Introduction
Lung cancer is the main cause of cancer-related
death worldwide. In China, approximately 2.5 million people die of
this disease each year, and lung cancer is the first leading cause
of cancer-related deaths in men and the third leading cause of
death in women (1). Incidence of lung
cancer in developing countries will continue to rise (1). Lung cancer at early stages has no
obvious influence on patient life, and most patients were diagnosed
at advanced stages, leading to poor treatment outcomes and
prognosis. Even with proper chemotherapy, the 5-year survival rate
is still unacceptably low. At present, gene-targeted therapy
provides a new approach for the treatment of lung cancer (2). EGFR-TKI resistance is main cause of poor
survival of patients with lung cancer. Therefore, other genes
related to lung tumors remains to be identified.
As a hot topic in recent years, pseudogenes have
been found in more and more eukaryotes since the concept of
pseudogene was introduced. Pseudogenes are a group of DNA sequences
that have similar sequences with functional genes, but they lack
protein-coding ability (3). However,
it was reported that not all pseudogenes can be transcribed
(4). A bioinformatics analysis
carried out by Harrison et al (5) found that there are 233 transcribable
pseudogenes in the human genome. CSDAP1 is one of the pseudogenes
of CSDA. Reverse transcription-polymerase chain reaction (RT-PCR)
and western blot analysis were used in this study to detect the
expression levels of messenger RNA (mRNA) and protein of cold shock
domain protein A intronless pseudogene (CSDAP1) in lung cancer
tissues and cancer-adjacent normal tissues. Methyl thiazolyl
tetrazolium (MTT) was used to detect the activity of lung cancer
cells. This study was conducted to explore the significance of
CSDAP1 in lung cancer.
Materials and methods
Clinical data
A total of 317 patients (age range 27–83 years;
median 59 years) with primary lung cancer who received surgeries in
Tianjin Nankai Hospital from January 2007 to January 2012 were
selected. Lung cancer tissues and normal lung tissues more than 3
cm away from the cancer were collected for study. Complete clinical
data were available for all the patients. None of the patients
received chemotherapy, radiotherapy or other anti-tumor therapies
prior to surgeries, and they were free from other malignant tumor
diseases or severe renal and hepatic damage. Pathologic types and
stages of the 317 patients with lung cancer were determined as per
imaging, B ultrasound and post-operative pathological biopsy
results. The patients included 219 males and 58 females, including
138 cases of pulmonary adenocarcinoma, 167 cases of lung squamous
cell carcinoma, 3 cases of large cell carcinoma and 9 cases of
small cell carcinoma. In total, 127 patients had lymph node
metastasis, while 190 cases had no lymph node metastasis. According
to tumor-node-metastasis (TNM) pathological staging, 179 patients
suffered from stage I–II lung cancer, and 138 patients suffered
from stage III–IV lung cancer. According to histological grading,
147 patients suffered from moderately to highly differentiated lung
cancer, and 170 patients suffered from poorly differentiated or
undifferentiated lung cancer. Statistics of the follow-up visit was
conducted on all the lung cancer patients up to January 2017, which
showed that 23 patients withdrew from the follow-up visit. The rate
of follow-up visit was 92.7%. All the lung cancer patients
underwent follow-up visits for 5 years.
This study was approved by the Ethics Committee of
Tianjin Nankai Hospital (Tianjin, China), and all the patients
signed informed consent. Pre-operative general data of the patients
are given in Table I.
 | Table I.Pre-operative general data of 317 lung
cancer patients. |
Table I.
Pre-operative general data of 317 lung
cancer patients.
| Patient
characteristics | n | % |
|---|
| Sex |
| Male | 219 | 69.1 |
|
Female | 98 | 30.9 |
| Age (years) |
| ≤40 | 49 | 15.5 |
|
40–60 | 113 | 35.6 |
|
>60 | 155 | 48.9 |
| Lymph node
metastasis |
| Yes | 127 | 40.1 |
| No | 190 | 59.9 |
| Diameter of
tumors |
| ≤3
cm | 128 | 40.4 |
| >3
cm | 189 | 59.6 |
| Body mass index |
| Less than
normal | 116 | 36.6 |
|
Normal | 103 | 32.5 |
| Higher
than normal | 98 | 30.9 |
| Smoking index
(cigarette/year) |
| No
smoking | 98 | 30.9 |
|
>400 | 112 | 35.3 |
|
<400 | 107 | 33.8 |
Cell lines and siRNA
Human alveolar basal epithelial cells (A549), human
lung adenocarcinoma cell line (Calu-3), human embryonic fibroblasts
(MRC-5), human small cell lung cancer cells (NCI-H446) and human
lung cancer cells (PC-9) were purchased from American type Culture
Collection (Manassas, VA). One siRNA was designed and chemically
synthesized based on the sequence characteristics of CSDAP1.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Lung cancer tissues and normal tissues more than 3
cm away from the cancer were taken from all the patients. TRIzol™
reagents (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) were used to extract total RNA from the lung cancer tissues
and normal lung tissues. CSDAP1-set small interfering RNA (siRNA)
Lentivector kits (Nanjing EnoGene Biotech. Co., Ltd., Nanjing,
China) were used to detect mRNA expression of CSDAP1 in lung cancer
tissues and normal lung tissues by RT-PCR method. Primer sequences
used were: CSDAP1 amplification (452 bp):
5′-TACGTCCAAGGTCGGGCAGGAAGA-3′; upstream: 5′-CACTGATAGGCAGTTCTC;
downstream: 5′-GGTTCTCAGTTGGTGCTTC. Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was taken as internal reference to detect
mRNA expression level of CSDAP1 in the cells. All the selected
mRNAs were pre-degenerated for 5 min at 95°C, and 1 min at 94°C for
35 cycles. Then they were pre-degenerated for 1 min at 56°C, 2 min
at 72°C and 10 min at 72°C. The relative expression level of mRNA
of CSDAP1 was expressed as 2−∆∆Cq (2−∆∆Cq ≥2
was regarded as high expression) (6).
