Introduction
Gastric cancer is a relatively common
gastrointestinal cancer. Relevant data have shown that the
incidence of gastric cancer is on the increase, and gastric cancer
tends to occur in the aging population. Gastric cancer is the third
most common cause of cancer-related deaths worldwide (1). The main clinical manifestations of type
2 diabetes mellitus (T2DM) include insulin resistance and
progressive decline of islet function, and it is commonly
associated with systemic lipid metabolism (2). With an aging population that continues
to grow, number of elderly patients with gastric cancer combined
with T2DM is progressively increasing.
The number of elderly patients affected by gastric
cancer has shown an increasing trend. Once diabetes is complicated
by other diseases, body function becomes worse, which in turn
increases the difficulty of treatment. Incidence of gastric cancer
is correlated with diabetes, possibly due to living habits and
family inheritance. The mechanism is complicated. Surgery is
currently an effective method in the treatment of gastric cancer,
but preoperative preparation, postoperative fasting, surgical
trauma, anesthesia and other factors may affect blood glucose
level, resulting in blood glucose fluctuations in patients, which
can negatively affect postoperative rehabilitation, and the life
and health of patients (3,4). Therefore, monitoring of blood glucose is
of great clinical significance for reducing the incidence of
complications and promoting the prognosis of gastric cancer
patients combined with T2MD.
A retrospective case-control study, which included
elderly patients with gastric cancer that underwent radical
operation, was conducted in this study to investigate changes in
blood glucose and the effects of medication adjustment on
rehabilitation and prognosis of those patients.
Materials and methods
General data
A total of 46 elderly patients with gastric cancer
combined with T2DM who underwent radical operation from January
2007 to January 2017 and 30 gastric cancer patients without T2DM
were included in the study. All the patients were subjected to
total abdominal computed tomography (CT) and nuclear magnetic
examination, and all of them were confirmed to be in line with the
indications of radical operation for gastric cancer. In addition,
all the patients received adjuvant chemotherapy. Patients combined
with other malignant tumors other than gastric cancer, severe
cardiovascular and cerebrovascular diseases, cachexia and inability
for self-care were excluded. All enrolled patients were informed of
the operation condition, and they or their family members signed
the informed consent. This study was approved by the Ethics
Committee of The First Hospital of Lanzhou University (Lanzhou,
China). Patients were divided into the DM group and non-DM group
according to the diagnosis of T2DM. T2DM diagnosis was in line with
the diagnostic criteria of the World Health Organization (WHO)
(5).
General clinical data of the 76 elderly patients,
including age, sex, tumor location, tumor-node-metastasis (TNM)
staging and pathological type were recorded and summarized.
Pathological type was determined based on the 4th edition of 2010
WHO Classification of Tumors of the Digestive System (6). Gastric cancer staging was performed
using the methods described in the 7th edition of 2010 Union for
International Cancer Control (UICC) TNM Staging System of Gastric
Cancer, and the tumor location was determined based on the 3rd
edition of Japanese gastric cancer treatment guidelines (7,8). The DM
group comprised 26 males and 20 females, with an age range of 60–70
years and a mean age of 65.4±9.5 years. The non-DM group comprised
16 males and 14 females, with an age range of 60–73 years and a
mean age of 67.2±10.3 years. Levels of preoperative blood glucose
of the two groups of patients were controlled to reach the
standard. As shown in Table I, no
significant differences in general data and preoperative blood
glucose control were found between the two groups of patients
(P>0.05). In 46 patients with gastric cancer combined with T2DM,
24 patients received adjustment of postoperative blood glucose with
insulin, and 22 patients received no drug intervention to control
blood glucose.
 | Table I.General data of study subjects. |
Table I.
General data of study subjects.
