Introduction
Gastric cancer is a common malignant tumor of the
gastrointestinal tract (1). Recent
statistics from China reported the incidence of gastric cancer to
be the second highest of all malignancies in 2015, while the
mortality rate of gastric cancer increased from the third to the
second highest in China (2). Chronic
inflammation contributes to the pathogenesis of gastric cancer. A
number of immune cells and cytokines causing chronic inflammation,
such as Th1 cells, Th2 cells, Th17 cells, regulatory T cells and
interleukin (IL)-17, have been reported to be involved in the
pathogenesis of gastric cancer (3–5).
Follicular helper T (Tfh) cells, were identified in
human tonsils by Schaerli et al (6) and belong to a class of T cells with B
cell helper functions. The characteristic cell phenotype of Tfh
cells is cluster of differentiation (CD)4+ chemokine C-X-C receptor
(CXCR)5+ inducible T cell co-stimulator (ICOS)+ (7). Tfh cells interact with B cells through
the ICOS and CD40 ligand on the surface of the cell, which not only
results in Tfh cell differentiation into effector Tfh cells, but
also promotes the proliferation and differentiation of B cells into
plasma cells (8). Circulating Tfh
(cTfh) cells exist in human peripheral blood and have the same
approximate cellular phenotype as Tfh cells (9). Unlike Tfh cells in follicles, CD69, ICOS
and programmed cell death 1 (PD-1) exhibit a low expression on the
surfaces of cTfh cells, suggesting that cTfh cells may function as
resting or memory Tfh cells (10).
cTfh cells also share functional properties with Tfh cells in
secondary lymphoid organs (11). The
percentage of cTfh cells is increased in infectious diseases,
including chronic hepatitis C infection, autoimmune diseases, such
as Sjögren's syndrome and systemic lupus erythematosus, and certain
malignancies, including non-small cell lung cancer, and was
associated with the severity of disease, indicating that cTfh cells
may serve a role in the progression of these diseases (12–14).
However, the role of cTfh cells in gastric cancer has not, to the
best of our knowledge, been reported.
IL-21 is the major effector of cTfh cells that is
critical for plasma cell generation and normal immunoglobulin
production (15,16). C-X-C motif chemokine ligand (CXCL) 13
may be one of the most important factors in the homing, migration
and accumulation of B lymphocytes by specifically binding to CXCR5
(17). The CXCL13/CXCR5 interaction
also promotes the homing of Tfh cells to lymphoid follicles,
facilitating contact between Tfh cells and B cells (18). IL-21 and/or CXCL13 also serve a role
as biomarker of progression and prognosis in various types of
cancer, including breast cancer, cervical cancer and colon cancer
(19–21); however, few studies have been
performed examining their effect on gastric cancer.
The aim of the present study was to investigate the
distribution of cTfh cells and serum concentrations of associated
cytokines (IL-21 and CXCL13) in patients with gastric cancer.
Correlations between the percentage of cTfh cells, IL-21 and CXCL13
were also investigated. The results of the present study attempted
to elucidate the effects of these cells and molecules in gastric
cancer and their probable mechanisms of action.
Materials and methods
Patients
The study group included 50 patients with gastric
cancer recruited from Qingdao Municipal Hospital (Qingdao, China)
from January 2013 to December 2013. The patients, including 32
males and 18 females, were aged between 40 to 78 years with a
median age of 62 years. Diagnosis was confirmed by two independent
pathologists blinded to the data from Qingdao Municipal Hospital
based on histological criteria by samples from endoscopic biopsy
and/or surgery resection. None of the patients had undergone
surgery, chemotherapy or radiotherapy prior to enrollment in the
present study. A total of 30 healthy controls were enrolled during
the aforementioned period. The controls were aged between 45 to 74
years with a median age of 60 years. No obvious inflammatory or
ulcerative lesions were identified by gastroscopy or biopsy
histology. All controls were matched with patients in terms of age
and sex (Table I).
Tumor-Node-Metastasis (TNM) staging system by American Joint
Committee on Cancer was used to evaluate the tumor stage (22). Informed consent was obtained from all
study participants according to the Declaration of Helsinki. The
present study was approved by the ethics committee of Qingdao
Municipal Hospital (Qingdao, China).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
Characteristics | Gastric cancer
(n=50) | Controls
(n=30) | P-value |
|---|
| Age, years | 62.8±1.3 | 59.8±1.5 | 0.137 |
| Sex |
|
| 0.721 |
|
Male | 32 | 18 |
|
|
Female | 18 | 12 |
|
| TNM stage |
|
|
|
| I | 11 |
|
|
| II | 9 |
|
|
|
III | 21 |
|
|
| IV | 9 |
|
|
Isolation of peripheral blood
mononuclear cells (PBMCs)
PBMCs were obtained by Ficoll-Hypaque
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to the
manufacturer's protocol following centrifugation at 800 × g at 25°C
for 10 min of heparinized blood. PBMCs were then resuspended in
RPMI-1640 tissue culture medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Flowcytometry
Human PBMCs were incubated with Fc receptor blocking
reagent (1:5; cat no. 14-9161-73; eBioscience; Thermo Fisher
Scientific, Inc.) for 20 min at 25°C and then stained with antibody
of phycoerythrin (PE)-conjugated anti-human CXCR5 (1:40; cat no.
