Introduction
Myelodysplastic syndrome (MDS) represents clonal
disorders primarily of the elderly, and is characterized by
ineffective hematopoiesis and an increased risk of transformation
into acute myeloid leukemia. Previous research has indicated that
changes in bone marrow niches, such as abnormal interactions of
hemopoietic stem cells (HSCs) and malignant clones, abnormal
secretion of hematopoietic growth factors and cytokines, may
contribute to the pathogenesis of MDS (1). Previous studies identified that
mesenchymal cells in the bone marrow niche, including osteoblasts,
osteoprogenitors, and reticular and fat cells, serve an important
role in hematopoiesis regulation (2).
Osteoblastic cells are critical for early differentiation of stem
cells, and the destruction of osteoblasts can accelerate the
progression of certain malignant hematological diseases (3).
Osteoblasts are bone-forming cells that secrete
calcium and synthesize the bone matrix and typically cover the
endosteal bone surface to form an interface between calcified bone
and marrow cells, which are reported to provide signals required
for HSC quiescence, long-term maintenance and bone marrow retention
(4). The study Raaijmakers et
al (5) provided evidence that
disturbance of the endosteal niche can result in MDS. Deletion of
Dicer 1, an RNase III endonuclease, in osteoprogenitors was
demonstrated to impair osteoblastic differentiation in vitro
and in vivo. These mice developed fatal neutropenia with
hyperplastic bone marrow and dysmyelopoiesis, which is highly
suggestive of MDS. Their research was the first to demonstrate that
a change of the bone marrow niche caused by the destruction of
osteoblasts led to the occurrence of MDS.
Osteocalcin (OCN) is a bone-specific protein that is
closely related to the mineralization of osteoblasts, and reflects
the osteoblast mineralization ability. OCN (also referred to as
bone γ-carboxyglutamic acid-containing protein) is the most
abundant non-collagenous protein found in bone (6). It is uniquely synthesized and secreted
by mature osteoblasts and osteocytes, and is widely used as a
marker for bone formation.
A number of studies have demonstrated that the Notch
signaling pathway contributes to the occurrence and development of
genetic diseases, autoimmune diseases and various types of cancer
(7). Jagged 1 (JAG1) is the most
highly expressed Notch ligand during skeletal development and
healing, and participates in the regulation of bone metabolism in a
compartment-dependent manner.
T-cell immunoglobulin and mucin domain-containing 3
(TIM3; also know as hepatitis A virus cellular receptor 2) is a
negative regulator of T cells. TIM3+ HSCs in MDS display
aberrant differentiation, over-proliferation and decreased
apoptosis (8).
Research regarding osteoblasts in MDS patients is
scarce. The present study aimed to address whether any changes in
osteoblasts occurred in patients with MDS, and to assess their role
in the bone marrow niche. The quantity and function of osteoblasts
and osteoprogenitors were investigated; furthermore, the
associations between changes in osteoblasts and the course of MDS,
and the pathogenesis of the interactions between osteoblasts and
bone marrow niches in MDS were explored.
Materials and methods
Patients
A total of 38 untreated patients (20 males and 18
females; median age, 63 years; age range, 27–77 years) with MDS,
who were treated at the Hematology Department of Tianjin Medical
University General Hospital (Tianjin, China) between September 2015
and September 2016 were enrolled in the present study (Table I). Patients were diagnosed according
to the World Health Organization (WHO) classification (9) and were divided in two groups according
to the WHO Classification-based Prognostic Scoring System (WPSS)
score. Group 1 (18 patients) consisted of patients with a WPSS
score of 0–2, and Group 2 (20 patients) consisted of patients with
a WPSS score of 3–6 (10). In
addition, 25 healthy donors (10 males and 15 females; median age,
56 years; age range, 19–72 years) were enrolled as controls. The
ethics committee of Tianjin Medical University General Hospital
approved this study. All patients provided written informed consent
for the use of their clinical specimens for medical research.
 | Table I.Characteristics of the patients with
MDS enrolled in this study (n=38). |
Table I.
Characteristics of the patients with
MDS enrolled in this study (n=38).
| Characteristic | Value |
|---|
| Sex, n |
|
| Male | 20 |
|
Female | 18 |
| Age, years |
|
|
Median | 63 |
|
Range | 27–77 |
| Cytogenetics |
|
| +8 | 2 |
| −7 | 1 |
|
20q- | 1 |
| Type of MDS, n
(%) |
|
|
RCUD | 1 (2.6) |
|
RARS | 2 (5.3) |
|
RCMD | 10 (26.3) |
|
RAEB-1 | 16 (42.1) |
|
RAEB-2 | 9
(23.7) |
| WPSS score, n
(%) |
|
| 0 | 0 |
| 1 | 3 (7.9) |
| 2 | 15 (39.5) |
| 3 | 10 (26.3) |
| 4 | 10 (26.3) |
|
5–6 | 0 |
Cell culture
A total of 5 ml fresh bone marrow specimens were
collected in heparin-anticoagulant tubes from patients with MDS as
specified previously. Specimens were added to Ficoll® PM
400 Histopaque®−1077 medium (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 5 ml and centrifuged (700 × g) for 20 min
at room temperature. Following centrifugation, there was obvious
stratification: The upper layer was the plasma, the middle was the
transparent separation liquid, and the buffy coat between the
plasma and the separation liquid was the bone marrow mononuclear
cells (BMMNC), collecting the buffy coat carefully with sterile
pipettes. The BMMNC were cultured at a density of 3×105
cells/cm2 in 6-well culture plates with Dulbecco's
modified Eagle's medium (DMEM), 15% fetal bovine serum (FBS) (both
from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
1×10−7 mmol/l dexamethasone, 0.01 mol/l
β-glycerophosphate, 0.05 g/l vitamin C (all from Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), 100 U/l penicillin and phytomycin
(Gibco; Thermo Fisher Scientific, Inc.), at 37°C with 5%
CO2. Cultures were refreshed by replacing the medium
bi-weekly. Until confluence, the cultures were isolated with 0.5 ml
0.25% parenzyme (Gibco; Thermo Fisher Scientific, Inc.) each well
for 3 min and subcultured at a density of 1×104
cells/cm2 in 6-well culture plates under identical
conditions. Following two subcultures, osteoblasts differentiated
from bone marrow were obtained. Osteoblasts were collected on the
6th day follwoing two subcultures. Osteoblasts from each well of
the culture plates were placed into 1 ml buffer (PBS plus 2% FBS).
