Introduction
Hepatoblastoma (HB) is an aggressive type of cancer
that has a high morbidity and mortality rate in children (1). Early diagnosis and treatment are
important for improving the survival rate of patients with HB. The
majority of HBs are hypervascular (2); therefore, angiogenesis may serve a vital
role in HB development and metastasis. Angiogenesis is the
formation of new blood vessels, and is a hallmark of early-stage
tumors (3). The growth of solid
tumors relies on neovascularization (4). Tumor and endothelial cells may
constitute a highly integrated system, which could cause a mutual
growth promotion (5,6). Tumor cells secrete specific cytokines to
stimulate the proliferation of vascular endothelial cells, and the
endothelial cells may modulate tumor cell growth by providing
oxygen and nutrients (6). During
angiogenesis, specific molecular markers are overexpressed on the
surface of endothelial cells (3).
Endoglin, also termed CD105, is a homodimeric
transmembrane glycoprotein that contains 561 amino acid residues in
its extracellular domain and 47 amino acids in a cytoplasmic tail
domain (7). It is a major component
of the transforming growth factor-β (TGF-β) receptor complex
(8). Endoglin is expressed in adult
vascular endothelial cells and stromal cells (7). In cultured endothelial cells, higher
levels of endoglin expression can be detected during proliferation
(9). In tumor tissues, endoglin is
overexpressed during remodeling in angiogenic vessels (10); therefore, endoglin is a biomarker for
angiogenesis (11–13). A previous study identified that
endoglin was expressed in 100% of surgically resected specimens, in
both tumor tissues and para-carcinomatous tissues (14).
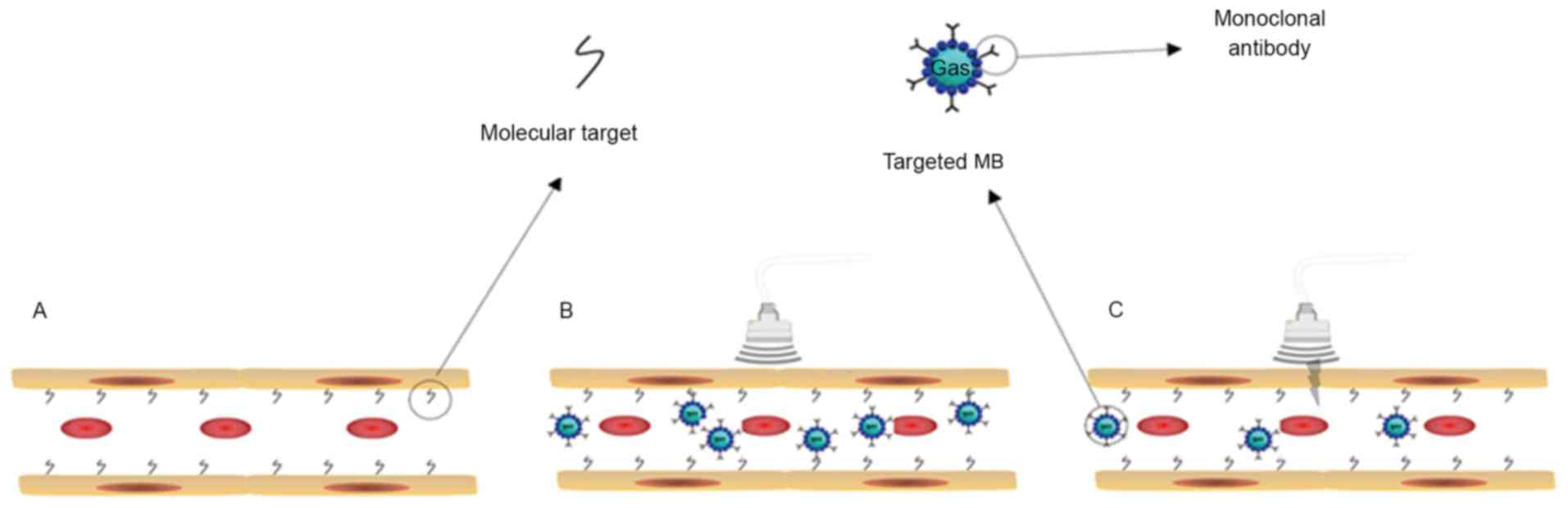
Recently, ultrasound molecular imaging has emerged
as a means of visualizing disease processes at the molecular level
in vivo. This technique has the ability to qualitatively and
quantitatively illustrate specific molecules in tissues, cells or
subcellular organelles. With the development of targeted ultrasound
contrast agents, ultrasound has the ability to detect molecular
changes in living cells. The targeted microbubbles (MB), ranging in
size from 1–4 µm (15), usually
consist of an insoluble gas, including perfluoropropane,
perfluorobutane, perfluorohexane, or sulfur hexafluoride and the
bubble's outer wall (16). The
ligands (antibody, peptide and scaffold) were bound to the shell by
covalent or non-covalent bonding (17). Following intravenous injection,
targeted MB bound to specific molecules in the circulatory system
(Fig. 1).