Analysis of protein expression of
CSDAP1 by western blot analysis
The protein-containing cell debris was washed twice
with phosphate buffer. Radio-immunoprecipitation assay (RIPA) lysis
buffer (Beyotime Institute of Biotechnology, Shanghai, China) was
added, and stored in a refrigerator at −80°C for standby after
centrifugation at 2,000 × g for 5 min at 4°C. Sodium
sulfate-polyacrylamide (Na2SO4-PAM) was used
to carry out protein gel electrophoresis, and the debris was
transferred to nitrocellulose (NC) fibrous membrane. Then the
membrane was blocked at room temperature for 1 h with 5% skimmed
milk. The membrane was incubated overnight with rabbit polyclonal
HMGA2 antibody (dilution: 1/500; cat. no: ab52039), rabbit
polyclonal Bcl-2 antibody (dilution, 1:500; cat. no.: ab59348),
rabbit polyclonal E-cadherin antibody (dilution, 1:500; cat. no.:
ab15148) and rabbit polyclonal β-actin antibody (dilution, 1:1,000;
cat. no.: ab8227) at 4°C, separately. The membrane was then
incubated with secondary goat anti-rabbit (HRP) IgG antibody
(dilution, 1:2,000; cat. no.: ab6721) at room temperature for 1 h.
All the antibodies were purchased from Abcam (Cambridge, MA, USA).
Then chemiluminescence was performed using a luminometer (Hitachi,
Tokyo, Japan).
Detection of in vitro proliferation of
lung cancer cells with methyl thiazolyl tetrazolium (MTT)
method
The accurately counted cells were inoculated in
96-well plates in a concentration of 1.5×103 cells/well.
At 24 h later, the tumor cells were taken out for experimental
culture when they were in logarithmic growth phase. After that, new
culture medium was added to carry out culturing for 48 h; then the
detection of cell activity was conducted. MTT was added to each
well, and the culture dish was placed in a thermostatic incubator
to incubate the cells for another 3 h. The supernatant was removed
by centrifugation at 2,000 × g for 5 min at 4°C, and dimethyl
sulfoxide was added. Incubation was conducted again for 15 min at
room temperature. The absorbance was measured at the wavelength of
570 nm.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
22.0 (IBM Corp., Armonk, NY, USA) was used for statistical
analysis. ANOVA test was used for comparison of multiple groups and
the comparison of multiple groups was carried out using SNK test.
The data were analyzed, and the measurement data were expressed as
(mean ± SD). The χ2 test was performed for comparison of
CSDAP1 expression levels and clinical factors. The post-operative
survival rate was calculated as per Kaplan-Meier survival curve.
Significance test was conducted using the log-rank test for
univariate analysis. Statistically significant variables
(P<0.01) in the univariate analysis were included in the Cox
model to carry out multivariate analysis. P<0.05 indicated that
the difference was statistically significant.
Results
Expression of CSDAP1 in lung cancer
tissues and that in normal tissues
The expression of CSDAP1 in lung cancer tissues was
obviously higher than that in cancer-adjacent normal tissues. The
difference had statistical significance (P<0.01) (Table II).
 | Table II.Expression of CSDAP1 in lung cancer
tissues and that in cancer-adjacent normal tissues. |
Table II.
Expression of CSDAP1 in lung cancer
tissues and that in cancer-adjacent normal tissues.
|
| CSDAP1 |
|
|---|
|
|
|
|
|---|
| Type of tissues | High expression | Low expression | P-value |
|---|
| Lung cancer
tissues | 105 | 212 | <0.01 |
| Cancer-adjacent
tissues | 7 | 310 |
|
In this study, the high expression of CSDAP1 had no
obvious differences in the comparison of age and sex of lung cancer
patients as well as the diameter and differentiation degree of the
cancer. The difference had no statistical significance (P>0.05).
