Introduction
Primary carcinoma of the Bartholin's gland (BGC) is
an extremely rare malignancy accounting for <5% of all vulvar
malignancies and representing 0.001% of gynecological cancers in
the United States (1–3). Adenocarcinoma (ACC) and squamous cell
carcinoma (SCC) are the predominating types of carcinoma at this
site, occurring alongside ~80% of cases of BGC (equal frequency
between ACC and SCC) (4). Di Donato
et al (2017) conducted a literature search for all
manuscripts discussing BGC, and identified only 3 reported cases of
small cell neuroendocrine carcinoma (SCNC) in the Bartholin's gland
(BG) (5–7). SCNC progresses aggressively, producing
early local recurrences and distant metastases, resulting in
eventual patient mortality. The present case report details a case
of well-characterized SCNC that arose in BG associated with a later
hepatic metastasis following 6 months' chemotherapy treatment with
no local recurrence or distant metastasis. Continued studies of
diagnosis and treatment are required in order to improve management
of this rare malignancy.
Case report
A 56-year-old postmenopausal female presented with a
1-month history of increasing pain and swelling on the left vulva
with consistent bleeding. The patient first sought medical
attention at Shanghai Fengai Hospital in September 2016. A biopsy
demonstrated an ACC that arouse in BG. The maximum diameter of the
tumor was ~30 mm. The patient sought additional treatment at
Zhongshan Hospital of Fudan University (Shanghai, China) in
December 2016. Gynecological examination revealed a 30 mm
ulcerated, indurated lump involving the left labium majus. No
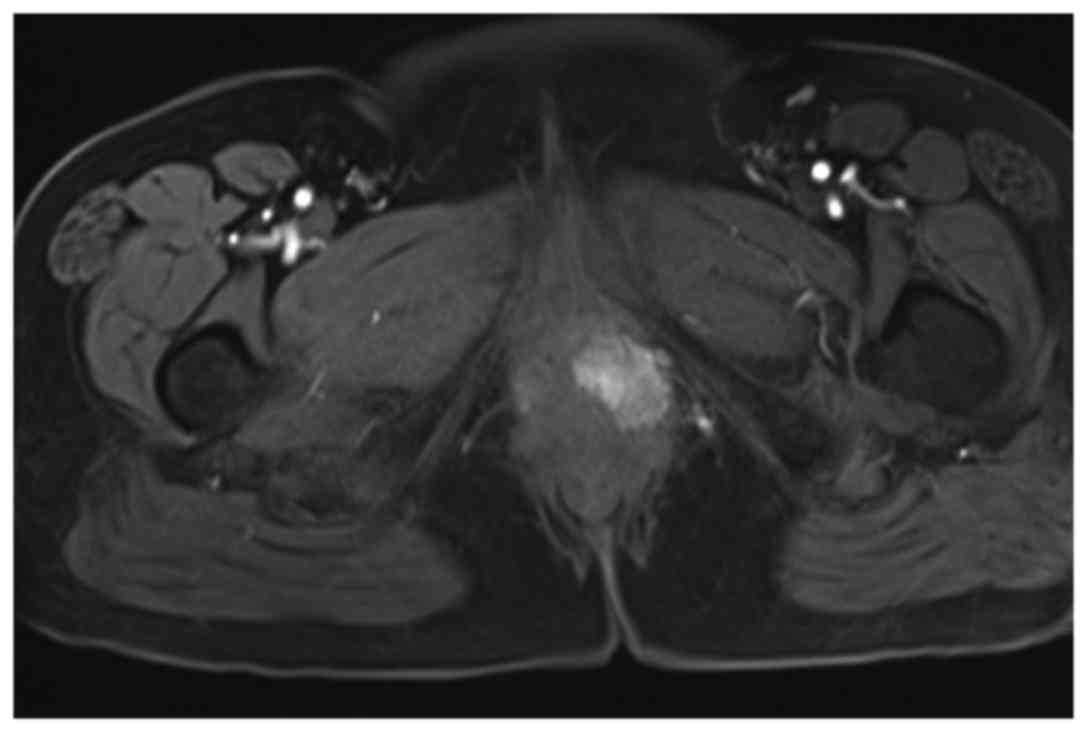
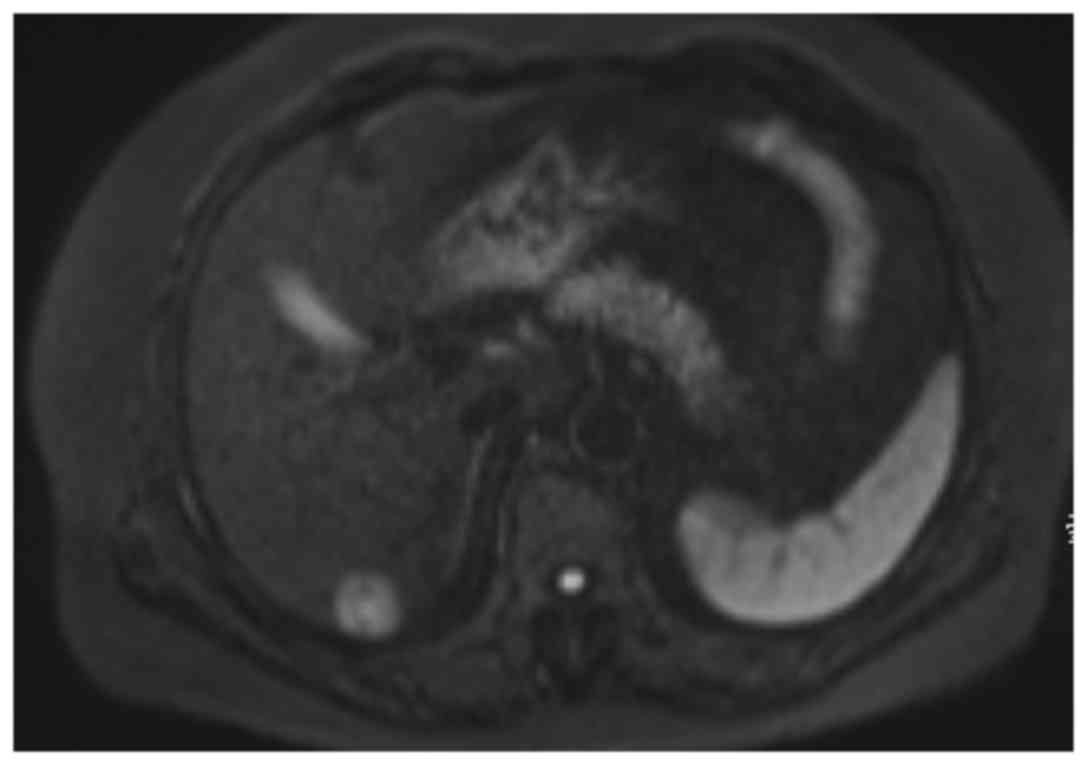
inguinal or supraclavicular nodes were palpable. Magnetic resonance
imaging of the pelvis identified a 30 mm solid mass with
intermediate signal on T1-weighted imaging and a slightly high
signal on T2-weighted imaging arising from the left BG (Fig. 1). A metastatic workup computed
tomography scan, which included examination of the whole torso, did
not demonstrate any metastatic disease. The patient's serum level
of neuron specific enolase (NSE) was also within the normal range
(normal level ~15.2 ng/ml). The biopsy specimen was reanalyzed by a
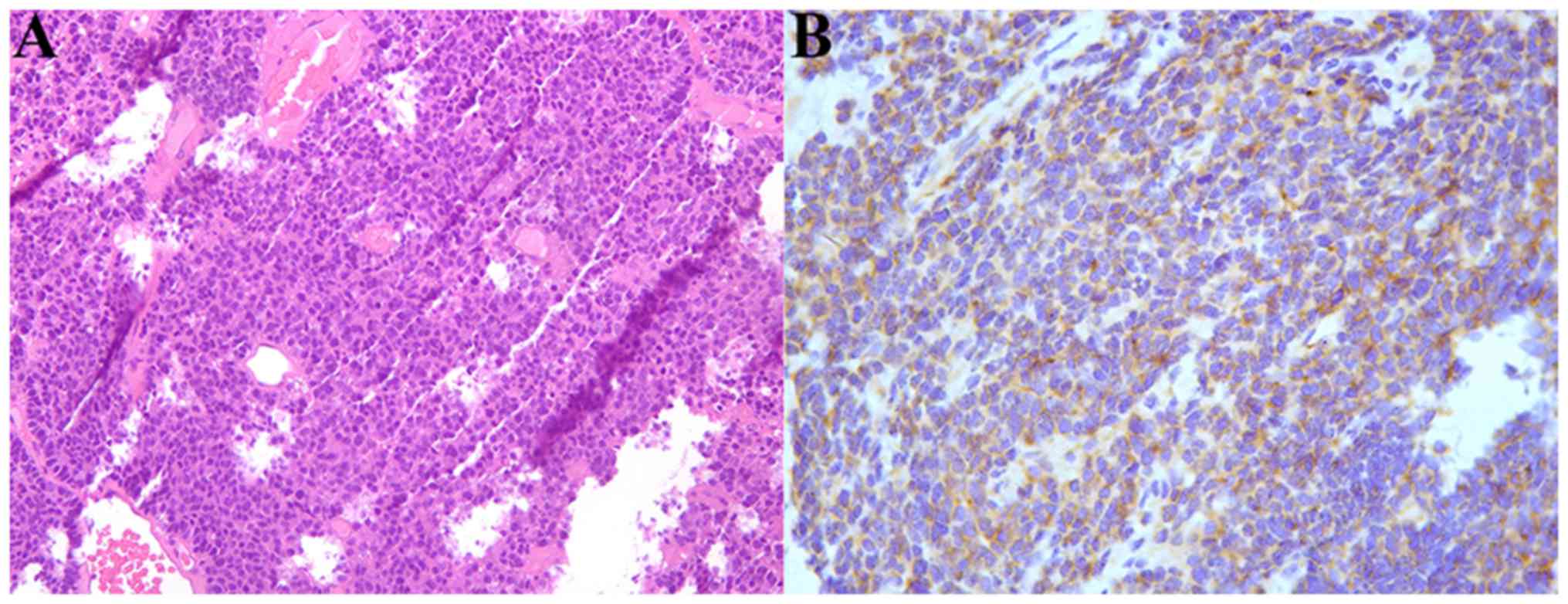
pathologist of Zhongshan Hospital of Fudan University and a SCNC
that arose in BG was identified (Fig.