12-9185-42; eBioscience; Thermo Fisher Scientific, Inc.),
fluorescein isothiocyanate-conjugated anti-human ICOS (1:5; cat no.
11-9948-42; eBioscience; Thermo Fisher Scientific, Inc.), PE-Texas
Red (ECD)-conjugated anti- human CD4 (1:100; cat no. 6604727;
Beckman Coulter, Inc., Brea, CA, USA) or isotype-matched IgG1,
including PE-conjugated IgG1 (cat no. 12-4714-82; Invitrogen;
Thermo Fisher Scientific, Inc.), FITC-conjugated IgG1 (cat no.
11-4714-42; Invitrogen; Thermo Fisher Scientific, Inc.) and
ECD-conjugated IgG1 (cat no. A07797; Beckman Coulter, Inc.) as the
control at 25°C away from light for 15 min. These antibodies were
used for both groups. Following washing with 1× PBS twice, the
cells were then subjected to analysis using a CytoFLEX cytometer
and CytExpert software version 2.0 (Beckman Coulter, Inc.). The
cells were gated on the forward scatter of living cells and then
centered on CD4+CXCR5+ICOS+ T cells.
ELISA
Following fasting for 8 h, 3 ml blood was taken from
the subjects and then centrifuged at 1,600 × g at 25°C for 10 min.
Serum was collected and stored at −20°C for further examination.
ELISA kits were used for detecting IL-21 (cat no. CHE0056; 4A
Biotec Co., Ltd, Beijing, China) and CXCL13 (cat no. A0336; Westang
Bio-TECH Co., Ltd, Shanghai, China). The procedures were performed
in strict accordance with the manufacturer's protocol.
Statistical analysis
SPSS17.0 software (SPSS, Inc., Chicago, IL, USA) was