The high expression rate of CSDAP1 in the cancer tissues of 138
patients with pulmonary adenocarcinoma was 42.8%, while that in 167
patients with lung squamous cell carcinoma was 27.5%. The
difference between them was significant (P=0.05). The high
expression rate of CSDAP1 in the cancer tissues of 127 lung cancer
patients with lymph node metastasis was 41.7%, while that in the
cancer tissues of 190 lung cancer patients without lymph node
metastasis was 27.4%. The difference between them was significant
(P<0.008). The high expression rate of CSDAP1 in patients with
stage I–II lung cancer was 25.1%, which was less than that in
patients with stage III–IV lung cancer (43.5%) P=0.001 (Table III).
 | Table III.Correlation of CSDAP1 expression in
lung cancer with clinicopathological features. |
Table III.
Correlation of CSDAP1 expression in
lung cancer with clinicopathological features.
|
|
|
| CSDAP1 |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Parameters | n | % | High expression | Low expression | χ2
value | P-value |
|---|
| Sex |
|
|
|
| 0.09 | 0.764 |
| Male | 219 | 38.4 | 84 | 135 |
|
|
|
Female | 58 | 36.2 | 21 | 37 |
|
|
| Age (years) |
|
|
|
| 0.461 | 0.794 |
| ≤40 | 49 | 36.7 | 18 | 31 |
|
|
|
40–60 | 113 | 33.6 | 38 | 75 |
|
|
|
