Introduction
The frequency of brain metastases (BM) from
non-small cell lung cancer (NSCLC) exceeds 20% at the time of
diagnosis of the primary tumor, and a significant proportion of
patients develop BM over the course of disease (1,2). In recent
years, the incidence of BM in patients with NSCLC has increased,
due to improved survival rates resulting from the
molecular-targeting therapy and improvements in diagnostic
neuroimaging modalities, including magnetic resonance imaging and
positron emission tomography (3).
Adenocarcinoma is the most common subtype of NSCLC, occurring in
~40% of all patients, and is characterized by rapid progression,
early distant metastasis and a significantly higher incidence of BM
compared with other subtypes of NSCLC (4–6). BM are
considered to be a poor prognostic factor in NSCLC, and are
associated with median overall survival (OS) times of 4–7 weeks in
untreated patients (6–8). In patients with BM, recursive
partitioning analysis (RPA) and the graded prognostic assessment
(GPA) score are still the most widely accepted models for
evaluation of disease prognosis (6,9).
In lung adenocarcinoma, mutations in the epidermal
growth factor receptor (EGFR) gene have been identified as a
driver mutation promoting tumor growth (10–12).
Therapies targeting mutant EGFR protein are associated with
relatively high overall response rates, and have been established
as standard-of-care treatments (10–12).
Furthermore, EGFR-targeted therapies have demonstrated efficacy in
the treatment of BM from NSCLC (13–15).
Histological and genetic evaluation is essential in
identifying patients with NSCLC that may benefit from EGFR-targeted
therapies. In cases in which tissue sampling from the primary tumor
is difficult, tissue samples from BM lesions may provide a useful
alternative for evaluation. In the majority of previous reports,
histological analysis and genetic evaluation in patients with NSCLC
have been performed using tissue from the primary tumor only
(10–15). There are a few reports describing a
histopathological and genetic evaluation using tissue samples from
BM and the primary lung adenocarcinoma together (16,17). Given
evidence that the presence of EGFR mutations is a positive
prognostic factor in patients with BM from lung adenocarcinoma
(13–15), it is feasible that analysis of tissue
from BM may be used to guide more aggressive and effective
treatments for patients with EGFR mutation-positive
NSCLC.
In the present study, the clinical features and
histopathological subtypes of BM from lung adenocarcinoma in a
patient population from a single institution were retrospectively
evaluated in order to identify prognostic factors. In addition, an
evaluation of EGFR mutations and their association with
disease prognosis was assessed. The results of the present study
suggest that the analysis of tissue from BM may inform the
treatment of patients with metastatic brain lesions arising from
lung adenocarcinoma.
Materials and methods
Study design and patients
A total of 232 BM samples in the histopathological
files of the Department of Pathology that had been surgically
resected at the Department of Neurosurgery, Fukuoka University
Hospital (Fukuoka, Japan), between January 1985 and December 2014
were reviewed. Tumor resection was performed to improve
neurological symptoms and quality of life, and aimed to prolong the
survival time of patients. Patients in which BM were resected
included those in which: i) A single large (>3 cm) or
symptomatic tumor was present; ii) the primary tumor was
well-controlled; iii) life expectancy exceeded 3 months; and iv)
written informed consent from the patient or family was obtained. A
total of 68 patients with BM from lung adenocarcinoma were
retrospectively analyzed. Analysis data from eight patients lacking
clinical data and specimens, and from one patient in which
polymerase chain reaction (PCR) amplification of EGFR failed
were excluded. Data from a total of 59 patients were included in
the present study. The current study was reviewed and approved by
the Ethics Committee of the Fukuoka University Hospital (approval
no. 15-5-14).
Pathologic evaluation
Surgically-resected specimens were fixed in 10%
formalin (room temperature for 24 h) and embedded in paraffin
blocks. Tissue sections were cut at a thickness of 4 µm, and
stained with hematoxylin and eosin for histopathological
examination. Tissue sections were stained with hematoxylin for 10
min, and with eosin 3 min at room temperature. Histological
subtyping was performed according to the 2015 World Health
Organization criteria for classification of lung tumors (18). The clinicopathological parameters
considered in this study included patient age, sex, location of BM,
histological subtype of BM, smoking status, Karnofsky performance
score (KPS), number of BM, presence of extracranial metastasis
(ECM), GPA, timing of diagnosis, tyrosine kinase inhibitor (TKI)
treatment prior to diagnosis of BM, presence of RAS
mutations or anaplastic lymphoma kinase (ALK) rearrangements
and re-operation.
Analysis of EGFR and KRAS mutations
and ALK rearrangement
EGFR and RAS gene mutations were
analyzed in paraffin-embedded tissue sections from BM only.
EGFR mutations (including G719X in exon 18, exon 19
deletion, T790M in exon 20, and L858R and L861Q in exon 21) and
RAS mutations (G12C and G12A in exon 2 and Q61H in exon 3)
were detected in matching formalin-fixed paraffin-embedded tissue
samples using cycleave PCR (which was performed by SRL Inc., Tokyo,
Japan, cobas® EGFR Mutation Test v2 kit and
MEBGEN™ RASKET kit according to the manufacture's
protocol). ALK rearrangement was analyzed by
immunohistochemistry (ALK Detection kit; Nichirei Bioscience,
Tokyo, Japan), according to the manufacturer's protocols. The
slides were observed under ×200 magnification using a BX50
fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
To identify clinical features associated with
EGFR mutations, categorical variables were compared using
Wilcoxon signed-rank test or Fisher's exact test. OS was defined as
duration between the date of BM diagnosis and the date of mortality
or last follow-up. The Kaplan-Meier estimator method was used for
survival curve analysis, and differences between survival curves
were analyzed using the log-rank test. Multivariate analysis with
the Cox proportional hazard model was used to determine independent
prognostic factors. P<0.05 were considered to indicate a
statistically significant difference. Statistical analysis was
performed with SPSS software (version 21.0; IBM Corp., Armonk, NY,
USA).