| Items | DM group n=46 | Non-DM group
n=30 | t/χ2 | P-value |
|---|
| Age (years) | 65.4±9.5 | 67.2±10.3 | t=0.7826 | P>0.05 |
| Sex (n) |
|
|
χ2=0.7826 | P>0.05 |
| Male | 26 | 16 |
|
|
|
Female | 20 | 14 |
|
|
| Tumor location
(n) |
|
|
χ2=0.304 | P>0.05 |
| Upper part of the
cancer (U part) | 16 | 14 |
|
|
| Medium part of the
cancer (M part) | 16 | 8 |
|
|
| Lower part of the
cancer (L part) | 14 | 8 |
|
|
| TNM staging |
|
|
χ2=0.075 | P>0.05 |
| I | 26 | 16 |
|
|
| II | 20 | 14 |
|
|
| III | 0 | 0 |
|
|
| Intraoperative blood
loss (ml) | 246.2±159.4 | 229.8±186.7 | t=0.098 | P>0.05 |
| Operation time
(min) | 210.4±96.5 | 205.7±97.3 | t=0.272 | P>0.05 |
| First defecation time
(days) | 6.0±2.6 | 5.2±2.1 | t=1.473 | P>0.05 |
Methods
In 46 patients combined with DM, 24 patients were
included in the insulin treatment group, and those patients were
treated with continuous injection of insulin pumps to adjust blood
glucose, and the initial injection dose was determined based on the
patient's blood glucose level, age, body weight, and regimens in
the early phase. Blood glucose was monitored 4 times a day, and the
dose of insulin was adjusted according to the blood glucose level
to avoid significant fluctuations in blood glucose, thus preventing
the occurrence of hypoglycemia. The remaining 22 patients combined
with T2DM were included in the non-insulin treatment group, and
were treated with postoperative routine treatment, and insulin
intervention was not performed.
According to condition of the clinical recovery
after operation, postoperative blood glucose was controlled at
8.4–11.2 mmol/l, and blood glucose level was measured every 6 h.
Blood glucose level higher than this range in two measurements
indicated poor blood glucose control. Fasting blood was extracted
from each patient in the early morning. Serum carbohydrate antigen
19-9 (CA19-9) was detected using Roche Cobas E601 electrochemical
luminescence spectrometer. Serum carcinoembryonic antigen (CEA) was
determined by enzyme-linked immunosorbent assay (ELISA). Fasting
elbow venous blood (6 ml) was extracted from each patient in the
early morning before and after treatment. Blood sample was stored
in tubes containing ethylenediamine tetraacetic acid (EDTA), and 3
ml blood from each sample was used for white blood cell counting,
while the remaining 3 ml was used to prepare serum. White blood
cells were counted using an automatic blood cell analyzer (XS800i,
Sysmex Corporation, Kobe, Japan). Serum markers were detected by an
automatic biochemical analyzer (Roche Modular P800, Roche
Diagnostics GmbH, Mannheim, Germany) and immunoturbidimetric
assay.
Follow-up
After the first postoperative chemotherapy, the
patients began to receive long-term follow-up through telephone
from the date of discharge to January 2017 or their death.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
21.0 software was used for all statistical analyses. Kaplan-Meier
method was used to calculate the 5-year survival rate. Measurement
data were expressed as mean ± SD and the t-test was used for
comparisons between two groups. The χ2 test was used for
comparisons of count data. Pairwise t-test was used for comparisons
of data at two time-points within a group, and analysis of variance
followed by post hoc test (Least Significant Difference) was used
for comparisons among multiple time points within a group.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Changes in perioperative blood glucose
in the DM and non-DM groups
Blood glucose levels in the DM group were
significantly higher than those in the non-DM group before and at
24 h and 3 days after operation (P<0.05). Blood glucose
concentrations after operation were significantly increased in DM
group compared with the preoperative levels (P<0.05) (Table II).
 | Table II.Changes in perioperative blood glucose
of patients with gastric cancer in the DM group and the non-DM
group (mmol/l, mean ±SD). |
Table II.
Changes in perioperative blood glucose
of patients with gastric cancer in the DM group and the non-DM
group (mmol/l, mean ±SD).