used for data processing. The measurement data are expressed as the
mean ± standard error. Comparisons between 2 groups were made by
unpaired Student's t-test and between ≥3 groups by one-way analysis
of variance followed by a Least-Significance-Difference post-hoc
test. Linear correlation analysis was performed to calculate the
Pearson's correlation coefficient. Categorical data were compared
by χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Distribution of cTfh cells in patients
with gastric cancer and its association with the examined
clinicopathological features
cTfh cells were identified as CXCR5+ ICOS+ T cells
within the CD4+ T cell population by flow cytometry (Fig. 1A and B). The percentage of cTfh cells
(cTfh%) was quantified by calculating the proportion of cells in
the right upper quadrant as depicted in Fig. 1C and D. The cTfh% represented by
density of the point in the right upper quadrant was higher in the
gastric cancer group (Fig. 1D) than
in the control group (Fig. 1C). The
cTfh% in patients with gastric cancer and in the control group were
11.00±0.62 and 3.03±0.29, respectively. The comparison of the mean
cTfh% between different groups is depicted in Fig. 1E. The cTfh% in patients with lymph
node metastasis, TNM III–IV and low differentiation were
significantly higher than in those without lymph node metastasis,
TNM I–II and well differentiated tumors (P<0.01). There were no
significant differences in cTfh% between individuals of different