Results
Patient characteristics
Patient characteristics are summarized in Table I. A total of 59 patients with BM from
lung adenocarcinoma were included in the present study. The median
patient age was 61.3±12.2 years old (range, 38–87 years). A total
of 45 patients were male (76.3%) and 14 patients were female
(23.7%). A total of 14 patients (23.7%) exhibited EGFR
mutations (Table II), of which
deletion of exon 19 was the most common genetic alteration (eight
patients, 57.1%), followed by L858R mutation in exon 21 (five
patients, 35.7%). In addition, one patient (7.1%) had a L861Q
mutation in exon 21. EGFR mutations occurred significantly
more often in female compared with male patients (64.3 vs. 35.7%;
P=0.0001). EGFR mutations also occurred significantly more
frequently in non-smokers compared with current or ex-smokers (57.1
vs. 42.9%; P=0.04). BM within the frontal lobe were significantly
more common compared with other locations in patients with
EGFR mutations [64.3 (frontal lobe) vs. 21.4 (cerebellum)
vs. 7.1% (posterior lobe); P=0.03]. The rate of surgery for tumor
recurrence occurred significantly more often in patients with
wild-type compared with mutated EGFR (37.8 vs. 7.1%;
P=0.04). The two groups did not significantly differ in GPA
measures of age, KPS, number of BM, presence of ECMs, timing of
diagnosis, and frequency of RAS mutation and ALK
rearrangements.
 | Table I.Patient baseline characteristics
(n=59). |
Table I.
Patient baseline characteristics
(n=59).
| Characteristic | Total (n=59) | EGFR MT (n=14) | WT (n=45) | P-value MT vs.
WT |
|---|
| Age,
yearsa | 61.3±12.2 | 61.6±12.0 | 61.1±12.4 | 0.80 |
| Sex (%) |
|
|
| 0.0001 |
|
Male | 45 (76.3) | 5 (35.7) | 40 (88.9) |
|
|
Female | 14 (23.7) | 9 (64.3) | 5 (11.1) |
|
| Location (%) |
|
|
|
|
| Frontal
lobe | 23 (39.0) | 9 (64.3) | 14 (31.1) | 0.03 |
|
Cerebellum | 11 (18.6) | 3 (21.4) | 8 (17.8) | 0.76 |
|
Posterior lobe | 8 (15.6) | 1 (7.1) | 7 (15.6) | 0.67 |
| Histological
subtype (%) |
|
Solid | 34 (57.6) | 4 (28.6) | 30 (66.7) | 0.03 |
|
Papillary | 13 (22.0) | 5 (35.7) | 8 (17.7) | 0.26 |
|
Acinar | 11 (18.6) | 4 (28.6) | 7 (15.6) | 0.43 |
| Smoking status
(%) |
|
|
| 0.04 |
|
Never | 19 (32.2) | 8 (57.1) | 11 (24.4) |
|
| Current
or past | 40 (67.8) | 6 (42.9) | 34 (75.6) |
|
| KPS (%) |
|
|
|
|
|
<70 | 19 (32.2) | 6 (42.9) | 13 (28.9) | 0.51 |
|
70–80 | 15 (25.4) | 5 (35.7) | 10 (22.2) | 0.48 |
|
81–100 | 25 (42.4) | 3 (21.4) | 22 (48.9) | 0.12 |
| Number of BM
(%) |
| 1 | 31 (52.5) | 5 (35.7) | 26 (45.8) | 0.22 |
|
2–3 | 17 (28.8) | 5 (35.7) | 12 (26.7) | 0.74 |
|
>3 | 11 (18.6) | 4 (28.6) | 7 (15.6) | 0.43 |
| ECM (%) |
|
|
| 0.80 |
|
Absent | 27 (45.8) | 6 (42.9) | 21 (46.7) |
|
|
Present | 32 (54.2) | 8 (57.1) | 24 (53.3) |
|
| GPA (%) |
|
0–1.0 | 13 (22.0) | 4 (28.6) | 9 (20.0) | 0.71 |
|
1.5–2.0 | 24 (40.7) | 7 (50.0) | 17 (37.8) | 0.54 |
|
2.5–3.0 | 18 (30.5) | 3 (21.4) | 15 (33.3) | 0.52 |
|
3.5–4.0 | 4 (6.8) | 0 (.0) | 4 (8.9) | 0.56 |
| Timing of
diagnosis |
| BM
diagnosed before | 27 (45.8) | 5 (35.7) | 22 (48.8) | 0.54 |
| PT (%) |
| TKI (prior to BM)
(%) | 2 (3.4) | 2 (14.3) | 0 (.0) | 0.05 |
| RAS (%) | 5 (8.5) | 0 (.0) | 5 (11.1) | 0.16 |
| ALK (%) | 3 (5.0) | 0 (.0) | 3 (6.7) | 0.57 |
| Re-operation
(%) | 18 (30.5) | 1 (7.1) | 17 (37.8) | 0.04 |
 | Table II.EGFR mutation analysis with brain
metastasis from lung adenocarcinoma (n=59). |
Table II.
EGFR mutation analysis with brain
metastasis from lung adenocarcinoma (n=59).
| EGFR mutation | No. of patients
(%) |
|---|
| Exon 19
deletion | 8 (57.1) |
| Exon 21 L858R | 5 (35.7) |
| Exon 21 L861Q | 1 (7.1) |
| Exon 18 G719X | 0 (0.0) |
| Exon 20 T790M | 0 (0.0) |
All patients received craniotomy followed by total
resection of the BM. In 32 patients (54.2%), ECMs were detected at
the time of BM diagnosis. The sites at which ECMs occurred in the
patients with EGFR mutation were similar to those in which
ECMs occurred in patients with WT. In patients with EGFR
mutations, six patients had ECMs (one patient had multiple
lesions). Of these ECMs, six occurred in the lymph nodes (100%),
one occurred in bone (16.7%), and one occurred in liver (16.7%). In
patients with WT, 21 patients had ECMs (4 patients had multiple
lesions). Of these lesions, 15 occurred in the lymph nodes (71.4%),
six occurred in bone (28.6%), two occurred in liver (9.5%) and two
occurred in the adrenal glands (9.5%). There was no statistically
significant difference in the sites at which ECMs occurred in
comparing patients with these two conditions. Regarding the number
of BM present, one in 31 patients (52.5%), two in 13 patients
(22.0%), three in four patients (6.8%), four in two patients (3.4%)
and ≥five in nine patients (15.3%). Following surgical resection of
BM, the majority of patients received local therapy (38 patients,
64.4%), consisting of whole brain radiation therapy (WBRT; 24
patients, 40.7%) or stereotactic radiosurgery (SRS; 14 patients,
23.7%). Following local therapy, 18 patients (30.5%) received
systemic chemotherapy. A total of five patients (8.5%) received
systemic chemotherapy only, while two patients (3.4%) with
EGFR mutations were treated with the EGFR-TKI gefitinib
prior to the diagnosis of BM. Patients with poor performance status
and patients that declined treatment (14 patients, 23.7%) were
provided with best supportive care.