| Items | DM group | Non-DM group | t value | P-value |
|---|
| Case (n) | 46 | 30 |
|
|
| Blood glucose before
operation | 16.98±1.64 | 4.01±1.75 | 10.21 | 0.009 |
| Blood glucose at 24 h
after operation | 24.09±1.42 | 5.71±1.03 | 8.12 | 0.02 |
| Blood glucose at 3 d
after operation | 22.52±2.08 | 5.09±1.22 | 11.43 | 0.001 |
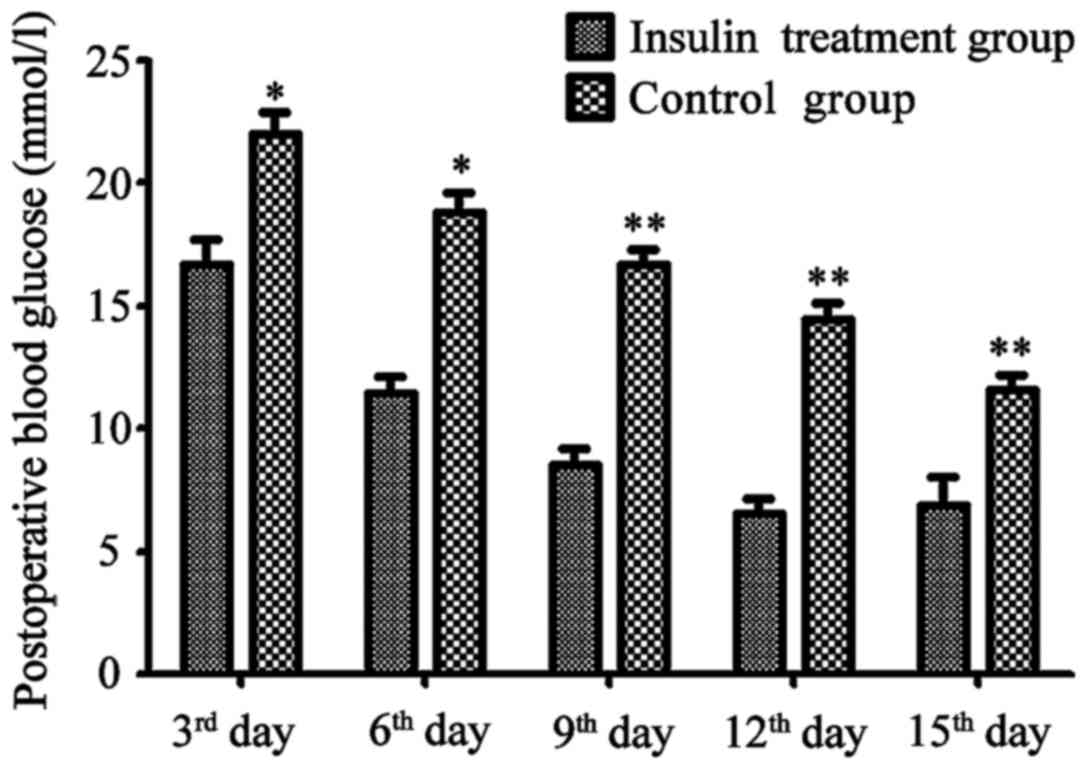
Changes in postoperative blood glucose
in the insulin treatment and non-insulin treatment groups
At 3 days after the treatment, concentration of
blood glucose in the treatment group was significantly lower than
that in non-treatment group (P<0.05). Blood glucose level of the
non-treatment group was slowly recovered, and significant decreases
were observed on the 3rd, 6th, 9th, 12th and 15th day after
operation. Decreases in level of blood glucose were more
significant in insulin treatment group than in non-insulin
treatment group (Fig. 1).
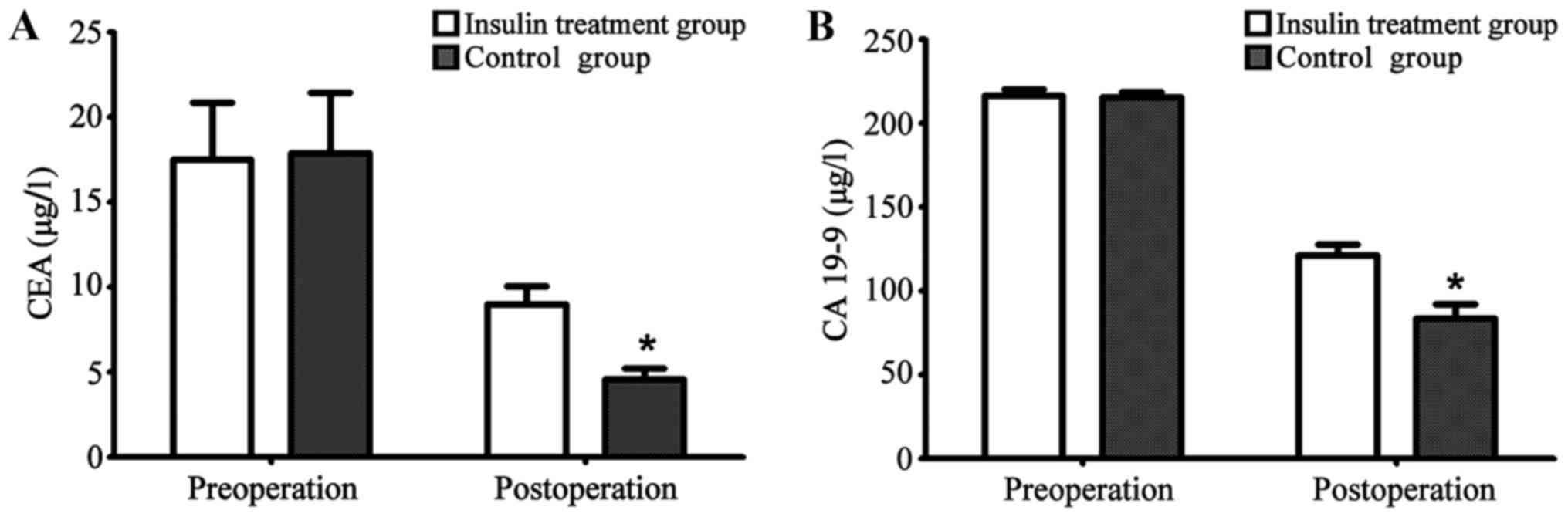
Changes in levels of gastric cancer
markers in insulin treatment group and non-insulin treatment group