age, sex, tumor location or tumor size (Fig. 1E).
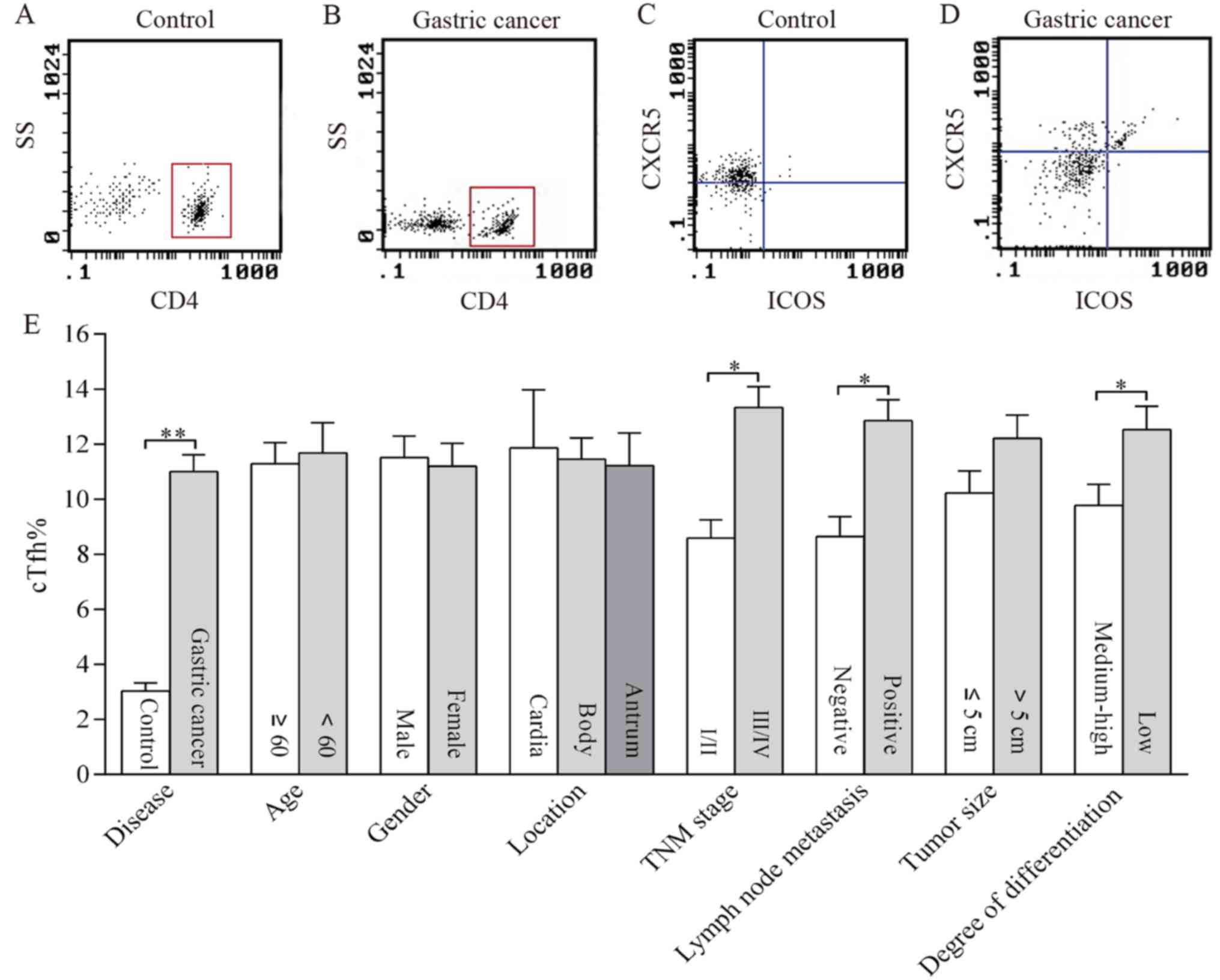 | Figure 1.Distribution of cTfh in the peripheral
blood of patients with gastric cancer and its association with
clinicopathological features. cTfh cells were identified as CXCR5+
ICOS+ T cells within the CD4+ T cell population by flow cytometry.
As presented in representative dotplots, CD4+ cells were
distinguished by setting the gate in (A) controls and in (B) the
patients. Then cTfh cells were recognized as CXCR5+ ICOS+ cells
shown in the right upper quadrant in (C) the controls and in (D)
the patients. The percentage of cTfh cells was compared in patients
with gastric cancer and controls as well as in patients with
different clinicopathological characteristics including age, sex,
location, TNM stage, lymph node metastasis, tumor size, and degree
of differentiation (E). *P<0.01; **P<0.001. cTfh, circulating
follicular helper T cells; TNM, Tumor-Node-Metastasis; CXCR5,
Chemokine C-X-C receptor 5; ICOS, inducible T cell co-stimulator;
SS, side scatter. |
Association between the concentration
of IL-21 and clinicopathological features in patients with gastric
cancer
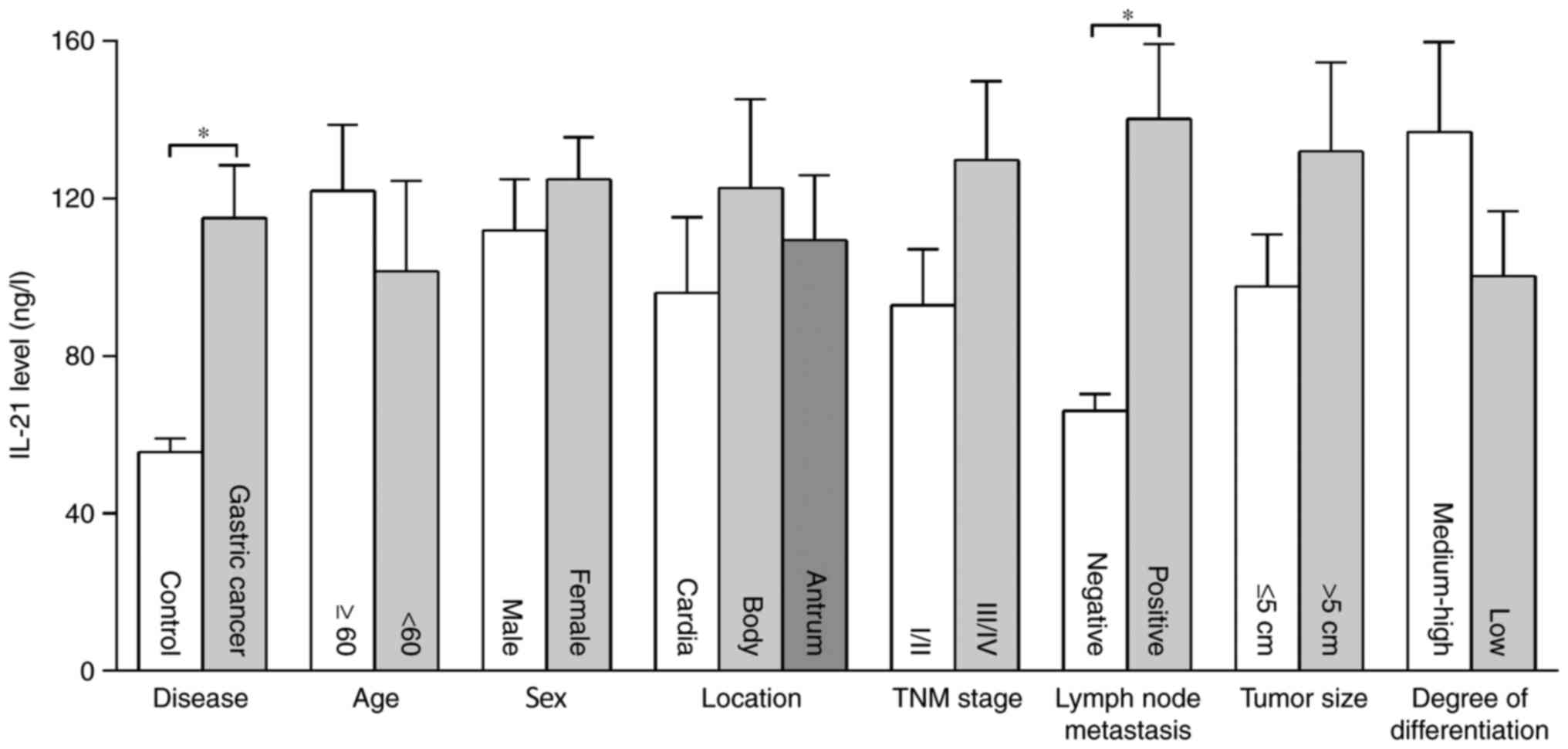
The serum levels of IL-21 in patients with gastric
cancer were significantly higher than those in the control group,
and the serum concentrations of IL-21 in the two groups were
114.95±13.51 and 55.66±3.39 ng/l, respectively (P<0.01). The
