Introduction
Cervical cancer is one of the most common malignant
tumors among women globally, exhibiting high morbidity and
mortality and most frequently occurring as cervical squamous cell
carcinoma (1). The etiology of
cervical cancer remains to be fully understood but may be
associated with certain factors, including engaging in sexual
intercourse with multiple partners or before the age of 16, giving
birth at a young age, multiple pregnancies, smoking, poor nutrition
and health and infection with persistent high-risk human papilloma
virus (2). Cervical cancer diagnosed
at an early stage may be treated effectively and detecting and
treating cervical cancer at an earlier stage is associated with an
improved prognosis (3). Therefore,
identifying the cells and molecular mechanisms facilitating the
development of cervical cancer is a major focus for researchers
globally (3).
Autophagy refers to the process of ‘self-digestion’
that occurs in eukaryotic cells and is characterized by the
formation of an autophagosome, which possesses a double-layer
membrane and wraps organelle and macromolecular proteins in the
cytoplasm (4). An autophagic vacuole
and a lysosome fuse to form an autophagosome, which subsequently
degrades the contents inside it, thereby supplying energy and
resources to support the cell metabolism and the renewal of
organelles (5). Autophagy is a
nonapoptotic form of eukaryotic cell death and is also known as
Type-II programmed cell death (6).
Autophagy is associated with numerous biological processes,
including the development and growth of cells, and is a crucial
biological phenomenon (7).
Apoptosis and autophagy are two causes of cell death
associated with different cell morphologies (8). Apoptosis is the predominant mechanism
underlying cell death, and is morphologically characterized by
membrane bubbles, cell shrinkage, nuclear fragmentation, chromatin
condensation, the breaking of chromosomal DNA and the formation of
apoptotic bodies. Autophagic cells may be sent to the mechanistic
target of rapamycin (mTOR) pathway (9). The activated mTOR kinase in mTOR complex
1 may phosphorylate, and inhibit the activity of, unc-51 like
autophagy activating kinase 1 (10).
mTOR complex l negatively regulates cell autophagy. Microtubule
associated protein 1 light chain 3 α (MAP1LC3A) and the mammalian
homologous yeast protein autophagy-related protein 8 (Atg8) serve
important functions in the transfer and maturity of autophagic
vacuoles (10).
Protein kinase B (Akt) serves a key function in
controlling the survival and apoptosis of cells, and may be
activated by insulin and numerous growth factors (11). Akt functions in wortmannin-sensitive
pathways with phosphoinositide 3-kinase (PI3K). Activated Akt
serves numerous functions, including supporting the combination of
phosphate esters, the phosphorylation and activation of Thr308 by
pyruvate dehydrogenase kinase 1 and the phosphorylation of the
C-terminal of Ser473 (8). The
phosphorylation of Akt may deactivate multiple target genes,
including BCL2 associated agonist of cell death, c-Raf and caspase
9, and thereby suppress apoptosis (12). Furthermore, Akt may promote the
phosphorylation of mTOR, serving a key function in cell growth.
Importantly, the phosphorylation of Akt may deactivate tuberin,
which inhibits the activity of regulatory associated protein of
MTOR complex 1 (13).
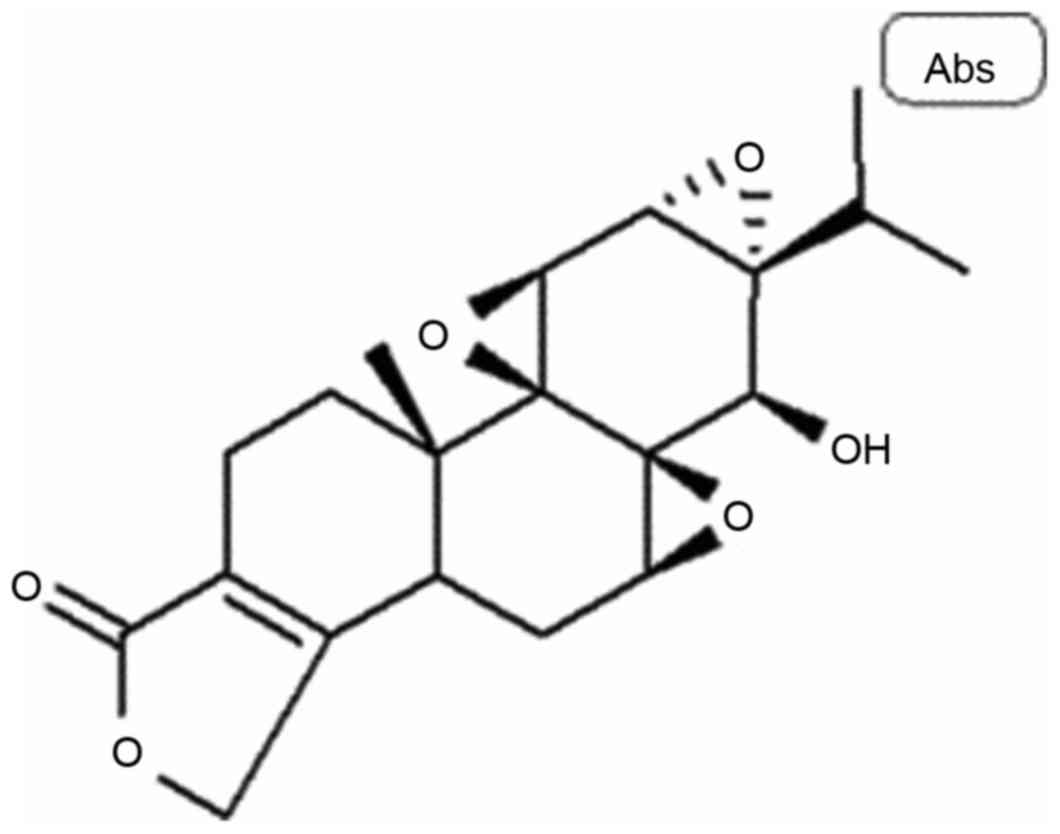
Triptolide (Fig. 1) is
a type of diterpene lactone epoxide compound isolated from
Tripterygium wilfordii (14).