before and after treatment
Levels of gastric markers, CEA and CA 19-9 in the
treatment and non-treatment groups before and 3 months after
operation were recorded and compared. Differences were not
significant (P>0.05). Compared with the preoperative levels,
levels of carcinoembryonic antigen in both groups were
significantly decreased after operation (P<0.05), and decreases
were more significant in insulin treatment group than in control
group (P<0.05) (Fig. 2A). Compared
with preoperative levels, levels of carcinoembryonic antigen in
both groups were significantly decreased after operation
(P<0.05), and decreases were more significant in the insulin
treatment group than in the control group (P<0.05) (Fig. 2B and Table
III).
 | Table III.Levels of gastric cancer markers in
the insulin treatment group and the non-insulin treatment group
before and after treatment. |
Table III.
Levels of gastric cancer markers in
the insulin treatment group and the non-insulin treatment group
before and after treatment.
| Items | Insulin treatment
group (n=24) | Non-insulin treatment
group (n=22) | t value | P-value |
|---|
| Preoperative CEA
(µg/l) | 17.4±6.5 | 16.4±9.5 | 2.032 | 0.541 |
| Postoperative CEA
(µg/l) | 1.4±9.6a | 6.9±9.7a | 10.765 | 0.014 |
| Preoperative CA 19-9
(kU/l) | 210.4±96.5 | 220.4±97.5 | 2.986 | 0.492 |
| Postoperative CA 19-9
(kU/l) |
90.4±87.5a |
110.4±77.8a | 8.231 | 0.026 |
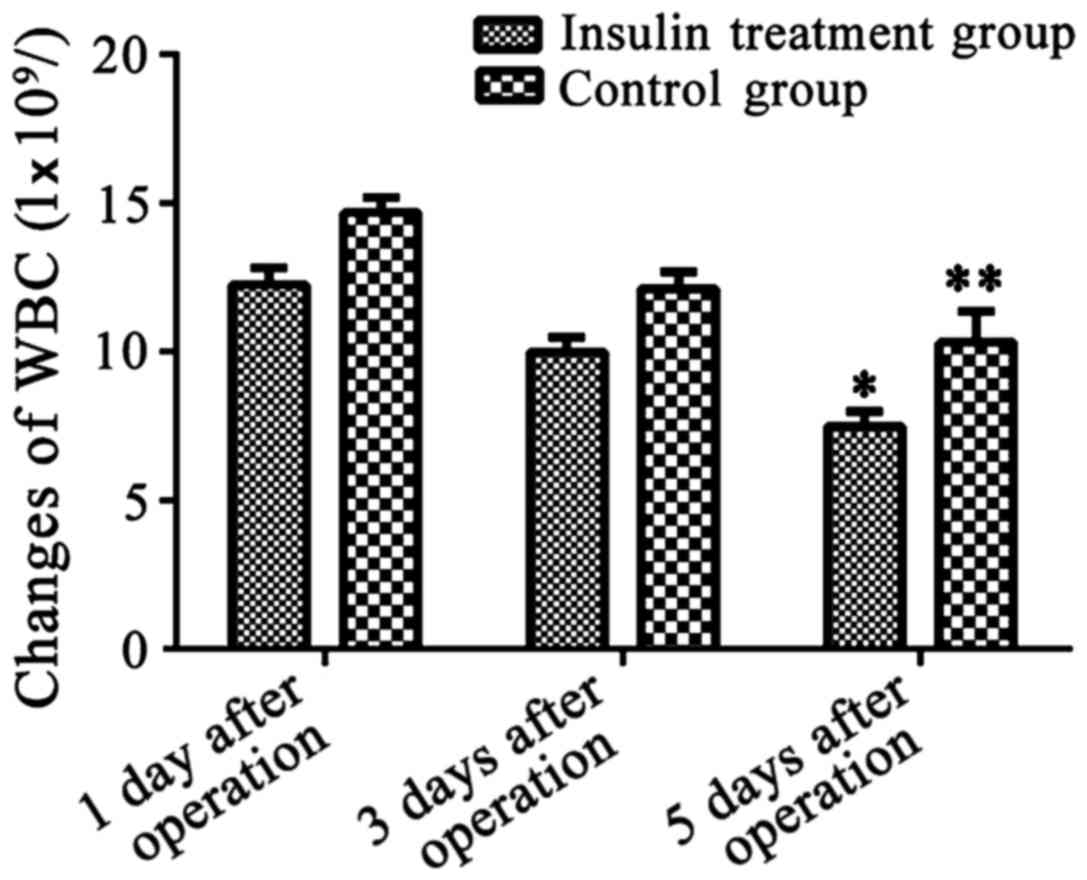
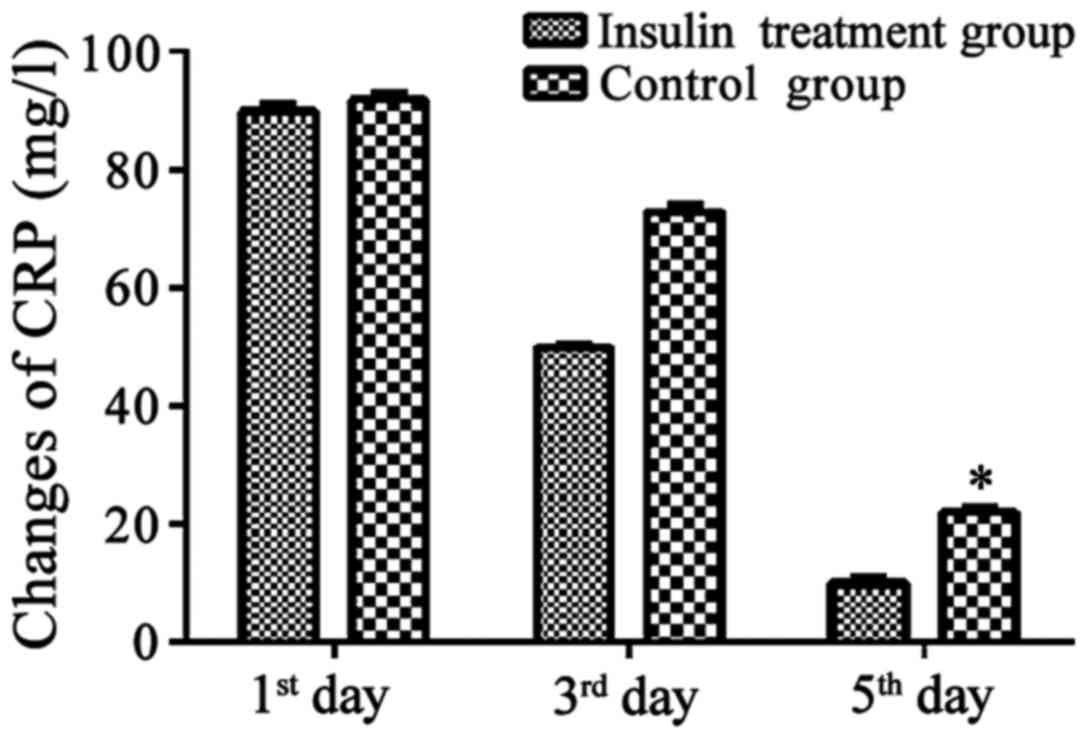
Changes in inflammatory response
indexes, white blood cells and CRP in the insulin and non-insulin
treatment groups
Changes in inflammatory response indexes, white
blood cells and CRP in insulin treatment group and non-insulin
treatment group on the 1st, 3rd and 5th day after operation were
recorded. Results showed that levels of inflammatory response
indexes in the two groups of patients on the 5th day after
operation were significantly decreased compared with those on the
1st day after operation. Decreases in white blood cells were more
significant in patients of the insulin treatment group than those
in the control group (P<0.05, Fig.