The vibrations of the MB compression and expansion
may be distinguished with background noise under the non-linear
contrast ultrasonic mode (18,19).
Pre-clinical studies demonstrated that molecular ultrasound imaging
may detect angiogenesis in tumors (20–23).
Deshpande et al (24) assessed
three types of molecular-targeted ultrasound MB:
MBintegrin, MBendoglin and
MBVEGFR2 in animal models with three subcutaneous cancer
xenografts (breast, ovarian and pancreatic cancer). Their results
indicated a significantly higher expression of endoglin than αvβ3
integrin and VEGFR2 expression in early stage breast and ovarian
cancers.
In the present study, MB and endoglin were combined
and injected into nude mice with HB to measure specific binding to
microvessels, for the purpose of tumor angiogenesis diagnosis via
non-linear harmonic imaging. In addition, the techniques used to
detect endoglin expression in experimental animals included western
blotting, reverse transcription-quantitative polymerase chain
reaction (RT-qPCR), and immunohistochemistry. Conditioned medium of
HB cells was applied to incubate human umbilical vein endothelial
cells (HUVECs) to detect changes of endoglin expression in
HUVECs.
Materials and methods
Experimental model
All the animals in the present study were provided
by Professor Yourong Duan from the Shanghai Cancer Institute
(Shanghai, China). HepG2 cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS; Lonza Group, Ltd., Basel, Switzerland) and 1% antibiotics
(100 IU/ml penicillin and 100 µg/ml streptomycin; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Four BALB/c male nude mice, which
were 3–4 weeks old and weighing 22–25 g, were used in the present
study and maintained in a pathogen-free environment. Mice were
maintained in standard transparent polycarbonate cages and fed with
Food pellets (Lab Diet 5010; LabDiet, Richmond, IN, USA) with
water. The room was maintained with clean atmosphere, the lighting
on a 12 h light/dark cycle, and the room temperature between 20°C
and 22°C. Allograft tumors were produced via a subcutaneous
injection of 5×106 HepG2 cells suspended in 0.2 ml
sterile PBS into the right flank of the nude mice. After three
weeks, the maximum diameter of the tumors was 0.5 cm, which was
suitable for molecular ultrasound imaging.
Molecular ultrasound imaging
The in vivo distribution of the isotype and
endoglin-targeted MB was examined in mice with the HepG2
subcutaneous tumors (n=4) by the Vevo® 2100 small animal
high-resolution ultrasound system with the MS-250 transducer
(VisualSonics, Inc., Toronto, ON, Canada). The frequency of the
transducer is 40 MHz. Isotype and targeted MB were prepared using
Vevo® MicroMarker® Target-Ready Contrast
Agent kits (VisualSonics, Inc.). Biotinylated anti-endoglin and
isotype anti-mouse-IgG antibodies were purchased from Abcam
(Cambridge, MA, USA). The MB were prepared according to the
manufacturer's instructions, and the ultrasound system was operated
using The Guide to Small Animal Nonlinear Contrast Imaging
(25). Capturing ultrasound imaging
was completed following the intravenous injection of
5×107 MBIsotype through the tail vein and followed by
sequence destruction. MBendoglin (5×107) was
injected 30 min after the isotype was cleared from circulation. The
echo intensity prior to the destruction pulse represents
bound/circulating microbubbles and tissue signal. The echo
intensity following the destruction pulse represents the
microbubbles that are still in circulation and the residual
tissue-echoes, not the binding process. In the Targeted model, Vevo
Contrast Quantification (Vevo CQ™) Software (version 1.3.12.0;
VisualSonics, Inc, Toronto, Ontario, Canada.) is able to express
the specific binding as a difference between the echo power
averaged in the segment before destruction pulse and the residual
echo power averaged in the segment after destruction pulse. The
Vevo CQ™ Software was used to visualize the spatial distribution of
perfusion parameters as color-coded parametric images. The
differential targeted enhancement (ΔTE) was computed by subtracting
the mean intensity detected after the destructive pulse from the
mean intensity detected prior to the destructive pulse. Liver and
tumor tissues were isolated for examination by
immunohistochemistry, western blotting and RT-qPCR.
RT-qPCR
Total RNA was isolated from cultured cells, and the
liver and tumor tissues of nude mice, using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). RNA isolation and cDNA synthesis were performed using an
RNeasy Mini kit (Qiagen GmbH, Hilden, Germany) and the High
Capacity RNA-to-cDNA Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) RT-qPCR was performed with a ViiA7 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
using SYBR-Green Master Mix (Thermo Fisher Scientific, Inc.). An
aliquot of 2 µg total RNA from each sample was used for the
synthesis of cDNA using a High-Capacity cDNA Reverse Transcription
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). cDNA was
amplified in a final volume of 20 µl with 1 U Taq DNA polymerase
(Invitrogen; Thermo Fisher Scientific, Inc.) and 10 pmol of each
primer. Reaction conditions were as follows: 95°C for 5 min, 40
cycles of 95°C for 10 sec and 60°C for 32 sec. All PCR reactions
were conducted in triplicate. Relative expression was calculated by
comparing the relative expression level of CD105 with the internal
reference GAPDH by the 2−ΔΔCt method (26). Primer sequences are listed in Table I.
 | Table I.RT-qPCR primer sequences. |
Table I.