concentration of IL-21 in patients with lymph node metastasis was
higher than in those without lymph node metastasis (P<0.01).
There were no significant differences in serum IL-21 levels among
individuals of different age, sex, tumor location, TNM stage,
histological differentiation and tumor size (Fig. 2).
Association between the concentration
of CXCL13 and clinicopathological features in patients with gastric
cancer
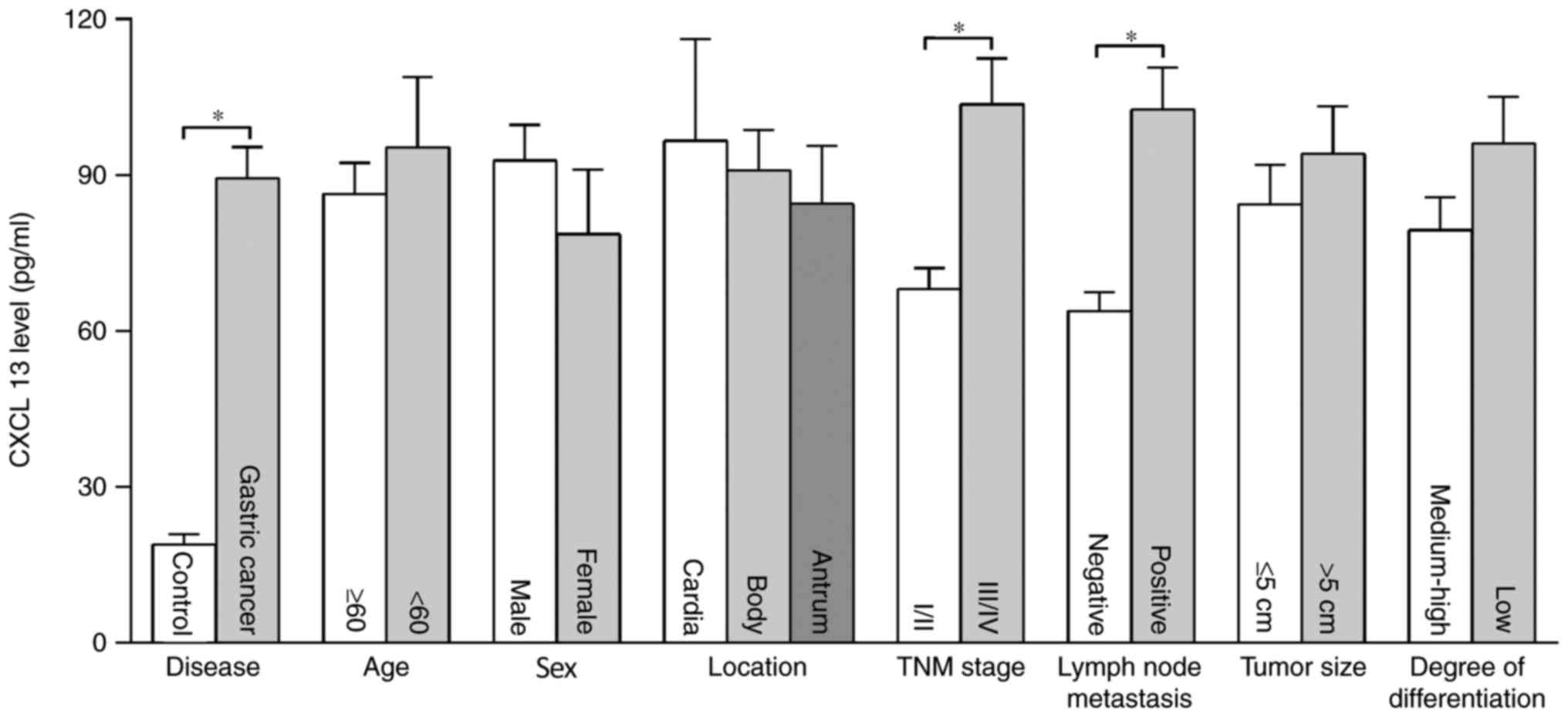
The concentration of serum CXCL13 in patients with
gastric cancer was significantly higher than in the control group
(P<0.01), and the concentrations of CXCL13 in the two groups
were 89.41±6.00 and 18.91±1.99 pg/ml, respectively. The
concentration of CXCL13 in patients with lymph node metastasis and
TNM III–IV was significantly higher than those without lymph node
metastasis and TNM I–II (P<0.01). There were no significant
differences in concentration of CXCL13 among patients of different
age, sex, tumor location, histological differentiation and tumor
size (Fig. 3).
Correlation between cTfh%, IL-21 and
CXCL13 in patients with gastric cancer
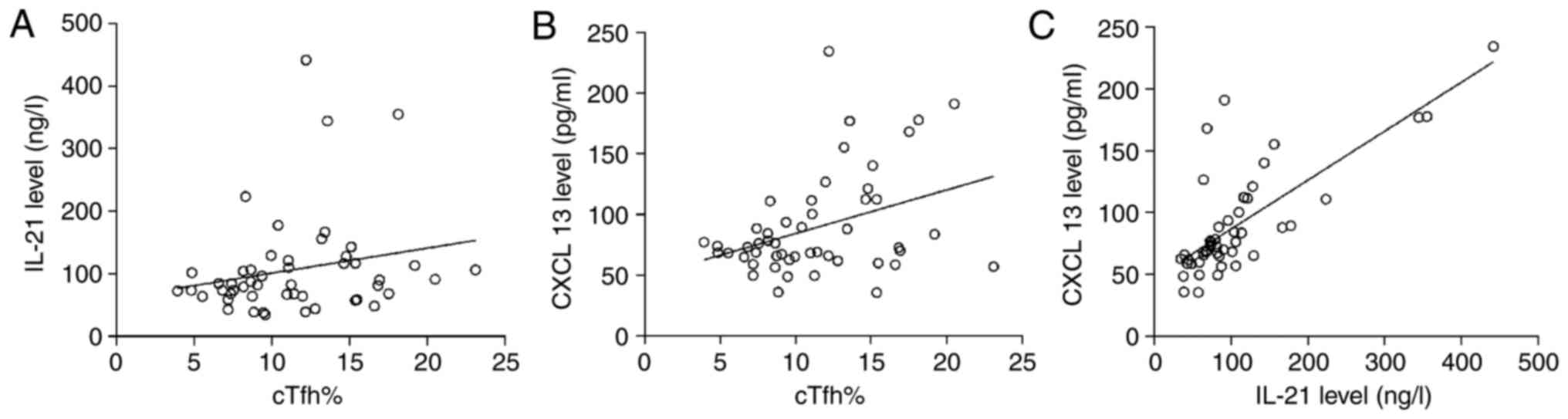
The results of linear correlation analysis
demonstrated that cTfh% had no correlation with the concentration
of IL-21 in patients with gastric cancer (r=0.217, P=0.130), while
a significant correlation was observed between cTfh% and CXCL13
(r=0.368, P=0.008), as well as between IL-21 and CXCL13 (r=0.748,
P=0.000; Fig. 4).