3). Decreases in CRP were more significant in patients of the
insulin treatment group than those in the control group.
(P<0.05, Fig. 4)
Comparisons of postoperative hospital
stay, poor postoperative blood glucose control, and poor incision
healing between the insulin and non-insulin treatment groups
In the non-treatment group, hospital stay of elderly
patients was 12±2 days, which was significantly longer than that in
treatment group (9±1 days) (P<0.05). Incidence rate of poor
incision healing was 12.5% in patients of the insulin treatment
group, and 63.6% in patients of the non-treatment group. The
incidence rate of poor incision healing in the non-insulin
treatment group (90.9%) was significantly higher than that in
treatment group (25.0%) (Table
III).
Comparison of the 5-year survival rate
of patients between insulin treatment group and non-insulin
treatment group
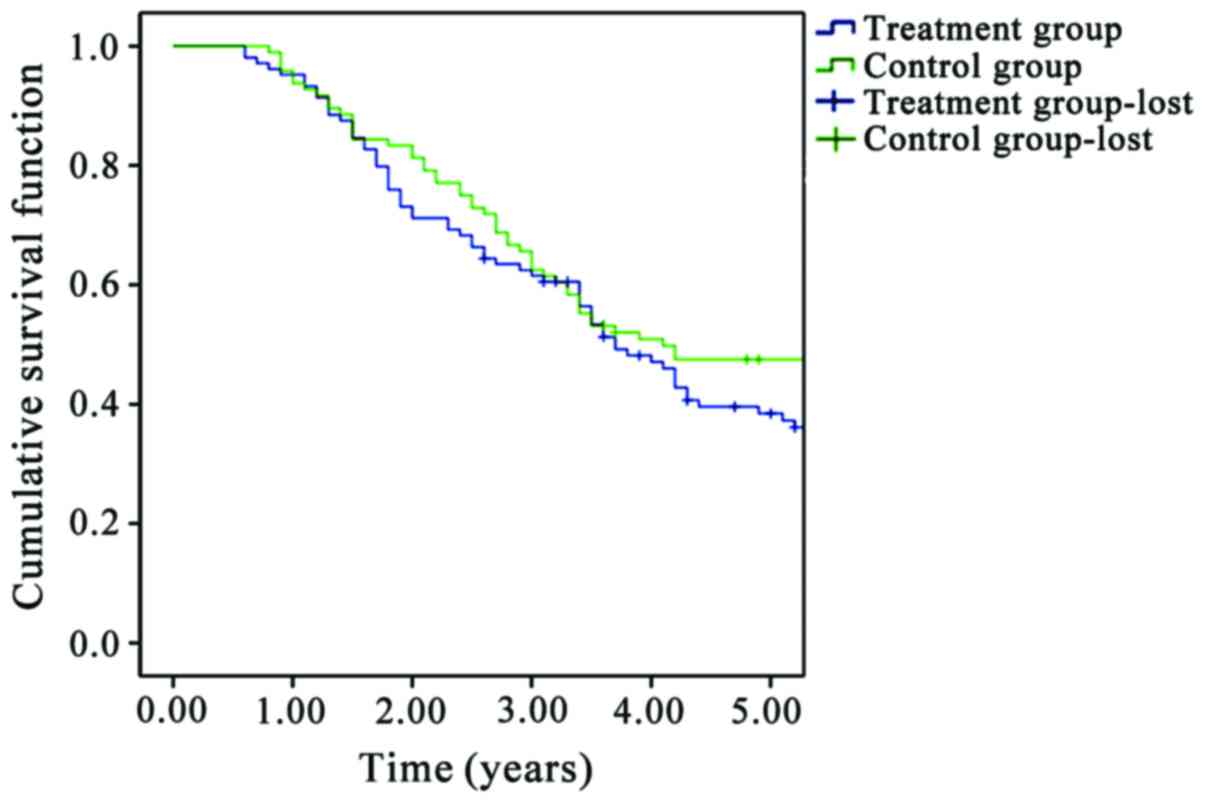
The 5-year survival rates in the insulin treatment
group and the non-insulin treatment group were 66.67 and 40.90%,
respectively. Survival time of patients in the insulin treatment
group was significantly longer than that of patients in non-insulin
treatment group (P<0.05) (Fig.
5).