RT-qPCR primer sequences.
| Gene | Sequence | Product size
(bp) | TM (°C) |
|---|
| CD105-mouse |
|
|
|
|
Forward |
5′-CCCTCTGCCCATTACCCTG-3′ | 11 | 59.5 |
|
Reverse |
5′-GTAAACGTCACCTCACCCCTT-3′ | 7 | 57.6 |
| CD105-human |
|
|
|
|
Forward |
5′-CGCCAACCACAACATGCAG-3′ | 15 | 57.3 |
|
Reverse |
5′-GCTCCACGAAGGATGCCAC-3′ | 8 | 59.5 |
| GAPDH-mouse |
|
|
|
|
Forward |
5′-AAGGATGAAGGAAGTGATTTG-3′ | 40 | 53.77 |
|
Reverse |
5′-AAGAGGAACATCGTGGTAAAG-3′ | 3 | 55.62 |
| GAPDH-human |
|
|
|
|
Forward |
5′-TGATGACATCAAGAAGGTGGTGAAG-3′ | 24 | 61.03 |
|
Reverse |
5′-TCCTTGGAGGCCATGTGGGCCAT-3′ | 0 | 68.32 |
Western blot analysis
Western blotting was performed as previous described
(27). Briefly, cells were lysed in
ice-cold RIPA buffer (20 mM Tris pH 7.5, 150 mM NaCl, 50 mM NaF, 1%
NP40, 0.1% DOC, 0.1% SDS, 1 mM EDTA and supplemented with 1 mM PMSF
and 1 µg/ml leupeptin). Equal amounts of protein (40 µg), as
determined by a BCA assay, were separated via 10% SDS-PAGE and then
transferred to a polyvinylidene fluoride membrane. Membranes were
blocked with 2.5% non-fat milk and incubated with an rabbit
polyclonal anti-CD105 antibody (dilution 1:400; cat. no. ab107595;
Abcam, Cambridge, MA, USA) in PBS with Tween 20 (TBST) at 4°C
overnight. Subsequent to washing with TBST 3 times, the membrane
was incubated with the secondary antibody at 37°C for 1 h. The
secondary antibody was horseradish peroxidase-conjugated
anti-rabbit IgG (dilution 1:6,000; cat. no. SA00001-2; Protein Tech
Group, Inc.; Wuhan Sanying Biotechnology, Wuhan, China). The
membranes were then visualized with an enhanced chemiluminescence
reagent (cat. no. WBKLS0500; Merck KGaA).
Immunohistochemical staining
Tissue preparation and immunohistochemistry was
performed as previously described (28). Briefly, 6 µm serial sections were cut
from the blocks of each mouse liver or tumor. The slides were
deparaffinized in xylene and rehydrated in gradient ethyl alcohol
of 100, 95, 85, 80 and 75%. Antigen retrieval was performed in a
citrate salt antigen repair solution for 15 min in a pressure
cooker at 120°C, then the sections were cooled to room temperature
and washed with PBS three times. Sections were incubated with
rabbit anti-mouse CD105 polyclonal antibody (dilution 1:50; cat.
no. ab107595; Abcam, Cambridge, UK) at 4°C overnight. Then the
sections were placed in room temperature for 40 min, washed with
PBS three times and incubated with biotinylated rabbit anti-rat IgG
(dilution 1:500; cat. no. BA4000; Vector Laboratories, Inc.,
Burlingame, CA, USA) at 37°C for 20 min. The sections were
incubated with 20× DAB substrate buffer (cat. no. ZLI-9017; Origene
Technologies, Inc., Beijing, China) for 1 min. The slides were then
counterstained with hematoxylin (Surgipath®; Leica
Microsystems GmbH, Wetzlar, Germany) at room temperature for 1 min
and dehydrated through five grades of alcohol (75, 80, 95 and 100%)
and xylene. The slides were observed under a light microscope and
images were captured with an Olympus FSX100 imaging system (Olympus
Corporation, Tokyo, Japan) and analyzed by Image Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA)
(magnification, ×400).
Conditioned medium cell culture
The human HB carcinoma cell line HepG2 and human
umbilical vein endothelial cells (HUVECs) purchased from American
Type Culture Collection (Manassas, VA, USA), were cultured in DMEM
supplemented with 10% FBS, 100 IU/ml penicillin and 100 µg/ml
streptomycin. All the cells were maintained at 37°C in a humidified
atmosphere containing of 5% CO2. HepG2 cells were
incubated with DMEM, containing 0.5% FBS, 100 IU/ml penicillin and
100 µg/ml streptomycin, and the conditioned media was collected
after 12 h. HUVECs were co-cultured with conditioned media from
HepG2 cells. The cells were lysed with TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The total mRNA and protein was
collected for western blotting and RT-qPCR detection after 12
h.