>60 | 155 | 31.6 | 49 | 106 |
|
|
| Diameter of
tumors |
|
|
|
| 0.683 | 0.409 |
| ≤3
cm | 128 | 30.5 | 39 | 89 |
|
|
| >3
cm | 189 | 34.9 | 66 | 123 |
|
|
| Differentiation
degree |
|
|
|
| 3.387 | 0.066 |
| Middle
and high differentiation | 147 | 27.9 | 41 | 106 |
|
|
| Low
(non) differentiation | 170 | 37.6 | 64 | 106 |
|
|
| Types of
tissues |
|
|
|
| 7.742 | 0.005 |
|
Pulmonary adenocarcinoma | 138 | 42.8 | 59 | 79 |
|
|
| Lung
squamous cell carcinoma | 167 | 27.5 | 46 | 121a |
|
|
| Lymph node
metastasis |
|
|
|
| 7.09 | 0.008 |
|
Yes | 127 | 41.7 | 53 | 74 |
|
|
| No | 190 | 27.4 | 52 | 138 |
|
|
| Clinical
staging |
|
|
|
| 11.83 | 0.001 |
|
I–II | 179 | 25.1 | 45 | 134 |
|
|
|
III–IV | 138 | 43.5 | 60 | 78 |
|
|
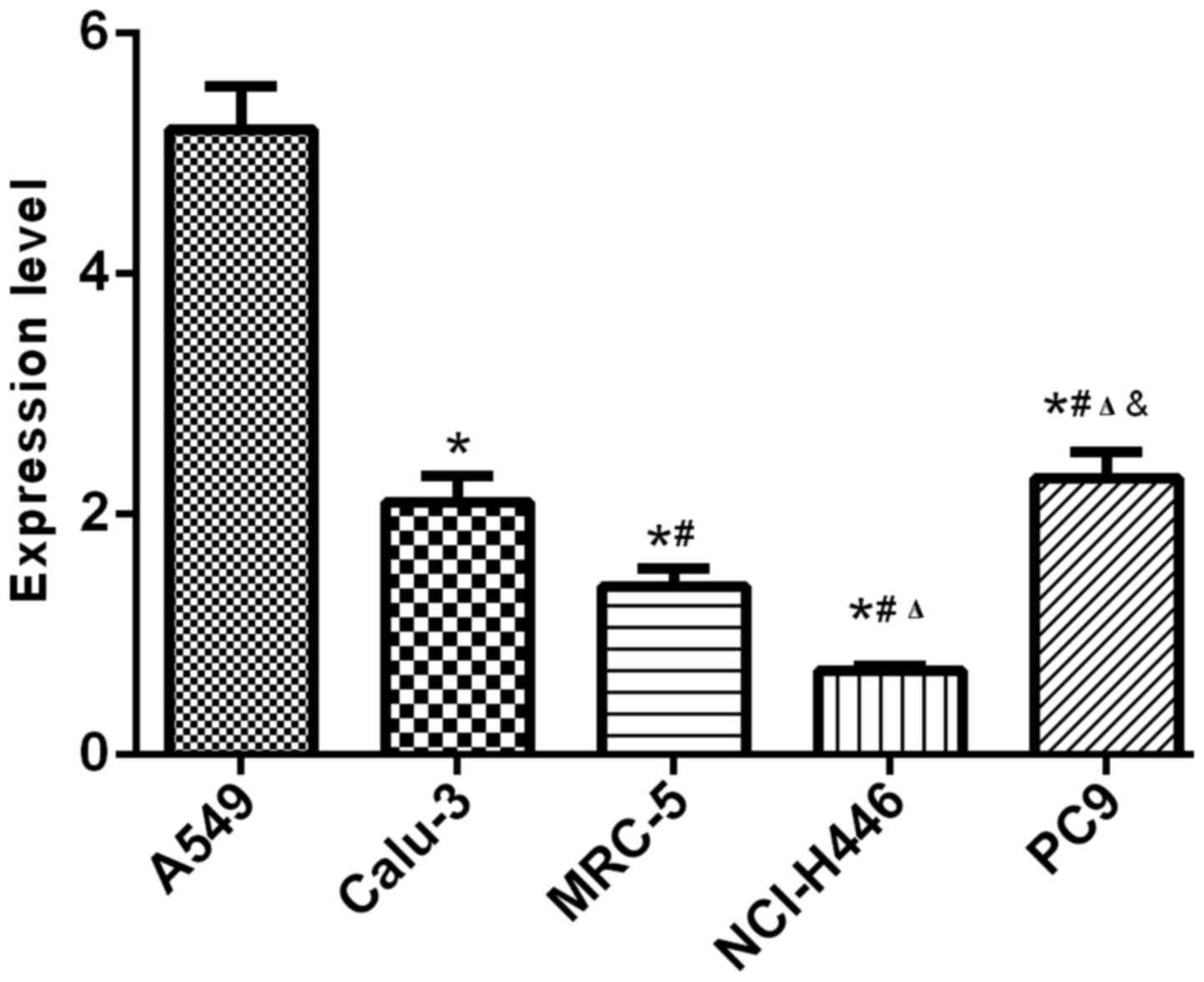
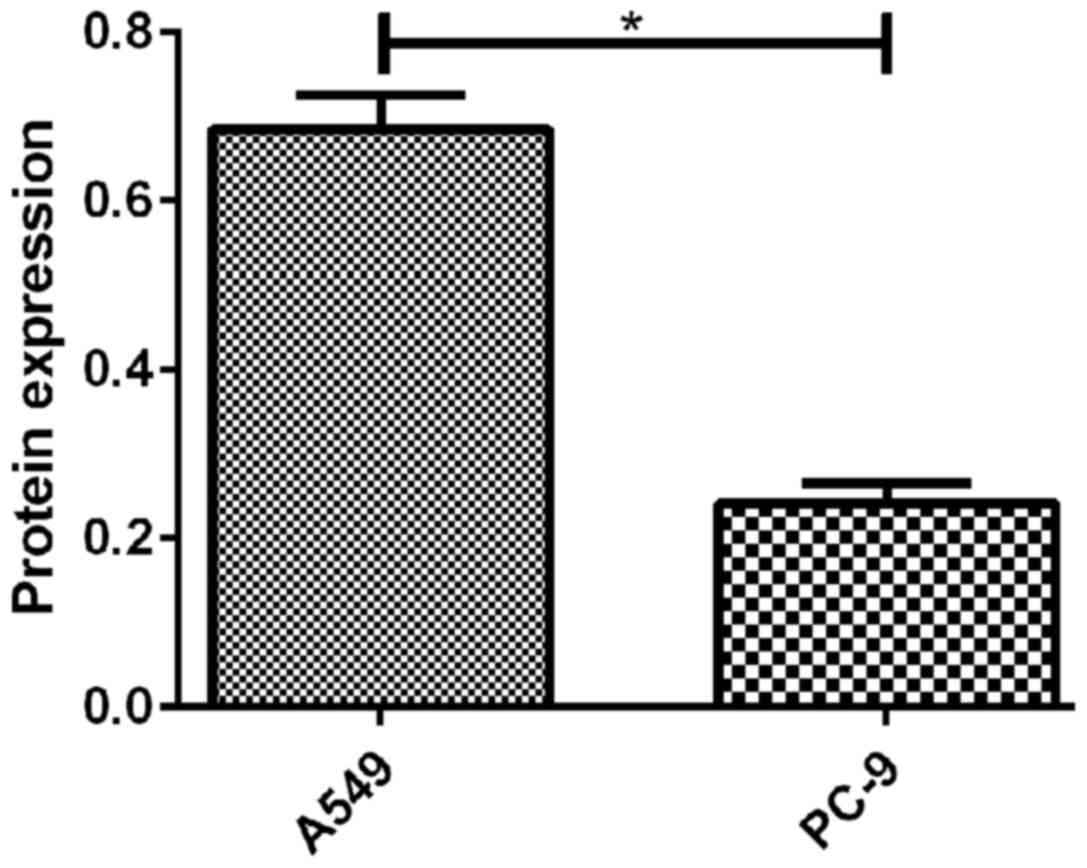
Results of RT-qPCR and western blot analysis showed
that the expression levels of CSDAP1 in lung cancer cells such as
human alveolar basal epithelial cells (A549), human lung
adenocarcinoma cell line (Calu-3), human embryonic fibroblasts
(MRC-5), human small cell lung cancer cells (NCI-H446) and human
lung cancer cells (PC-9) were different in transcription and
translation, among which, A549 and PC-9 had relatively high
expression levels (Figs. 1 and.