The numbers of cells were determined under a light microscope (×100
magnification) (BX53; Olympus Corporation, Tokyo, Japan). Cultures
were observed by inverted microscopy, with images captured daily. A
cell growth curve was drawn to calculate the cell doubling
time.
Immunohistochemical staining
Following two subcultures, osteoblasts were cultured
at a density of 1×105 cells/cm2 in 6-well
culture plates with cover slips for 3 days. Following washing with
PBS, the osteoblasts on cover slips were identified by type-I
collagen staining and intracellular alkaline phosphatase (ALP)
activity (qualitatively and quantitatively assessed by fast violet
staining). Osteoblasts showing mineralization (identified by von
Kossa staining) were also measured.
Type-I collagen staining
Osteoblasts were fixed by 10% formaldehyde for 15
min at 4°C, washed with Hank's balanced salt solution (HBSS) for 5
min, stained at 4°C with rabbit anti-human collagen type I antibody
(cat. no. BS-0578R; Bioss, Beijing, China; dilution, 1:100) for 30
min and developed with an SP kit, according to the manufacturer's
protocol (cat. no. SP-0023; Zymed, Waltham, MA, USA). Under light
microscopy, brown-yellow was considered to indicate a positive
result.
ALP staining
Osteoblasts were fixed in 10% formaldehyde for 15
min at 4°C and washed with distilled water for 5 min. Staining was
performed according to the ALP Kit (no. 507N; Sunbio, Beijing,
China). Under light microscopy, brown-red was considered to
indicate a positive result.
von Kossa staining
Following a 3-week culture, osteoblasts were fixed
in 10% formaldehyde for 1 h, washed with distilled water for 5 min,
and fixed in 10% formaldehyde for 1 h; 2% silver nitrate was then
added and the cells were incubated in the dark at room temperature
for 30 min. Finally, the cells were washed with distilled water for
5 min and exposure to sunlight and left to air-dry for 15 min.
Under light microscopy, black nodules were considered to indicate a
positive result.
Flow cytometry (FCM)
Bone marrow samples (0.5 ml) were collected in
heparin sodium-anticoagulant tubes then incubated with 1 ml
erythrocyte lytic solution (BD Biosciences, Franklin Lakes, NJ,
USA) at room temperature for 10 min, then washed twice with PBS.
Osteoblasts cultured in vitro were harvested. At harvesting,
0.5 ml 0.25% parenzyme (Gibco; Thermo Fisher Scientific, Inc.) was
added to each well for 3 min, and then 1 ml PBS was added.
Following gentle mixing with a plastic pipette. Cells were washed
with PBS three times, the samples remained suspended in PBS and
were incubated with 5 µg mouse anti-human direct-labeled
immunoglobulin G antibodies. All antibodies were incubated at 4°C
for 30 min in the dark. The dilution of all direct-antibodies were
used at 1:10. No secondary antibody was used in this experiment.
Anti-CD34-PerCP (cat. no. 555823 BD Biosciences), anti-Lin-FITC
(cat. no. 562722 BD Biosciences) and anti-OCN-allophycocyanin (APC)
(cat. no. 557833 BD Biosciences) staining was used to measure the
normal osteogenic function by osteoblasts cultured in vitro.
Anti-CD34-PerCP (cat. no. 555823 BD Biosciences) and anti-OCN-PE
(cat. no. 564146, BD Biosciences) staining was used to identify
osteoprogenitor cells in the bone marrow. The percentages of
JAG1+ osteoblasts and TIM3+ HSCs were
measured by FCM using anti-JAG1-PE (cat. no. 565495 BD Biosciences)
and anti-TIM3-PE antibodies (cat. no. 563422, BD Biosciences). A
minimum of 30,000 cells were acquired. Data acquisition and
analysis were performed using fluorescence-activated cell sorting
analysis (FACS; FACScalibur™; BD Biosciences) and
CellQuest™ software version 6.0 (BD Biosciences).
Statistical analysis
All statistical analyses were performed using SPSS
19.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as
the mean ± standard deviation. Groups of data were compared by
Student's t-test or one-way analysis of variance (ANOVA). Multi
group comparisons of the means were carried out by one-way ANOVA
test with post hoc contrasts by Student-Newman-Keuls test.
Pearson's correlation analysis was used to assess the correlations
between the activity of osteoblasts and severity of MDS P<0.05
was considered to indicate a statistically significant
difference.
