Introduction
Surgical excision is the gold standard therapy for
early-stage non-small cell lung cancer (NSCLC), However, the
increasing number of elderly patients with comorbidities
demonstrates the need for less-invasive therapies (1).
Stereotactic body radiotherapy (SBRT) for peripheral
lung tumors has emerged as a safe and noninvasive alternative to
surgical resection with equivalent rates of local tumor control,
and has been established as a standard of care in patients with
inoperable lung tumors oad in those declining surgery (2–4). Recently,
the role of SBRT in oligo-recurrence and sync-oligometastases in
the lung parenchyma has also come under investigation, with
promising results (5–9).
However, for both surgery and SBRT, established
adaptation is limited to peripheral lesions. Surgical resection of
central tumors requires a larger resection area than peripheral
lesions, and carries a high risk of complications (10–12).
Likewise, central SBRT remains a challenge, because the central
thoracic structures are considered to have multiple organs at risk
(OARs), increasing the risk of adverse events (AEs). Timmerman
et al reported in 2006 that SBRT of central tumors carried
an increased risk of severe toxicity, up to 11 times higher that of
peripheral tumors (13). Although
multiple centers have reported various dose divisions in the search
for a safe and effective regimen, it is unknown whether SBRT can be
applied to all centrally located tumors or whether there are
locations which are too close to OARs.
In our hospital, SBRT for central lung lesions is
actively performed as an alternative to surgery when the patient is
not a good surgical candidate or surgery is declined. Since 2011,
we have treated central lung tumors with a 56 Gy/7 fr prescription
[Biological effective dose (BED10)=100.8 Gy]. The
primary purpose of this study was to assess the toxicity of SBRT
with 56 Gy/7 fr in central lesions, and to evaluate the validity of
this treatment in our institution.
Materials and methods
Patients and materials
From October 2011 to October 2016, 35 patients with
36 central lesions, either NSCLC or pulmonary/mediastinal
oligo-recurrence, were treated with stereotactic body radiation
therapy (SBRT) with or without volumetric modulated arc therapy
(VMAT) at the University of Tokyo Hospital. All patients provided
written informed consent, Data from the electronic medical record
were retrospectively analyzed.
We excluded tumors located at or involving the hilar
structures and those invading the bronchial tree or mediastinum,
which are not considered safe targets for central SBRT regimens
(14), as well as those that required
additional fractions, such as 50 Gy in 10 fractions (fr). We also
excluded cases of obvious idiopathic pulmonary fibrosis on computed
tomography (CT). We defined a central lesion as a tumor within 2 cm
of the proximal bronchial tree, as described in RTOG 0236 (15,16), or
within 2 cm in any directions of any critical mediastinal
structure, including the bronchial tree, esophagus, heart, brachial
plexus, major vessels, spinal cord, phrenic nerve, and recurrent
laryngeal nerve (5,17,18).
Treatment planning
Patients were immobilized in a stereotactic body
frame and underwent a four-dimensional (4D) CT scan (2 mm
sections). Scans were performed using an external respiratory
monitoring system (AZ-733 V®; Anzai Medical, Tokyo,
Japan) with free breathing or with abdominal compression in cases
where tumor excursion exceeded 1 cm. In our institution, 4D-CT for
planning divides the respiratory cycle into 10 sections.
Respiratory phase data were transferred to a treatment planning
system (TPS) (Pinnacle3®, version 9.10; Philips, Best,
The Netherlands). Gross tumor volume (GTV) was delineated in each
respiratory phase using the lung window (window, 1,600 HU; level,
−300 HU). These 10 GTVs were fused to form the internal target
volume (ITV). A uniform 5 mm margin was then added to create the
planning target volume (PTV) (19).
For the main OARs (heart, lungs, esophagus, spinal cord, proximal
tracheobronchial tree, and brachial plexus) were contoured
consistent with guidelines provided by Radiation Therapy Oncology
Group Trial (RTOG) 0236 (15,16).
Treatment procedure and dose
Patients treated between October 2011 and March 2013
received a conventional SBRT plan using 6–11 beams. Patients
treated between April 2013 and October 2016 received volumetric
modulated arc therapy (VMAT-SBRT) with 6 or 10 MV beams. VMAT plans
were designed using a single partial arc with angle ranges of −40°
to 180° (left lung) or −180° to 40° (right lung), which has been
previously described in detail (19,20).