Statistical analyses
Statistical significance was assessed using
paired-sample student's t-tests. All statistical analyses were
performed using SPSS 17.0 statistical software (SPSS Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
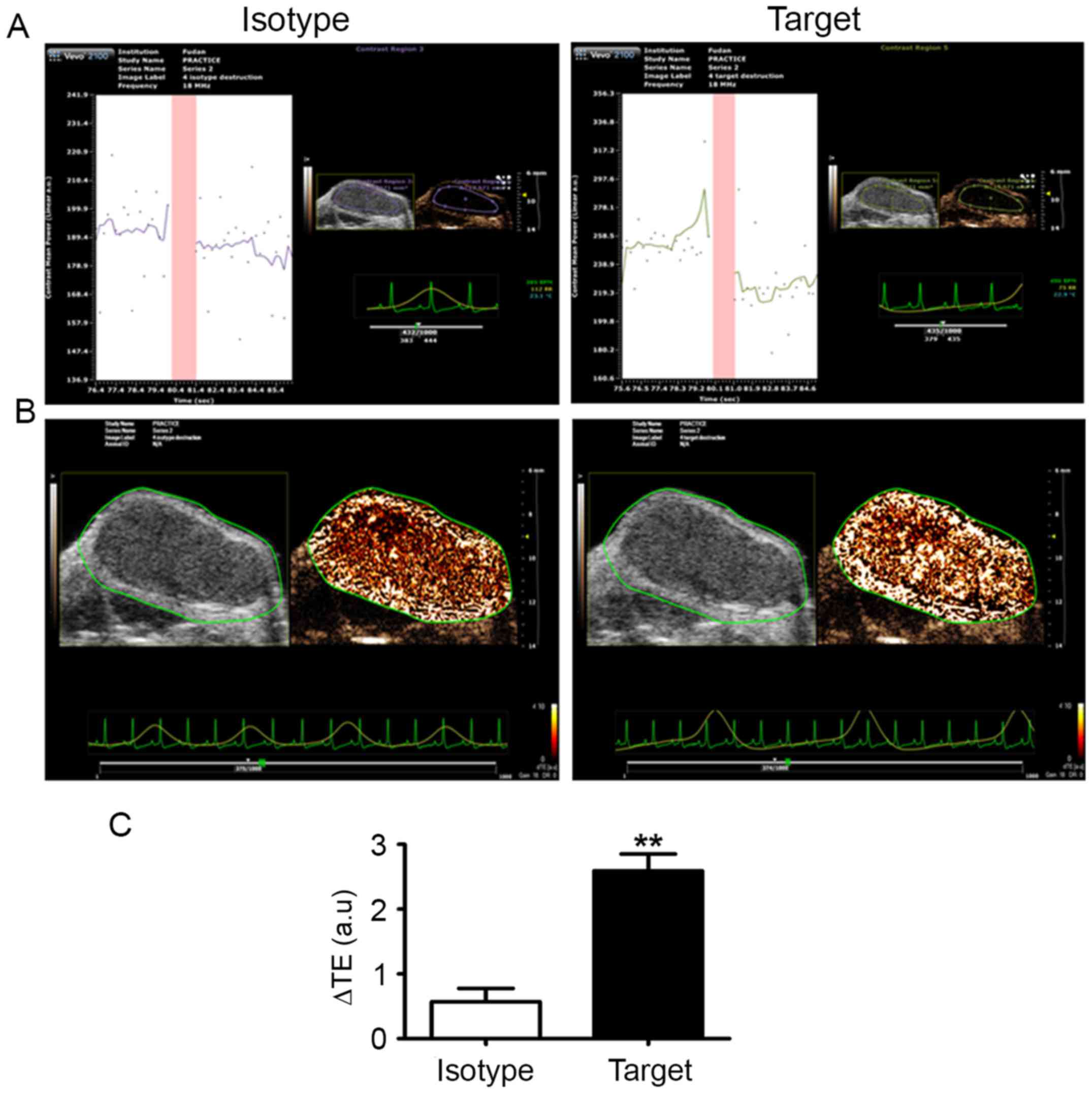
Targeted ultrasound imaging
The experimental animals were tolerant of ultrasound
contrast imaging, with no acute toxic reactions observed. To
confirm the specific binding of MBendoglin,
MBisotype was injected to eliminate non-specific
binding. Following ultrasound imaging capturing, the ΔTE of
MBendoglin and MBisotype was calculated
automatically using the built-in software (VisualSonics, Inc.).
Previous studies demonstrated that endoglin is an excellent
biomarker of angiogenesis (9–13). In the present study, anti-endoglin
monoclonal antibodies were bound to MB to target overexpressed
endoglin on the surface of tumor vessel endothelial cells in
subcutaneous HepG2 xenografts in nude mice. The echo intensity
taken prior to the destruction pulse represent bound and
circulating MB, and the tissue signal. The echo intensity following
the destruction pulse correspond to MB that remained in circulation
and to any residual tissue-echoes, which are not representative of
the binding process. The difference between prior to and following
destruction is the intensity of bound MB. The ΔTE of
MBendoglin was higher than that of MBisotype
(Fig. 2A and B), with the difference
being statistically significant (P<0.001; Fig. 2C).