Discussion
Gastric cancer is one of the most common malignant
gastrointestinal tumors with increasing incidence rate (9). Radical resection is the only effective
mean for treating gastric cancer. With the development of surgical
techniques, incidence rate of postoperative complications of
gastric cancer has been significantly reduced. However,
surgery-related factors may increase the risk of infections,
leading to postoperative death (10).
At present, with an aging population that continues to grow,
incidence rate of T2DM is increased year by year, and blood glucose
level is an important factor affecting the recovery of gastric
cancer after radical operation (11).
Incidence rate of poor postoperative blood glucose
control in elderly patients of non-treatment group (90.9%) was
significantly higher than that in insulin treatment group (25%),
which was consistent with previous studies. At the same time,
radical operation performed for DM patients can easily trigger or
aggravate the complications of DM, and also can greatly increase
the incidence of complications after gastrectomy. Mortality rate of
surgery and incidence rate of complications are approximately 5
times higher in DM patients than in patients without DM (12). In addition, poor blood glucose control
and surgical stimulations will trigger the stress responses and
increase the level of blood glucose. Insulin can be secreted to
resist the elevated blood glucose, while patients with islet
dysfunction or a history of DM usually have poor hypoglycemic
ability.
A study has shown that blood glucose controlling
through perioperative drug intervention can significantly reduce
the incidence rate of complications (10), which is consistent with our study
findings. Comparison of the 5-year survival rate of elderly
patients between insulin treatment group and non-insulin treatment
group showed that survival time is significantly longer in insulin
treatment group than that in non-insulin treatment group. At the
same time, a meta-analysis showed that the combination of DM with
malignant tumors could significantly increase the mortality rate of
patients (13). Another recent
meta-analysis in 2017 revealed that patients with T2DM treated with
melbine have a lower risk of gastric cancer than those who were not
treated with melbine (14). In
addition, perioperative glycemic control can not only reduce the
incidence rate of postoperative complications, but also can benefit
patients in the long-term prognosis because early blood glucose
control can delay the deterioration of tumor to some extent and
improve the survival of patients (15,16). These
studies are consistent with our conclusions.
Elderly patients have special clinical
characteristics, such as dormant clinical manifestations, more
preoperative complications, poor postoperative tolerance and weak
cardiovascular and respiratory system functions. Thus, early
intervention and treatment are needed for those patients. DM and
poor postoperative glycemic control are common causes of infection,
and a study has shown that prolonged hospital stay may even cause
death (17). Infection is mainly
reflected by incision healing condition. Our results showed that
the incidence rates of poor incision healing in insulin treatment
group and the non-insulin treatment group were 12.5 and 63.6%,
respectively, and the difference was not statistically significant.
It was believed that the inconsistency of the results might be
related to the relatively small sample size. Among 46 elderly
patients combined with T2DM underwent radical operation, incidence
rate of poor incision healing in the drug intervention group was
significantly lower than that in non-drug treatment group, which
was likely to be related to stress hyperglycemia of the body.
Stress hyperglycemia may lead to immune dysfunction
to promote the release of inflammatory factors, aggravate the
systemic inflammatory responses and increase the incidence of
infection, thus leading to a series of complications (18). Drug intervention in blood glucose
control can not only improve the utilization of glucose in the
body, but also can enhance the metabolism, reduce catabolism, and
indirectly reduce the incidence rate of inflammatory responses.
Therefore, blood glucose control with drug intervention can
significantly promote the rehabilitation of patients and reduce the
occurrence of complications (19). A
comparison of prognosis between the elderly patients and
non-elderly patients after radical operation has shown that,
although elderly patients with gastric cancer have relatively more
preoperative complications, strengthened perioperative treatment
could significantly reduce incidence rate of complications and
mortality rate to reach normal level (20). Therefore, postoperative blood glucose
control through drug intervention is of great clinical significance
for improvement of treatment outcomes, especially for elderly
patients.
In this study, elderly patients were selected as
subjects, and the changes in blood glucose of these elderly
patients with gastric cancer combined with T2DM after radical
operation were investigated, and the effects of medication
adjustment on the recovery and prognosis of gastric cancer were
explored. Our study provided reference for the treatment of gastric
cancer combined with T2DM. It was believed that DM might be an
independent risk factor for poor prognosis of gastric cancer
patient underwent radical operation. For the elderly patients
combined with DM, comprehensive preoperative assessment and
positive postoperative adjustment of blood glucose can
significantly reduce incidence of postoperative complications and
mortality rate, thereby enhancing the long-term survival rate.
