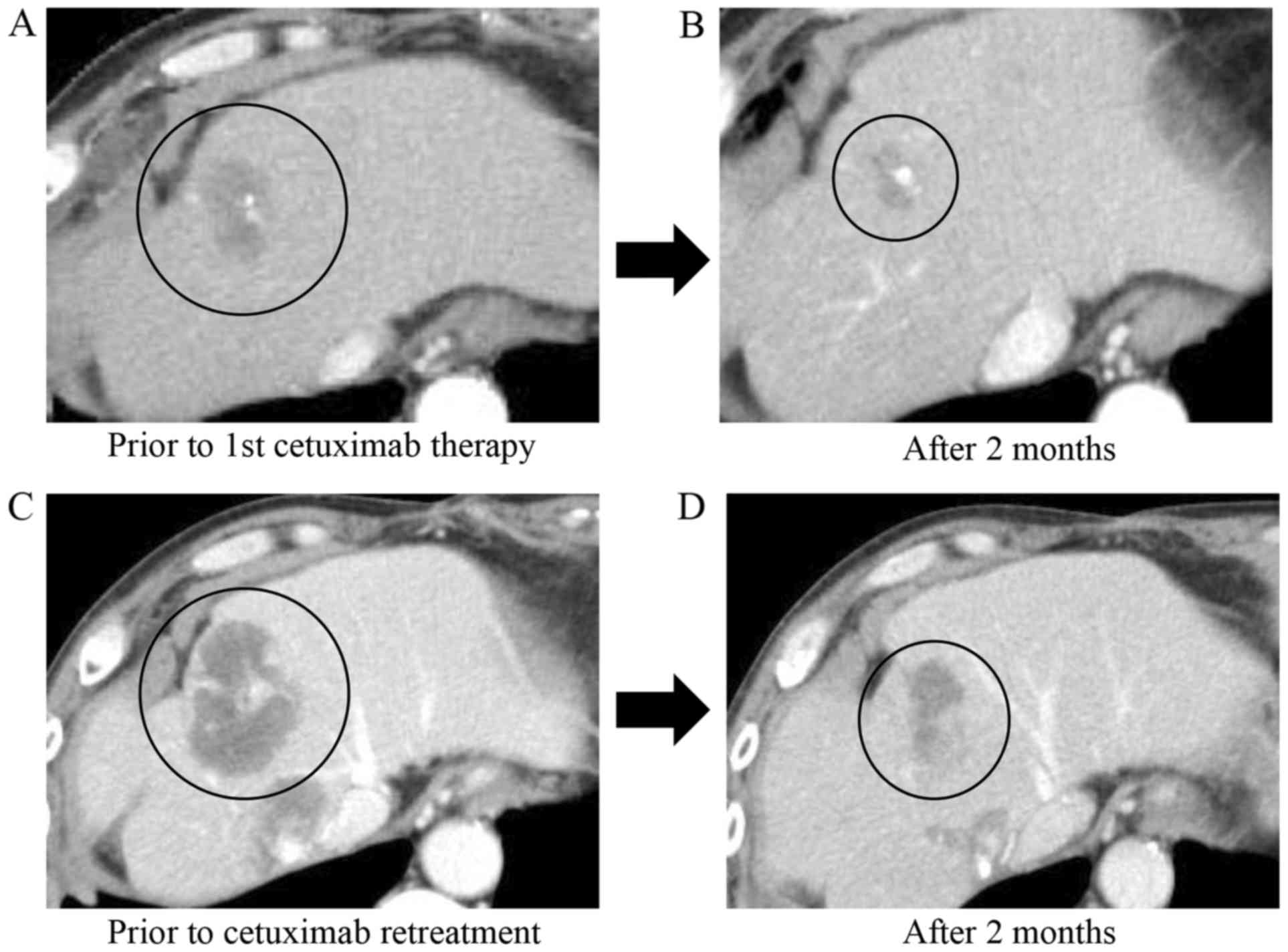
|
1
|
Matsuda A, Matsuda T, Shibata A, Katanoda
K, Sobue T and Nishimoto H: Japan Cancer Surveillance Research
Group: Cancer incidence and incidence rates in Japan in 2008: A
study of 25 population-based cancer registries for the monitoring
of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol.