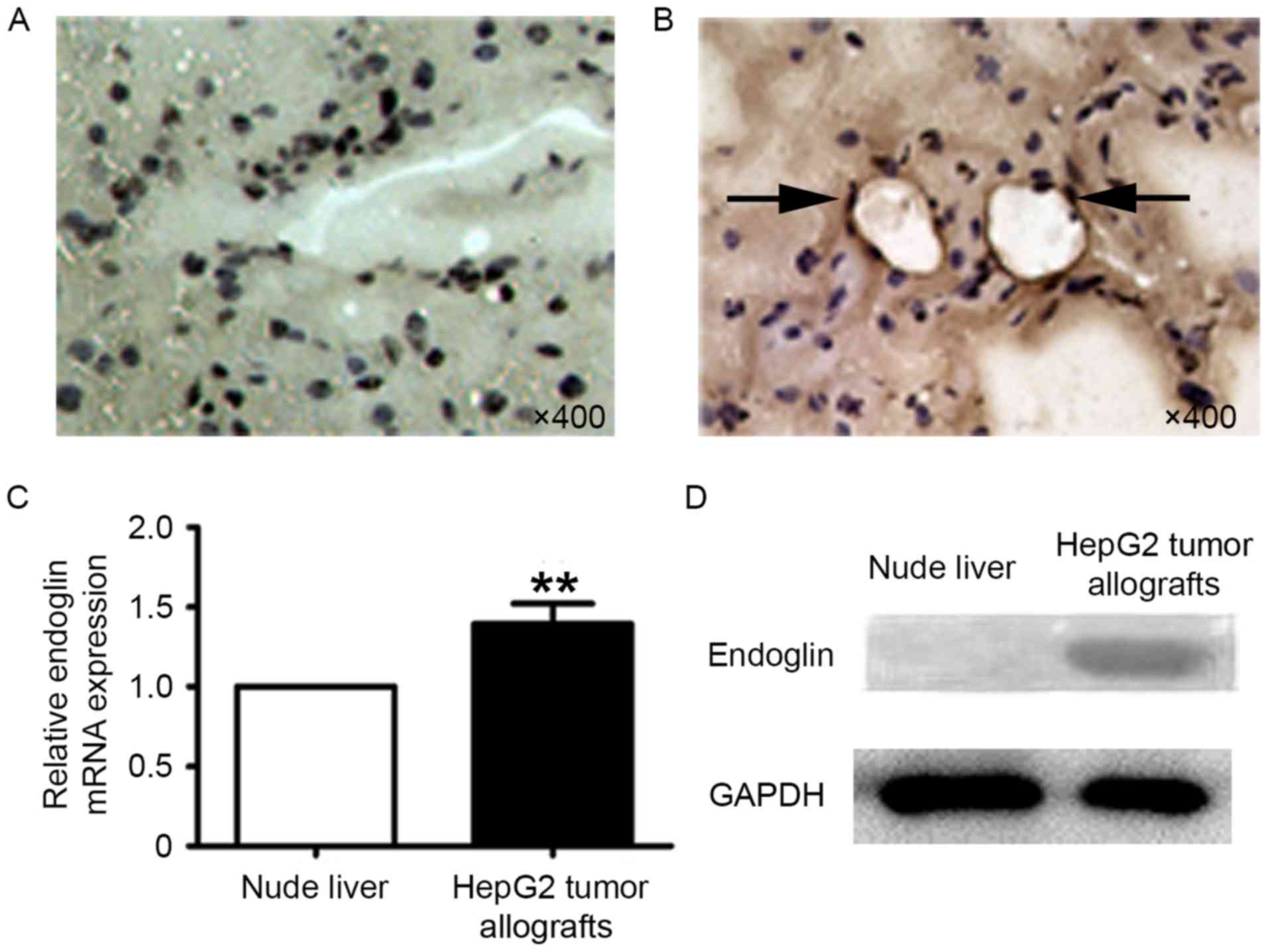
Endoglin expression in the liver
tissues and HepG2 tumor allografts of nude mice
Tissues extracted from experimental nude mice liver
and subcutaneously allografted HepG2 tumors underwent
immunohistochemical staining, RT-qPCR analysis and western blot
analysis. Endoglin is not expressed on the endothelium of
microvessels in the normal liver tissue and blood vessels (Fig. 3A), but is expressed in the HepG2
tumors (Fig. 3B, as the black arrow
indicates). RT-qPCR analysis demonstrated that endoglin mRNA
expression in the HepG2 tumors was significantly higher than in the
liver tissues of the nude mice (n=4; P<0.001; Fig. 3C). Western blot analysis was performed
to evaluate endoglin expression. Endoglin was present in the
protein extracts of HepG2 tumors; however, it was absent in the
liver tissues of nude mice (Fig.
3D).