Thirty-five patients received 56 Gy in 7 fr to cover 95% of the PTV
(D95%). This dose was set in 2011 with the intention of
increasing the number of fractions above that for peripheral
lesions (48 Gy/4 Fr) while maintaining BED >100 Gy (21). Doses to OARs were required to meet
explicit objectives as follows: V20 <10% (less than 10% of the
volume receiving 20 Gy) and V5 <25% for the ipsilateral lung,
V20=0% and V5 <15% for the contralateral lung, V15=0% for spinal
cord, V30=0% for heart and liver, and V50=0% for body (15,20,22).
Treatment planning was performed using a3D RTP
(Pinnacle3, New Version 7.4i; Philips). The collapsed
cone convolution method together with the superposition algorithm
were used for heterogeneity correction for the lungs. All final
calculations were performed with a grid size of 2.0 mm. Dose
distributions were calculated using peak exhalation CT data.
Planning target coverage aimed to cover the PTV with
95% of the prescribed dose. The main OARs were healthy lung, spinal
cord, heart, and esophagus. Treatment plans were required to meet
explicit objectives as follows: V20 <10% (less than 10% of the
volume receiving 20 Gy) and V5 <25% for the ipsilateral lung,
V20 <0% and V5 <15% for the contralateral lung, V15=0% for
spinal cord, V30=0% for heart and liver, and V50=0% for body
(23).
Follow-up/chart review
Follow-up consisted of a history and physical
examination and non-contrast chest CT scan, beginning 2 months
after SBRT, then every 3 months for 2 years, and at least every 6
months thereafter. In cases of suspected tumor relapse or
progression, a contrast-enhanced CT scan or a
18F-fluorodeoxyglucose positron emission tomography/CT
(FDG-PET/CT) was performed. Local recurrence was defined as
progressive and increasing CT scan abnormalities which were
confirmed by progressive and incremental increases in the maximum
standardized uptake value (SUVmax) of a lesion on serial PET
imaging, with or without biopsy. The SUVmax was calculated as the
most intense voxel within the volume of interest. All controversial
cases were discussed at a tumor board and either verified by biopsy
or by consensus.
All hospital records, follow-up notes, and imaging
data were reviewed. Acute and late AEs were assessed according to
the Common Terminology Criteria for Adverse Events Version 4.0
(CTCAE v 4.0). Dosimetric quality of treatments was measured from
dose volume histogram (DVH) analysis. Doses to OARs were calculated
for the following structures: Point dose maximum to the proximal
tracheobronchial tree (proximal tracheobronchial tree point),
maximum dose received by 5 cc of the proximal tracheobronchial tree
(proximal tracheobronchial tree 5 cc), mean total lung dose (MLD
total), volume of lung receiving 5/10/20 Gy or more (V5/V10/V20),
and maximum dose to spinal cord/esophagus/heart/brachial
plexus.
Statistical analysis
Descriptive statistics for categorical variables are
reported as frequency and percentage, whereas continuous variables
are reported as median (range). For categorical variables,
comparisons between groups were made using Pearson's χ2
tests. The 1-year local control rate (LCR), overall survival (OS),
and relapse-free survival (RFS) were defined over the period from
the first day of SBRT until death, recurrence, or last patient
contact, and were calculated using Kaplan-Meier curves. The
statistical analyses were performed using R software (https://www.r-project.org/), and significance of
univariate analyses was set at P<0.05.
Results
Patient and treatment
characteristics
A total of 35 patients with 36 lesions were
evaluated. All cases were treated with 56 Gy in 7 fr
(BED10=100.8 Gy). Patients and treatment characteristics
are listed in Table I. The median age
of patients was 74 years (range 45–89 years), and the median of
Karnofsy performance scale (KPS) was 90% (range 80–100). SBRT
treatment characteristics and tumor volumes for the study
population are summarized in Table
II. Fifteen lesions were primary NSCLC, 13 were local
recurrence or mediastinal lymph nodes involved in NSCLC, and 8 were
non-NSCLC pulmonary oligo-recurrences. Eighteen of the 35 patients
(51%) had undergone surgery for the lung tumor before SBRT, 13
(37%) of which were salvage cases for a postoperative pulmonary
recurrence. We usually distinguish ‘ultra-central’ tumors directly
abutting the central airway (14);
most of these tumors were treated with a different protocol, namely
50 Gy in 10 fr, but in this analysis, four ‘ultra-central’ cases
receiving 56 Gy in 7 fr were included.
 | Table I.Patient and treatment
characteristics. |
Table I.
