Introduction
Esophageal cancer is one of the most common
malignancies, with an incidence rate that ranks sixth in China and
fourth among the causes of cancer-related mortality (1). Esophageal cancer is also one of the most
common lethal tumors worldwide with the sixth highest mortality
rate (2). Esophageal squamous cell
carcinoma (ESCC) accounts for >90% of the cases of esophageal
cancer, and has a high degree of aggressiveness and complexity of
biological behavior, making its clinical course variable and
heterogeneous. A considerable number of ESCC patients have already
metastasized at diagnosis. The metastasis of ESCC is the main cause
of poor prognosis and mainly involves lymph node and distant
metastasis (3). Previous studies
demonstrated that the 5-year survival rate of ESCC patients without
metastasis was 70–92%, whereas it was only 18–47% in those with
metastasis (3,4). Therefore, an in-depth understanding of
the mechanism underlying ESCC metastasis is of high scientific and
clinical value for improving the survival rate of ESCC.
Long non-coding (lnc)RNAs are a class of mature RNAs
with a transcription length of >200 bases, which is
significantly longer compared with that of mRNA. Despite having no
coding ability, lncRNAs are involved in several processes,
including gene imprinting, chromatin remodeling, cell cycle
regulation, splicing regulation, mRNA degradation and translation
regulation (5). They can regulate
gene expression at the genetic, transcriptional and
post-transcriptional level, and are widely implicated in most
physiological as well as pathological processes (6). An increasing number of studies have
demonstrated that differentially expressed lncRNAs are closely
associated with tumor proliferation, invasion, metastasis and
prognosis, and have great potential of becoming new targets for
tumor therapy (7,8). It was demonstrated that lncRNAs are
closely related to ESCC (9–11). However, little is known on their
effect on ESCC metastasis. Therefore, it is crucial to identify
differentially expressed lncRNAs in ESCC and investigate their
function and underlying mechanism in ESCC metastasis.
LncRNA-ENST00000589379 has been found to be highly
expressed in ESCC tissues with undefined function by gene chips
(12). In the present study, this
novel lncRNA is referred to as lncRNA-ECM. To further investigate
the role of lncRNA-ECM in ESCC metastasis, we investigated the
correlation between its expression and the clinical pathology of
ESCC using real-time fluorescence quantitative PCR. Changes in cell
invasion and migration capacity were detected following lncRNA-ECM
knockdown/overexpression in the ESCC TE-1 and Eca109 cell lines by
classical Transwell assay. Bioinformatics analysis and rescue
experiments were used to examine the expression changes in the
downstream geneintercellular adhesion molecule 1 (ICAM1) in order
to elucidate the mechanism of action of lncRNA-ECM.
Materials and methods
Cases and specimens
Tissue samples from 62 cases with ESCC and matched
paracancerous tissues were collected in Taizhou People's Hospital
affiliated to Nantong University (Nantong, China) between September
2014 and December 2016. All the cases were confirmed by
pathological examination. The patients included 43 men and 19
women, with a mean age at surgery of 65.75±6.39 years. None of the
patients had received any radiotherapy or chemotherapy prior to
surgery. The fresh specimens were immediately placed in liquid
nitrogen; 8–10 min later, they were packed into RNAfixer (Takara,
Dalian, China) and preserved at −80°C. The tumors were classified
according to the 7th edition of the American Joint Committee on
Cancer TNM staging guidelines for esophageal cancer. The study was
approved by the Ethics Committee of Taizhou People's Hospital
affiliated to Nantong University and the informed consent of the
patients was obtained.
RNA extraction and RT-qPCR
Total RNA was extracted from ESCC samples and ESCC
cell lines using the TRIzol reagent kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in accordance with the
manufacturer's protocol; The total RNA quality and concentration
was determined by UV spectrophotometry. Double-stranded
complementary DNA was synthesized by reverse transcription in
accordance with the cDNA synthesis kit; qPCR was performed using
the Script SYBR Green PCR kit (both Toyobo, Osaka, Japan). The
primer sequences of lncRNA-ECM were as follows: Forward,
5′-CAATATGTCCGTGGGACCCT-3′ and reverse, 5′-GGAGGCCAAACAACTGTGGA-3′.
The primer sequences for ICAM1 were as follows: Forward,
5′-TTGAGGGCACCTACCTCTGT-3′ and reverse, 5′-GATAGGTTCAGGGAGGCGTG-3′.
β-actin was used as reference (forward, 5′-CTGGGACGACATGGAGAAAA-3′
and reverse, 5′-AAGGAAGGCTGGAAGAGTGC-3′). Each experiment was
duplicated three times. Relative expression levels were calculated
using the 2−∆∆Ct method.
Microarray analysis (12)
LncRNA gene chip service was provided by Outdo
Biotech [Agilent Human lncRNA Microarray V2.0 (4×180K; design ID
062918; containing 46,506 lncRNAs); Agilent Technologies, Inc.,
Santa Clara, CA, USA]. The operating process of the gene chip was
as follows: First, RNA was prepared and double-stranded cDNA was
synthesized by reverse transcription; next, a fluorescently labeled
cRNA was synthesized with cyanine-3-CTP (Cy3). Then, chip
hybridization between the cRNA and the chip was conducted after
measuring their concentration and purity. After elution, the
original image was obtained by scanning through the Agilent Scanner
G2505C (Agilent Technologies, Inc.). The Feature Extraction
Software (version 10.7.1.1; Agilent Technologies, Inc.) was used to
process the original image and extract the data. The GeneSpring
software (version 12.5; Agilent Technologies, Inc.) was used to
analyze normalization and variance. Finally, all the differentially
expressed lncRNAs were screened out with the selection criteria of
fold change (FC)>2 and P<0.05.