Results
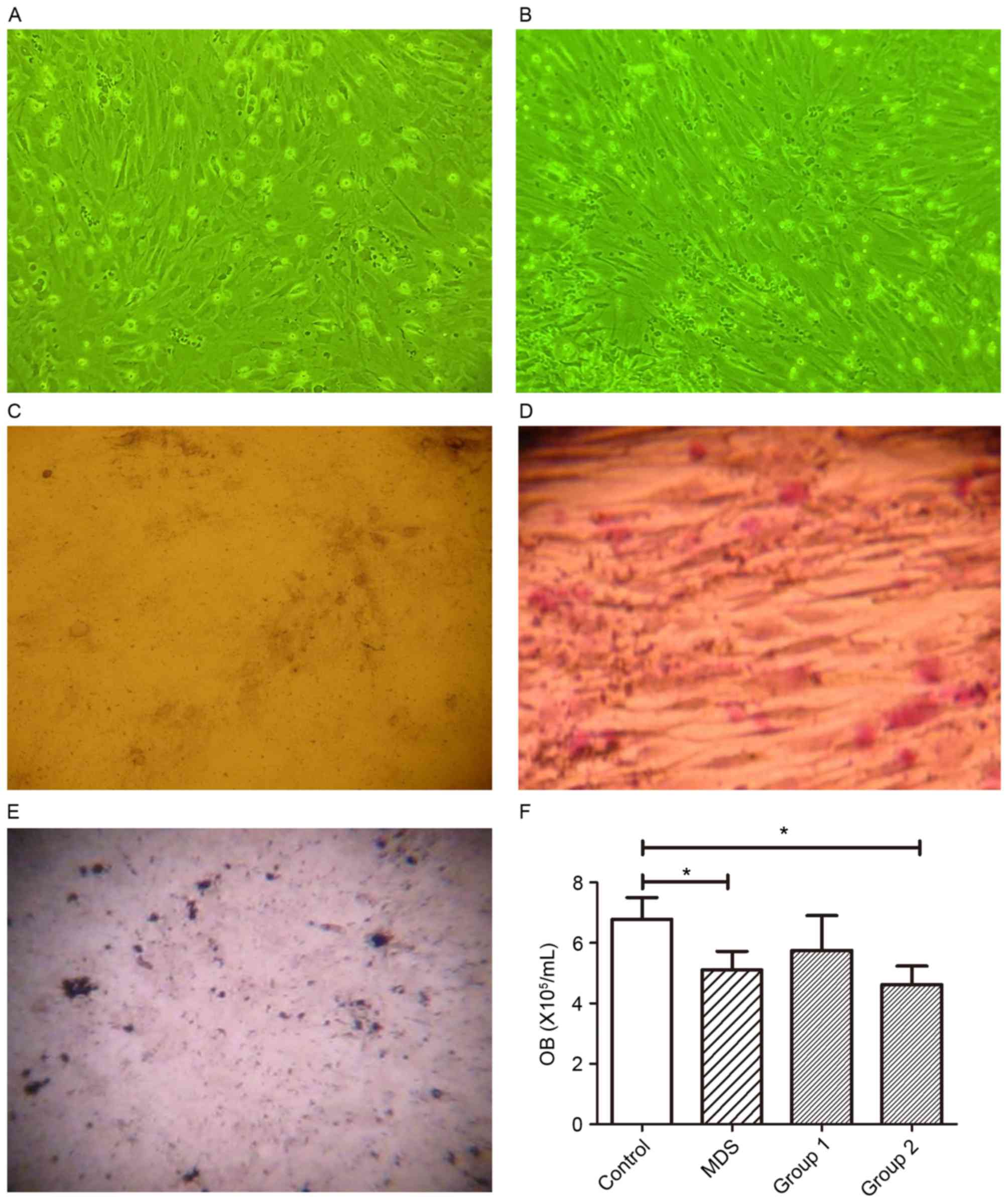
Osteoprogenitors are significantly
fewer in MDS patients than in normal controls
The phenotypes and growth characteristics of
osteoblasts are shown in Fig. 1. The
osteoblasts were characterized as large, fusiform and as growing in
parallel or spiral patterns. Cells adhered in 24 h and the cell
doubling time was ~36 h. After 2 weeks, the cells concentrated and
formed nodes. The quantity of osteoblasts from normal controls were
increased compared with that of osteoblasts from MDS patients
(Fig. 1A and B). Type-I collagen
staining (Fig. 1C), ALP staining
(Fig. 1D) and von Kossa staining
(indicating mineralization) (Fig. 1E)
were positive, thereby identifying osteoblasts. The quantity of
osteoblasts from patients with MDS (5.11±0.61×105/ml)
and Group 2 (4.62±0.62×105/ml) was less than that of
normal controls (6.79±0.79×105/ml) (P<0.05), whereas
that of osteoblasts from Group 1 (5.75±1.16×105/ml) was
close to that of normal controls (P>0.05) (Fig. 1F).