Patient and treatment
characteristics.
| Patient
characteristics | No. (%) |
|---|
| Age, years |
|
|
≥75 | 17 (49) |
|
<75 | 18 (51) |
| Sex |
|
|
Male | 25 (71) |
|
Female | 10 (29) |
| KPS, % |
|
|
≥90 | 27 (77) |
|
<90 | 8 (23) |
| Surgical
history |
|
|
Yes | 18 (51) |
| No | 17 (49) |
| Chest RT
history |
|
|
Yes | 2 (6) |
| No | 33 (94) |
| COPD |
|
|
Yes | 11 (31) |
| No | 24 (69) |
| KL-6, U/ml |
|
|
>500 | 2 (6) |
|
≤500 | 27 (77) |
| No
data | 6 (17) |
| Smoking
history |
|
|
Current | 9 (26) |
| Past
only | 14 (40) |
|
Never | 12 (34) |
| Cancer
typea |
|
| Primary
NSCLC | 15 (28) |
|
Recurrent NSCLC | 13 (36) |
|
Recurrent non-NSCLC | 8 (16) |
| Definition of
‘Central’ |
|
| RTOG
0236 definition | 20 (56) |
|
Others | 16 (44) |
| Tumor diameter,
cm |
|
| ≥3 | 18 (50) |
|
<3 | 18 (50) |
 | Table II.SBRT treatment characteristics and
tumor volumes of 36 tumors. |
Table II.
SBRT treatment characteristics and
tumor volumes of 36 tumors.
|
Characteristics | Median (range) |
|---|
| Tumor diameter,
cm | 29 (11–70) |
| PTV,
cm3 | 60.13
(7.2–388.8) |
| ITV,
cm3 | 21.16
(0.99–217.2) |
| Lung dose |
|
| V5,
% | 29.61
(16.4–63.7) |
| V10,
% | 18.9
(7.02–43.9) |
| V20,
% | 11.31
(2.1–17.91) |
| MLD,
cGy | 679.9
(299.6–1256.5) |
| Trachea |
|
| Max
dose (point), cGy | 548.6
(20.0–5736.1) |
| Max
dose (5cc), cGy | 135.7
(30.2–2563.8) |
| Carina |
|
| Max
dose (point), cGy | 5,090.9
(142.0–9527.9) |
| Max
dose (5cc), cGy | 1,145.7
(1206–2366.8) |
| Esophagus |
|
| Max
dose (point), cGy | 1,699.8
(463.2–6551.2) |
| Max
dose (5cc), cGy | 1,296.5
(101–2335.7) |
| Heart |
|
| V30,
% | 1.715
(0–24.42) |
| Max
dose (point), cGy | 5,618.9
(40.3–6505.1) |
| Spine |
|
| Max
dose (point), cGy | 1,742.4
(211.6–3453.1) |
| Chest wall |
|
| Max
dose (point), cGy | 5,842.2
(2537.2–7299.4) |
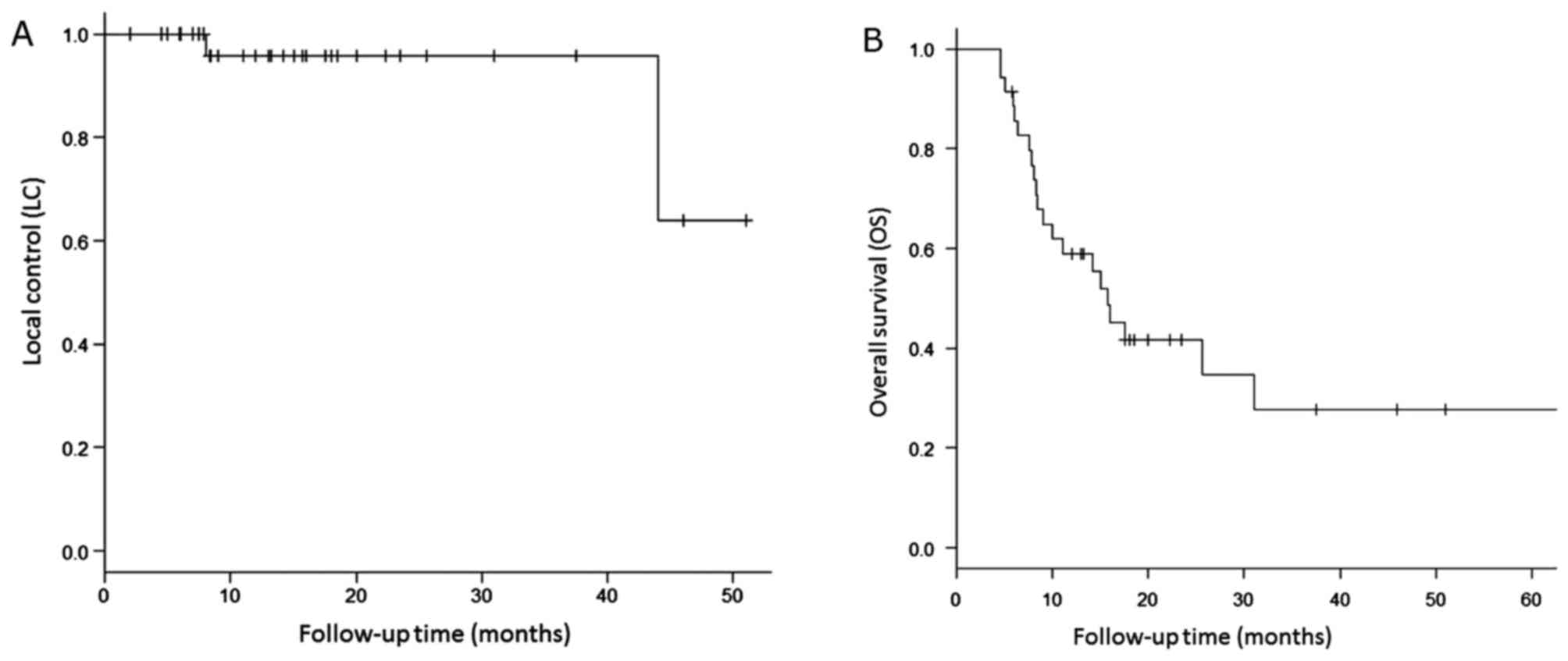
LC and survival
The median follow-up period for all patients was
13.1 months (range, 4.5–64 months) and that for survivors was 18.3
months (range, 5.8–51 months). During follow-up, local recurrence
occurred in only two lesions (6%). The first was a case of
pulmonary oligo-recurrence from esophageal cancer, with LR