Cell culture and transfection
The human ESCC cell lines TE-1, KYSE150, Eca109,
KYSE30, EC9706 and the normal human esophageal mucosal epithelial
HET-1A cell line were purchased from Shanghai Meixuan Biotechnology
(Shanghai, China) and cultured in RPMI-1640 (Corning Inc., Corning,
NY, USA) medium containing 10% fetal bovine serum (GE Healthcare
Life Sciences, Logan, UT, USA) in 5% CO2 at 37°C. In the
logarithmic growth phase, TE-1 and Eca109 cells were inoculated
into 6-well plates (4×105 cells/ml). When the cell healing degree
reached 90–95%, the plasmids of lncRNA-ECM silencing and
overexpression were transfected into the cells by Lipofectamine
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The lncRNA-ECM overexpression plasmids were synthesized
according to the NCBI reference sequence. PcDNA3.1 vectors were
used as null controls. After transfection for 48 h, the cells were
used for the experiments. The sequences of si-lncRNA-ECM were as
follows: si-lncRNA-ECM 1#, sense, 5′-CAGGAAAGCCAUACCAUGATT-3′ and
antisense, 5′-UCAUGGUAUGGCUUUCCUGTT-3′; si-lncRNA-ECM 2# sense,
5′-CCUAUAGAGCGACUGUCAATT-3′ and antisense,
5′-UUGACAGUCGCUCUAUAGGTT-3′; si-lncRNA-ECM 3# sense,
5′-CAGCAAACUCGUAGGUCAATT-3′ and antisense,
5′-UUGACCUACGAGUUUGCUGTT-3′ (Sangon Biotech, Shanghai, China).
Cell invasion and migration
assays
Transwell plates (Costar, New York, NY, USA) were
used for the Transwell assays. After 12 h of transfection, the
cells were inoculated into the 24-well Transwell plate (Corning
Inc.) at an inoculation volume of 4×103/well. The
membrane at the bottom of the upper chamber of the Transwell plate
was coated with 100 µl (~25 µg) diluted Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA) and dried at 4°C; 50 µl serum-free culture
medium was added to each well and cultured at 37°C for 30 min. To
digest and collect transfected cells, the cells were suspended in
serum-free medium at a density of 105/ml; 100 µl of the
cell suspension was added to the upper chamber of the 24-well
Transwell plate. Complete medium with 500 µl 20% FBS was added to
the lower chamber. The plates were incubated in an incubator with
5% CO2 at 37°C for 24 h, washed with PBS twice, the
upper layer of the cells was wiped with a cotton swab, fixed for 20
min in methanol, dyed for 10 min with 0.1% crystal violet solution,
and rinsed with distilled water until there was no stain under the
optical microscope. Finally, five random ×20 visual fields were
observed, and the number of tumor cells was counted. The migration
assay was performed in a similar manner, without the addition of
Matrigel. All the experiments were performed in triplicate.
Statistical analysis
Student's t-test was used to evaluate the
significance of the difference between the two groups of lncRNA-ECM
expression in ESCC at the tissue level and the cellular level.
One-way ANOVA was used to compare the means of the multiple groups
data. When the result is significant, the LSD post hoc test was
then used to compare the means between two pairs in this group. The
clinicopathological characteristics were analyzed by the
Chi-squared test or Wilcoxon's rank-sum test. The correlations were
assessed by Pearson's correlation analysis. Data are expressed as
mean ± standard deviation of three independent experiments.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed by SPSS 22.0
(IBM Corp., Armonk, NY, USA).
Results
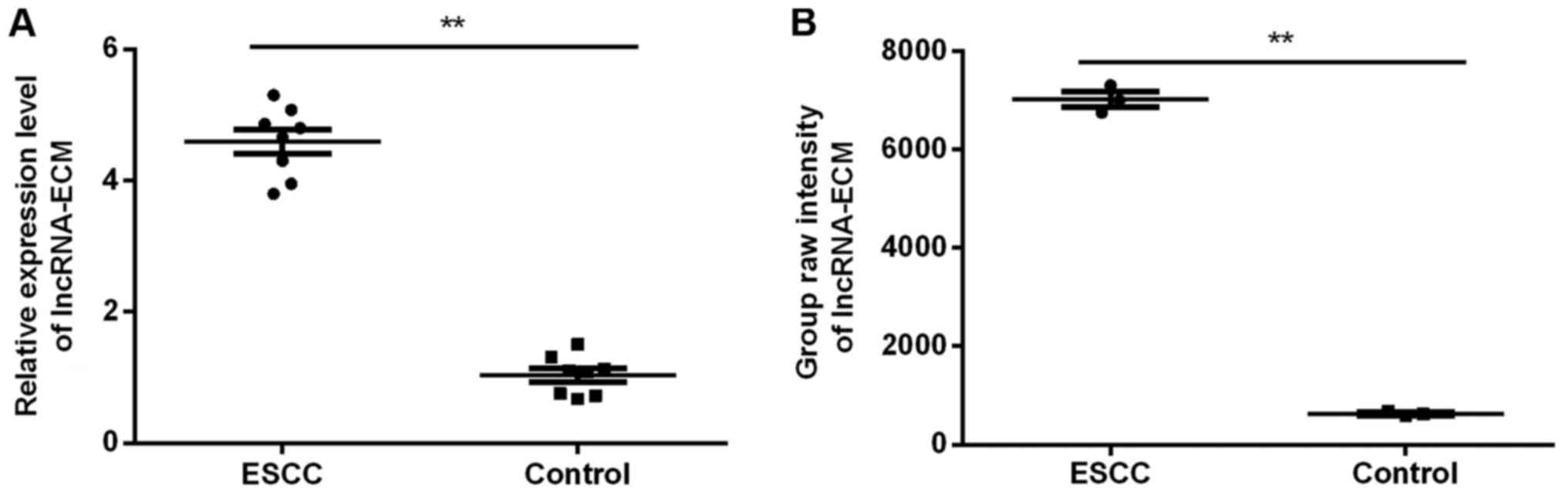
Microarray analysis
Among the 39 lncRNAs with significant differences
(FC>10) reported previously (12),
due to the significant between-group difference, non-significant
in-group difference (Fig. 1A) and
higher abundance compared with its origin (Fig. 1B) in the chip, lncRNA-ECM was
selected.