Triptolide exhibits numerous pharmacological effects, including
immunosuppression, antineoplastic activity and conferring
resistance to certain types of infection (15). Triptolide is used in the treatment of
arthritis, autoimmune disorders, certain types of cancer, kidney
disease and asthma and to suppress immune rejection following organ
transplantation (16,17). The present study further examined
whether triptolide, a naturally occurring compound, exhibited
antineoplastic activity and assessed the mechanism underlying the
effect of triptolide on the growth and apoptosis of human cervical
cancer cells.
Materials and methods
Cell culture
The human cervical cancer cell line SiHa was
purchased from the Shanghai Cell Bank of the Chinese Academy of
Sciences (Shanghai, China) and cultured in Dulbecco's modified
Eagle's medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) with 10% fetal bovine serum (Hyclone; GE Healthcare Life
Sciences) at 37°C under 5% CO2 conditions with saturated
humidity.
Cell viability assay
SiHa cells were treated with 0–100 nM triptolide
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 12, 24 or 48 h.
Cell viability was assessed using an MTT dye reduction assay. SiHa
cells were seeded (1×104 cells/ml) onto 96-well plates
and incubated overnight at 37°C. Subsequently, 40 µl MTT was added
onto the cells and the plates were incubated for 4 h at 37°C. DMEM
was then removed and dimethyl sulfoxide was added onto the cells
and the plates were incubated for 20 min at 37°C. Optical density
was measured using an ELISA reader (Apollo LB 9110; Berthold
Technologies GmbH & Co. KG, Bad Wildbad, Germany) at 490 nm.
This experiment was repeated three times.
Immunofluorescence of autophagy
staining
SiHa cells were incubated with triptolide (0, 12.5,
25 and 50 nM) for 48 h at 37°C and washed with PBS, fixed with 75%
ethanol on ice for 30 min. SiHa cells perforated with 0.25%
Tris-100 in PBS for 15 min and blocked with 5% bovine serum albumin
in PBS for 1 h at 37°C. SiHa cells were stained with MAP1LC3A
antibodies (cat no. 3868; 1:500; Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C overnight and then incubated with Alexa
Fluor® 488 conjugate-anti-rabbit immunoglobulin G (H+L)
(cat no. 4412; 1:1,000; Cell Signaling Technology, Inc.) and
observed using a LSM 780 NLO confocal microscope (magnification,
×40; Zeiss GmbH, Jena, Germany).
Flow cytometry
SiHa cells (2.5×105 cells/ml) were seeded
onto 6-well plates with DMEM and incubated with triptolide (0,
12.5, 25 and 50 nM) for 48 h at 37°C. Fluorescein
isothiocyanate-Annexin V (BD Biosciences, Franklin Lakes, NJ, USA)
was used to stain SiHa cells for 30 min according to the
manufacturer's protocol. Propidium iodide (BD Biosciences) was also
used to stain SiHa cells for 30 min according to the manufacturer's
protocol. Flow cytometry (BD Biosciences) was used to analyze
apoptosis and analyzed by CellQuest software version 3.1 (BD
Biosciences, San Jose, CA, USA). This experiment was repeated 3
times.
Western blot analysis
SiHa cells (2.5×105 cells/ml, n=3) were
seeded onto 6-well plates and incubated with triptolide (0, 12.5,
25 and 50 nM) for 48 h at 37°C. The cells were subsequently washed
with PBS and resuspended in radioimmunoprecipitation lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) for 30 min on
ice. Protein content was quantified using a Bradford protein assay
(Beyotime Institute of Biotechnology). Protein (50 µg/lane) was
separated using SDS-PAGE on a 10–12% gel and transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were blocked using 5% nonfat dried milk dissolved
in TBS for 1 h at 37°C and incubated overnight at 4°C with
antibodies against phosphorylated (p)-Akt (cat. no. sc-293125,
1:500, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), p-mTOR
(cat. no. sc-293133, 1:500, Santa Cruz Biotechnology, Inc.),
p-p70S6K (cat. no. 9204, 1:2,000, Cell Signaling Technology, Inc.,
Danvers, MA, USA), p-p38 (cat. no. sc-81621, 1:500, Santa Cruz
Biotechnology, Inc.), p53 (cat. no. sc-126, 1:500, Santa Cruz
Biotechnology, Inc.), p-forkhead box O3 (Foxo3a; cat. no. 5538,
1:2,000, Cell Signaling Technology, Inc.) and GAPDH (cat. no.