2).
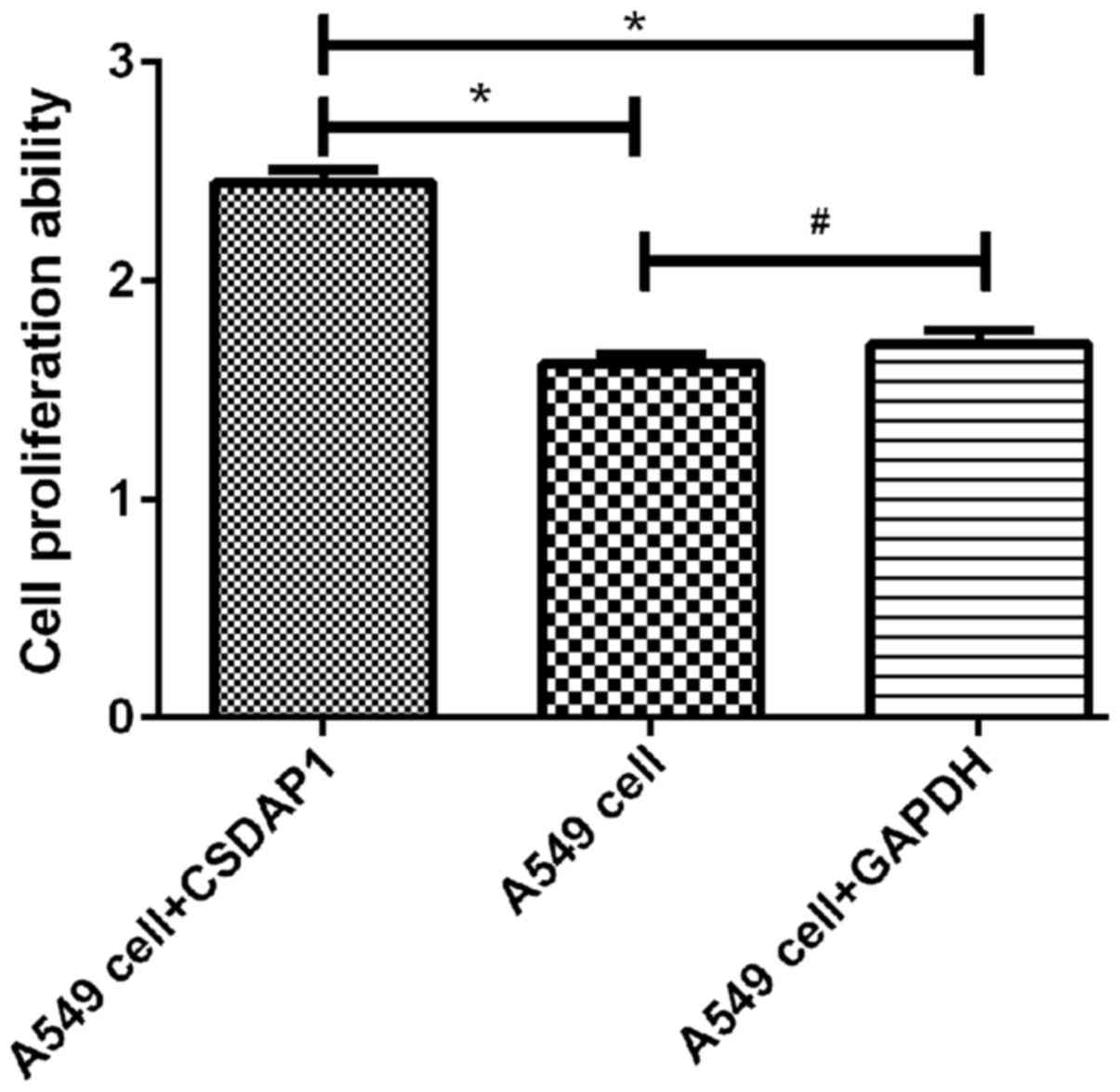
Detection of activity of A549 in lung
cancer with MTT method
The activity of A594 containing CSDAP1 was
(2.45±0.1), which was increased significantly compared with that of
the unprocessed A549 (1.62±0.08) and that of A549 containing GAPDH
(1.71±0.11). The differences had statistical significance
(P<0.05) (Fig. 3).