Discussion
In the present study, the distribution of cTfh cells
and the concentrations of IL-21 and CXCL13 in the peripheral blood
of patients with gastric cancer were investigated. Compared with
the control group, the cTfh% in the peripheral blood and the
concentration of IL-21 and CXCL13 in the serum were significantly
higher in patients with gastric cancer. A higher cTfh% and a higher
concentration of IL-21 and CXCL13 were also associated with the
characteristics of tumor progression, including the late tumor
stage, lymph node metastasis and poor differentiation.
cTfh may serve a role in the pathogenesis and
progression of various types of malignancies. A study undertaken by
Cha et al (16), reported that
in primary diffuse large B cell lymphoma (DLBCL), CD4+ CXCR5+ cells
in the peripheral blood promoted the proliferation and inhibited
the apoptosis of DLBCL cells. Additionally, a study undertaken by
Shi et al (14) demonstrated
the percentage of cTfh to be significantly higher in non-small cell
lung cancer (NSCLC) and in advanced-stage cases. An additional
study reported that when Crohn's disease was accompanied by
colorectal cancer, the number of cTfh cells was increased 1.59-fold
(23). To the best of our knowledge,
at present, there are no reports on the role of cTfh in gastric
cancer. The results of the present study demonstrated that the
percentage of cTfh in patients with gastric cancer was
significantly higher than in the control group, and was associated
with lymph node metastasis, advanced stages of cancer and a low
degree of differentiation. Therefore, cTfh may be considered as an
indicator of diagnosis/prognosis in patients with gastric
cancer.
The leading studies of IL-21 in tumors have focused
on its inhibitory effect on tumor growth, and consequently IL-21
has become a target for immunotherapy (24–26).
However, in breast cancer, the IL-21 gene polymorphism may be an
important index for assessing the prognosis of disease (27). While the role of IL-21 in gastric
cancer has rarely been reported, a previous study reported that the
concentration of IL-21 in gastric cancer tissues has been increased
(28). The serum concentration of
IL-21 in patients with gastric cancer was demonstrated to be
significantly higher when compared with the control group. By
comparing the levels of IL-21 expression between different
clinicopathological features, it was identified that the serum
concentration of IL-21 in patients with lymph node metastasis was
significantly higher than in those without lymph node metastasis.
This suggested that IL-21 may serve a role in the development and
progression of gastric cancer.
The impact of CXCL13/CXCR5 on various types of
cancer, including breast cancer, colorectal cancer and prostate
cancer has been the focus of a number of studies (29–31).
CXCL13 and CXCR5 may be associated with unfavorable clinical
characteristics in tumors, including invasive pathological type,
positive lymph node, metastasis and the relapse of cancer at an
advanced stage of disease. The results of the present study
demonstrated that the concentration of CXCL13 in the peripheral
blood of patients with gastric cancer was significantly higher,
when compared with the control group, and the concentration of
CXCL13 in patients with lymph node metastasis and TNM III–IV was
higher than in those without lymph node metastasis and TNM I–II.
These results suggested that the increased concentration of CXCL13
may be associated with the progression of gastric cancer.
IL-21 is a well-established effector of Tfh cells,
and can also bind with IL-21R on the surface of Tfh cells to
promote the expression of CXCR5 and ICOS through the autocrine
signaling pathway, thereby inducing further differentiation in Tfh
cells (32). As the principal ligand
of CXCR5, CXCL13 can induce the homing and migration of Tfh cells
by combining with CXCR5 (33). In
this way, there may be a correlation between Tfh, IL-21 and CXCL13.
In the present study, the correlations between these three
parameters were examined. A study undertaken by An et al
(34), reported that the proportion
of Tfh cells in the peripheral blood was positively associated with
the concentration of IL-21 in patients with unexplained
infertility. In the present study, no correlation was determined
between the percentage of cTfh and the concentration of IL-21 in
serum of patients with gastric cancer. The result of no correlation
between cTfh and IL-21 in peripheral blood may be associated with
the multiple sources of IL-21 in the serum. In addition to Tfh
cells, Th17 cells and natural killer T cells are capable of
secreting a small amount of IL-21 (35,36). Th17
cells in the peripheral blood appeared to be notably amplified in
gastric cancer (29). Whether this
affects the synthesis and secretion of IL-21 remains to be
established. The association between Tfh cells and CXCL13, as well
as CXCL13 and IL-21, has not, to the best of our knowledge, been
reported previously. The results of the present study demonstrated
a positive association between cTfh% and CXCL13, and a positive
correlation between CXCL13 and IL-21. CXCL13 and IL-21 are the key
factors in the survival of the Tfh/B cell axis. CXCL13 can direct
Tfh cell migration into B cell follicles through binding to CXCR5
on the surface of Tfh (37). Tfh
cells located in lymphoid follicles express ICOS, PD-1 and IL-21,
which not only provide markers for identification of Tfh cells but
also serve important functions in their interactions with B cells
(18,38,39). The
association between cTfh, CXCL13 and IL-21 was determined in
peripheral blood. Whether this association can indirectly reflect
the interaction between these three parameters in lymphoid
follicles remains to be studied.
To conclude, the findings of the present study
demonstrated that an increased percentage of cTfh cells, IL-21 and
CXCL13 were observed in patients with gastric cancer, and that all
three factors may be involved in the development and progression of
cancer. Furthermore, there may be mutual regulation among cTfh
cells, IL-21 and CXCL13; however, the direct association between
cTfh cells and associated factors was not reflected in the present
study. In the future, the direct associations between them may be
analyzed through intervention experiments in vitro in order
to fully elucidate their effects on gastric cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XM and CZ produced the concept of the present study.