AF0006, 1:5,000, Beyotime Institute of Biotechnology). Membranes
were subsequently washed three times in TBS-Tween (0.1%) and
incubated with horseradish peroxidase-conjugated goat anti-mouse
secondary antibodies (cat. no. A0216, 1:5,000, Beyotime Institute
of Biotechnology) at 37°C for 1 h. Membranes were subsequently
visualized using a SuperSignal™ West Pico Chemiluminescent
Substrate kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and quantified using Carestream Molecular Imaging software version
5.3.4 (Carestream Health, Inc., Rochester, NY, USA). Experiments
were performed in triplicate.
Statistical analysis
Data were presented as mean ± standard error of the
mean. Statistical analyses were performed using SPSS version 19.0
(IBM Corp., Armonk, NY, USA). Significant differences between the
groups were determined using one-way ANOVA by Bonferroni post-hoc
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Triptolide inhibits viability in human
cervical cancer cells
The present study assessed the effect of triptolide
on the viability of human cervical cancer cells using an MTT assay.
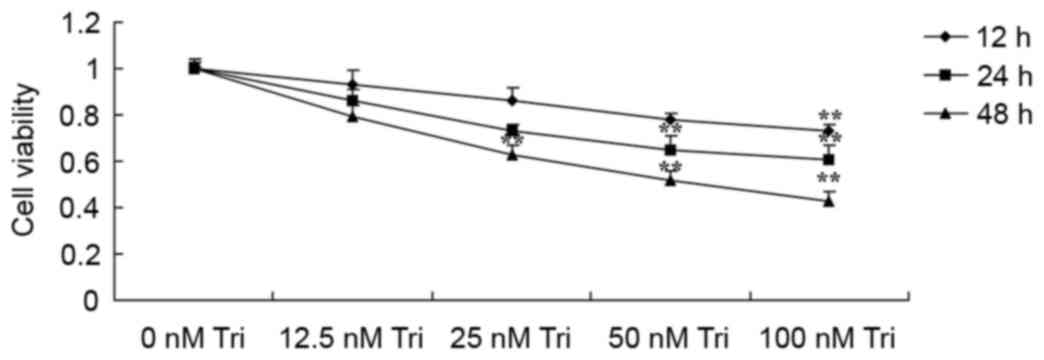
Treatment with triptolide significantly inhibited the viability of
SiHa cells dose- and time-dependently (Fig. 2). The viability of SiHa cells was
significantly inhibited by treatment with 100 nM triptolide for 12,
24 and 48 h compared with the control cells (Fig. 2). The viability of SiHa cells was
significantly inhibited by 50 nM triptolide at 24 and 48 h compared
with the control cells (Fig. 2). The
viability of SiHa cells was significantly inhibited by 25 nM
triptolide at 48 h compared with the control cells (Fig. 2).
Triptolide induces apoptosis in human
cervical cancer cells
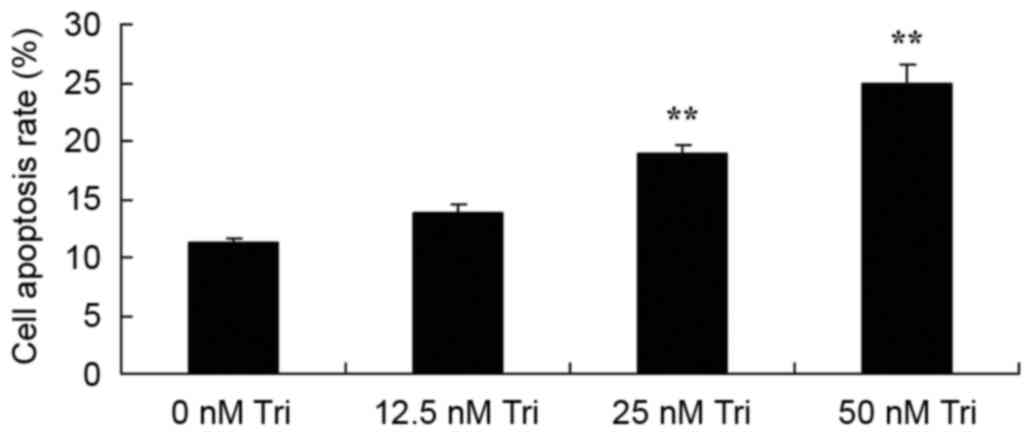
To assess the effect of triptolide on the apoptosis
of human cervical cancer cells, the apoptotic rate was measured
using flow cytometry. Treatment with 25 or 50 nM triptolide
significantly increased the apoptotic rate of SiHa cells
dose-dependently, compared with the control cells (Fig. 3).