2). The patient was surgically treated with a wide local
excision and a bilateral inguinal lymph node dissection. An
intraoperative frozen section was sent to the laboratory to confirm
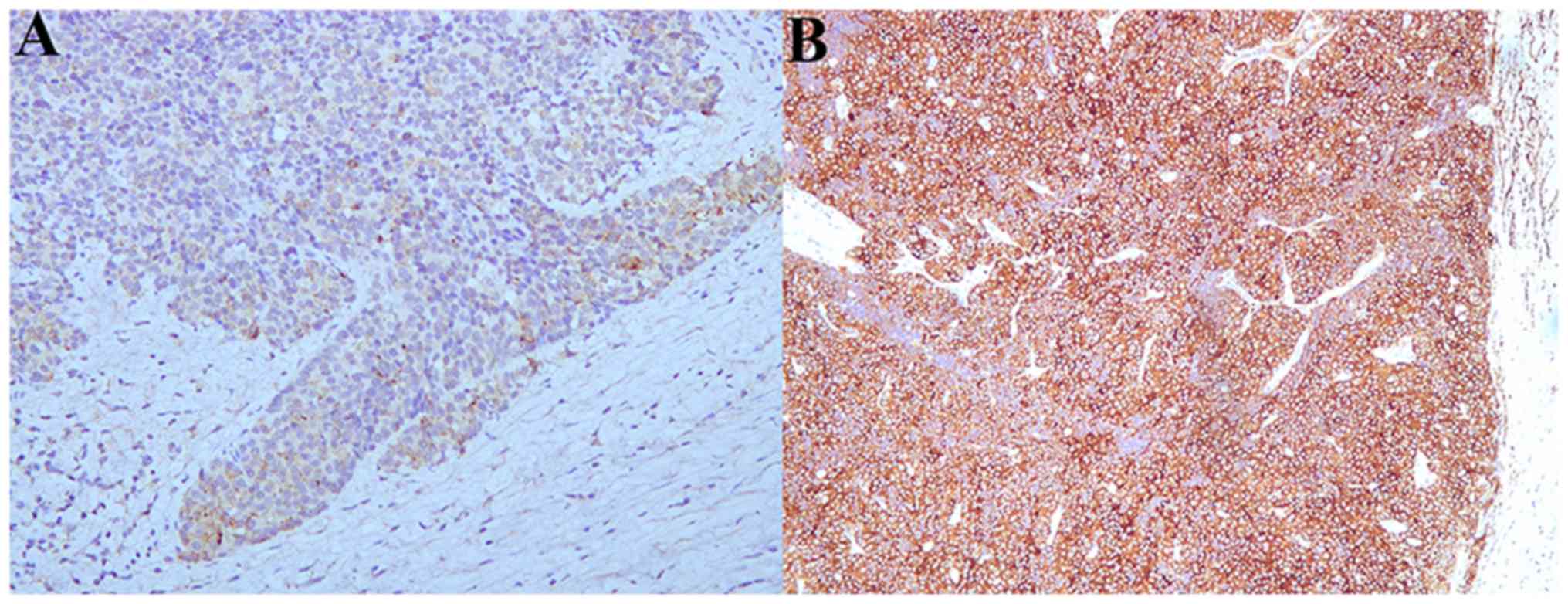
the free resection margin. Final pathology confirmed the diagnosis
of a SCNC of BG with free surgical margin (Fig. 3). No inguinal lymph nodes were
positive for metastatic tumor growth (Fig. 4). Following surgery, six courses of
adjuvant chemotherapy (cisplatin 2 mg/kg and etoposide 5 mg/kg,
every 21 days) were performed. After 1 month, the patient
maintained regular outpatient surveillance. Unfortunately, distant
metastasis was identified in June 2017. Magnetic Resonance
Cholangiopancreatography identified an 18 mm ovoid shape with low
signal on T1-weighted imaging and high signal on T2-weighted
imaging in the right hepatic lobe (Fig.