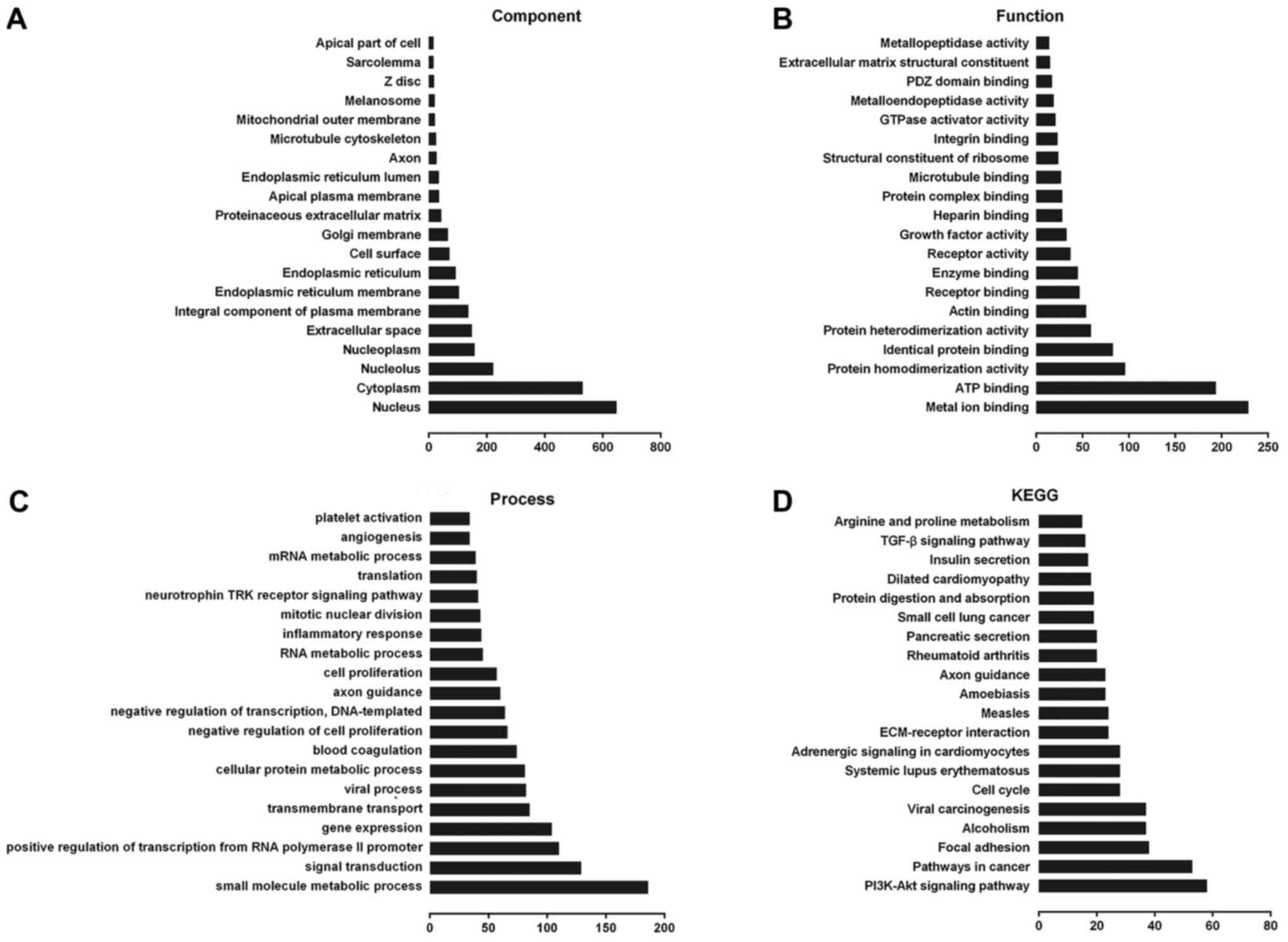
Potential functional analysis of
lncRNA-ECM
Using Gene Ontology (GO) and Kyoto Encyclopedia of
Genes and Genomes (KEGG) analysis, we found that lncRNA-ECM and its
potential target genes are closely associated with cell cycle
regulation, cell differentiation and other processes (Fig. 2A-C), including extracellular matrix
receptor interaction, cell cycle regulation, adhesive junctions,
and other multiple signaling pathways associated with cancer
(Fig. 2D), and it may also be
involved in the pathogenesis of ESCC.