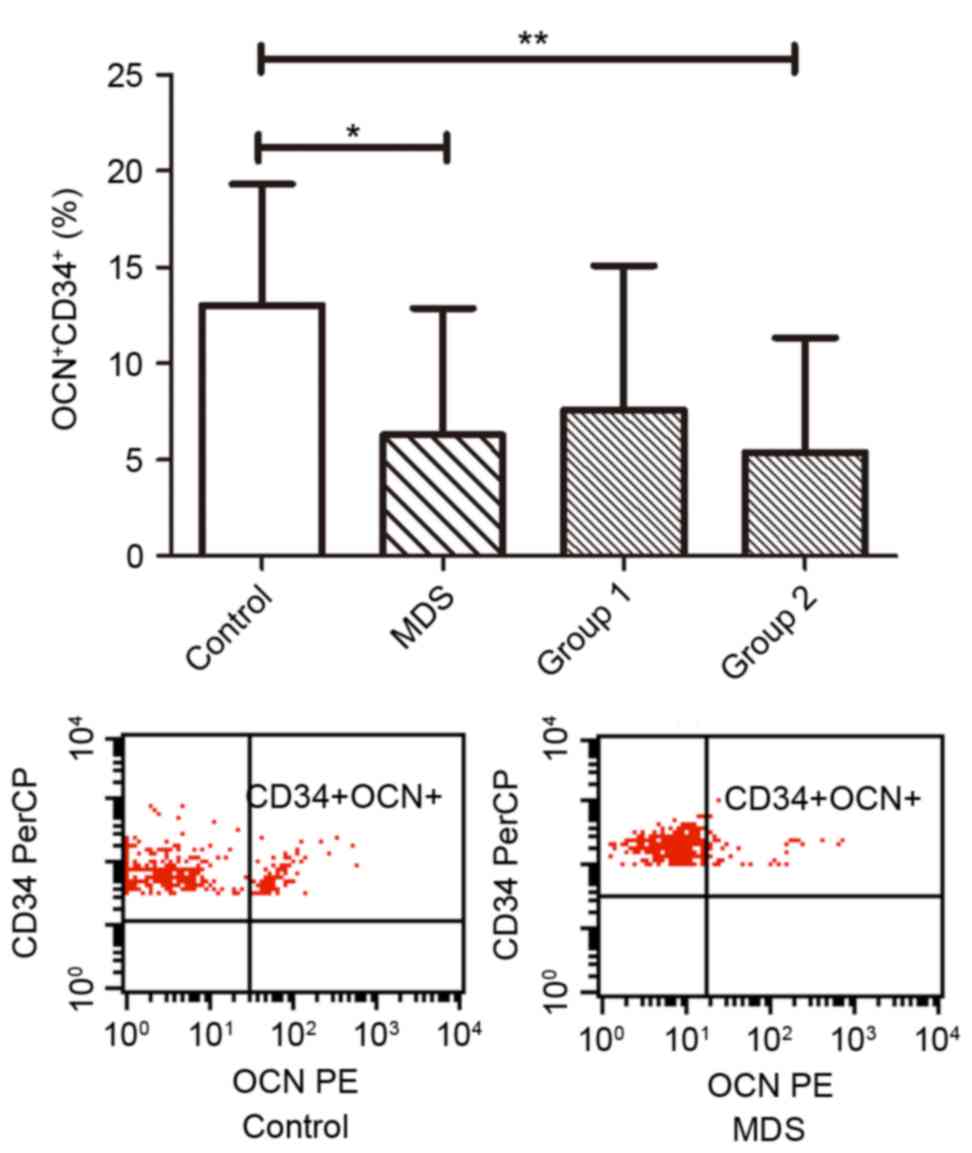
The percentage of osteoprogenitors
(CD34+OCN+) out of all the CD34+
cells in patients with MDS was also measured (Fig. 2). The ratio of osteoprogenitors in MDS
patients (6.29±1.27%) was less than that in normal controls
(13.01±1.26%; P<0.05). The percentage of osteoprogenitors in
Group 1 (7.59±2.26%) was not significantly different from that in
normal controls (P>0.05); however, Group 2 exhibited a
significantly decreased percentage (5.39±1.49%) as compared with
normal controls (P<0.01; Fig.
2).
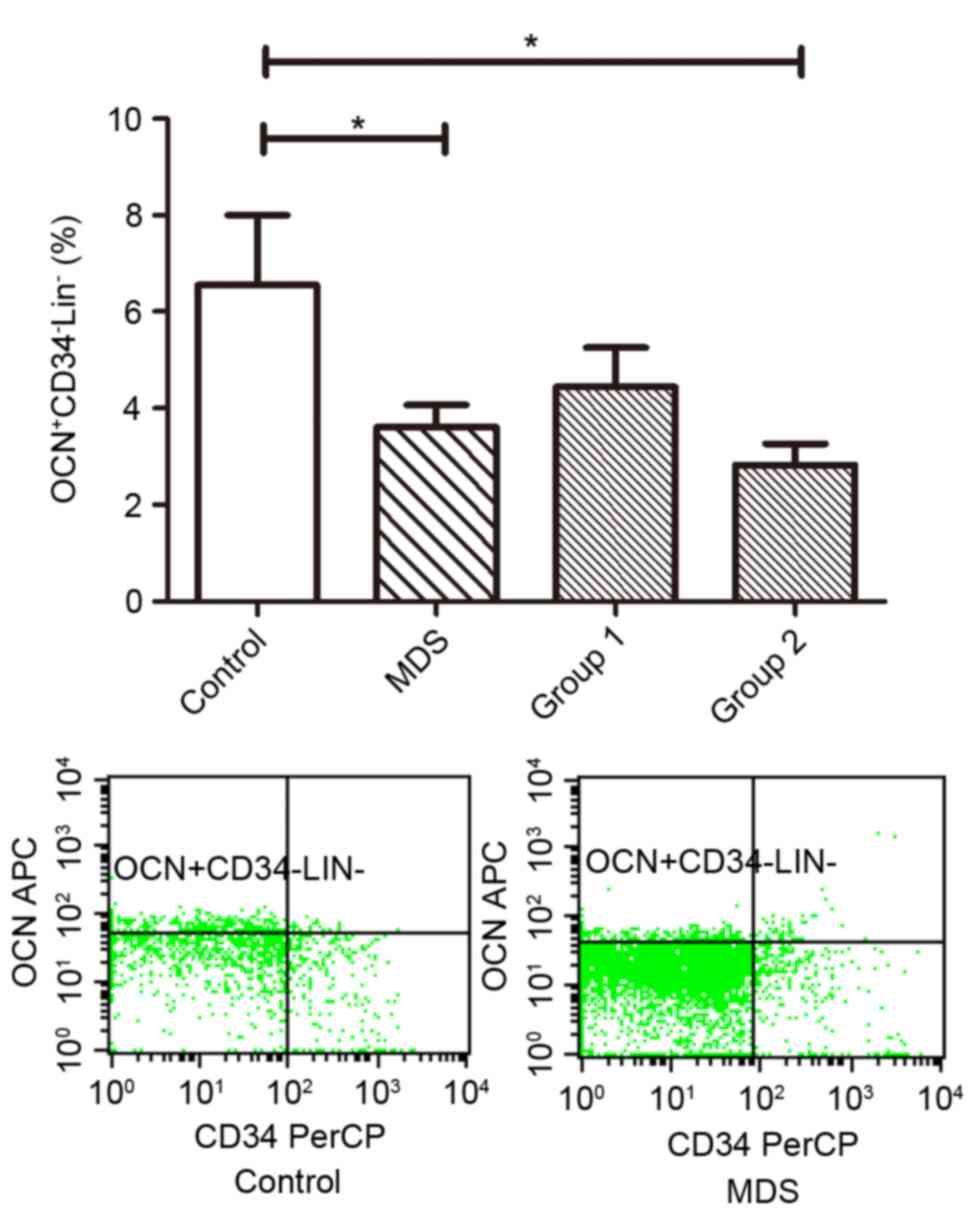
Osteoblast activity is significantly
downregulated in patients with MDS and significantly correlates
with the severity of MDS
The ratio of
OCN+CD34−Lin− osteoblasts of MDS
patients (3.61±0.47%) was less than that in normal controls
(6.55±1.46%; P<0.05). Group 1 (4.67±0.85%) did not differ
significantly from normal controls (P>0.05), whereas Group 2
(2.83±0.43%) showed a significant decrease in
OCN+CD34−Lin− osteoblasts compared
with normal controls (P<0.05; Fig.