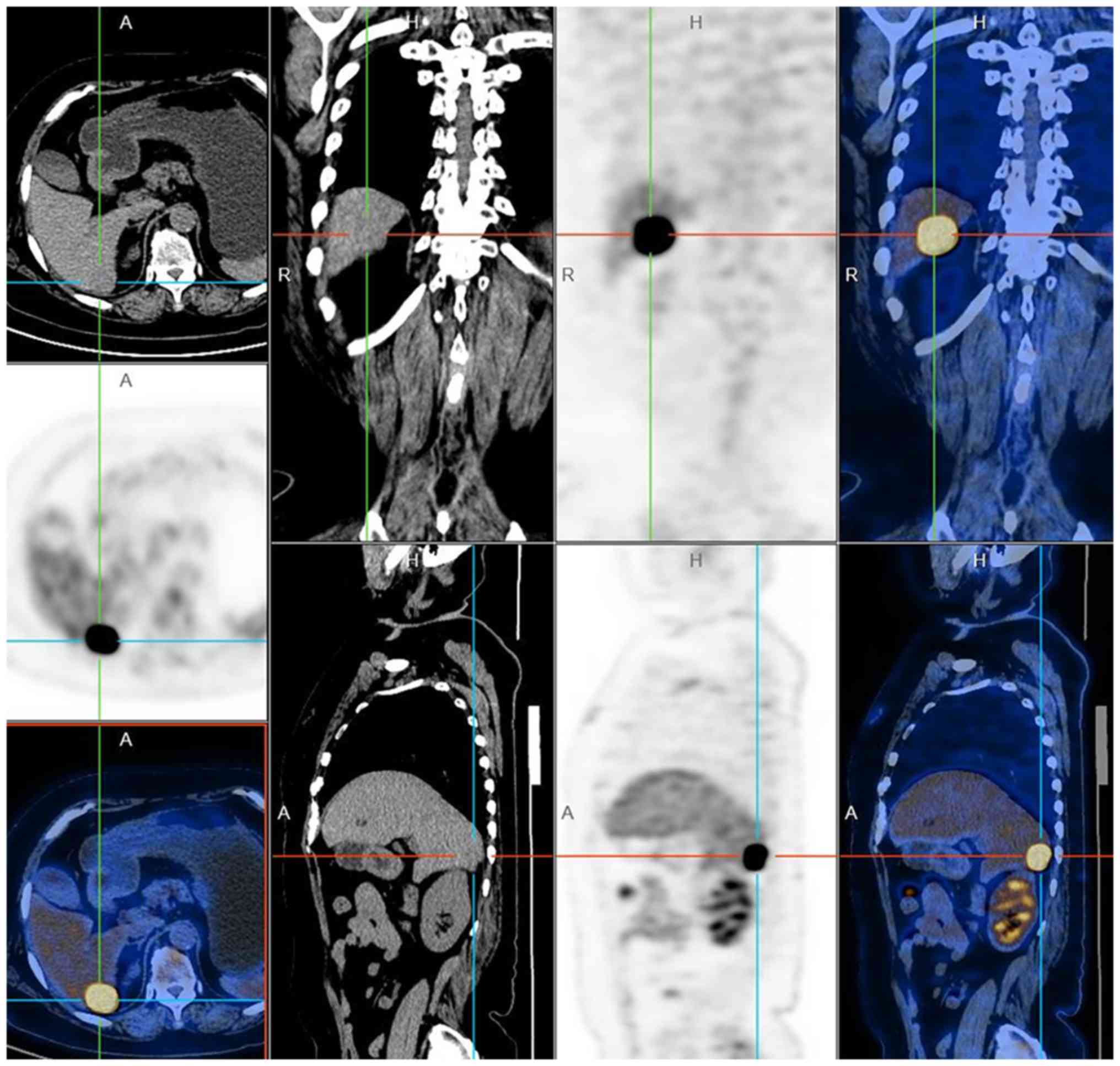
5). Positron emission tomography scanning detected
fluorodeoxyglucose-avid lesions in the right hepatic lobe and did
not demonstrate any additional distant disease including the local
vulva (Fig. 6). The serum NSE and
α-fetoprotein (AFP) were also in the normal range. A VI hepatic
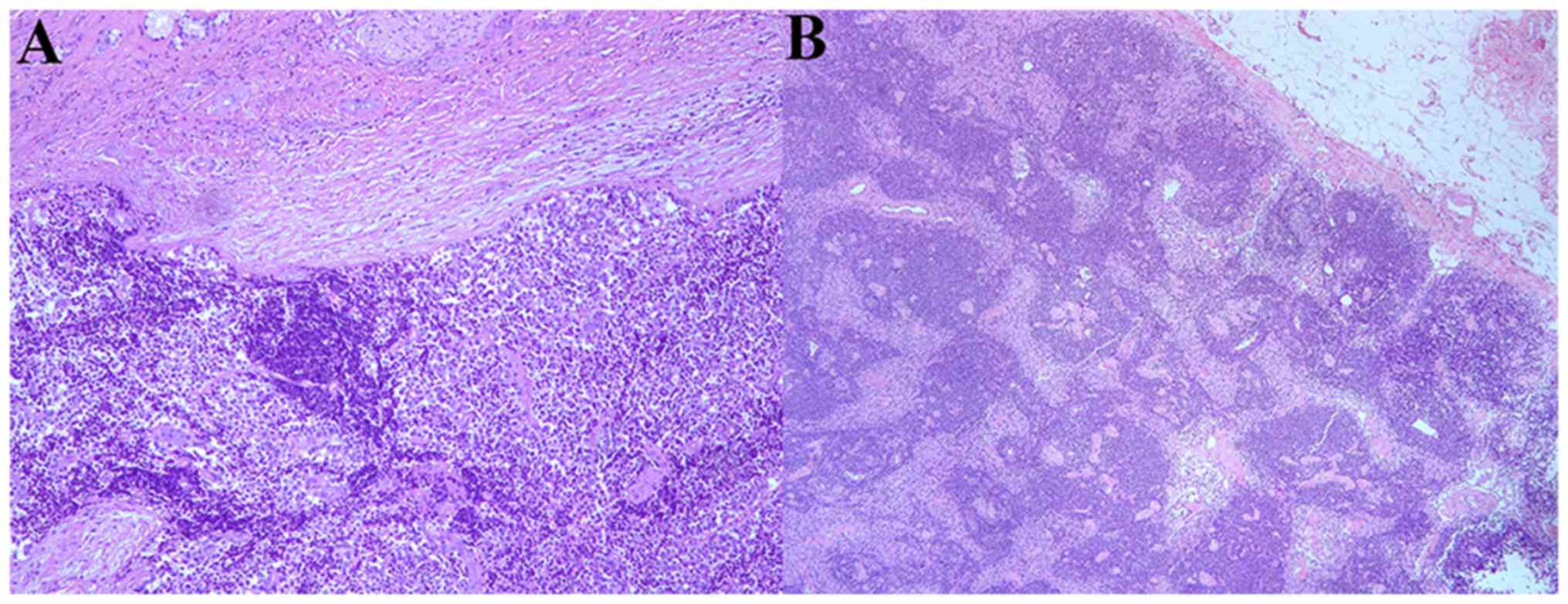
lobectomy was performed. Postoperative pathology identified a SCNC
that arose in BG with hepatic metastasis (Fig. 7). The patient was administered regular
adjuvant chemotherapy (using the treatment regimen described above)
every month under outpatient surveillance. The patient provided
written informed consent for the publication of their data.
Immunohistochemical procedures were performed as
follows: Tissue was fixed with 10% buffered formalin (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 30 min at room temperature,
then embedded in paraffin wax for 3 h at 60°C and cut into 5 µm
thick sections. Dewaxing was performed with 5% xylene (Thermo
Fisher Scientific, Inc.) for 20 min twice at room temperature, and
tissue sections were rehydrated through a graded series of ethanol
solutions (100, 95, 90 and 80% each for 5 min; Thermo Fisher
Scientific, USA). Endogenous peroxidase activity was blocked by
incubating slides in 1% H2O2 (Thermo Fisher
Scientific, Inc.) in methanol for 15–20 min at room temperature.
Slides were washed with running deionized water for 2 min.