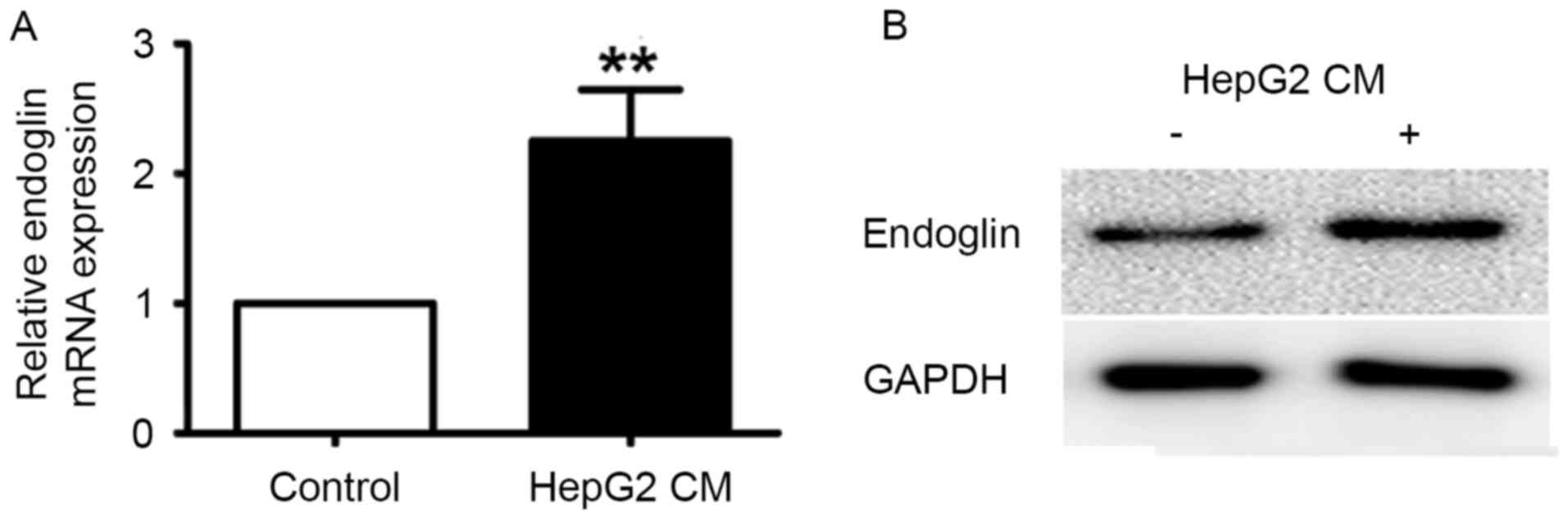
Endoglin expression in HUVECs treated
with the conditioned media of HepG2 cells
In cultured endothelial cells in vitro, high
endoglin expression could be detected in the cells during
proliferation and activation. To mimic the microenvironment of
tumor vessels, conditioned media from HepG2 cells were collected
and applied to HUVECs. Following culturing in HepG2-conditioned
media for 12 h, endoglin mRNA levels were significantly upregulated
in HUVECs (P<0.001; Fig. 4A).
Protein extracts of HUVECs were also collected for examination of
protein expression by western blotting. As depicted in Fig. 4B, endoglin protein levels were
significantly greater in HUVECs treated with HepG2-conditioned
media (P<0.001).
Discussion
The growth of HB is dependent on angiogenesis, which
is stimulated by vaso-active substances from HB cells (29). Blood supply to liver tumors is from
the hepatic artery and forms disordered nourishing vessels
(30). In the present study, MB were
bound to an anti-endoglin antibody, and then injected into HB
xenograft tissues in nude mice in order to detect specific binding
to tumor microvasculature, via non-linear harmonic imaging. It was
determined that the ΔTE of MBendoglin was significantly
higher than that of MBisotype. In addition, increased
expression of endoglin was identified in the HB xenografts, but not
in the livers of recipient nude mice. In vitro, treatment
with the conditioned media of HepG2 cells increased endoglin
expression in HUVECs; therefore, endoglin is specifically
overexpressed in HB vascular endothelial cells, which can be
detected by endoglin-bound MB.
In the past ten years, a novel technique for
ultrasound has been utilized for molecular imaging, which
visualizes disease processes at the molecular level at target
locations (25,31). With the introduction of a novel
targeted ultrasound contrast agent, disease processes could be
visualized at molecular level qualitatively and quantitatively
(18). Currently, the most widely
used ultrasound contrast agents are MB designed in micrometers to
detect and monitor the expression of molecules on the endothelium
(32,33). Targeted enhanced-contrast ultrasound
could improve the detection rate of early-stage HB by the specific
targeted combination of MB concentrating on tumor angiogenesis.
Several newly discovered angiogenesis markers,
including VEGFR2 and CD34, were verified by molecular ultrasound
imaging in vivo (25,34–36).
Endoglin is overexpressed in angiogenic endothelial cells of
various tumors (12,33,35,37). It is
part of the TGF-β receptor complex (11–13).