3).
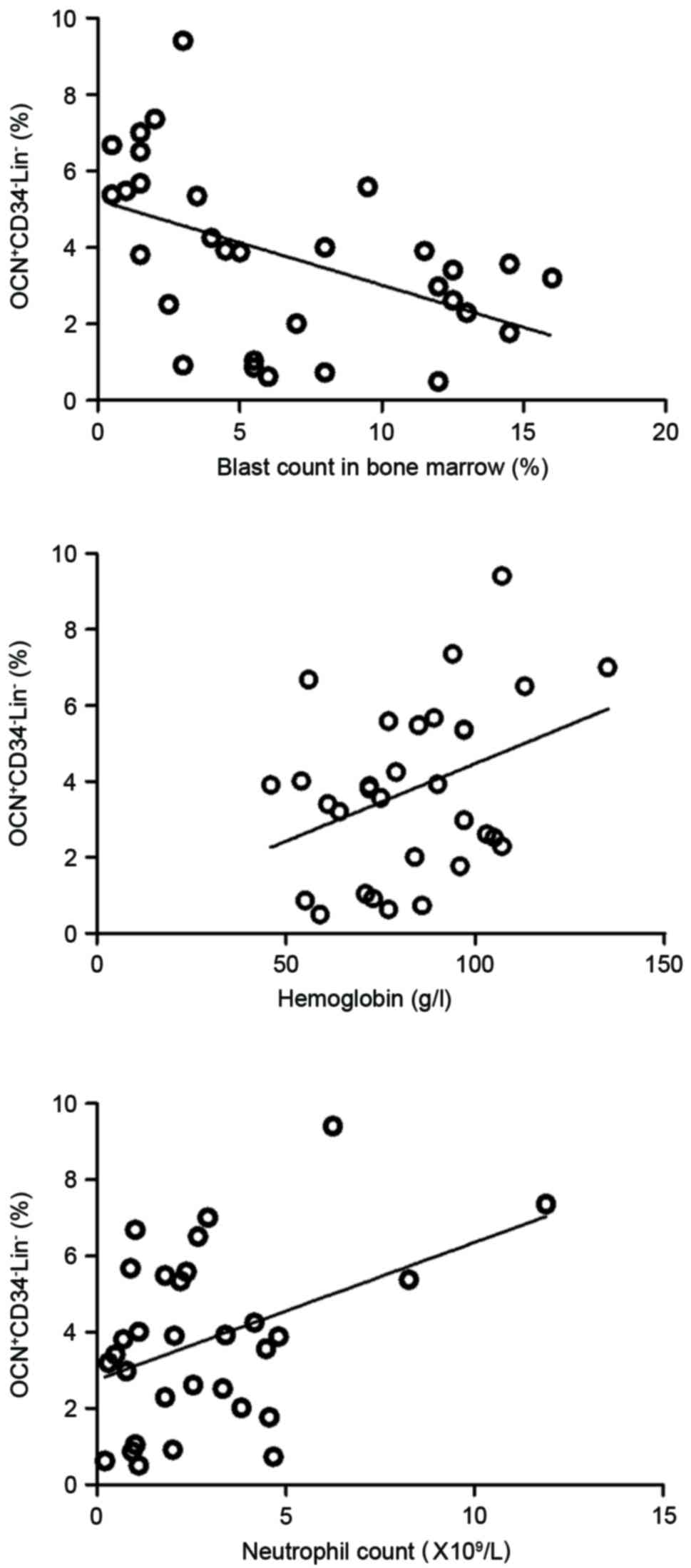
This study also observed the association between the
activity of osteoblasts and severity of MDS. The results of this
study demonstrated that the ratio of
OCN+CD34−Lin− osteoblasts in MDS
patients was negatively correlated with the blast count in the bone
marrow smear (r=−0.480, P<0.01), and was positively correlated
with hemoglobin level (r=0.367, P<0.05) and neutrophil count
(r=0.402, P<0.05; Fig. 4) in the
peripheral blood.