Acknowledgements
Not applicable.
Funding
This study was supported by Natural Science
Foundation of Gansu Province (1308RJZA219).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ and SL designed the study. JW and XY collected
the patient data. LZ and YZ analyzed the patient data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All enrolled patients were informed of the operation
condition, and they or their family members signed the informed
consent. This study was approved by the Ethics Committee of The
First Hospital of Lanzhou University (Lanzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang-Chen KJ, Mullur R and
Bernal-Mizrachi E: Beta-cell failure as a complication of diabetes.
Rev Endocr Metab Disord. 9:329–343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan RP, Galas FR, Hajjar LA, Bello CN,
Piccioni MA and Auler JO Jr: Intensive perioperative glucose
control does not improve outcomes of patients submitted to
open-heart surgery: A randomized controlled trial. Clinics (Sao
Paulo). 64:51–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lavernia F: Treating hyperglycemia and
diabetes with insulin therapy: Transition from inpatient to
outpatient care. Medscape J Med. 10:216quiz 216. 2008.PubMed/NCBI
|
|
5
|
Rongli Q: The significance of new
diagnostic criteria and typing of diabetes mellitus. J Clin Int
Med. 17:1332000.
|
|
6
|
Teng XD and Lai DM: New progress of
gastric pathology - 2010 WHO Classification of Tumors of the
Digestive System. Chin J Clin Exp Pathol. 28:121–123. 2012.
|
|
7
|
Kwon SJ: Evaluation of the 7th UICC TNM
staging system of gastric cancer. J Gastric Cancer. 11:78–85. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forman D and Burley VJ: Gastric cancer:
Global pattern of the disease and an overview of environmental risk
factors. Best Pract Res Clin Gastroenterol. 20:633–649. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sugisawa N, Tokunaga M, Tanizawa Y, Bando
E, Kawamura T and Terashima M: Intra-abdominal infectious
complications following gastrectomy in patients with excessive
visceral fat. Gastric Cancer. 15:206–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dronge AS, Perkal MF, Kancir S, Concato J,
Aslan M and Rosenthal RA: Long-term glycemic control and
postoperative infectious complications. Arch Surg. 141:375–380.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jacober SJ and Sowers JR: An update on
perioperative management of diabetes. Arch Intern Med.
159:2405–2411. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barone BB, Yeh HC, Snyder CF, Peairs KS,
Stein KB, Derr RL, Wolff AC and Brancati FL: Long-term all-cause
mortality in cancer patients with preexisting diabetes mellitus: A
systematic review and meta-analysis. JAMA. 300:2754–2764. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou XL, Xue WH, Ding XF, Li LF, Dou MM,
Zhang WJ, Lv Z, Fan ZR, Zhao J and Wang LX: Association between
metformin and the risk of gastric cancer in patients with type 2
diabetes mellitus: A meta-analysis of cohort studies. Oncotarget.
8:55622–55631. 2017.PubMed/NCBI
|
|
15
|
Pettit S, Cresta E, Winkley K, Purssell E
and Armes J: Glycaemic control in people with type 2 diabetes
mellitus during and after cancer treatment: A systematic review and
meta-analysis. PLoS One. 12:e01769412017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miao ZF, Xu H, Xu YY, Wang ZN, Zhao TT,
Song YX and Xu HM: Diabetes mellitus and the risk of gastric
cancer: A meta-analysis of cohort studies. Oncotarget.
8:44881–44892. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sah BK, Zhu ZG, Chen MM, Yan M, Yin HR and
Zhen LY: Gastric cancer surgery and its hazards: Post operative
infection is the most important complication.
Hepatogastroenterology. 55:2259–2263. 2008.PubMed/NCBI
|
|
18
|
Orsenigo E, Tomajer V, Palo SD, Carlucci
M, Vignali A, Tamburini A and Staudacher C: Impact of age on
postoperative outcomes in 1118 gastric cancer patients undergoing
surgical treatment. Gastric Cancer. 10:39–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bagry HS, Raghavendran S and Carli F:
Metabolic syndrome and insulin resistance: Perioperative
considerations. Anesthesiology. 108:506–523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Egi M and Bellomo R: Reducing glycemic
variability in intensive care unit patients: A new therapeutic
target? J Diabetes Sci Technol. 3:1302–1308. 2009. View Article : Google Scholar : PubMed/NCBI
|