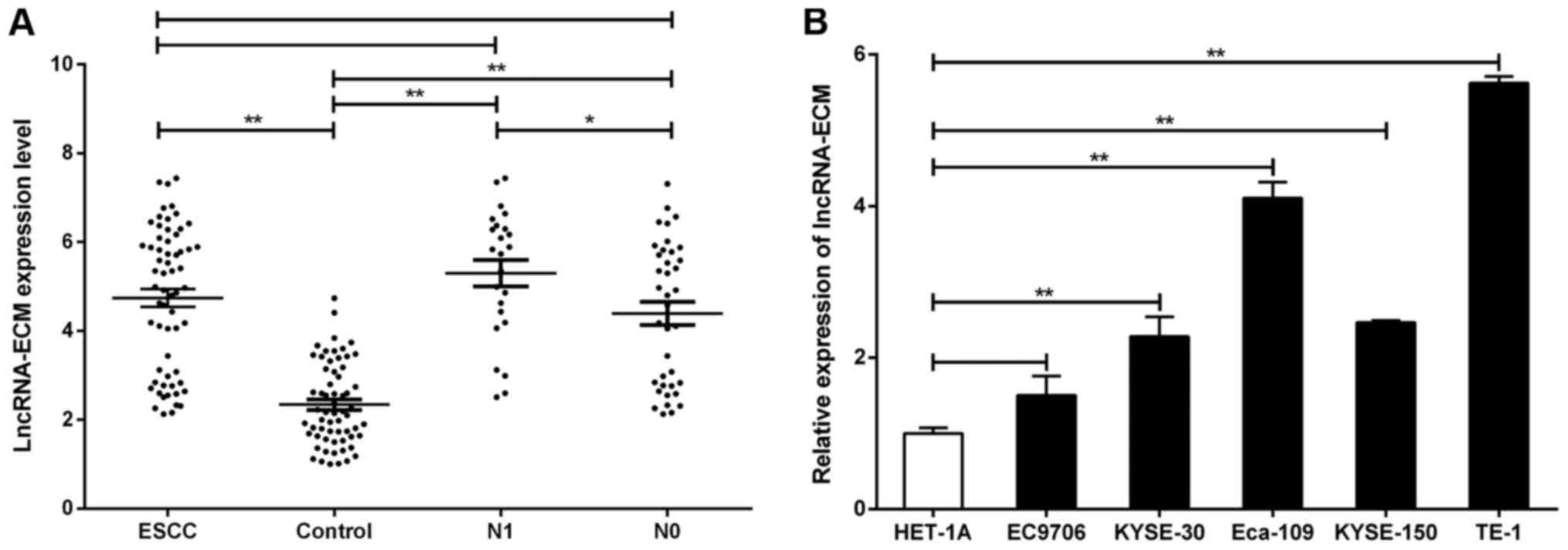
Expression of lncRNA-ECM in ESCC
tissues and association with clinicopathological parameters
To further investigate the biological behavior of
lncRNA-ECM in ESCC, we examined the expression of lncRNA-ECM in
cancer tissues from 62 ESCC patients and corresponding
paracancerous tissues by RT-PCR. The results demonstrated that the
expression of lncRNA-ECM in ESCC tissues was significantly higher
compared with that in adjacent tissues (Fig. 3A), which was in agreement with the
microarray results. The experiments on cultured ESCC cell lines
also yielded similar results: Compared with the normal human
esophageal mucosal epithelial HET-1A cell line, the expression of
lncRNA-ECM was significantly higher in all human ESCC cell lines
(TE-1, KYSE150, Eca109 and KYSE30; Fig.
3B). The clinicopathological characteristics of the 62 ESCC
cases are listed in Table I.
LncRNA-ECM was significantly associated with lymph node metastasis
(P=0.029; Table I and Fig. 3A) and TNM stage (P=0.036, Table I), but not with age, sex, tumor size,
pathological differentiation or T stage (all P>0.05; Table I). These findings indicate that
overexpression of lncRNA-ECM may be associated with ESCC
metastasis.
 | Table I.Association between long non-coding
RNA-ECM expression and the clinicopathological features of patients
with ESCC. |
Table I.
Association between long non-coding
RNA-ECM expression and the clinicopathological features of patients