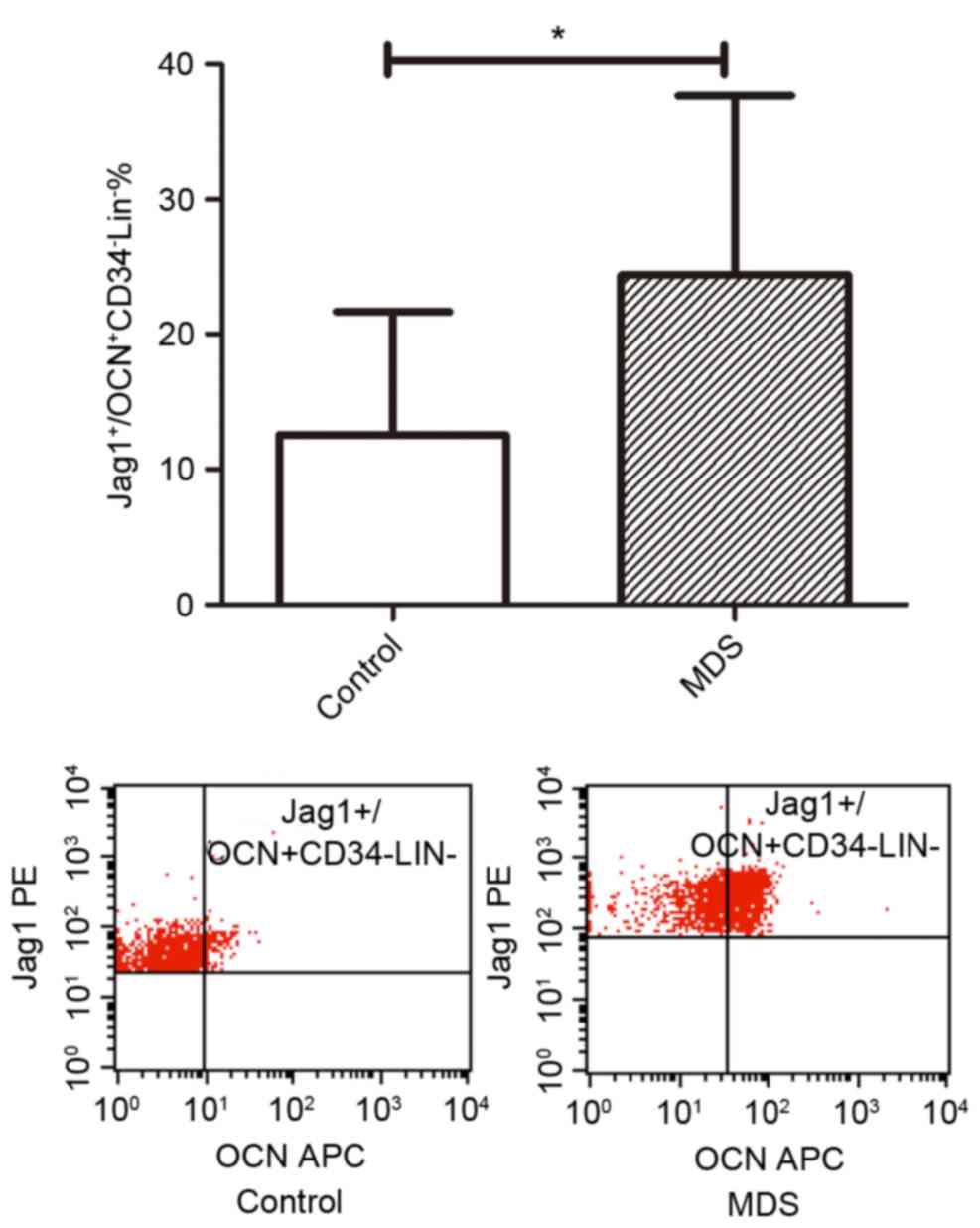
TIM3 and JAG1 are overexpressed on
osteoblasts from patients with MDS
The percentage of JAG1+
OCN+CD34−Lin−cells out of the
total number of osteoblasts in patients with MDS (24.34±3.55%) were
higher than that of normal controls (12.54±3.04%; P<0.05;
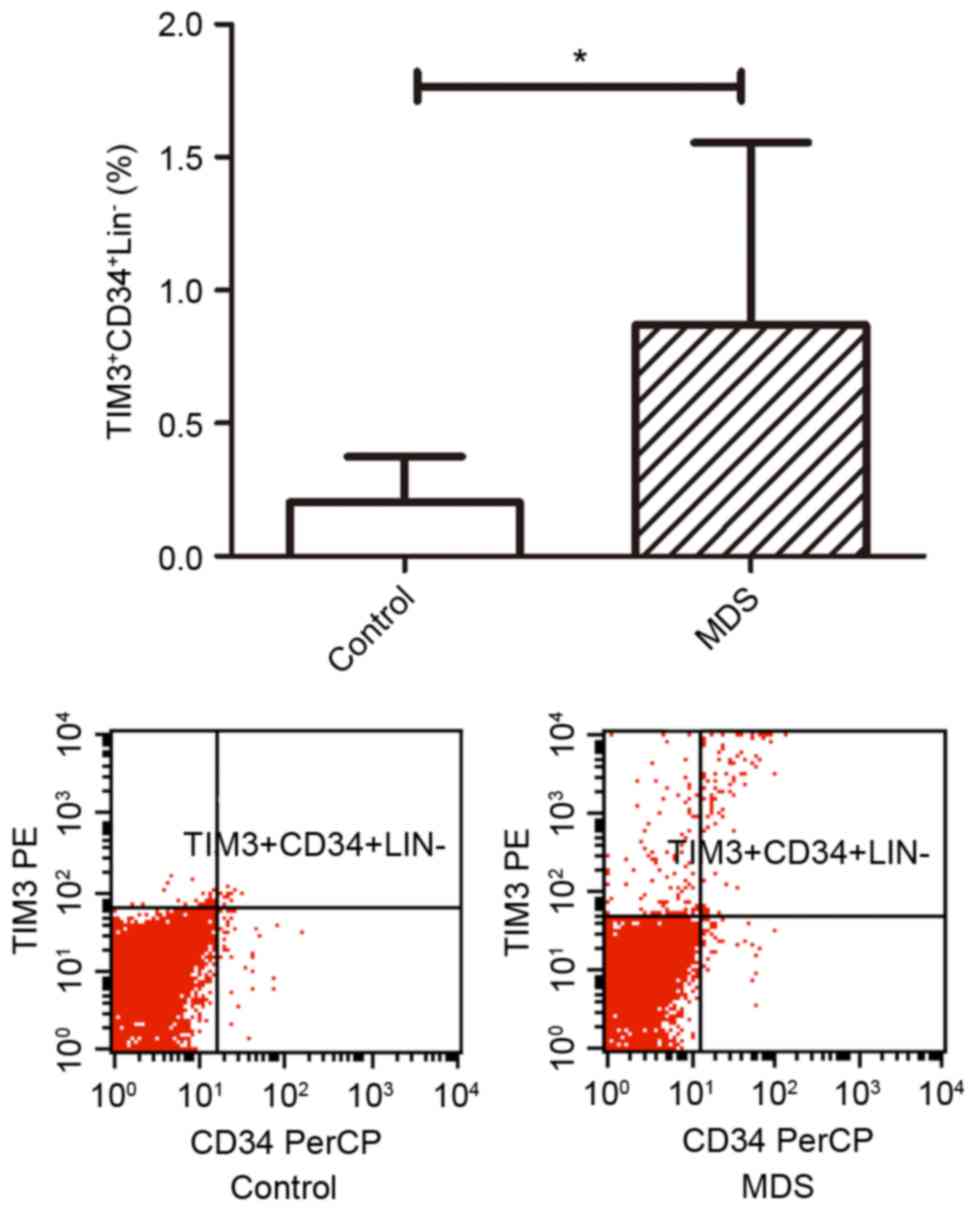
Fig. 5). In addition, the percentage
of TIM3+CD34+Lin− cells out of
osteoblasts in patients with MDS (0.87±0.23%) was higher than that
of normal controls (0.20±0.07%; P<0.05; Fig. 6).