Interaction of endoglin with activated activin receptor-like kinase
1 (ALK1), ALK5 and TGF-β Receptor 2 results in phosphorylation of
serine and threonine residues in the cytoplasmic domain of endoglin
(36). ALK1 is exclusively expressed
in endothelial cells and induces the phosphorylation of endoglin,
which regulates endothelial cell proliferation (36). In the present study, it was
demonstrated that endoglin is overexpressed in vasculature in HB
xenografts and endoglin expression can be upregulated by co-culture
with HB cells. In addition, endoglin-antibody-bound MB to detect
angiogenic vessels in HB xenografts in nude mice were successfully
used. Ultrasound molecular imaging is particularly valuable in
early detection, differentiation of focal lesions and treatment
monitoring. Several biomarkers in ultrasound molecular imaging have
been reported for detecting early-stage tumors (37). Endoglin could be considered a novel
marker for early-stage tumors detectable via targeted ultrasound
imaging.
To summarize, it was demonstrated that
endoglin-targeted ultrasound imaging recognizes subcutaneous HepG2
xenografts in nude mice, and that endoglin expression in
endothelial cells is upregulated by co-culture with HepG2 cells
in vitro.
In conclusion, endoglin is upregulated in angiogenic
vessels in HepG2 cell xenografts in nude mice. Endoglin-targeted
ultrasound imaging has been verified as a potential novel method
for the diagnosis of liver carcinoma.
Acknowledgements
The authors are grateful for the support from the
Shandong Taishan Scholarship (Professor Ju Liu, Shandong
University, Jinan, China).
Funding
The present study was supported by the grants from
the Science and Technology Development Plan of Shandong Province
(grant no. 2014GSF118062), the Special Research Program for Public
Services of Shandong Province (grant no. 2014fwyyd04).
Availability of data and materials
The datasets generated and analyzed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JL, RS and HS conceived and designed the
experiments; RS, BW, FD, AW, ZS performed the experiments; JL, RS,
HS analyzed the data; JL, RS, HS wrote the paper.
Ethics approval and consent to
participate
The procedures in the present study were approved by
the ethics committee of Shandong Provincial Qianfoshan Hospital
(Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HB
|
hepatoblastoma
|
|
MB
|
microbubbles
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Tomlinson GE and Kappler R: Genetics and
epigenetics of hepatoblastoma. Pediatr Blood Cancer. 59:785–92.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu CH, Chiu NC, Yeh YC, Kuo Y, Yu SS, Weng
CY, Liu CA, Chou YH and Chiou YY: Uncommon liver tumors: Case
report and literature review. Medicine (Baltimore). 95:e49522016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: Angiogenesis. Annu Rev Med.
57:1–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folkman J and Haudenschild C: Angiogenesis
in vitro. Nature. 288:551–556. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bry M, Kivelä R, Leppänen VM and Alitalo
K: Vascular endothelial growth factor-B in physiology and disease.
Physiol Rev. 94:779–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dallas NA, Samuel S, Xia L, Fan F, Gray
MJ, Lim SJ and Ellis LM: Endoglin (CD105): A marker of tumor
vasculature and potential target for therapy. Clin Cancer Res.
14:1931–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheifetz S, Bellón T, Calés C, Vera S,
Bernabeu C, Massagué J and Letarte M: Endoglin is a component of
the transforming growth factor-beta receptor system in human
endothelial cells. J Biol Chem. 267:19027–19030. 1992.PubMed/NCBI
|
|
9
|
López-Novoa JM and Bernabeu C: The
physiological role of endoglin in the cardiovascular system. Am J
Physiol Heart Circ Physiol. 299:H959–H974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagatsuka H, Hibi K, Gunduz M, Tsujigiwa
H, Tamamura R, Sugahara T, Sasaki A and Nagai N: Various
immunostaining patterns of CD31, CD34 and endoglin and their
relationship with lymph node metastasis in oral squamous cell
carcinomas. J Oral Pathol Med. 34:70–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fonsatti E, Nicolay HJ, Altomonte M, Covre
A and Maio M: Targeting cancer vasculature via endoglin/CD105: A
novel antibody-based diagnostic and therapeutic strategy in solid
tumours. Cardiovasc Res. 86:12–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duff SE, Li C, Garland JM and Kumar S:
CD105 is important for angiogenesis: Evidence and potential
applications. FASEB J. 17:984–992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miller DW, Graulich W, Karges B, Stahl S,
Ernst M, Ramaswamy A, Sedlacek HH, Müller R and Adamkiewicz J:
Elevated expression of endoglin, a component of the
TGF-beta-receptor complex, correlates with proliferation of tumor
endothelial cells. Int J Cancer. 81:568–572. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang LY, Lu WQ, Huang GW and Wang W:
Correlation between CD105 expression and postoperative recurrence
and metastasis of hepatocellular carcinoma. BMC Cancer. 6:1102006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deshpande N, Needles A and Willmann JK:
Molecular ultrasound imaging: Current status and future directions.