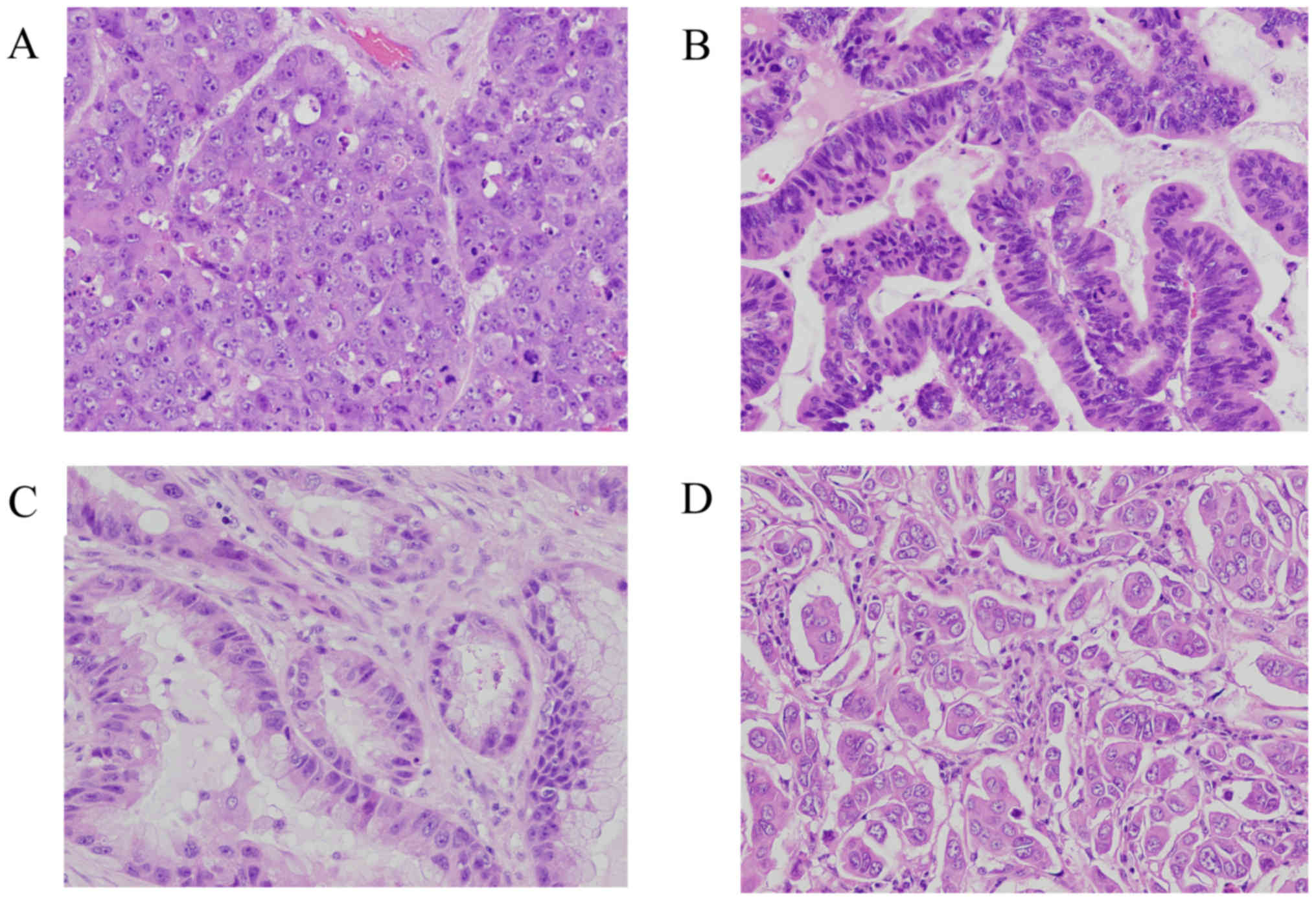
Pathological subtypes
The predominant subtypes of BM associated with lung
adenocarcinoma were solid adenocarcinoma (34 patients, 57.6%),
followed by papillary adenocarcinoma (13 patients, 22.0%), acinar
adenocarcinoma (11 patients, 18.6%) and micropapillary
adenocarcinoma (one patient, 1.8%) (Table
I). EGFR mutations occurred most commonly in papillary
adenocarcinoma (five patients, 35.7%), followed by acinar
adenocarcinoma (four patients, 26.7%), solid adenocarcinoma (four
patients, 26.7%) and micropapillary adenocarcinoma (one patient,
7.1%). No significant association was identified between the
occurrence of an EGFR mutation and histological subtype.
However, the wild-type EGFR gene was significantly more
common in patients with solid adenocarcinoma compared with other
histological subtypes. Fig. 1 shows
representative histological subtypes of BM from lung
adenocarcinoma.
Tissue from the primary lung adenocarcinoma together
with BM were available for 20 patients. Characteristics of primary
lung adenocarcinoma and BM are summarized in Table III. The most common histological
subtype in patients with primary lung adenocarcinoma who also had
BM was solid adenocarcinoma (10 patients, 50.0%), followed by
acinar adenocarcinoma (seven patients, 35.0%), and papillary
adenocarcinoma (three patients, 15.0%). Histopathological subtypes
of lung adenocarcinoma and BM matched each other in 75% of all of
cases.
 | Table III.Pathological subtype of primary lung
adenocarcinoma and metastatic brain tumor (n=20). |
Table III.