Discussion
Approximately 30% of MDS patients develop acute
leukemia, and the bone marrow niche is associated with the
apoptosis, proliferation and migration of leukemia cells.
Osteoblasts serve an important role in the stability of normal HSCs
via intracellular expression of JAG1 and other cytokines. Schepers
et al (11) indicated that
osteoblasts were remodeled in myeloproliferative neoplasia.
Following abnormal remodeling, osteoblasts could better support
tumor cell survival, but inhibited normal HSCs significantly. The
present study used CD34+OCN+ as a marker for
osteoprogenitor cells (12,13), and identified that the percentage of
osteoprogenitors was significantly decreased in MDS patients,
thereby demonstrating that the differentiation pathway of HSC into
osteoprogenitors may be abnormal in MDS.
A previous study demonstrated that dexamethasone,
β-glycerophosphate and vitamin C could induce the differentiation
of osteoblasts from bone marrow mesenchymal stem cells and their
osteogenic function in vitro (14). In the present study, bone marrow cells
in culture were induced to differentiate into osteoblasts by a
nutrient solution with conditional factors such as dexamethasone,
β-glycerophosphate and vitamin C in vitro. Patients in Group
1 were those with low- and intermediate-risk MDS (WPSS score 0–2),
and patients in Group 2 had high- and very high-risk MDS (WPSS
score 3–6). The results demonstrated osteoblasts were decreased in
patients with MDS especially in high-risk MDS, when compared with
the normal control group.
OCN reflects the mineralization ability of
osteoblasts. Carboxylated OCN, produced by bone-forming
osteoblasts, has a high binding affinity for mineralized bone
matrix (4,15). The present study used
OCN+CD34−Lin− as a marker for
mineralization and osteogenic potential of mature osteoblasts in
vitro. The quantity and osteogenic potential of osteoblasts in
MDS was lower, as indicated by a significantly decreased percentage
of OCN+CD34−Lin− cells. The
quantity and activity of osteoblasts in low-risk MDS patients was
close to normal levels. Furthermore, the ratio of
OCN+CD34−Lin− osteoblasts was
negatively correlated with the blast count in the bone marrow and
positively correlated with hemoglobin level and neutrophil count in
the peripheral blood of the patients. The association between
higher blast count in the bone marrow and decreased activity of
osteoblasts demonstrated that there is a close association between
the activity of osteoblasts in MDS patients and the severity of
MDS.
In MDS, Notch signaling serves an important role in
the development of drug resistance. Activation of this pathway
occurs when a Notch ligand (JAG1 or 2, or Delta-like 1, 3 or 4)
expressed on the surface of a signaling cell interacts with a Notch
receptor (Notch 1–4) expressed on the surface of a receiving cell
(16). Activating mutations of
β-catenin in osteoblasts, which are commonly identified in patients
with MDS, lead to increased synthesis of the Notch ligand JAG1,
which in turn activates Notch signaling in HSCs, leading to
alteration of the differentiation potential of hematopoietic
progenitors and acute myeloid leukemia development (17). In the present study, the Notch ligand
JAG1 was found to be increased in osteoblasts from MDS patients,
indicating that the Notch pathway in osteoblasts was altered
abnormally in MDS.
Bone formation and the immune system are closely
associated through cellular and molecular interactions (18). The interaction of TIM3 with its
ligand, Galectin-9, promotes apoptosis of T-helper 1 cells, and
induces CD8+ T cell exhaustion with decreased production
of interferon γ; it also induces the expansion of myeloid-derived
suppressor cells, which suppress immune responses indirectly. TIM3,
as a negative regulator of anti-tumor immunity (19), is highly expressed on HSCs in MDS
patients, and the high level of TIM3 expression on HSCs in MDS
patients is closely associated with the WPSS score (20). In the present study, the percentage of
TIM3+CD34+Lin− cells among the
osteoblasts of MDS patients was higher than that of normal
controls. The results showed the osteoblasts and HSC were
homologous in bone marrow niche, and that the differentiation of
osteoblasts is abnormal in MDS. The osteoblasts with abnormal
immune expression may be one of the reasons the bone marrow niche
is affected in patients with MDS.
In conclusion, the quantity of osteoprogenitors in
the bone marrow of MDS patients was decreased when compared with
that of normal controls. When cultured in vitro, the
quantity of osteoblasts derived from high- and very high-risk MDS
patients was decreased significantly. The osteogenic potential of
osteoblasts was also decreased in MDS patients compared with normal
controls, particularly those patients with high- and very high-risk
MDS (WPSS score 3–6). The activity of osteoblasts from patients was
correlated with the severity of MDS. JAG1 and TIM3 were highly
expressed on the osteoblasts in vitro. Osteoblasts and HSCs
are homologous in the bone marrow niche of MDS patients. These
results indicated that, as an important part of the bone marrow
niche, osteoblasts were abnormal in MDS. Certain abnormal changes
were associated with the severity of MDS. Further study is required
in order to determine whether the bone marrow niche can be altered
by correcting the abnormal activity of osteoblasts, which can be
used to assist the treatment of MDS.
Acknowledgements
Not applicable.