Non-specific binding was blocked by incubating sections in 2.5%
fetal bovine serum (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) diluted in Tris-buffered saline (TBS; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) with 0.1% bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA) for 30 min at room temperature. Excess
serum was removed without washing and replaced with 100–300 µl
mouse monoclonal anti-SYN (1:100; cat. no. 4329), mouse monoclonal
CAM 5.2 (1:200; cat. no. 3362S), rat monoclonal CD20 (1:200; cat.
no. 23543) or rat monoclonal anti-CHG (1:100 cat. no. 85339; all
Cell Signaling Technology, Inc., Danvers, MA, USA) diluted in TBS
with 0.1% BSA. Samples were incubated at room temperature for 60
min in a humid chamber. Subsequent secondary antibodies, including
Cy3-conjugated goat anti-mouse, or anti-rat which were diluted in
TBS with 0.1% BSA (anti-mouse IgG, 1:300, cat. no. 4410; anti-rat
IgG, 1:400 or 1:500, cat. no. 4417; Cell Signaling Technology,
Inc.) was placed on the sections and incubated for 30 min at room
temperature, then washed off with PBS (Hyclone; GE Healthcare Life
Sciences) for 5 min twice and exposed to streptavidin-peroxidase
conjugate (Vector Laboratories, Ltd., Peterborough, UK) for 30 min
at room temperature. The bound antibody-peroxidase complexes on the
sections were visualized using a 3,3-diaminobenzidine
tetrahydrochloride (DAB; Cell Signaling Technology) substrate
solution consisting of 1.5 mg DAB and 50 µl of 30% hydrogen
peroxide in 10 ml of 0.1 M Tris-HCl (both Thermo Fisher Scientific,
Inc.), pH 7.6. The sections were incubated in the dark until brown
staining appeared for 12 h at 4°C, washed in PBS, counterstained
with hematoxylin, dehydrated, and mounted with Permount (both
Thermo Fisher Scientific, Inc.). A low-power view of the tumor
biopsy was obtained using a light microscope (magnification, ×20;
Thermo Fisher Scientific, Inc.).
Discussion
BGC is a rare malignancy and accounts for fewer than
5% of all vulvar carcinomas (7). SCNC
arising in BG is extremely rare, and only 3 cases have been
reported in English literature (3). A
foreign language literature search was not performed. Due to the
potentially aggressive behavior of SCNC, prompt diagnosis is
required (8). However, SCNC of the BG
is often diagnosed late since the lesions are deep within the vulva
and present with similar symptoms to most vulvar diseases,
including abscesses or cysts (9).
Patients usually complain of pain, swelling on the vulva,
dyspareunia and bleeding (10,11). In
cases where BGC is suspected, the clinical diagnostic criteria is
as follows: The tumor must be primarily located in the BG area; the
surrounding skin must be undamaged; areas of apparent transition
from normal to neoplastic elements must be observed; the
histological tumor type must be consistent with the BG origin;
there must be no evidence of a previous or subsequent primary tumor
of similar histologic type elsewhere (12,13). Di
Donato et al (7) collected all
published manuscripts regarding BGC and the median age of patients
was 52.99±13.94 years. Therefore, independent of whether a patient
is pre- or post-menopausal, every mass within the BG area should be
considered as a potential carcinoma until proven to be benign with
biopsies of adequate size and depth (4,14).
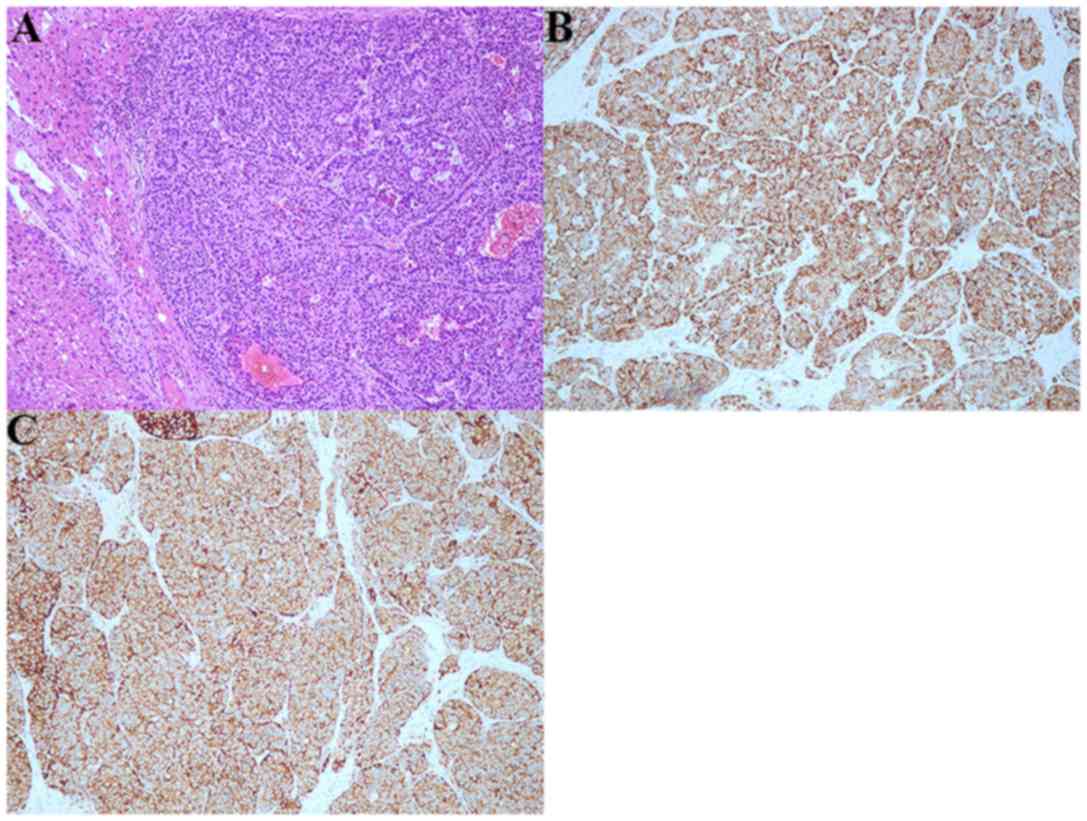
Diagnosis of BGC is established based upon histological
examination. Abundant apoptotic debris and mitotic figures may be