Pathological subtype of primary lung
adenocarcinoma and metastatic brain tumor (n=20).
| No. | Stage | Size (mm) | Primary | BM |
|---|
| 1 | T1aN0Mx | 11×10×15 | Solid (solid +
acinar) | Solid |
| 2 | T1bN0M0 | 20×15×20 | Solid (solid +
acinar) | Acinar |
| 3 | T2aNxMx | 40×30×25 | Acinar | Acinar |
| 4 | T1cN0M0 | 28×26×24 | Acinar (acinar +
solid) | Acinar |
| 5 | T1bN0Mx | 20×15×15 | Acinar (acinar +
papillary) | Papillary |
| 6 | T1bNxMx | 20×10×8 | Solid | Solid |
| 7 | T1bNxMx | 20×8×8 | Solid (acinar +
papillary + MPP) | Solid |
| 8 | T2aNxMx | 30×38×35 | Solid (solid +
acinar + papillary) | Acinar |
| 9 | T4N2M1 | 40×35×35 | Acinar (acinar +
MPP) | Solid |
| 10 | T1cN0Mx | 30×25×20 | Solid (solid +
acinar) | Solid |
| 11 | T2NxM1 | 15×10×10 | Solid (solid +
acinar + MPP) | Solid |
| 12 | T3N0M1 | 40×35×35 | Solid (solid +
acinar) | Solid |
| 13 | T4N0Mx | 70×70×55 | MPP (MPP + acinar +
solid) | Solid |
| 14 | T1bN0Mx | 16×15×15 | Solid (solid +
acinar) | Papillary |
| 15 | T2aN2M0 | 18×17×24 | MPP (MPP + acinar +
papillary) | Solid |
| 16 | T4N2Mx | 85×60×50 | Solid (solid + MPP
+ acinar) | Solid |
| 17 | T1cNxMx | 22×18×10 | Acinar (acinar +
MPP + papillary) | MPP |
| 18 | T2bN2Mx | 50×50×30 | Acinar (acinar +
solid + papillary) | Solid |
| 19 | T1cNxMx | 30×20×27 | Acinar (acinar +
papillary) | Papillary |
| 20 | T2aNxMx | 35×40×25 | MPP (MPP +
acinar) | Papillary |
Patient survival
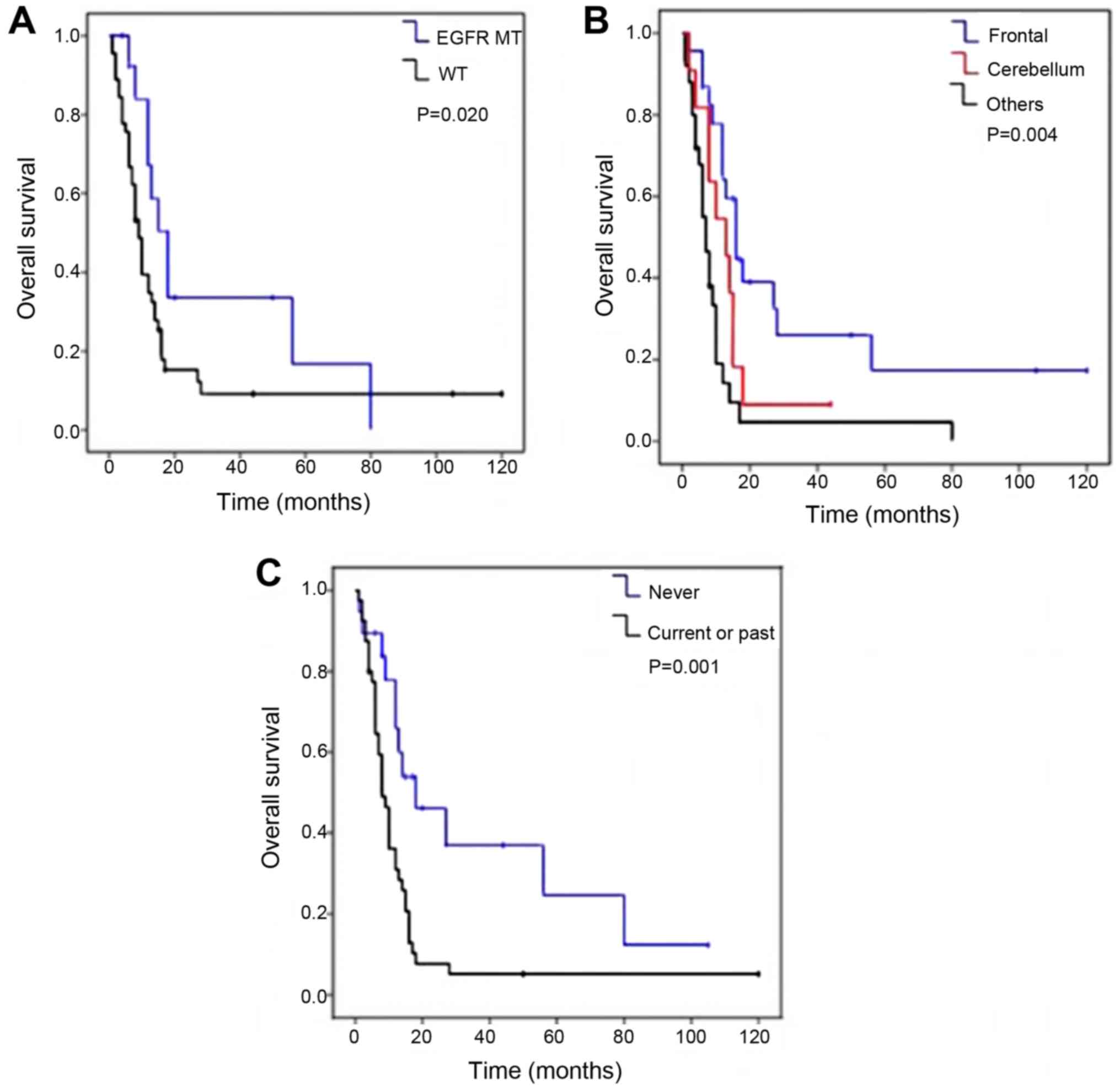
After a mean follow-up duration of 17.9 months,
39/59 patients (66.1%) had succumbed, 10 patients (16.9%) survived
and 10 patients (16.9%) were lost to follow-up. The median OS was
19.2 months. Table IV presents the
results of the OS univariate analysis. The OS time was
significantly increased in patients with EGFR mutations
(median OS of 31.5 months vs. 15.6 months; P=0.02; Fig. 2A). No significant difference was
observed in OS in patients with exon 19 deletion compared with
those with L858R mutations in exon 21 (P=0.31, data not shown). In
addition, the OS time was significantly increased in patients with
BM in the frontal lobe compared with patients with BM the
cerebellum or in other regions [median OS, 28.2 (BM in the frontal
lobe) vs. 13.7 (BM in the cerebellum) vs. 12.7 months (BM in other
brain regions); P=0.004] (Fig. 2B)
and in non-smokers (33.1 months in non-smokers vs. 12.5 months in
current or ex-smokers; P=0.001; Fig.