Funding
This work was partly supported by the National
Natural Science Foundation of China (grant nos. 81570111, 81500101,
81400088, 81570106 and 81370607), the Natural Science Foundation of
Tianjin (grant nos. 14JCYBJC27200 and 15JCYBJC24300), and the
Tianjin Anti-Cancer Major Special Research Program (grant no.
12ZCDZSY17900).
Availability of data and materials
The datasets supporting the conclusions of this
article are included within the article and its accompanying
images.
Authors' contributions
SG carried out the cell culture and statistical
analyses and drafted the manuscript. SG, HY, JT and CL conducted
the flow cytometry. HW and HJ assisted in the design of the study
and performed the statistical analysis. RF oversaw the collection
of the data and performed the statistical analysis. ZS conceived
the study, participated in its design and coordination, and helped
to draft the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The ethics committee of Tianjin Medical University
General Hospital approved this study. All patients provided written
informed consent for the use of their clinical specimens for
medical research.
Patient consent for publication
All of the patients in this study provided consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bulycheva E, Rauner M, Medyouf H, Theurl
I, Bornhäuser M, Hofbauer LC and Platzbecker U: Myelodysplasia is
in the niche: Novel concepts and emerging therapies. Leukemia.
29:259–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Méndez-Ferrer S, Michurina TV, Ferraro F,
Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A,
Enikolopov GN and Frenette PS: Mesenchymal and haematopoietic stem
cells form a unique bone marrow niche. Nature. 466:829–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arai F and Suda T: Maintenance of
quiescent hematopoietic stem cells in the osteoblastic niche. Ann N
Y Acad Sci. 1106:41–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bowers M, Zhang B, Ho Y, Agarwal P, Chen
CC and Bhatia R: Osteoblast ablation reduces normal long-term
hematopoietic stem cell self-renewal but accelerates leukemia
development. Blood. 125:2678–2688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raaijmakers MH, Mukherjee S, Guo S, Zhang
S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian
RP, Scadden EO, et al: Bone progenitor dysfunction induces
myelodysplasia and secondary leukaemia. Nature. 464:852–857. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brennan-Speranza TC and Conigrave AD:
Osteocalcin: An osteoblast-derived polypeptide hormone that
modulates whole body energy metabolism. Calcif Tissue Int. 96:1–10.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Youngstrom DW, Dishowitz MI, Bales CB,
Carr E, Mutyaba PL, Kozloff KM, Shitaye H, Hankenson KD and Loomes
KM: Jagged1 expression by osteoblast-lineage cells regulates
trabecular bone mass and periosteal expansion in mice. Bone.
91:64–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakuishi K, Jayaraman P, Behar SM,
Anderson AC and Kuchroo VK: Emerging Tim-3 functions in
antimicrobial and tumor immunity. Trends Immunol. 32:345–349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A and Bloomfield CD: The 2008
revision of the World Health Organization (WHO) classification of
myeloid neoplasms and acute leukemia: Rationale and important
changes. Blood. 114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malcovati L, Della Porta MG, Strupp C,
Ambaglio I, Kuendgen A, Nachtkamp K, Travaglino E, Invernizzi R,
Pascutto C, Lazzarino M, et al: Impact of the degree of anemia on
the outcome of patients with myelodysplastic syndrome and its
integration into the WHO classification-based Prognostic Scoring
System (WPSS). Haematologica. 96:1433–1440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schepers K, Pietras EM, Reynaud D, Flach
J, Binnewies M, Garg T, Wagers AJ, Hsiao EC and Passegué E:
Myeloproliferative neoplasia remodels the endosteal bone marrow
niche into a self-reinforcing leukemic niche. Cell Stem Cell.
13:285–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manavalan JS, Cremers S, Dempster DW, Zhou
H, Dworakowski E, Kode A, Kousteni S and Rubin MR: Circulating
osteogenic precursor cells in type 2 diabetes mellitus. J Clin
Endocrinol Metabol. 97:3240–3250. 2012. View Article : Google Scholar
|
|
13
|
Fu R, Peng F, Liu H, Wang Y, Li L, Wang G,
Song J and Shao Z: Clinical significance of osteoblast precursors
and osteoclast precursors in earlier diagnosis and monitoring of
myeloma bone disease. Ann Hematol. 95:1099–1106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flynn CM and Kaufman DS: Donor cell
leukemia: Insight into cancer stem cells and the stem cell niche.
Blood. 109:2688–2692. 2007.PubMed/NCBI
|
|
15
|
Dong M, Jiao G, Liu H, Wu W, Li S, Wang Q,
Xu D, Li X, Liu H and Chen Y: Biological silicon stimulates
collagen Type 1 and osteocalcin synthesis in human osteoblast-like
cells through the BMP-2/Smad/RUNX2 signaling pathway. Biol Trace
Elem Res. 173:306–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grochowski CM, Loomes KM and Spinner NB:
Jagged1 (JAG1): Structure, expression, and disease associations.
Gene. 576:381–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kode A, Manavalan JS, Mosialou I, Bhagat
G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R,
et al: Leukaemogenesis induced by an activating β-catenin mutation
in osteoblasts. Nature. 506:240–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu R, Gao S, Peng F, Li J, Liu H, Wang H,
Xing L and Shao Z: Relationship between abnormal osteoblasts and
cellular immunity in multiple myeloma. Cancer Cell Int. 14:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakuishi K, Jayaraman P, Behar SM,
Anderson AC and Kuchroo VK: Emerging Tim-3 functions in
antimicrobial and tumor immunity. Trends Immunol. 32:345–349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tao JL, Li LJ, Fu R, Wang HQ, Jiang HJ,
Yue LZ, Zhang W, Liu H, Ruan EB, Qu W, et al: Elevated TIM3+
hematopoietic stem cells in untreated myelodysplastic syndrome
displayed aberrant differentiation, overproliferation and decreased
apoptosis. Leuk Res. 38:714–721. 2014. View Article : Google Scholar : PubMed/NCBI
|