seen in SCNC of BG, and immunohistochemical stains were strongly
positive for CAM 5.2, NSE, SYN, CHG, CD56 and CD10 (7,15–17). In the present case report,
immunohistochemical stains obtained by the Shanghai Fengai Hospital
were strongly positive for CAM 5.2 and CD20 and demonstrated an ACC
that arose in BG. The biopsy was reanalyzed by pathologists of
Zhongshan Hospital of Fudan University, and the postoperative
pathology supported the diagnosis of SCNC via the positive presence
of SYN and CHG.
There is presently no consensus on the treatment of
BGC due to the lack of randomized controlled trials and large cases
in the literature. The treatment of BGC may include extensive
vulvar surgery, and inguinal and pelvic lymphadenectomy, similar to
treatment of SCC of the vulva. SCNC is a subtype of neuroendocrine
cancer, resembling small cell carcinoma of the lung (18). It has a poor prognosis and the use of
cisplatin and etoposide is recommended as in small cell pulmonary
tumors (19–21). In the present case, one month after
primary treatment, distant metastasis of the liver was diagnosed,
however the patient did not exhibit any other distance disease,
including disease of the local vulva. Furthermore, the serum NSE
and AFP levels, (normal ~20 ng/ml) were also in the normal range.
As a result of the distant metastasis, regular outpatient
surveillance was not limited to the pelvic cavity and tumor
markers; general examinations were also carried out. When the
metastasis of the liver was diagnosed, a VI hepatic lobectomy was
performed and primary chemotherapy was performed every 21 days
(cisplatin 2 mg/kg and etoposide 5 mg/kg, daily). The treatment
strategy remained unchanged since SCNC of BG is rare, thus there
was no definitive treatment guideline available to follow. As a
result, physicians utilized the pulmonary strategy of adjuvant
chemotherapy (cisplatin and etoposide) (21). Additionally, the lesions of the liver
were completely excised, meaning the patient had no local
recurrence or distant metastasis, confirmed via regular outpatient
surveillance.
To conclude, primary BGC is a rare form of vulvar
cancer. BGC remains a challenge for gynecologic oncologists to
treat. The initial diagnosis is often delayed because of the
absence of specific symptoms and its potential for misdiagnosis as
a benign disease. A case of early stage SCNC arising from BG is
reported based on morphological and immunohistochemical criteria. A
delay in the diagnosis is not uncommon but prompt recognition may
aid treatment. The present case underscores a potential need for
biopsy and excision of BG cysts when they present, in order to
screen for the clinical diagnostic criteria of BGC. Additional
education for patients and primary providers is required in order
to avoid misdiagnosis and improve early diagnosis of BGC. Due to
the rarity of SCNC, current therapeutic guidelines have not been
standardized. Presently, the treatment modalities used are similar
to other forms of vulvar carcinoma and the outcomes appear to be
similar and do not have good recurrence-free survival or overall
survival rates. Therefore, it is important to report additional
cases of BGC and conduct clinical trials to obtain a clinical
consensus on treatment.
Acknowledgements
The present study was supported by Zhongshan
Hospital of Fudan University.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
Conception and design: YQZ, JCW, MLX and WBT.