with ESCC.
| Variable | Patients | Mean ± SD | P-value |
|---|
| Age (years) |
|
| 0.528 |
| ≥60 | 52 | 4.64±1.58 |
|
|
<60 | 10 | 5.26±1.68 |
|
| Sex |
|
| 0.744 |
| Male | 43 | 4.91±1.51 |
|
|
Female | 19 | 4.35±1.76 |
|
| Tumor size (cm) |
|
| 0.867 |
| ≤3 | 25 | 4.51±1.78 |
|
|
>3 | 37 | 4.89±1.47 |
|
| Pathological
differentiation grade |
|
| 0.609 |
| Well | 6 | 4.75±1.53 |
|
|
Moderately | 35 | 4.74±1.55 |
|
|
Poorly | 21 | 4.72±1.77 |
|
| T stage |
|
| 0.471 |
| T1-2 | 30 | 4.37±1.61 |
|
| T3-4 | 32 | 5.08±1.54 |
|
| N stage |
|
| 0.029 |
| N0 | 38 | 4.20±1.56 |
|
| N1 | 24 | 5.59±1.27 |
|
| TNM stage |
|
| 0.036 |
|
I–II | 43 | 4.23±1.54 |
|
|
III–IV | 19 | 5.87±1.07 |
|
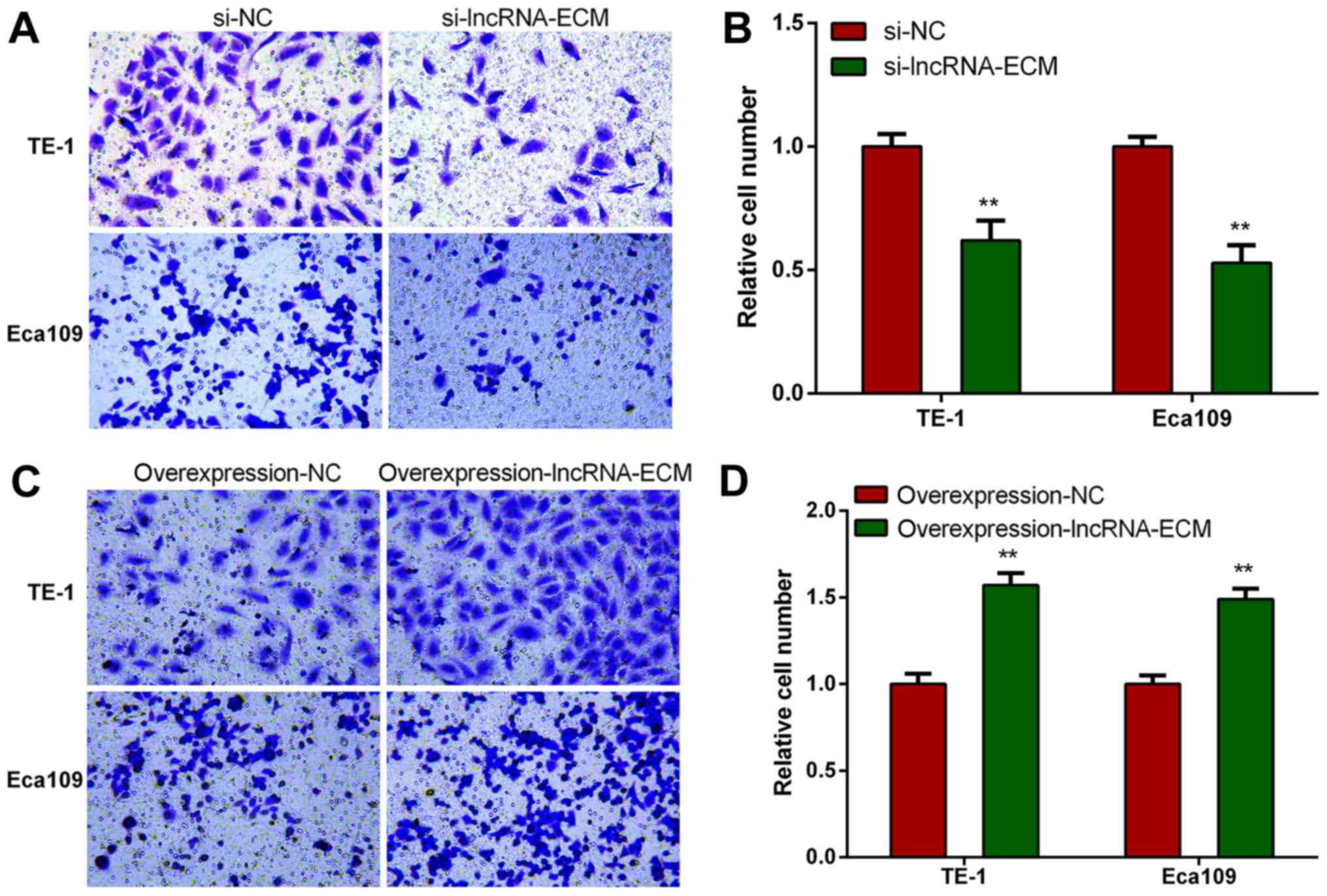
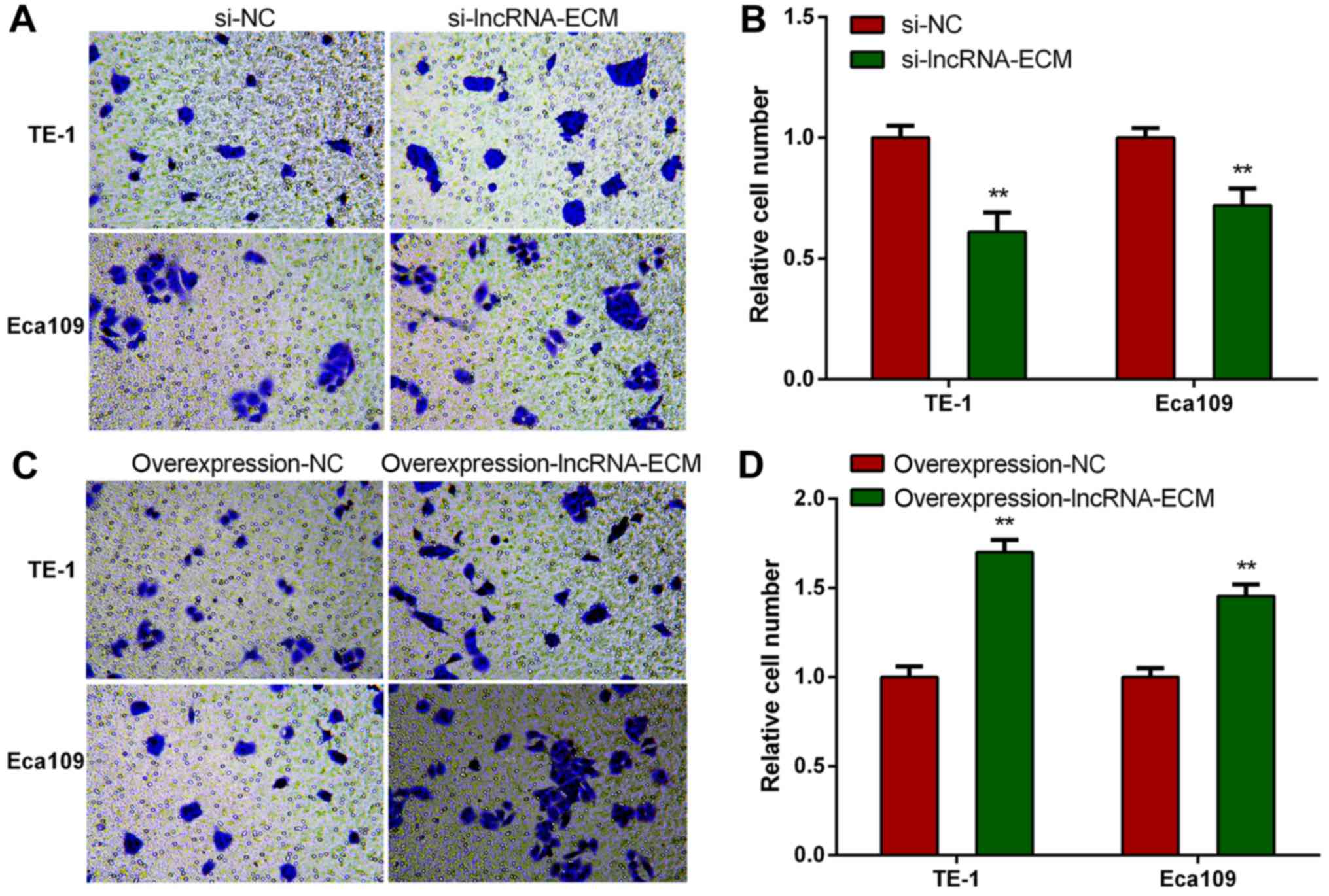
Knockdown of lncRNA-ECM decreases
invasion and migration of ESCC cells
To explore the function of lncRNA-ECM in ESCC
invasion and migration, we selected two highest cell lines of TE-1
and Eca109 to perform loss or gain of function assays. We
transfected the specific siRNA of lncRNA-ECM into TE-1 and Eca109
cell lines and utilized a classical Transwell assay to assess the
effect of lncRNA-ECM on the invasion and migration. The results
revealed that the invasion and migration of ESCC cell lines
decreased significantly after lncRNA-ECM silencing (Fig. 4). The result indicated that lncRNA-ECM
is involved in the regulation of ESCC cell invasion and
migration.