2C).
 | Table IV.Univariate analysis of overall
survival with brain metastasis from lung adenocarcinoma. |
Table IV.
Univariate analysis of overall
survival with brain metastasis from lung adenocarcinoma.
| Variable | No. of
patients | Median OS (m) | P-value |
|---|
| Age |
|
| 0.729 |
|
<70 | 45 | 22.7
(11.8–33.7) |
|
|
≥70 | 14 | 19.4
(9.1–29.7) |
|
| Sex |
|
| 0.131 |
|
Male | 45 | 16.9
(11.4–22.4) |
|
|
Female | 14 | 27.8
(15.7–39.9) |
|
| Location |
|
| 0.004 |
|
Frontal | 23 | 28.2
(19.0–37.5) |
|
|
Cerebellum | 11 | 13.7
(7.4–20.0) |
|
|
Others | 25 | 12.7
(5.9–19.5) |
|
| Smoking |
|
| 0.001 |
|
Never | 19 | 33.1
(22.1–33.1) |
|
| Current
or past | 40 | 12.5
(8.3–16.7) |
|
| Histological
subtype |
|
| 0.236 |
|
Solid | 34 | 17.4
(11.4–23.5) |
|
|
Papillary | 13 | 26.9
(13.0–40.8) |
|
|
Aciner | 11 | 11.2
(5.9–16.6) |
|
| EGFR |
|
| 0.020 |
|
Mutation | 14 | 31.5
(19.3–43.8) |
|
| Wild
type | 45 | 15.6
(10.3–20.8) |
|
| KPS |
|
| 0.551 |
|
<70 | 19 | 18.2
(8.8–27.6) |
|
|
70–80 | 15 | 17.1
(8.3–25.9) |
|
|
81–100 | 25 | 21.9
(13.4–30.4) |
|
| GPA |
|
| 0.180 |
|
0–1.0 | 13 | 22.8
(10.0–35.6) |
|
|
1.5–2.0 | 24 | 12.8
(7.8–17.8) |
|
|
2.5–3.0 | 18 | 20.2
(11.5–28.8) |
|
|
3.5–4.0 | 4 | 37.0
(12.7–61.2) |
|
| Timing of
diagnosis |
|
| 0.079 |
| BM diagnosed before
PT |
|
Yes | 27 | 12.5
(8.3–16.9) |
|
| No | 32 | 23.4
(15.6–31.3) |
|
| RAS |
|
| 0.074 |
|
Mutation | 5 | 7.8 (4.7–10.8) |
|
| Wild
type | 54 | 23.7
(13.8–33.5) |
|
| Re-operation |
|
| 0.712 |
|
Present | 18 | 17.7
(8.8–26.7) |
|
|
Absent | 41 | 19.7
(13.3–26.1) |
|
Patients diagnosed with BM following initial
diagnosis of the primary lung adenocarcinoma primarily had a longer
OS, compared with patients with an initial diagnosis of BM prior to
lung cancer (median OS, 23.4 vs. 12.5 months; P=0.079), but this
did not reach statistical significance. Patient age, histological
subtype of BM, KPS and re-operation were not identified to be
associated with OS. Statistical analysis of patients with
ALK rearrangement was not feasible as only three patients
with this genetic change were present in the patient
population.
Multivariate analysis suggested that BM located in
the frontal lobe and a non-smoker status were independent and
significant prognostic factors (P=0.007 and P=0.008, respectively;
Table V).
 | Table V.Multivariate analysis of overall
survival with brain metastasis of lung adenocarcinoma. |
Table V.
Multivariate analysis of overall
survival with brain metastasis of lung adenocarcinoma.
| Variable | HR | 95% CI | P-value |
|---|
| Location,
frontal | 0.552 | 0.215–0.781 | 0.007 |
| Never smoking | 2.654 | 1.295–5.442 | 0.008 |
| EGFR mutation | 0.552 | 0.260–1.171 | 0.121 |
Discussion
In the present study, the clinical and histological
features of patients that developed BM from lung adenocarcinoma
were evaluated in order to identify prognostic factors for such
patients. Previous studies have investigated the incidence of
EGFR mutations in a large population of patients with lung
cancer (19,20). EGFR mutations have been
detected in 30% of patients with adenocarcinoma, 38% of female
patients, 47% of non-smokers and 32% of patients of East Asian
origin in the patients with lung adenocarcinoma (19,21). It
has also been reported to be easier to detect EGFR mutations
in smaller primary tumors (22).
Deletion of exon 19 and L858R mutations in exon 21 account for ~85%
of EGFR mutations and have been recognized as important
markers in determining the appropriate treatment strategy for
patients with advanced lung cancer (21). BM from lung cancer were detected at
the time of diagnosis of the primary tumor in 8–23% of patients
with EGFR mutations, and in 24% of patients with NSCLC
during follow-up care (23,24). In patients with NSCLC in which
EGFR mutations were present, 34.2% were identified to have
developed BM 1 year after diagnosis, 38.4% at 2 years, 46.7% at 3
years, 48.7% at 4 years and 52.9% at 5 years in the USA (25).
At the time of the initial diagnosis of primary lung
adenocarcinoma, the incidence of EGFR mutation is reported
to vary according to the site of BM (24,26).