Triptolide induces protective
autophagy in human cervical cancer cells
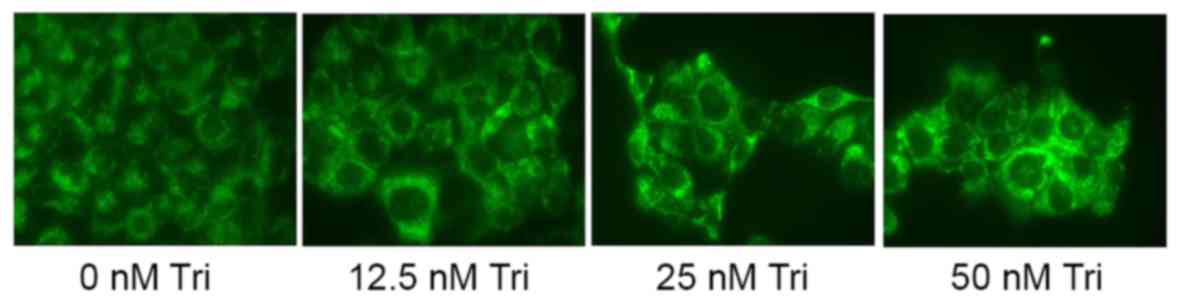
The present study examined the anticancer effect of
triptolide on autophagy in human cervical cancer cells by staining
and observing SiHa cells. Treatment with 25 or 50 nM triptolide
induced autophagy in SiHa cells (Fig.
4).
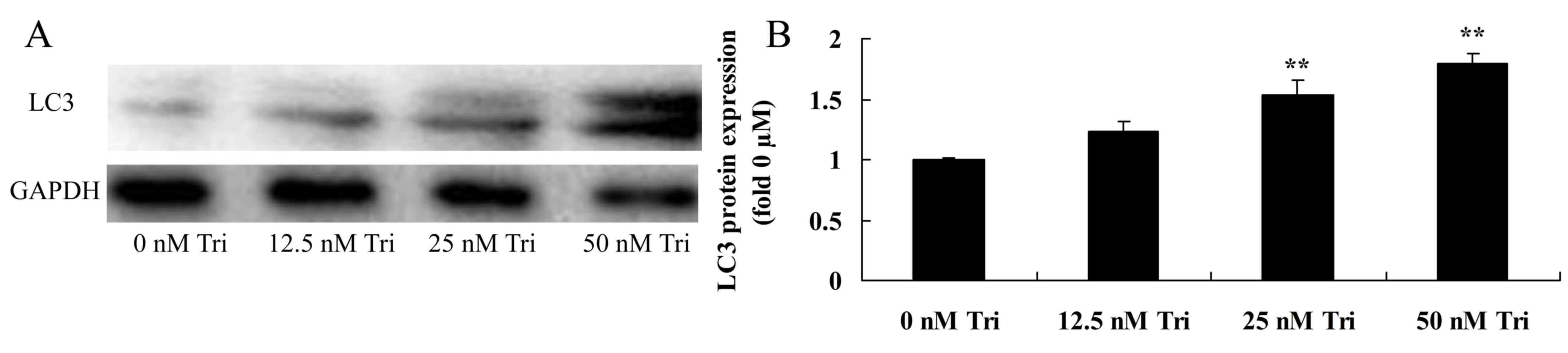
Triptolide regulates MAP1LC3A protein
expression in human cervical cancer cells
Western blot analysis demonstrated that triptolide
regulated MAP1LC3A protein expression in SiHa cells. However,
treatment with 25 or 50 nM triptolide significantly increased
MAP1LC3A protein expression in SiHa cells (Fig. 5).
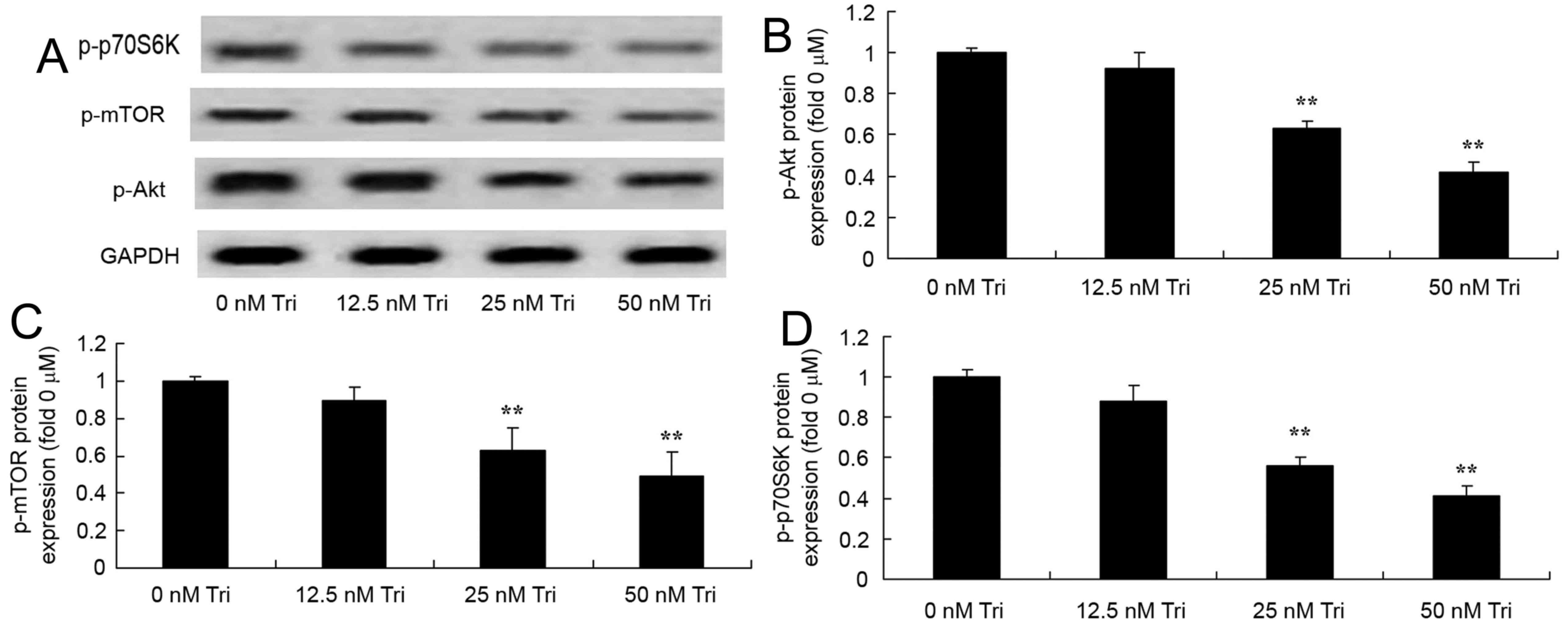