XM and QD designed the present study. CZ and QD supervised the
present study. XM, XY and JM provided the materials. XM, QX, XY, XX
and JL conducted data collection and/or processing.XM, XY and JM
conducted analysis and/or interpretation. XM, JM and XY conducted
the literature review. XM and XY wrote the manuscript. QD and CZ
critically reviewed the manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all study
participants according to the Declaration of Helsinki. The present
study was approved by the ethics committee of Qingdao Municipal
Hospital (Qingdao, China).
Patient consent for publication
The patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Li Q, Chen J, Liu Y, Zhao X, Tan B,
Ai J, Zhang Z, Song J and Shan B: Prevalence of Th17 and Treg cells
in gastric cancer patients and its correlation with clinical
parameters. Oncol Rep. 30:1215–1222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meng XY, Zhou CH, Ma J, Jiang C and Ji P:
Expression of interleukin-17 and its clinical significance in
gastric cancer patients. Med Oncol. 29:3024–3028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Y, Gao J, Su Z, Dai X, Li Y, Liu Y,
Chen J, Tong J, Zhang Y, Wu C, et al: Downregulation of Hlx closely
related to the decreased expressions of T-bet and Runx3 in patients
with gastric cancer may be associated with a pathological event
leading to the imbalance of Th1/Th2. Clin Dev Immunol.
2012:9498212012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schaerli P, Willimann K, Lang AB, Lipp M,
Loetscher P and Moser B: CXC chemokine receptor 5 expression
defines follicular homing T cells with B cell helper function. J
Exp Med. 192:1553–1562. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Zhou Y, Yu Q, Zhao Z, Wang H, Luo
X, Chen Y, Zhu Z, Chen G, Wu M and Qiu L: Higher frequency of
CD4+CXCR5+ICOS+PD1+ T follicular helper cells in patients with
infectious mononucleosis. Medicine (Baltimore). 94:e20612015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma CS and Deenick EK: Human T follicular
helper (Tfh) cells and disease. Immunol Cell Biol. 92:64–71. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitt N, Bentebibel SE and Ueno H:
Phenotype and functions of memory Tfh cells in human blood. Trends
Immunol. 35:436–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simpson N, Gatenby PA, Wilson A, Malik S,
Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, et
al: Expansion of circulating T cells resembling follicular helper T
cells is a fixed phenotype that identifies a subset of severe
systemic lupus erythematosus. Arthritis Rheum. 62:234–244. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forcade E, Kim HT, Cutler C, Wang K, Alho
AC, Nikiforow S, Ho VT, Koreth J, Armand P, Alyea EP, et al:
Circulating T follicular helper cells with increased function
during chronic graft-versus-host disease. Blood. 127:2489–2497.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang M, Zhang L, Li H, Chen Z, Luo A, Liu
B, Chen M, Peng M, Ren H and Hu P: Circulating T follicular helper
cells are associated with rapid virological response in chronic
hepatitis C patients undergoing peginterferon therapy. Int
Immunopharmacol. 34:235–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szabo K, Papp G, Szanto A, Tarr T and
Zeher M: A comprehensive investigation on the distribution of
circulating follicular T helper cells and B cell subsets in primary
Sjogren's syndrome and systemic lupus erythematosus. Clin Exp
Immunol. 183:76–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi W, Li X, Cha Z, Sun S, Wang L, Jiao S,
Yang B, Shi Y, Wang Z, Wu Z and Dai G: Dysregulation of circulating
follicular helper T cells in nonsmall cell lung cancer. DNA Cell
Biol. 33:355–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davis MR, Zhu Z, Hansen DM, Bai Q and Fang
Y: The role of IL-21 in immunity and cancer. Cancer Lett.
358:107–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cha Z, Qian G, Zang Y, Gu H, Huang Y, Zhu
L, Li J, Liu Y, Tu X, Song H and Qian B: Circulating CXCR5+CD4+ T
cells assist in the survival and growth of primary diffuse large B
cell lymphoma cells through interleukin 10 pathway. Exp Cell Res.
350:154–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dupuis J, Boye K, Martin N, Copie-Bergman
C, Plonquet A, Fabiani B, Baglin AC, Haioun C, Delfau-Larue MH and
Gaulard P: Expression of CXCL13 by neoplastic cells in
angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker
providing evidence that AITL derives from follicular helper T
cells. Am J Surg Pathol. 30:490–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin L, Yu D, Li X, Yu N, Li X and Wang Y
and Wang Y: CD4+CXCR5+ follicular helper T cells in salivary gland
promote B cells maturation in patients with primary Sjogren's
syndrome. Int J Clin Exp Pathol. 7:1988–1996. 2014.PubMed/NCBI
|
|
19
|
Zhu S, Lin J, Qiao G, Wang X and Xu Y:
Tim-3 identifies exhausted follicular helper T cells in breast
cancer patients. Immunobiology. 221:986–993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian Y, Yuan C, Ma D, Zhang Y, Liu Y,
Zhang W, Hou F and Cui B: IL-21 and IL-12 inhibit differentiation
of Treg and TH17 cells and enhance cytotoxicity of peripheral blood
mononuclear cells in patients with cervical cancer. Int J Gynecol
Cancer. 21:1672–1678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Z, Zhang X, Guo H, Fu L, Pan G and Sun
Y: CXCL13-CXCR5 axis promotes the growth and invasion of colon
cancer cells via PI3K/AKT pathway. Mol Cell Biochem. 400:287–295.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng J, Zhang R, Zhang L, Liu Y, Hao X and
Liang H: Negative node count improvement prognostic prediction of
the seventh edition of the TNM classification for gastric cancer.