Patients with lung adenocarcinoma with EGFR mutations have
been suggested to have a higher incidence rate of BM compared with
patients without mutations (24,26).
However, another study reported that the timeline of development of
BM was not significantly longer in EGFR-mutated NSCLC
compared with wild-type EGFR NSCLC (27). Therefore, the association between the
presence of EGFR mutations and BM is controversial. Multiple
BM (≥2) have been reported to occur more frequently in patients
with EGFR mutations, but the size of the largest BM does not
appear to be associated with the presence of EGFR mutations
(28).
Several studies have compared EGFR status in
the primary tumor and BM in patients with NSCLC (26,29). These
results suggested high concordance rates in EGFR mutation
status (93.3–100%) between the primary tumor and BM (26,29). In
the current study, 5/20 patients examined had EGFR mutations
in the primary lung adenocarcinoma and BM. Five patients had the
same EGFR mutation in both sites (three patients had exon 19
deletions and one patient each had had L858R and L861Q
substitutions in exon 21). In the present study, the frequency of
EGFR mutations in patients with BM (23.7%) was lower
compared with previously reported (19,21). This
discrepancy may be due to the characteristics of the patient
population in the current study. EGFR mutations in lung
adenocarcinoma are most common in female patients and non-smokers,
but the present study cohort included predominantly male patients
(76.3% of all patients).
The International Association for the Study of Lung
Cancer/American Thoracic Society/European Respiratory Society
criteria for lung adenocarcinoma classification (30), and the 2015 WHO criteria lung tumor
classification (18) recommend the
identification of pathological patterns. Primary invasive lung
adenocarcinoma has five architectural growth patterns, including
lepidic, acinar, papillary, solid and micropapillary patterns.
Tsuta et al (31) reported
that the most prevalent subtype of adenocarcinoma was
papillary-predominant (37.1%), followed by lepidic-predominant
(15.1%), solid with mucin production-predominant (13.7%),
acinar-predominant (10.8%) and micropapillary-predominant (6.7%) in
a study of 757 cases of invasive lung adenocarcinoma (31). In a previous study of 119 patients
with primary lung adenocarcinoma, the most common histopathological
subtype reported was papillary-predominant (58%), followed by
acinar-predominant (16%), micropapillary-predominant (9%),
solid-predominant (4%) and lepidic-predominant (2%) (32). EGFR mutation have been reported
to occur significantly more often in adenocarcinoma papillary
predominant, compared with all other subtypes (31).
To the best of our knowledge, only one report has
demonstrated the clinical relevance of pathological subtypes in
metastatic lung adenocarcinoma (16).
In this study of 100 patients with metastatic lung adenocarcinoma,
the most frequent histological pattern was solid with mucin (50%),
followed by acinar (29%), micropapillary (20%) and papillary (1%).
This study included 30 tissue samples from BM. The study authors
hypothesized that this difference in predominant components in
primary and metastatic sites was based on the function of the
metastatic potential of each adenocarcinoma subtype. The major
solid histopathological subtype of metastatic lung adenocarcinoma
was associated with poorer OS compared with other histopathological
subtypes (16). Furthermore, this
histopathological subtype was less likely to harbor EGFR
mutations and occurred less frequently in never smokers (16). In a separate study, Amin et al
(17) reported micropapillary
predominant adenocarcinoma may be more likely to metastasize. The
present study also identified that the most frequent subtype of BM
from lung adenocarcinoma was solid predominant (57.6%). OS did not
vary as a function of histological subtype. However, wild type
EGFR was significantly more common in patients with the
solid predominant subtype. This data is consistent with previous
reports (30,31), but these results require validation in
larger cohorts.
In recent years, TKIs targeting mutated EGFR
proteins have been established as the standard treatment for NSCLC.
This treatment is associated with longer progression-free survival
and OS compared with treatment with conventional chemotherapy
(11,33), particularly in certain populations,
including Eastern Asian, female and non-smoker patients with
adenocarcinoma (14). In addition to
demonstrating improved efficacy, TKI treatment is associated with
less toxicity relative to conventional chemotherapy. Several
studies have reported that the TKI treatment of patients with NSCLC
with BM results in intracranial response rates ranging between 26.7
and 83% (14,15), and rates of 64–87% when combined with
WBRT (34). TKI treatment for the
patients with BM resulted in reduced tumor size and significantly
improved OS (18.8 vs. 11.1 months) (13–15). In
the present study, only two patients received TKI treatment prior
to surgical resection, as the majority of patients were treated
prior to the development of TKIs. Excluding these two patients,
there was no statistically significant difference in OS between
patients with and without EGFR mutations (17.1 vs. 15.6
months). The presence of an EGFR mutation prolonged OS, but
improved outcome may be due to the efficacy of treatment with TKIs.
In agreement with the current results, for patients with BM from
lung adenocarcinoma, the presence of EGFR mutations has been
associated with improved survival from the time of development of
BM (15–25 vs. 7 months) (35–37). However, no significant difference was
observed in the timeline of BM development in patients treated with
EGFR-TKI compared with those receiving conventional chemotherapy
(24).
To the best of our knowledge, no previous study has
reported an association between EGFR mutation and the site
of BM. Thus, the findings in the present study of a significant
association between EGFR mutations and BM in the frontal
lobe is novel. In low-grade gliomas, lesions localized in the
frontal lobe may be vigorously resected without causing persistent
functional deficits (38). The
frontal lobe, which does not contain vital brain regions
controlling speech and motor areas, is a brain region in which
relatively aggressive surgical treatment may be pursued with lower
risk of causing lasting and serious functional deficits.
In patients with BM, RPA and GPA scores are still
the most widely accepted variables considered in evaluating disease
prognosis (6,9). However, the present study indicated that
the presence of EGFR mutations, BM within the frontal lobe
and non-smoker status are additional independent prognostic
factors.
A long time period was investigated for
retrospective analysis to evaluate the clinicopathological and
genetic changes of BM from lung adenocarcinoma. The diagnostic
neuroimaging and the treatment modalities, including WRRT, SRS and
chemotherapy have been developed. Nonetheless, these results remain
valid. The most common histological subtype in patients with
primary lung adenocarcinoma was the papillary subtype. However, the
predominant subtype of BM from lung adenocarcinoma was solid
adenocarcinoma in this study. The clinicopathological
characteristics of patients are not affected by the development of
treatment. While the interval between detection of the primary
cancer and detection of BM increased over the period covered in
this study, the interval elapsed between detection of BM and the
date of patient mortality or last follow-up did not change
significantly. Therefore, the prognostic factors identified in this
study are reliable.