Parameters showing P<0.1 in univariate analysis
were included in Cox proportional hazard regression model. The
results showed that CSDAP1, lymph node metastasis and clinical
staging were independent risk factors for prognosis of lung cancer
(P<0.05) (Table IV).
 | Table IV.Multivariate analysis of prognosis in
patients with lung cancer. |
Table IV.
Multivariate analysis of prognosis in
patients with lung cancer.
| Factors | Estimated
value | Standard error | Wald value | P-value | OR value | 95% confidence
interval (CI) |
|---|
| Expression of
CSDAP1 | −1.503 | 0.278 | 39.523 | <0.001 | 3.702 | 0.121–0.630 |
| TNM staging | 2.823 | 1.124 |
5.021 | 0.007 | 1.963 | 0.623–2.477 |
| Lymph node
metastasis | −2.017 | 0.896 |
7.431 | 0.001 | 2.305 | 0.503–2.135 |
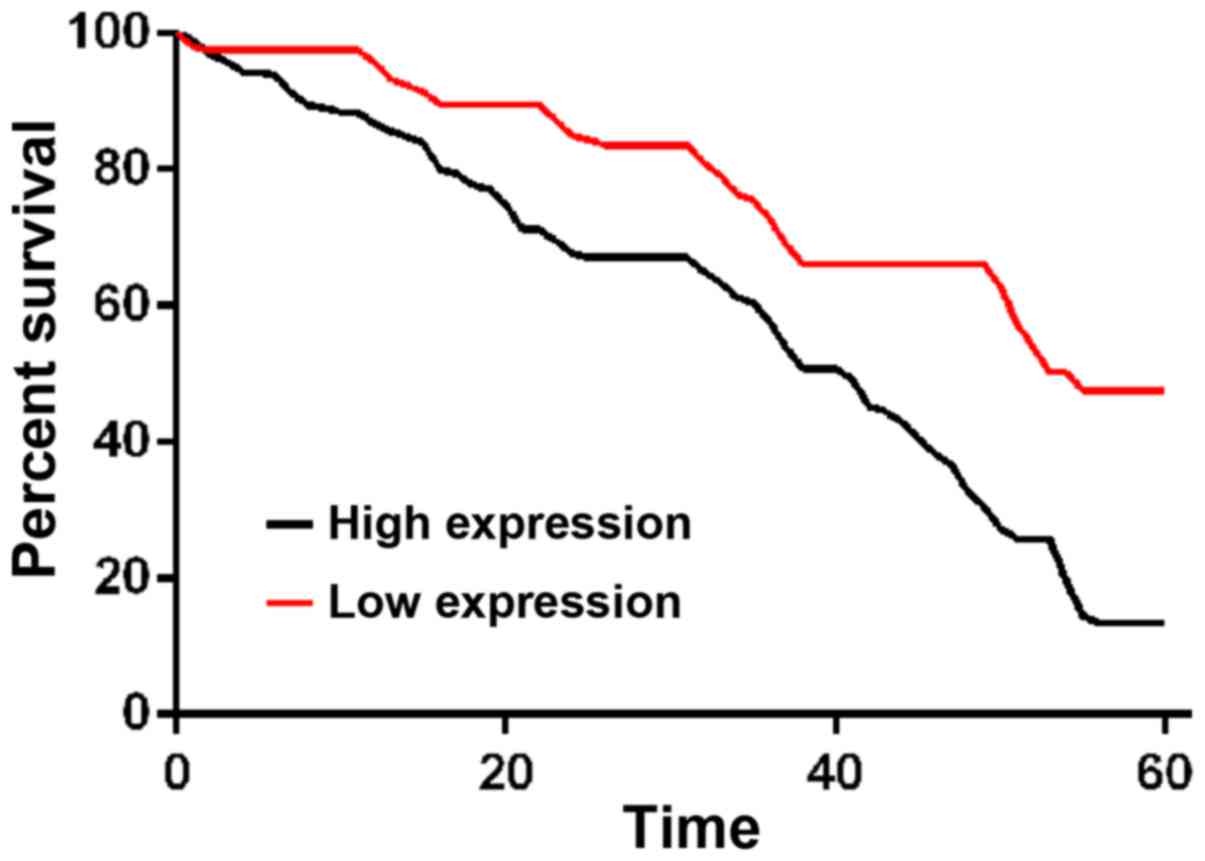
The comparison of 1-, 3- and 5-year survival rates
between patents with a high expression of CSDAP1 and those with a
low expression of CSDAP1 in lung cancer tissues showed that 1-, 3-
and 5-year survival rates of patients with high expression of
CSDAP1 were lower than those of patients with low expression of
CSDAP1 in lung cancer tissues (Fig.
4).