Overexpression of lncRNA-ECM increases
invasion and migration of ESCC cells
To elucidate whether lncRNA-ECM promotes ESCC cell
invasion and migration, lncRNA-ECM overexpression plasmids were
transfected into ESCC TE-1 and Eca109 cell lines, and the invasion
and migration ability of the cell lines was evaluated after
transfection. It was observed that the invasion and migration
ability of ESCC cells was markedly increased in cells
overexpressing lncRNA-ECM (Fig. 5).
This result confirmed that lncRNA-ECM plays an important role in
promoting ESCC cell migration.
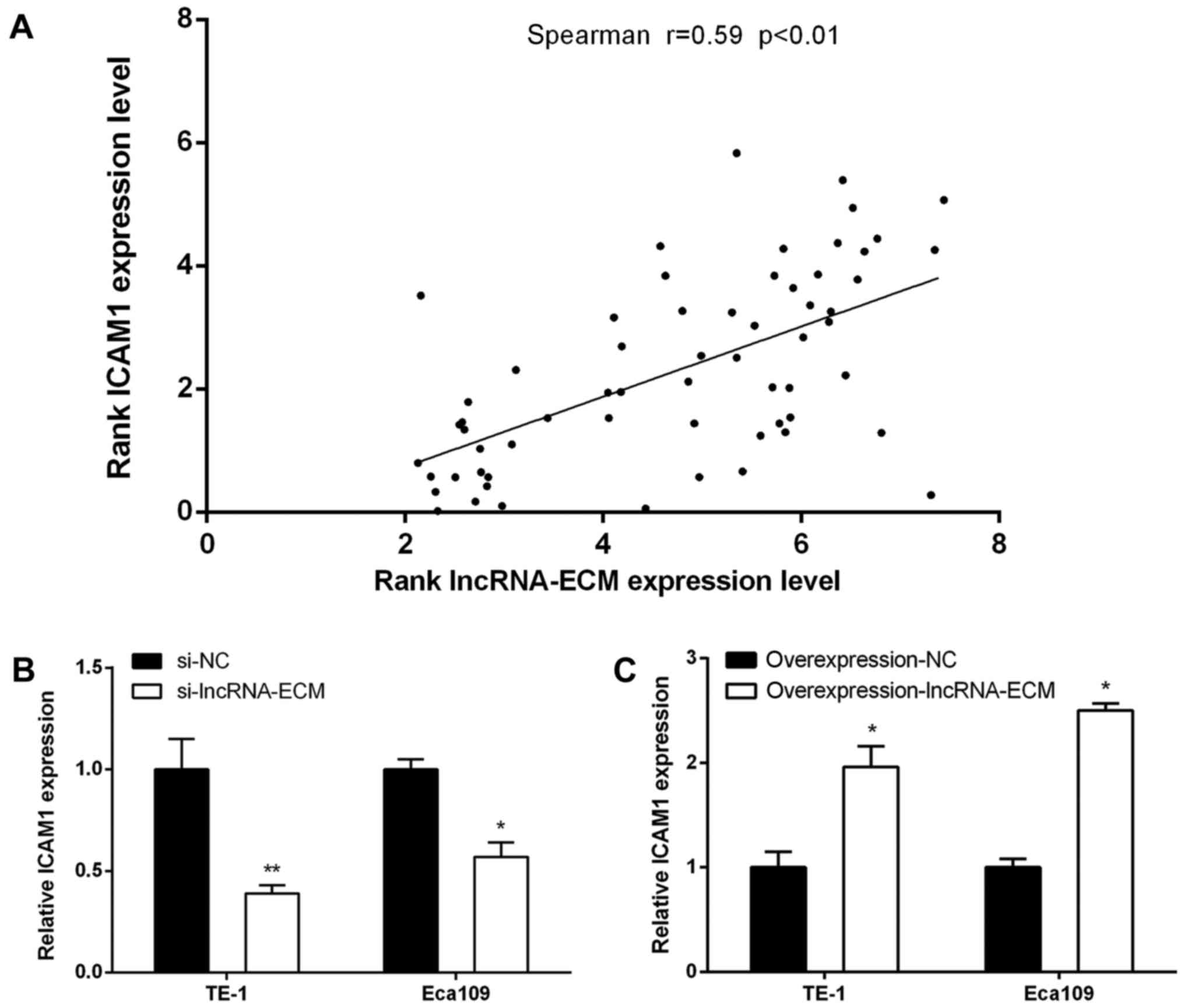
LncRNA-ECM may regulate ICAM1 in ESCC
cells
As genes with similar spatial location are often the
targets of lncRNA regulation (6), the
location of lncRNA-ECM and nucleic acid sequences was analyzed by
UCSC and BLAST, and we found that ICAM1 (intercellular cell
adhesion molecule-1) is adjacent to lncRNA-ECM. We detected the
expression level of ICAM1 in the 62 cases of ESCC using RT-qPCR in
the present study, and the results demonstrated that the expression
level of ICAM1 was positively correlated with lncRNA-ECM expression
(Fig. 6A; Pearson's correlation,
R=0.59, P<0.01). We further investigated whether the expression
of ICAM1 changed according to the level of lncRNA-ECM in ESCC
cells. It was observed that, after knocking down lncRNA-ECM, the
expression of ICAM1 also decreased, whereas the level of ICAM1
increased following transfection of lncRNA-ECM overexpression
plasmids (Fig. 6B and C), suggesting
that ICAM1 may be a downstream target of lncRNA-ECM.
Discussion
Despite a slight decrease in its incidence in recent
years, ESCC remains one of the most common malignant tumors in
China. Due to its high invasiveness and propensity for metastasis,
the prognosis of ESCC is not satisfactory. Over the last decades,
the association between biomolecules and cancer has attracted
extensive attention, and a number of studies on ESCC have been
performed (13,14). However, only few sensitive and
specific biomarkers for ESCC have been identified. Therefore,
active research on the development of effective molecular markers
will be beneficial in improving the diagnosis and prognosis of
esophageal cancer.