Triptolide regulates p-Akt, p-mTOR and
p-p70S6K protein expression in human cervical cancer cells
To determine whether Akt serves a function in the
anticancer effect of triptolide on cervical cancer cells, western
blot analysis was used to measure the protein expression of p-Akt,
p-mTOR and p-p70S6K in SiHa cells. Treatment with 25 or 50 nM
triptolide significantly suppressed the expression of p-Akt, p-mTOR
and p-p70S6Kprotein in SiHa cells (Fig.
6).
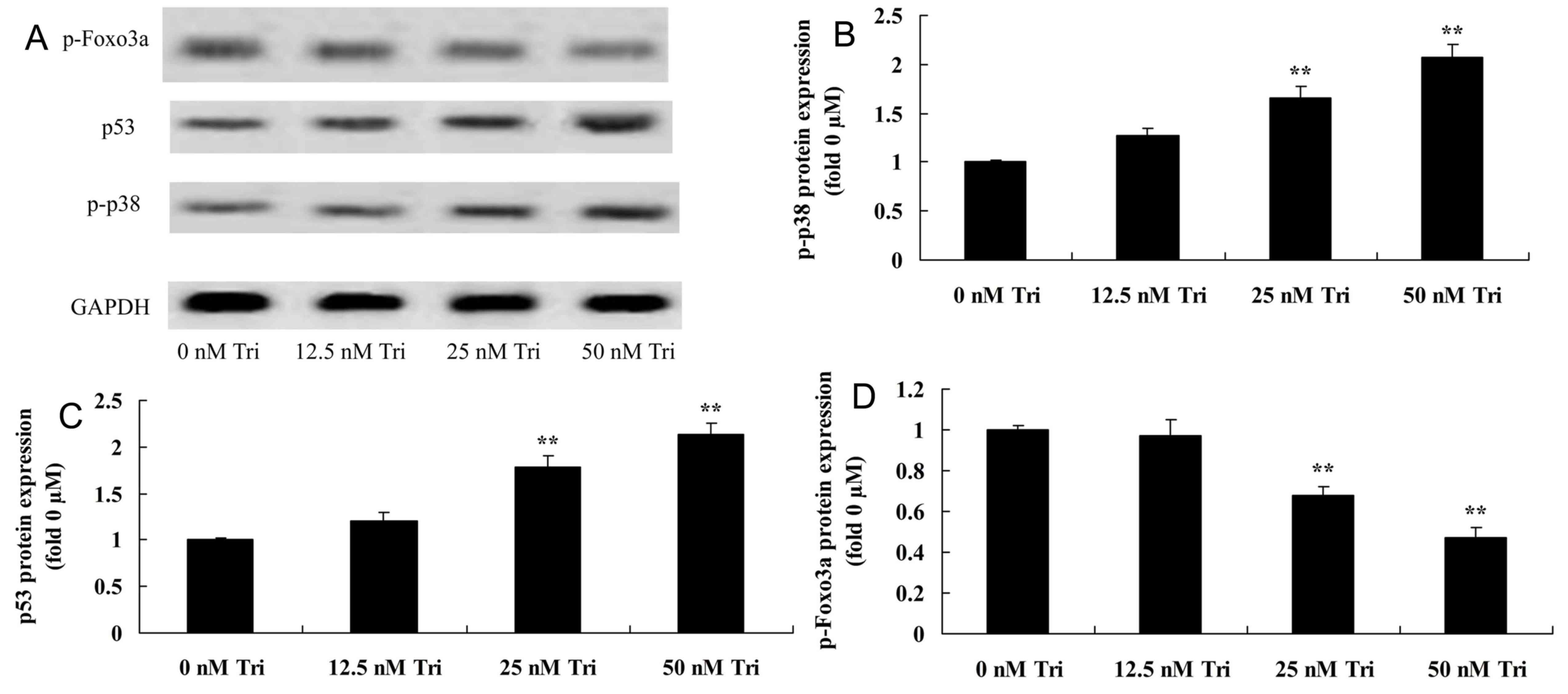
Triptolide regulates p-p38, p53 and
p-Foxo3a protein expression in human cervical cancer cells
Expression of p-Akt, p-mTOR and p-p70S6K protein in
triptolide-treated SiHa cells was detected using western blotting.