PLoS One. 8:e800822013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Wang Z, Diao Y, Qian X, Zhu N and
Dong W: Circulating follicular helper T cells in Crohn's disease
(CD) and CD-associated colorectal cancer. Tumour Biol.
35:9355–9359. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sondergaard H and Skak K: IL-21: Roles in
immunopathology and cancer therapy. Tissue Antigens. 74:467–479.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skak K, Kragh M, Hausman D, Smyth MJ and
Sivakumar PV: Interleukin 21 combination strategies for cancer
therapy. Nat Rev Drug Discov. 7:231–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kannappan V, Butcher K, Trela M, Nicholl
I, Wang W and Attridge K: Interleukin 21 inhibits cancer-mediated
FOXP3 induction in naïve human CD4 T cells. Cancer Immunol
Immunother. 6:637–645. 2017. View Article : Google Scholar
|
|
27
|
You Y, Deng J, Zheng J, Hu M, Li N, Wu H,
Li W, Lu J and Zhou Y: IL-21 gene polymorphism is associated with
the prognosis of breast cancer in Chinese populations. Breast
Cancer Res Treat. 137:893–901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su Z, Sun Y, Zhu H, Liu Y, Lin X, Shen H,
Chen J, Xu W and Xu H: Th17 cell expansion in gastric cancer may
contribute to cancer development and metastasis. Immunol Res.
58:118–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Biswas S, Sengupta S, Chowdhury Roy S,
Jana S, Mandal G, Mandal PK, Saha N, Malhotra V, Gupta A, Kuprash
DV and Bhattacharyya A: CXCL13-CXCR5 co-expression regulates
epithelial to mesenchymal transition of breast cancer cells during
lymph node metastasis. Breast Cancer Res Treat. 143:265–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qi XW, Xia SH, Yin Y, Jin LF, Pu Y, Hua D
and Wu HR: Expression features of CXCR5 and its ligand, CXCL13
associated with poor prognosis of advanced colorectal cancer. Eur
Rev Med Pharmacol Sci. 18:1916–1924. 2014.PubMed/NCBI
|
|
31
|
El-Haibi CP, Singh R, Gupta P, Sharma PK,
Greenleaf KN, Singh S and Lillard JW Jr: Antibody microarray
analysis of sSignaling networks regulated by cxcl13 and cxcr5 in
prostate cancer. J Proteomics Bioinform. 5:177–184. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spolski R and Leonard WJ: IL-21 and T
follicular helper cells. Int Immunol. 22:7–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Havenar-Daughton C, Lindqvist M, Heit A,
Wu JE, Reiss SM, Kendric K, Bélanger S, Kasturi SP, Landais E,
Akondy RS, et al: CXCL13 is a plasma biomarker of germinal center
activity. Proc Natl Acad Sci USA. 113:2702–2707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
An LF, Zhang XH, Sun XT, Zhao LH, Li S and
Wang WH: Unexplained infertility patients have increased serum
IL-2, IL-4, IL-6, IL-8, IL-21, TNFα, IFNγ and increased Tfh/CD4 T
cell ratio: Increased Tfh and IL-21 strongly correlate with
presence of autoantibodies. Immunol Invest. 44:164–173. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duan MC, Huang Y, Zhong XN and Tang HJ:
Th17 cell enhances CD8 T-cell cytotoxicity via IL-21 production in
emphysema mice. Mediators Inflamm. 2012:8980532012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Coquet JM, Kyparissoudis K, Pellicci DG,
Besra G, Berzins SP, Smyth MJ and Godfrey DI: IL-21 is produced by
NKT cells and modulates NKT cell activation and cytokine
production. J Immunol. 178:2827–2834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gunn MD, Ngo VN, Ansel KM, Ekland EH,
Cyster JG and Williams LT: A B-cell-homing chemokine made in
lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature.
391:799–803. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hardtke S, Ohl L and Förster R: Balanced
expression of CXCR5 and CCR7 on follicular T helper cells
determines their transient positioning to lymph node follicles and
is essential for efficient B-cell help. Blood. 106:1924–1931. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Linterman MA and Vinuesa CG: Signals that
influence T follicular helper cell differentiation and function.
Semin Immunopathol. 32:183–196. 2010. View Article : Google Scholar : PubMed/NCBI
|