With the rapid development of genome microarray and
genome sequencing technology, a type of transcription without
protein encoding, originally considered to be non-RNA genome
encoding transcription ‘noise’, has become a research focus in
recent years (15). Considering the
abundance, variability, function and mechanism of lncRNAs, they may
be the regulatory core of RNA, and an increasing number of studies
have demonstrated that the differential expression of lncRNAs is
closely associated with tumorigenesis and tumor development
(7,8,16), which
provides a new basis for understanding the mechanism underlying
these processes. Since lncRNA AFAP1-AS1 was found to be highly
expressed in esophageal adenocarcinoma and silencing its expression
significantly inhibited the proliferation, invasion and metastasis
of esophageal adenocarcinoma cell lines (17), the research of lncRNAs and ESCC has
attracted the attention of many scholars, and a few types of
lncRNAs have been identified in ESCC. For example, lncRNA AFAP1-AS1
(9), HOTAIR (10) and POU3F3 (18) are highly expressed in ESCC, and their
high expression can promote the development of ESCC and adversely
affect the prognosis. Following knockdown of these lncRNAs, the
proliferation, invasion and migration ability of ESCC cells was
significantly inhibited. In addition, the expression of lncRNA-LET
(19), UCA1 (20) and LOC285194 (21) is low in ESCC tissues, and their
expression level is inversely associated with tumor size, TNM
stage, lymph node metastasis, distant metastasis, survival rate and
prognosis, whereas their overexpression can inhibit the migration
of ESCC cells.
LncRNA-ECM (lncRNA ENST00000589379) is a newly
discovered lncRNA, which exhibited high expression in the ESCC gene
chip (12). Sequence analysis
demonstrated that lncRNA-ECM was composed of 3,218 bp, and there
were a number of cis-regulatory elements, binding sites and
hypersensitive sites of DNase I in the region of the chromosome,
indicating that the transcription status of this region was
relatively active. There are abundant DNA hypermethylation regions
and epigenetic regulatory loci in this region, such as histone
H3K4me1, H3K4me3 and H3K36me3, among others. To some extent,
histone modification and DNA methylation play important roles in
the development and progression of ESCC (22,23),
suggesting the functional significance of lncRNA-ECM. In the
present study, we investigated the expression of lncRNA-ECM in ESCC
at the cellular and tissue level. Combining the lncRNA microarray
data and RT-PCR results, the expression of lncRNA-ECM in ESCC
tissues was found to be significantly higher compared with that in
the corresponding paracancerous tissues and nomal control cells,
indicating that lncRNA-ECM may be a biomarker for ESCC detection.
In addition, we found that the expression level of lncRNA-ECM in
cancers with lymph node metastasis was significantly higher. The
results demonstrated that the expression of lncRNA-ECM was
correlated with lymph node metastasis. We also investigated the
association between the expression of lncRNA-ECM and TNM stage, and
observed that a higher expression of lncRNA-ECM in patients with
ESCC was associated with a more advanced TNM stage. Therefore,
these findings suggest that lncRNA-ECM may be a potential marker
for ESCC prognosis. Subsequently, we transfected siRNA plamids with
lncRNA-ECM knockdown into ESCC TE-1 and Eca109 cells and found that
the invasion and migration ability of the two cell lines was
diminished; by contrast, after transfecting lncRNA-ECM
overexpression plasmids, the invasion and migration ability of ESCC
cells was enhanced, suggesting lncRNA-ECM plays an important role
in ESCC metastasis and may be a characteristic molecule for
diagnosing ESCC and predicting its prognosis.
LncRNA signaling is associated with integrin
pathways, extracellular pathways and local adhesion pathways.
ICAM1, a member of the immunoglobulin superfamily (IGSF), is an
important adhesion molecule (24)
involved in cell metastasis, differentiation and proliferation.
Recently, ICAM1 was reported to serve as a liver cancer and ESCC
stem cell marker (25,26); it can also promote ESCC
epithelial-to-mesenchymal transition (EMT) (27) and cause metastasis of ESCC cells by
regulating the expression of tumor metastasis-related genes, such
as P53 (26). We predicted the
possible role of ICAM1 as a target gene for lncRNA-ECM according to
bioinformatics analysis. The expression of ICAM1 in ESCC tissues
was detected by RT-PCR, and its expression level was found to be
positively correlated with the expression of lncRNA-ECM.
Furthermore, after knocking down lncRNA-ECM in ESCC cells, the
level of ICAM1 decreased, while the expression level of ICAM1
increased following lncRNA-ECM overexpression. These data indicate
that lncRNA-ECM plays a regulatory role in promoting lymph node
metastasis in ESCC, and this regulation may be mediated by
ICAM1.
In conclusion, we investigated the biological
behavior of lncRNA-ECM in ESCC and found it to be overexpressed in
ESCC tissues. It may be deduced that lncRNA-ECM plays an important
role in oncogenesis and progression of ESCC by regulating ICAM1,
and it may promote ESCC metastasis. Therefore, lncRNA-ECM may be a
new biomarker for diagnosing ESCC and predicting patient prognosis,
and it may also represent a novel molecular target for the
treatment of ESCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jiangsu
Provincial Medical Innovation Team (grant no. CXTDA2017042) of
Jiangsu Province, and the 333 Plan Foundation (grant no.