Treatment with 25 or 50 nM triptolide significantly increased
p-Akt, p-mTOR and p-p70S6K protein expression in SiHa cells
(Fig. 7).
Discussion
Cervical cancer is one of the most common
gynecological malignant tumors globally, increasing by 500,000
cases since 2011 and responsible for 280,000 mortalities in 2012
(18). In China, 100,000 cases of
cervical cancer-associated mortality are estimated annually,
accounting for ~1/3 of the global total (19). Due to the improvement in screening for
cervical cancer in medical and healthcare institutions in China,
the rates of cervical cancer-associated morbidity and mortality are
decreasing (20). However, young
patients diagnosed with cervical cancer suffer from a relatively
high mortality rate (21). The
present study revealed that triptolide significantly inhibited
viability and induced apoptosis in SiHa cells.
Autophagy may remove damaged organelles and proteins
in cells and regulate cell growth to maintain the stability of
cells and certain genes (22).
However, when the expression of certain autophagy-associated
proteins is altered, autophagy may be inhibited, resulting in the
instability of certain genes and potentially promotion of tumor
growth; at this point, active autophagy may inhibit tumor growth
(23). However, tumor cells possess
an increased proliferation and metabolic rate. Where tumors are not
provided with sufficient blood, oxygen or nutrition, autophagy may
be activated to produce recycled adenosine triphosphate, which is
conducive to tumor survival (23).
The results of the present study suggested that triptolide induced
autophagy in SiHa cells.
Located in the anterior of autophagic vacuoles and
on the surface of autophagic vacuolar membranes, MAP1LC3A is a
mammalian homologue of yeast Atg8 and a common marker of the
autophagic vacuolar membrane. Newly synthetized MAP1LC3A may form
soluble MAP1LC3A-I following processing (24). MAP1LC3A-I may be modified by
ubiquitin-like modifiers to combine with phosphatidyl ethanolamine
on the surface of autophagic vacuolar membrane to form MAP1LC3A-II.
Since the amount of MAP1LC3A-II is directly proportional to the
number of autophagic vacuoles, the change in MAP1LC3A-II content
may partly reflect the change in autophagic activity of cells
(25). The present study demonstrated
that triptolide increased MAP1LC3A protein expression in SiHa
cells. Mujumdar et al (26)
reported that triptolide induced death in pancreatic cancer cells
via autophagic pathways. Further studies are required to examine
the mechanisms underlying triptolide-induced autophagy and to
provide novel insight to improve the treatment of cervical
cancer.
The PI3K/Akt/mTOR pathway may serve a key function
in the autophagic pathway and in the pathogenesis and clinical
phenotype manifestation of cervical cancer. The PI3K/Akt/mTOR
pathway may regulate the growth and proliferation of cells, but its
function in patients with cervical cancer may be inhibited due to
mutations, amplification or the absence of methylation or abnormal
translation of genes following reformation (13). The activation of the PI3K/Akt/mTOR
pathway in patients with cervical cancer may result in lesions of
increased malignancy, which are more difficult to diagnose and
treat (25). Numerous selective
inhibitors of PI3K/Akt/mTOR pathway regulators are being developed,
but their functions are limited due to a high rate of intrinsic or
acquired drug resistance (27). Tumor
cells may induce autophagy by inhibiting the activation of Akt to
induce Foxo3a to translocate to the nucleus and increase
autophagy-associated MAP1LC3A expression (27). In the present study, triptolide
suppressed p-Akt, p-mTOR and p-p70S6K expression in SiHa cells.
Zhao et al (28) reported that
triptolide induces protective autophagy by suppressing mTOR
expression in human prostate cancer cells. Therefore, the results
of the present study suggest that triptolide induced the
PI3K/Akt/mTOR/p70S6K-associated pathway to induce autophagy, which
exploit in order to treat cervical cancer.
The autophagy of mammals comprises six main steps:
Activation, nucleation, prolonging, closing, maturity and
degeneration or dying (29). The mTOR
pathway is one autophagy-associated pathway but numerous other
signaling pathways and translations may regulate the autophagy of
cells (30). Depending on its
subcellular location, p53 may activate or inhibit autophagy
(31). Autophagy may also be
regulated by certain proteins, including AMP-activated proteases,
Akt, p38, mitogen-activated protein kinase/extracellular
signal-regulating enzymes and protease C (32). The results of the present study
indicated that triptolide increased p-p38 and p53 protein
expression and suppressed Foxo3a protein expression in SiHa cells.
Xiong et al (33) suggested
that triptolide exhibits an anticancer effect in human breast
cancer by downregulating activated Akt and upregulating p53
expression. Park et al (34)
demonstrated that triptolide inhibited the growth of THP-1 cells by
inducing apoptosis through the mitogen-activated protein kinase
pathway. Therefore, p38, p53 and Foxo3a may be pivotal targets in
the anticancer effect of triptolide on cervical cancer.