Discussion
Drug resistance in lung cancer is closely related to
therapeutic effects. Although there are many new treatments such as
biotherapy and targeted therapy (7),
and the level of treatment of lung cancer continues to increase,
the survival rate of lung cancer patients remains unsatisfactory.
Moreover, how we can effectively predict the occurrence and
development of lung cancer, and how to choose treatment programs
constitute challenges in the treatment of lung cancer at present.
Therefore, it is particularly critical to identify more effective
genes against tumor cells.
CSDA is widely found in animals, plants and
micro-organisms. It is a member of the cold shock domain protein
gene (8–10), which can express lung, ovary,
prostate, colorectum, in various tissues. Its expression is
realized through response to cold shock (11), especially in the response to
cold-shock stimulation. Currently, cold shock domain proteins have
been used as tumor markers.
Current findings showed that the expression of
CSDAP1 in normal tissues is very small, while that in various
malignant tumors is increased. For example, CSDAP1 is closely
related to tumors such as breast, ovarian, prostate and colon
cancer (12), and it can be used as
risk factors for prognosis in these tumors. At present, the
relationship between the expression of CSDAP1 in lung cancer and
the prognosis is not confirmed yet. In this study, an analysis on
the expression level of CSDAP1 and different pathologic features of
lung cancer patients was conducted. There are few studies on the
relationship between CSDAP1 and clinicopathological features of
lung cancer. In this study, the correlation of CSDAP1 with lung
cancer is analyzed. The experiment using RT-qPCR method and western
blot analysis showed that the expression of CSDAP1 in lung cancer
tissues was obviously higher than that in cancer-adjacent normal
tissues. The difference had statistical significance. Diamond et
al (13) suggested that the cold
shock domain protein is transferred to the nucleus through
coordinating the expression of P53 and is involved in the
occurrence and drug resistance of bladder cancer. A study conducted
by Tang et al (14) showed
that CSDAP1 can be used as the function of growth stimulus in tumor
cells, and indicated that the overexpression of CSDAP1 in colon
cancer and prostate cancer and the downregulation of mRNA can
significantly reduce the survival of tumor cells and enhance the
response of prostate cancer cells to chemotherapy. The
aforementioned results indicated that the high expression of CSDAP1
may be closely related to the occurrence and development of tumors
such as lung cancer (15,16). The mechanism of CSDAP1 with diseases
such as tumors needs to be further verified in future studies.
In this study, it was found that the median survival
time of lung cancer patients with high expression of CSDAP1 was
significantly shorter than that of lung cancer patients with low
expression of CSDAP1, and that the survival prognosis of lung
cancer patients with high expression of CSDAP1 was significantly
lower than that of lung cancer patients with low expression of
CSDAP1, which confirmed that the expression of CSDAP1 may be
closely related to the prognosis of lung cancer. Multivariate
analysis revealed that CSDAP1, lymph node metastasis and clinical
staging could be used as independent risk factors of prognosis in
lung cancer (17,18), which is basically consistent with the
conclusion drawn in large clinical trials conducted by Zimmermann
et al (19). The
aforementioned results suggested that patients with high expression
of CSDAP1 may have relatively poor prognosis. Further studies on
the value of CSDAP1 in molecular-targeted therapy (20,21) may be
the direction of current studies.
This experiment has shortcomings, for example, it is
a scientific study conducted in only one medical center, the sample
size is insufficient and there are geographical differences in the
samples used for the experiment. We studied this pseudogenic gene
for the first time, and then we found that there was no related
literature to describe it before, and most of them were screened by
gene sequencing and GO enrichment. The functional mechanism was not
well studied. If a large sample size with patients from different
regions and ethnicities is available for future investigations, the
significance of this experiment may be confirmed. In addition,
future studies are to focus on the functional mechanism.
In conclusion, the expression level of CSDAP1 can be
used as an important factor for predicting prognosis in lung cancer
patients, especially those in the early stage. However, as the
expression of CSDAP1 in normal tissues is too low or absent, its
clinical effects can be proven further by combining with the
expression level of CSDAP1 protein. In this study, CSDAP1 may play
an important role in the occurrence, development and the judgement
of prognosis of lung cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TX and DL conceived and designed the study. TX, YH
and FZ were responsible for the collection and analysis of data.
TX, MQ and YC interpreted the data and drafted the manuscript. DL
and YH revised the manuscript critically for important intellectual
content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Tianjin Nankai Hospital (Tianjin, China). Signed informed consent
was obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tang H, Wang S, Xiao G, Schiller J,
Papadimitrakopoulou V, Minna J, Wistuba II and Xie Y: Comprehensive
evaluation of published gene expression prognostic signatures for
biomarker-based lung cancer clinical studies. Ann Oncol.
28:733–740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu C, Xie S, Song C, Huang L and Jiang Z:
Lin28 mediates cancer chemotherapy resistance via regulation of
miRNA signaling. Hepatogastroenterology. 61:1138–1141.