Collection and assembly of data: MLX and YQW. Data analysis and
interpretation: YQZ, JCW, MLX, WBT and YQW. Manuscript writing:
YQZ, JCW, MLX, WBT and YQW. Final approval of manuscript: YQZ, JCW,
MLX, WBT and YQW.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
DePasquale SE, McGuinness TB, Mangan CE,
Husson M and Woodland MB: Adenoid cystic carcinoma of Bartholin's
gland: A review of the literature and report of a patient. Gynecol
Oncol. 61:122–125. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chamlian DL and Taylor HB: Primary
carcinoma of Bartholin's gland. A report of 24 patients. Obstet
Gynecol. 39:489–494. 1972.PubMed/NCBI
|
|
3
|
Nasu K, Kawano Y, Takai N, Kashima K and
Miyakawa I: Adenoid cystic carcinoma of Bartholin's gland. Case
report with review of the literature. Gynecol Obstet Invest.
59:54–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ouldamer L, Chraibi Z, Arbion F, Barillot
I and Body G: Bartholin's gland carcinoma: Epidemiology and
therapeutic management. Surg Oncol. 22:117–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Obermair A, Koller S, Crandon AJ, Perrin L
and Nicklin JL: Primary Bartholin gland carcinoma: A report of
seven cases. Aust N Z J Obstet Gynaecol. 41:78–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jones MA, Mann EW, Caldwell CL, Tarraza
HM, Dickersin GR and Young RH: Small cell neuroendocrine carcinoma
of Bartholin's gland. Am J Clin Pathol. 94:439–442. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Donato V, Casorelli A, Bardhi E, Vena
F, Marchetti C, Muzii L and Panici Benedetti P: Bartholin gland
cancer. Crit Rev Oncol Hematol. 117:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khoury-Collado F, Elliott KS, Lee YC, Chen
PC and Abulafia O: Merkel cell carcinoma of the Bartholin's gland.
Gynecol Oncol. 97:928–931. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhalwal AB, Nick AM, Dos Reis R, Chen CL,
Munsell MF, Ramalingam P, Salcedo MP, Ramirez PT, Sood AK and
Schmeler KM: Carcinoma of the Bartholin gland: A Review of 33
cases. Int J Gynecol Cancer. 26:785–789. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang SY, Lee JW, Kim WS, Jung KL, Lee SJ,
Lee JH, Bae DS and Kim BG: Adenoid cystic carcinoma of the
Bartholin's gland: Report of two cases and review of the
literature. Gynecol Oncol. 100:422–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frable WJ and Goplerud DR: Adenoid cystic
carcinoma of Bartholin's gland diagnosis by aspiration biopsy. Acta
Cytol. 19:152–153. 1975.PubMed/NCBI
|
|
12
|
Copeland LJ, Sneige N, Gershenson DM, Saul
PB, Stringer CA and Seski JC: Adenoid cystic carcinoma of Bartholin
gland. Obstet Gynecol. 67:115–120. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee MY, Dalpiaz A, Schwamb R, Miao Y,
Waltzer W and Khan A: Clinical pathology of Bartholin's glands: A
Review of the literature. Curr Urol. 8:22–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosén C and Malmström H: Invasive cancer
of the vulva. Gynecol Oncol. 65:213–217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Groben P, Reddick R and Askin F: The
pathologic spectrum of small cell carcinoma of the cervix. Int J
Gynecol Pathol. 4:42–57. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fried-Oginski W, Lovecchio JL, Farahani G
and Smilari T: Malignant myxoid sarcoma of the Bartholin gland in
pregnancy. Am J Obstet Gynecol. 173:1633–1635. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
González-Bugatto F, Añón-Requena MJ,
López-Guerrero MA, Báez-Perea JM, Bartha JL and Hervías-Vivancos B:
Vulvar leiomyosarcoma in Bartholin's gland area: A case report and
literature review. Arch Gynecol Obstet. 279:171–174. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dakhil CS, Wick JA, Kumar AK, Satyan MT
and Neupane P: Extrapulmonary small cell carcinoma: The University
of Kansas experience and review of literature. Med Oncol.
31:1872014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yildirim Y, Elagoz S, Koyuncu A, Aydin C
and Karadayi K: Management of neuroendocrine carcinomas of the
breast: A rare entity. Oncol Lett. 2:887–890. 2011.PubMed/NCBI
|
|
20
|
Watrowski R, Jäger C, Mattern D and Horst
C: Neuroendocrine carcinoma of the breast-diagnostic and clinical
implications. Anticancer Res. 32:5079–5082. 2012.PubMed/NCBI
|
|
21
|
Ramirez RA, Chauhan A, Gimenez J, Thomas
KEH, Kokodis I and Voros BA: Management of pulmonary neuroendocrine
tumors. Rev Endocr Metab Disord. 18:433–442. 2017. View Article : Google Scholar : PubMed/NCBI
|