To conclude, the results from the present study
demonstrated that triptolide inhibited viability and increased the
apoptosis rate in SiHa cells by targeting the autophagy-inducing
PI3K/Akt/mTOR, p38, p53 and Foxo3a pathways. The present study
further examined the PI3K/Akt/mTOR pathway and provided novel
insight into the mechanism by which triptolide may function as an
anticancer agent for cervical cancer therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
GQ designed the experiment; PL and ZX performed the
experiment. GQ analyzed the data and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li JM, Shao JL, Zeng WJ and Liang RB:
General/epidural anesthesia in combination preserves NK cell
activity and affects cytokine response in cervical carcinoma
patients undergoing radical resection: A cohort prospective study.
Eur J Gynaecol Oncol. 36:703–707. 2015.PubMed/NCBI
|
|
2
|
Herrero R, Quint W, Hildesheim A, Gonzalez
P, Struijk L, Katki HA, Porras C, Schiffman M, Rodriguez AC,
Solomon D, et al: Reduced prevalence of oral human papillomavirus
(HPV) 4 years after bivalent HPV vaccination in a randomized
clinical trial in Costa Rica. PLoS One. 8:e683292013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frumovitz M, Querleu D, Gil-Moreno A,
Morice P, Jhingran A, Munsell MF, Macapinlac HA, Leblanc E,
Martinez A and Ramirez PT: Lymphadenectomy in locally advanced
cervical cancer study (LiLACS): Phase III clinical trial comparing
surgical with radiologic staging in patients with stages IB2-IVA
cervical cancer. J Minim Invasive Gynecol. 21:3–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu Y, Yu H, Qin H, Kang J, Yu C, Zhong J,
Su J, Li H and Sun L: Inhibition of autophagy enhances cisplatin
cytotoxicity through endoplasmic reticulum stress in human cervical
cancer cells. Cancer Lett. 314:232–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen TC, Hung YC, Lin TY, Chang HW, Chiang
IP, Chen YY and Chow KC: Human papillomavirus infection and
expression of ATPase family AAA domain containing 3A, a novel
anti-autophagy factor, in uterine cervical cancer. Int J Mol Med.
28:689–696. 2011.PubMed/NCBI
|
|
6
|
Xu L, Liu JH, Zhang J, Zhang N and Wang
ZH: Blockade of autophagy aggravates endoplasmic reticulum stress
and improves Paclitaxel cytotoxicity in human cervical cancer
cells. Cancer Res Treat. 47:313–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Q, Luo XY, Jiang H, Yang MH, Yuan GH,
Tang Z and Wang H: Hydroxychloroquine facilitates autophagosome
formation but not degradation to suppress the proliferation of
cervical cancer SiHa cells. Oncol Lett. 7:1057–1062. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim A, Yim NH and Ma JY: Samsoeum, a
traditional herbal medicine, elicits apoptotic and autophagic cell
death by inhibiting Akt/mTOR and activating the JNK pathway in
cancer cells. BMC Complement Altern Med. 13:2332013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Ji X, Wang W, Liu J, Liang X, Wu H,
Liu J, Eggert US, Liu Q and Zhang X: Ammonia Induces autophagy
through dopamine receptor D3 and MTOR. PLoS One. 11:e01535262016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Belzile JP, Sabalza M, Craig M, Clark E,
Morello CS and Spector DH: Trehalose, an mTOR-independent inducer
of autophagy, inhibits human cytomegalovirus infection in multiple
cell types. J Virol. 90:1259–1277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Feng Z, Li J, Chen L and Tang W:
High glucose induces autophagy of MC3T3-E1 cells via ROS-AKT-mTOR
axis. Mol Cell Endocrinol. 429:62–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv S, Xu QY, Sun EC, Zhang JK and Wu DL:
Dissection and integration of the autophagy signaling network
initiated by bluetongue virus infection: Crucial candidates ERK1/2,
Akt and AMPK. Sci Rep. 6:231302016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SJ, Ryu J, Kim IH, Choi YH and Nam
TJ: Activation of the mTOR signaling pathway in breast cancer MCF7
cells by a peptide derived from porphyra yezoensis. Oncol Rep.
33:19–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu G, Gong X, Wang L, Liu M, Liu Y, Fu X,
Wang W, Zhang T and Wang X: Triptolide promotes the clearance of
α-synuclein by enhancing autophagy in neuronal cells. Mol
Neurobiol. 54:2361–2372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ziaei S and Halaby R: Immunosuppressive,
anti-inflammatory and anti-cancer properties of triptolide: A mini
review. Avicenna J Phytomed. 6:149–164. 2016.PubMed/NCBI
|
|
16
|
Zhu W, Hu H, Qiu P and Yan G: Triptolide
induces apoptosis in human anaplastic thyroid carcinoma cells by a
p53-independent but NF-kappaB-related mechanism. Oncol Rep.