2014.PubMed/NCBI
|
|
3
|
De Martino M, Forzati F, Arra C, Fusco A
and Esposito F: HMGA1-pseudogenes and cancer. Oncotarget.
7:28724–28735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lister N, Shevchenko G, Walshe JL, Groen
J, Johnsson P, Vidarsdóttir L, Grander D, Ataide SF and Morris KV:
The molecular dynamics of long noncoding RNA control of
transcription in PTEN and its pseudogene. Proc Natl Acad Sci USA.
114:9942–9947. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harrison PM, Zheng D, Zhang Z, Carriero N
and Gerstein M: Transcribed processed pseudogenes in the human
genome: An intermediate form of expressed retrosequence lacking
protein-coding ability. Nucleic Acids Res. 33:2374–2383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. METHODS. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu YJ, Zhang HB, Liu YH, Zhu YZ, Chen J,
Li Y, Bai JP, Liu LR, Qu YC, Qu X, et al: Association of mutant
EGFR L858R and exon 19 concentration in circulating cell-free DNA
using droplet digital PCR with response to EGFR-TKIs in NSCLC.
Oncol Lett. 14:2573–2579. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Che H, Zhang W, Wang J, Ke T, Cao
R, Meng S, Li D, Weiming O, Chen J, et al: Effects of mild chronic
intermittent cold exposure on rat organs. Int J Biol Sci.
11:1171–1180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dal Piaz F, Terracciano S, De Tommasi N
and Braca A: Hsp90 activity modulation by plant secondary
metabolites. Planta Med. 81:1223–1239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanshin E, Kubiniok P, Thattikota Y,
D'Amours D and Thibault P: Phosphoproteome dynamics of
Saccharomyces cerevisiae under heat shock and cold stress.
Mol Syst Biol. 11:8132015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanabe Y, Nagatoishi S and Tsumoto K:
Thermodynamic characterization of the interaction between the human
Y-box binding protein YB-1 and nucleic acids. Mol Biosyst.
11:2441–2448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai CJ, Ye SJ, Zhu W, Sun LY, Wan HS, Feng
ZH, Ma L and Li L: Study on invasion and metastasis-associated
genes of lung cancer related with NM23-H1 gene. Sichuan Da Xue Xue
Bao Yi Xue Ban. 41:941–945. 2010.(In Chinese). PubMed/NCBI
|
|
13
|
Diamond P, Shannon MF, Vadas MA and Coles
LS: Cold shock domain factors activate the granulocyte-macrophage
colony-stimulating factor promoter in stimulated Jurkat T cells. J
Biol Chem. 276:7943–7951. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang C, Wang Y, Lan D, Feng X, Zhu X, Nie
P and Yue H: Analysis of gene expression profiles reveals the
regulatory network of cold-inducible RNA-binding protein mediating
the growth of BHK-21 cells. Cell Biol Int. 39:678–689. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalamida D, Karagounis IV, Mitrakas A,
Kalamida S, Giatromanolaki A and Koukourakis MI: Fever-range
hyperthermia vs. hypothermia effect on cancer cell viability,
proliferation and HSP90 expression. PLoS One. 10:e01160212015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hung MS, Lin YC, Mao JH, Kim IJ, Xu Z,
Yang CT, Jablons DM and You L: Functional polymorphism of the
CK2alpha intronless gene plays oncogenic roles in lung cancer. PLoS
One. 5:e114182010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen SJ, Lin PW, Lin HP, Huang SS, Lai FJ,
Sheu HM, Hsu LJ and Chang NS: UV irradiation/cold shock-mediated
apoptosis is switched to bubbling cell death at low temperatures.
Oncotarget. 6:8007–8018. 2015.PubMed/NCBI
|
|
18
|
Yamashita T, Higashi M, Momose S, Morozumi
M and Tamaru JI: Nuclear expression of Y box binding-1 is important
for resistance to chemotherapy including gemcitabine in
TP53-mutated bladder cancer. Int J Oncol. 51:579–586. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimmermann M, Traxler D, Simader E, Bekos
C, Dieplinger B, Lainscak M, Ankersmit HJ and Mueller T: In vitro
stability of heat shock protein 27 in serum and plasma under
different pre-analytical conditions: Implications for large-scale
clinical studies. Ann Lab Med. 36:353–357. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Hu W, Murakawa Y, Yin J, Wang G,
Landthaler M and Yan J: Cold-induced RNA-binding proteins regulate
circadian gene expression by controlling alternative
polyadenylation. Sci Rep. 3:20542013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu B, Wang J, He C, Wang W, Tang J, Zheng
R, Zhou C, Zhang H, Fu Z, Li Q, et al: Cytokine-induced killer cell
therapy for modulating regulatory T cells in patients with
non-small cell lung cancer. Exp Ther Med. 14:831–840. 2017.
View Article : Google Scholar : PubMed/NCBI
|