The limitations of the current study include its
retrospective nature, and the potential bias in treatment strategy
following resection of BM cannot be excluded. Furthermore, other
clinically important genetic changes, including ALK
rearrangement, were not assessed due to the limited number of
patients with this genetic alteration in the present cohort.
Despite these limitations, to the best of our knowledge, this is
the first report to investigate the histopathological subtypes of
BM from lung adenocarcinoma, and the association between these
subtypes, EGFR mutations and disease prognosis in a large
patient cohort.
In the present study, univariate analysis was used
to demonstrated that three factors, EGFR mutation, BM localized to
the frontal lobes and non-smoker status, may be used as prognostic
indicators. In addition, multivariate analysis confirmed these
results, and demonstrated that the presence of BM in the frontal
lobes and non-smoker status were the most informative prognostic
factors. However, these conclusions are primarily applicable to
postoperative assessment of BM. The present study suggests that
tissue from the BM may be useful as an alternative tissue for
analysis, as resectable BM from lung adenocarcinoma cases exhibit
characteristics that are similar to those of the primary tumor
tissue. TKI treatment for BM from lung adenocarcinoma should be
considered if indicated by histopathological and genetic
examination.
Acknowledgements
The authors would like to thank Ms. M. Onitsuka and
Ms. H. Fukagawa from the Department of Pathology, Faculty of
Medicine, Fukuoka University (Fukuoka, Japan) for excellent
technical assistance with immunohistochemistry.
Funding
The present study was supported in part by a grant
from the Research Center for Advanced Molecular Medicine, Fukuoka
University, Fukuoka, Japan.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
HK and MH analyzed statistics, and drafted the
manuscript. HK, MH, MY HA and MN analyzed and interpreted the
patient data. TM TI and KN contributed to the conception of the
study, critically reviewed the manuscript and supervised the study.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained, and the study
design was approved by our institutional review board (IRB protocol
#15-5-14).
Patient consent for publication
Patients provided written informed consent for the
publication of any data and associated images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALK
|
anaplastic lymphoma kinase
|
|
BM
|
brain metastases
|
|
ECM
|
extracranial metastasis
|
|
EGFR
|
epidermal growth factor receptor
|
|
GPA
|
graded prognostic assessment
|
|
H&E
|
hematoxylin and eosin
|
|
KPS
|
Karnofsky performance score
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
RPA
|
recursive partitioning analysis
|
|
TKI
|
tyrosine kinase inhibitor
|
|
TTF-1
|
Thyroid transcription factor-1
|
|
WBRT
|
whole brain radiation therapy
|
|
WHO
|
World health organization
|
References
|
1
|
Shi AA, Digumarthy SR, Temel JS, Halpern
EF, Kuester LB and Aquino SL: Does initial staging or tumor
histology better identify asymptomatic brain metastases in patients
with non-small cell lung cancer? J Thorac Oncol. 1:205–210. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heon S, Yeap BY, Britt GJ, Costa DB, Rabin
MS, Jackman DM and Johnson BE: Development of central nervous
system metastases in patients with advanced non-small cell lung
cancer and somatic EGFR mutations treated with gefitinib or
erlotinib. Clin Cancer Res. 16:5873–5882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barnholtz-Sloan JS, Sloan AE, Davis FG,
Vigneau FD, Lai P and SAwaya RE: Incidence proportions of brain
metastases in patients diagnosed (1973 to 2001) in the metropolitan
detroit cancer surveillance system. J Clin Oncol. 22:2865–2872.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cox JD, Scott CB, Byhardt RW, Emami B,
Russell AH, Fu KK, Parliament MB, Komaki R and Gasper LE: Addition
of chemotherapy to radiation therapy alters failure patterns by
cell type within non-small cell carcinoma of lung (NSCCL): Analysis
of radiation therapy oncology group (RTOG) trials. Int Radiat Oncol
Biol Phys. 43:505–509. 1999. View Article : Google Scholar
|
|
5
|
Wang SY, Ye X, Ou W, Lin YB, Zhang BB and
Yang H: Risk of cerebral metastases for postoperative locally
advanced non-small-cell lung cancer. Lung Cancer. 64:238–243;.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gaspar L, Scott C, Rotman M, Asbell S,
Phillips T, Wasserman T, McKenna WG and Byhardt R: Recursive
partitioning analysis (RPA) of prognostic factors in three
radiation therapy oncology group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sperduto PW, Berkey B, Gasper LE, Mehta M
and Curran W: A new prognostic index and comparison to three other
indices for patients with brain metastases: An anlysis of 1,960
patients in the RTOG database. Int J Radiat Oncol Biol Phys.
70:510–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang DB, Yang PC, Luh KT, Kuo SH, Hong RL
and Lee LN: Late survival of non-small cell lung cancer patients
with brain metastases. Influence of treatment. Chest.