Clin Radiol. 65:567–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klibanov AL: Ligand-carrying gas-filled
microbubbles: Ultrasound contrast agents for targeted molecular
imaging. Bioconjug Chem. 16:9–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abou-Elkacem L, Bachawal SV and Willmann
JK: Ultrasound molecular imaging: Moving toward clinical
translation. Eur J Radiol. 84:1685–1693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goodwin AP, Nakatsuka MA and Mattrey RF:
Stimulus-responsive ultrasound contrast agents for clinical
imaging: Motivations, demonstrations, and future directions. Wiley
Interdiscip Rev Nanomed Nanobiotechnol. 7:111–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmitz G: Ultrasonic imaging of molecular
targets. Basic Res Cardiol. 103:174–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toaldo Baron M, Salvatore V, Marinelli S,
Palamà C, Milazzo M, Croci L, Venerandi L, Cipone M, Bolondi L and
Piscaglia F: Use of VEGFR-2 targeted ultrasound contrast agent for
the early evaluation of response to sorafenib in a mouse model of
hepatocellular carcinoma. Mol Imaging Biol. 17:29–37. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Streeter JE, Herrera-Loeza SG, Neel NF,
Yeh JJ and Dayton PA: A comparative evaluation of ultrasound
molecular imaging, perfusion imaging, and volume measurements in
evaluating response to therapy in patient-derived xenografts.
Technol Cancer Res Treat. 12:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sirsi SR, Flexman ML, Vlachos F, Huang J,
Hernandez SL, Kim HK, Johung TB, Gander JW, Reichstein AR, Lampl
BS, et al: Contrast ultrasound imaging for identification of early
responder tumor models to anti-angiogenic therapy. Ultrasound Med
Biol. 38:1019–1029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anderson CR, Hu X, Zhang H, Tlaxca J,
Declèves AE, Houghtaling R, Sharma K, Lawrence M, Ferrara KW and
Rychak JJ: Ultrasound molecular imaging of tumor angiogenesis with
an integrin targeted microbubble contrast agent. Invest Radiol.
46:215–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deshpande N, Ren Y, Foygel K, Rosenberg J
and Willmann JK: Tumor angiogenic marker expression levels during
tumor growth: Longitudinal assessment with molecularly targeted
microbubbles and US imaging. Radiology. 258:804–811. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu H, Chen Y, Yan F, Han X, Wu J, Liu X
and Zheng H: Ultrasound molecular imaging of vascular endothelial
growth factor receptor 2 expression for endometrial receptivity
evaluation. Theranostics. 5:206–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Li L, Wang Y, Dong F, Chen X, Liu
F, Xu D, Yi F, Kapron CM and Liu J: NF-κB signaling maintains the
survival of cadmium-exposed human renal glomerular endothelial
cells. Int J Mol Med. 38:417–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu J, Kanki Y, Okada Y, Jin E, Yano K,
Shih SC, Minami T and Aird WC: A +220 GATA motif mediates basal but
not endotoxin-repressible expression of the von Willebrand factor
promoter in Hprt-targeted transgenic mice. J Thromb Haemost.
7:1384–1392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu AX, Duda DG, Sahani DV and Jain RK:
HCC and angiogenesis: Possible targets and future directions. Nat
Rev Clin Oncol. 8:292–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baek HJ, Lim SC, Kitisin K, Jogunoori W,
Tang Y, Marshall MB, Mishra B, Kim TH, Cho KH, Kim SS and Mishra L:
Hepatocellular cancer arises from loss of transforming growth
factor beta signaling adaptor protein embryonic liver fodrin
through abnormal angiogenesis. Hepatology. 48:1128–1137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grouls C, Hatting M, Rix A, Pochon S,
Lederle W, Tardy I, Kuhl CK, Trautwein C, Kiessling F and Palmowski
M: Liver dysplasia. US molecular imaging with targeted contrast
agent enables early assessment. Radiology. 267:487–495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ferrante EA, Pickard JE, Rychak J,
Klibanov A and Ley K: Dual targeting improves microbubble contrast
agent adhesion to VCAM-1 and P-selectin under flow. J Control
Release. 140:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Minhajat R, Mori D, Yamasaki F, Sugita Y,
Satoh T and Tokunaga O: Endoglin (CD105) expression in angiogenesis
of colon cancer: Analysis using tissue microarrays and comparison
with other endothelial markers. Virchows Arch. 448:127–134. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Foygel K, Wang H, Machtaler S, Lutz AM,
Chen R, Pysz M, Lowe AW, Tian L, Carrigan T, Brentnall TA and
Willmann JK: Detection of pancreatic ductal adenocarcinoma in mice
by ultrasound imaging of thymocyte differentiation antigen 1.
Gastroenterology. 145(885–894): e32013.
|
|
35
|
Tian H, Mythreye K, Golzio C, Katsanis N
and Blobe GC: Endoglin mediates fibronectin/alpha5beta1 integrin
and TGF-beta pathway crosstalk in endothelial cells. EMBO J.
31:3885–3900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park S, Sorenson CM and Sheibani N:
PECAM-1 isoforms, eNOS and endoglin axis in regulation of
angiogenesis. Clin Sci (Lond). 129:217–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bzyl J, Lederle W, Rix A, Grouls C, Tardy
I, Pochon S, Siepmann M, Penzkofer T, Schneider M, Kiessling F and
Palmowski M: Molecular and functional ultrasound imaging in
differently aggressive breast cancer xenografts using two novel
ultrasound contrast agents (BR55 and BR38). Eur Radiol.
21:1988–1995. 2011. View Article : Google Scholar : PubMed/NCBI
|