22:1397–1401. 2009.PubMed/NCBI
|
|
17
|
Jiang XH, Wong BC, Lin MC, Zhu GH, Kung
HF, Jiang SH, Yang D and Lam SK: Functional p53 is required for
triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB
activation in gastric cancer cells. Oncogene. 20:8009–8018. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verhoef VM, van Kemenade FJ, Rozendaal L,
Heideman DA, Bosgraaf RP, Hesselink AT, Melchers WJ, Massuger LF,
Bekkers RL, Steenbergen RD, et al: Follow-up of high-risk HPV
positive women by combined cytology and bi-marker CADM1/MAL
methylation analysis on cervical scrapes. Gynecol Oncol. 137:55–59.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rangwala R, Chang YC, Hu J, Algazy KM,
Evans TL, Fecher LA, Schuchter LM, Torigian DA, Panosian JT, Troxel
AB, et al: Combined MTOR and autophagy inhibition: Phase I trial of
hydroxychloroquine and temsirolimus in patients with advanced solid
tumors and melanoma. Autophagy. 10:1391–1402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hogdal N, Juhl C, Aadahl M and Gluud C:
Early preventive exercises versus usual care does not seem to
reduce trismus in patients treated with radiotherapy for cancer in
the oral cavity or oropharynx: A randomised clinical trial. Acta
Oncol. 54:80–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Ou J, Guo Y, Dai T, Li X, Liu J,
Xia M, Liu L and He M: TBLR1 is a novel prognostic marker and
promotes epithelial-mesenchymal transition in cervical cancer. Br J
Cancer. 111:112–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei B, Huang Q, Huang S, Mai W and Zhong
X: Trichosanthin-induced autophagy in gastric cancer cell MKN-45 is
dependent on reactive oxygen species (ROS) and NF-kB/p53 pathway. J
Pharmacol Sci. 131:77–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leisching G, Loos B, Botha M and
Engelbrecht AM: A nontoxic concentration of cisplatin induces
autophagy in cervical cancer: Selective cancer cell death with
autophagy inhibition as an adjuvant treatment. Int J Gynecol
Cancer. 25:380–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Florey O, Gammoh N, Kim SE, Jiang X and
Overholtzer M: V-ATPase and osmotic imbalances activate
endolysosomal LC3 lipidation. Autophagy. 11:88–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duan J, Yu Y, Yu Y, Li Y, Wang J, Geng W,
Jiang L, Li Q, Zhou X and Sun Z: Silica nanoparticles induce
autophagy and endothelial dysfunction via the PI3K/Akt/mTOR
signaling pathway. Int J Nanomedicine. 9:5131–5141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mujumdar N, Mackenzie TN, Dudeja V, Chugh
R, Antonoff MB, Borja-Cacho D, Sangwan V, Dawra R, Vickers SM and
Saluja AK: Triptolide induces cell death in pancreatic cancer cells
by apoptotic and autophagic pathways. Gastroenterology.
139:598–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu CC, Huang HB, Hung SK, Liao HF, Lee CC,
Lin HY, Li SC, Ho HC, Hung CL and Su YC: AZD2014 radiosensitizes
oral squamous cell carcinoma by inhibiting AKT/mTOR axis and
inducing G1/G2/M cell cycle arrest. PLoS One. 11:e01519422016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao F, Huang W, Zhang Z, Mao L, Han Y,
Yan J and Lei M: Triptolide induces protective autophagy through
activation of the CaMKKβ-AMPK signaling pathway in prostate cancer
cells. Oncotarget. 7:5366–5382. 2016.PubMed/NCBI
|
|
29
|
Kang EB and Cho JY: Effect of treadmill
exercise on PI3K/AKT/mTOR, autophagy, and Tau hyperphosphorylation
in the cerebral cortex of NSE/htau23 transgenic mice. J Exerc
Nutrition Biochem. 19:199–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baena M, Sanguesa G, Hutter N, Sánchez RM,
Roglans N, Laguna JC and Alegret M: Fructose supplementation
impairs rat liver autophagy through mTORC activation without
inducing endoplasmic reticulum stress. Biochim Biophys Acta.
1851:107–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen L, Jiang Z, Ma H, Ning L, Chen H, Li
L and Qi H: Volatile oil of acori graminei Rhizoma-induced
apoptosis and autophagy are dependent on p53 status in human glioma
cells. Sci Rep. 6:211482016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li JP, Yang YX, Liu QL, Pan ST, He ZX,
Zhang X, Yang T, Chen XW, Wang D, Qiu JX and Zhou SF: The
investigational Aurora kinase A inhibitor alisertib (MLN8237)
induces cell cycle G2/M arrest, apoptosis, and autophagy via p38
MAPK and Akt/mTOR signaling pathways in human breast cancer cells.
Drug Des Devel Ther. 9:1627–1652. 2015.PubMed/NCBI
|
|
33
|
Xiong J, Su T, Qu Z, Yang Q, Wang Y, Li J
and Zhou S: Triptolide has anticancer and chemosensitization
effects by down-regulating Akt activation through the MDM2/REST
pathway in human breast cancer. Oncotarget. 7:23933–23946. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park SW and Kim YI: Triptolide induces
apoptosis of PMA-treated THP-1 cells through activation of
caspases, inhibition of NF-kB and activation of MAPKs. Int J Oncol.
43:1169–1175. 2013. View Article : Google Scholar : PubMed/NCBI
|