101:1293–1297. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kepka L, Cieslak E, Bujko K, Fijuth J and
Wierzchowski M: Results of the whole-brain radiotherapy for
patients with brain metastases from lung cancer: The RTOG RPA
intra-classes analysis. Acta Oncol. 44:389–398. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carbonplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oisumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimato S, Mitsudomi T, Kosaka T, Yutabe
Y, Wakabayashi T, Mizuno M, Nakahara N, Hatano H, Natsume A, Ishii
D and Yoshida J: EGFR mutations in patients with brain metastases
from lung cancer: Association with the efficacy of gefitinib. Neuro
Oncol. 8:137–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JE, Lee DH, Choi Y, Yoon DH, Kim SW,
Suh C and Lee JS: Epidermal growth factor receptor tyrosine kinase
inhibitors as a first-line therapy for never-smokers with
adenocarcinoma of the lung having asymptomatic synchronous brain
metastasis. Lung Cancer. 65:351–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SJ, Kim HT, Lee DH, Kim KP, Kim SW,
Suh C and Lee JS: Efficacy of epidermal growth factor receptor
tyrosine kinase inhibitors for brain metastasis in non-small cell
lung cancer patients harboring either exon 19 or 21 mutation. Lung
Cancer. 77:556–560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clay TD, Do H, Sundararajan V, Moore MM,
Conron M, Wright GM, McLachlan SA, Dobrovic A and Russell PA: The
clinical relevance of pathologic subtypes in metastatic lung
adenocarcinoma. J Thorac Oncol. 9:654–663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amin MB, Tamboli P, Merchant SH, Ordonez
NG, Ro J, Ayala AG and Ro JY: Micropapillary component in lung
adenocarcinoma: A distinctive histologic feature with possible
prognostic significance. Am J Surg Pathol. 26:358–364. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Travis WD BE, Burke AP, Marx A and
Nicholson AG: WHO Classification of Tumors of the Lung, Pleura,
Thymus and Heart. 4th edition. IARC press; Lyon: 2015
|
|
19
|
Mitsudomi T and Yatabe Y: Mutations of the
epidermal growth factor receptor gene and related genes as
determinants of epidermal growth factor receptor tyrosine kinase
inhibitors sensitivity in lung cancer. Cancer Sci. 98:1817–1824.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitsudori T, Kosaka T and Yatabe Y:
Biological and clinical implications of EGFR mutations in lung
cancer. Int J Clin Oncol. 11:190–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shigematsu H, Lin L, Takahasi T, Nomura M,
Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et al:
Clinical and biological features associated with epidermal growth
factor receptor gene mutations in lung cancers. J Natl Cancer Inst.
97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yano M, Sasaki H, Kobayashi Y, Yukiue H,
Haneda H, Suzuki E, Endo K, Kawano O, Hara M and Fujii Y: Epidermal
growth factor receptor gene mutation and computed tomographic
findings in peripheral pulmonary adenocarcinoma. J Thorac Oncol.
1:413–416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doebele RC, Lu X, Sumey C, Maxson DA,
Weickhardt AJ, Oton AB, Bunn PA Jr, Barón AE, Franklin WA, Aisner
DL, et al: Oncogene status predicts patterns of metastatic spread
in treatment-naïve nonsmall cell lung cancer. Cancer.
118:4502–4511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hendriks LE, Smit EF, Vosse BA, Mellema
WW, Heideman DA, Bootsma GP, Westenend M, Pitz C, de Vries GJ,
Houben R, et al: EGFR mutated non-small cell lung cancer patients:
More prone to development of bone and brain metastases? Lung
Cancer. 84:86–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rangachari D, Yamaguchi N, VanderLaan PA,
Folch E, Mahadevan A, Floyd SR, Uhlmann EJ, Wong ET, Dahlberg SE,
Huberman MS and Costa DB: Brain metastases in patients with
EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung
Cancer. 88:108–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsumoto S, Takahasi K, Iwakawa R,
Matsuno Y, Nakanishi Y, Kohno T, Shimizu E and Yokota J: Frequent
EGFR mutations in brain metastases of lung adenocarcinoma. Int J
Cancer. 119:1491–1494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eichler AF, Kahle KT, Wang DL, Joshi VA,
Willers H, Engelman JA, Lynch TJ and Sequist LV: EGFR mutation
status and survival after diagnosis of brain metastasis in nonsmall
cell lung cancer. Neuro Oncol. 12:1193–1199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shin DY, Na II, Kim CH, Park S, Baek H and
Yang SH: EGFR mutation and brain metastasis in pulmonary
adenocarcinomas. J Thorac Oncol. 9:195–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun M, Behrens C, Feng L, Ozburn N, Tang
X, Yin G, Komaki R, Varella-Garcia M, Hong WK, Aldape KD and
Wistuba II: HER family receptor abnormalities in lung cancer brain
metastases and corresponding primary tumors. Clin Cancer Res.
15:4829–4837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/American thoracic society/European respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsuta K, Kawago M, Inoue E, Yoshida A,
Takahashi F, Sakurai H, Watanabe S, Takeuchi M, Furuta K, Asamura H
and Tsuda H: The utility of the proposed IASLC/ATS/ERS lung
adenocarcinoma subtypes for disease prognosis and correlation of
driver gene alternations. Lung Cancer. 81:371–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koga K, Hamasaki M, Kato F, Aoki M,
Hayashi H, Iwasaki A, Kataoka H and Nabeshima K: Association of
c-Met phosphorylation with micropapillary pattern and small cluster
invasion in pT1-size lung adenocarcinoma. Lung Cancer. 82:413–419.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicenter, open-label, randomized, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Welsh JW, Komaki R, Amini A, Munsell MF,
Unger W, Allen PK, Chang JY, Wefel JS, McGovern SL, Garland LL, et
al: Phase II trial of erlotinib plus concurrent whole-brain
radiation therapy for patients with brain metastases from
non-small-cell lung cancer. J Clin Oncol. 31:895–902. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stanic K, Zwitter M, Hitij NT, Kern I,
Sadikov A and Cufer T: Brain metastases in lung adenocarcinoma:
Impact of EGFR mutation status on incidence and survival. Radiol
Oncol. 48:173–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee DW, Shin DY, Kim JW, Keam B, Kim TM,
Kim HJ, Kim DW, Wu HG, Paek SH, Kim YW, et al: Additional
prognostic role of EGFR activating mutations in lung adenocarcinoma
patients with brain metastasis: Integrating with lung specific GPA
score. Lung Cancer. 86:363–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sekine A, Satoh H, Iwasawa T, Tamura K,
Hayashihara K, Saito T, Kato T, Arai M, Okudela K, Ohashi K and
Ogura T: Prognostic factors for brain metastases from non-small
cell lung cancer with EGFR mutation: Influence of stable
extracranial disease and erlotinib therapy. Med Oncol. 31:2282014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanriverdi T, Kemerdere R, Baran O,
Sayyahmelli S, Ozlen F, Isler C, Uzan M and Ozyurt E: Long-term
surgical and seizure outcomes of frontal low-grade gliomas. Int J
Surg. 33:60–64. 2016. View Article : Google Scholar : PubMed/NCBI
|