44:388–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
National Comprehensive Cancer Network
(NCCN): NCCN Guidelines for Colon cancer (version 2, 2017).
http://www.nccn.org/professionals/physician_gls/f_guidelines.aspNovember
09–2017
|
|
4
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aguilar Aranda E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stintzing S, Modest DP, Rossius L, Lerch
MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U,
Al-Batran SE, Heintges T, et al: FOLFIRI plus cetuximab versus
FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3):
A post-hoc analysis of tumour dynamics in the final RAS wild-type
subgroup of this randomised open-label phase 3 trial. Lancet.
Oncol. 17:1426–1434. 2016.
|
|
7
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeuchi N, Koike K, Yoshida S, Kudo A,
Sekiguchi N, Nakayama A, Kubota K, Rokuhara T and Kitahara M:
Treatment of metastatic refractory colorectal cancer following
regorafenib failure. Mol Clin Oncol. 7:308–312. 2017.PubMed/NCBI
|
|
10
|
Rossini D, Santini D, Cremolini C,
Salvatore L, Lonardi S, Aquila ED, Aprile G, Loupakis F, Vincenzi
B, Battaglin F, et al: Rechallenge with cetuximab + irinotecan in
3rd-line in RAS and BRAF wild-type metastatic colorectal cancer
(mCRC) patients with acquired resistance to 1st-line cetuximab +
irinotecan: The phase II CRICKET study by GONO. Ann Oncol. 28 Suppl
3:iii1–iii12. 2017. View Article : Google Scholar
|
|
11
|
Santini D, Vincenzi B, Addeo R, Garufi C,
Masi G, Scartozzi M, Mancuso A, Frezza AM, Venditti O, Imperatori
M, et al: Cetuximab rechallenge in metastatic colorectal cancer
patients: How to come away from acquired resistance? Ann Oncol.
23:2313–2318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuji A, Eto T, Masuishi T, Satake HS,
Segawa Y, Tanioka H, Hara H, Kotaka M, Sagawa T, Watanabe T, et al:
Phase II study of third-line cetuximab rechallenge in patients with
metastatic wild-type K-RAS colorectal cancer who achieved a
clinical benefit in response to first-line cetuximab plus
chemotherapy (JACCRO CC-08). Ann Oncol. 27:510P2016. View Article : Google Scholar
|
|
13
|
Liu X, George GC, Tsimberidou AM, Naing A,
Wheler JJ, Kopetz S, Fu S, Piha-Paul SA, Eng C, Falchook GS, et al:
Retreatment with anti-EGFR based therapies in metastatic colorectal
cancer: Impact of intervening time interval and prior anti-EGFR
response. BMC Cancer. 15:7132015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE). v4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40May
17–2010
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Diaz LA Jr, Williams RT, Wu J, Kinde I,
Hecht JR, Berlin J, Allen B, Bozic I, Reiter JG, Nowak MA, et al:
The molecular evolution of acquired resistance to targeted EGFR
blockade in colorectal cancers. Nature. 486:537–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Price T, Kim TW, Li J, Cascinu S, Ruff P,
Suresh AS, Thomas A, Tjulandin S, Guan X and Peeters M: Final
results and outcomes by prior bevacizumab exposure, skin toxicity,
and hypomagnesaemia from ASPECCT: Randomized phase 3
non-inferiority study of panitumumab versus cetuximab in
chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer.
Eur J Cancer. 68:51–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taniguchi H, Komori A, Narita Y, Kadowaki
S, Ura T, Andoh M, Yatabe Y, Komori K, Kimura K, Kinoshita T and
Muro K: A short interval between bevacizumab and anti-epithelial
growth factor receptor therapy interferes with efficacy of
subsequent anti-EGFR therapy for refractory colorectal cancer. Jpn
J Clin Oncol. 46:228–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wadlow RC, Hezel AF, Abrams TA,
Blaszkowsky LS, Fuchs CS, Kulke MH, Kwak EL, Meyerhardt JA, Ryan
DP, Szymonifka J, et al: Panitumumab in patients with KRAS
wild-type colorectal cancer after progression on cetuximab.
Oncologist. 17:142012. View Article : Google Scholar : PubMed/NCBI
|