BRA2017173) of Jiangsu Province, China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY was responsible for writing the manuscript and
performing the lncRNA-ECM cell experiments. XS was responsible for
collecting the ESCC and matched para-cancerous tissues and
performing the RT-qPCR experiments. HL, JX and SS were responsible
for collecting the ESCC and matched para-cancerous tissues. JXH
wrote the paper and performed the bioinformatics analysis. ML
assisted with the experiments and revised the paper.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Taizhou People's Hospital affiliated to Nantong University and
written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
References
|
1
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bogoevski D, Onken F, Koenig A, Kaifi JT,
Schurr P, Sauter G, Izbicki JR and Yekebas EF: Is it time for a new
TNM classification in esophageal carcinoma? Ann Surg. 247:633–641.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kayani B, Zacharakis E, Ahmed K and Hanna
GB: Lymph node metastases and prognosis in esophageal carcinoma-a
systematic review. Eur J Surg Oncol. 37:747–753. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engreitz JM, Pandya-Jones A, McDonel P,
Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander
ES, et al: The Xist lncRNA exploits three-dimensional genome
architecture to spread across the X chromosome. Science.
341:12379732013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X,
Lin L, Yao H, Su F, Li D, et al: A cytoplasmic NF-κB interacting
long noncoding RNA blocks IκB phosphorylation and suppresses breast
cancer metastasis. Cancer Cell. 27:370–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xing Z, Lin A, Li C, Liang K, Wang S, Liu
Y, Park PK, Qin L, Wei Y, Hawke DH, et al: lncRNA directs
cooperative epigenetic regulation downstream of chemokine signals.
Cell. 159:1110–1125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou XL, Wang WW, Zhu WG, Yu CH, Tao GZ,
Wu QQ, Song YQ, Pan P and Tong YS: High expression of long
non-coding RNA AFAP1-AS1 predicts chemoradioresistance and poor
prognosis in patients with esophageal squamous cell carcinoma
treated with definitive chemoradiotherapy. Mol Carcinog.
55:2095–2105. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen FJ, Sun M, Li SQ, Wu QQ, Ji L, Liu
ZL, Zhou GZ, Cao G, Jin L, Xie HW, et al: Upregulation of the long
non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma
metastasis and poor prognosis. Mol Carcinog. 52:908–915. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song W and Zou SB: Prognostic role of
lncRNA HOTAIR in esophageal squamous cell carcinoma. Clin Chim
Acta. 463:169–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao J, Huang JX, Lin M, Wu ZD, Yu H, Wang
PC, Ye J, Chen P, Wu J and Zhao GJ: Microarray expression profile
analysis of aberrant long noncoding RNAs in esophageal squamous
cell carcinoma. Int J Oncol. 48:2543–2557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu M, Hu Y, Zhang MF, Luo KJ, Xie XY, Wen
J, Fu JH and Yang H: MMP1 promotes tumor growth and metastasis in
esophageal squamous cell carcinoma. Cancer Lett. 377:97–104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Lin C, Liang W, Wu S, Liu A, Wu J,
Zhang X, Ren P, Li M and Song L: TBL1×R1 promotes lymphangiogenesis
and lymphatic metastasis in esophageal squamous cell carcinoma.
Gut. 64:26–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Svoboda M, Slyskova J, Schneiderova M,
Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z,
Protivankova M, et al: HOTAIR long non-coding RNA is a negative
prognostic factor not only in primary tumors, but also in the blood
of colorectal cancer patients. Carcinogenesis. 35:1510–1515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu W, Bhagat TD, Yang X, Song JH, Cheng Y,
Agarwal R, Abraham JM, Ibrahim S, Bartenstein M, Hussain Z, et al:
Hypomethylation of noncoding DNA regions and overexpression of the
long noncoding RNA, AFAP1-AS1, in Barrett's esophagus and
esophageal adenocarcinoma. Gastroenterology. 144(956–966):
e42013.
|
|
18
|
Li W, Zheng J, Deng J, You Y, Wu H, Li N,
Lu J and Zhou Y: Increased levels of the long intergenic
non-protein coding RNA POU3F3 promote DNA methylation in esophageal
squamous cell carcinoma cells. Gastroenterology. 146(1714–1726):
e52014.
|
|
19
|
Wang PL, Liu B, Xia Y, Pan CF, Ma T and
Chen YJ: Long non-coding RNA-Low Expression in Tumor inhibits the
invasion and metastasis of esophageal squamous cell carcinoma by
regulating p53 expression. Mol Med Rep. 13:3074–3082. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Gao Z, Liao J, Shang M, Li X, Yin
L, Pu Y and Liu R: lncRNA UCA1 inhibits esophageal squamous-cell
carcinoma growth by regulating the Wnt signaling pathway. J Toxicol
Environ Health A. 79:407–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong YS, Zhou XL, Wang XW, Wu QQ, Yang TX,
Lv J, Yang JS, Zhu B and Cao XF: Association of decreased
expression of long non-coding RNA LOC285194 with chemoradiotherapy
resistance and poor prognosis in esophageal squamous cell
carcinoma. J Transl Med. 12:2332014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu C, Liu M, Zhang W, Xu Q, Ma K, Chen L,
Wang Z, He S, Zhu H and Xu N: Upregulation of KLF4 by
methylseleninic acid in human esophageal squamous cell carcinoma
cells: Modification of histone H3 acetylation through HAT/HDAC
interplay. Mol Carcinog. 54:1051–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kishino T, Niwa T, Yamashita S, Takahashi
T, Nakazato H, Nakajima T, Igaki H, Tachimori Y, Suzuki Y and
Ushijima T: Integrated analysis of DNA methylation and mutations in
esophageal squamous cell carcinoma. Mol Carcinog. 55:2077–2088.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kluger HM, Hoyt K, Bacchiocchi A, Mayer T,
Kirsch J, Kluger Y, Sznol M, Ariyan S, Molinaro A and Halaban R:
Plasma markers for identifying patients with metastatic melanoma.
Clin Cancer Res. 17:2417–2425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J,
Wang J, Zhang D, Cheng S and Liu S: Expression of intercellular
adhesion molecule 1 by hepatocellular carcinoma stem cells and
circulating tumor cells. Gastroenterology. 144(1031–1041): e102013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai ST, Wang PJ, Liou NJ, Lin PS, Chen CH
and Chang WC: ICAM1 is a potential cancer stem cell marker of
esophageal squamous cell carcinoma. PLoS One. 10:e01428342015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|