occurring 44 months after SBRT. The second was a postoperative case
for a recurrent NSCLC. Local recurrence occurred 8 months after
salvage SBRT and the patient died 2 months later. This was the only
case of recurrence within 2 years, and for primary NSCLC cases
received SBRT as the initial treatment, there has not been any LR
at the present time. The 1-/2-year LCR were both 96% (95% CI:
74.8–99.4%), and the median LCR has not been reached. The survival
curves for LC are shown in Fig.
1A.
Twenty-two patients (62.9%) died, of whom 10 died
due to primary disease (including 4 NSCLC, and 6 non-NSCLC), 2 were
treatment-related, and 10 were due to cause-specific death. Distant
metastases occurred in 10 cases, 6 of which were the cases with
pulmonary oligo-recurrences from non-NSCLC. Recurrence or death
occurred in 24 patients (68.6%). The 1- and 2-year OS of the whole
cohort was 59.0% (95% CI: 40.8–73.3%) and 41.6% (95% CI:
24.5–58.0%), respectively. The median OS was 15.7 months (range:
8.4–31 months). The 1- and 2-year OS of the primary NSCLC subgroup
was 66.7% (95% CI: 37.5–84.6%) and 50.0% (95% CI: 22.2–72.6%); that
of the recurrent NSCLC subgroup was 50.0% (95% CI: 20.9–73.6%) and
30.0% (95% CI: 7.7–56.9%); and that of non-NSCLC subgroup
(pulmonary oligo-metastases/recurrence of other cancers) was 60%
(95% CI: 19.6–85.2%) and 45% (95% CI: 10.8–75.1%), respectively.
The 1- and 2-year RFS of the NSCLC subgroup was 51.9% (95% CI:
31.9–68.5%) and 38.9% (95% CI: 20.5–57.0%), respectively. Median
RFS has not been reached. The survival curves for OS are shown in
Fig. 1B.
AEs
Table III describes
the AEs occurring in the patients. Nine patients experienced grade
≥3 toxicity, representing 26% of the subjects. Two of these were
grade 5, one pneumonitis and one hemoptysis.
 | Table III.Adverse events of patients. |
Table III.
Adverse events of patients.
|
| Grade (CTCAE4.0),
no. (%) |
|---|
|
|
|
|---|
| Adverse events | I | II | III | IV | V |
|---|
| Acute |
|
|
|
|
|
|
Esophagitis | 6
(17) | 1 (3) | – | – | – |
|
Dermatitis | 2 (6) | 3 (9) | – | – | – |
|
All | 8
(23) | 4
(12) | – | – | – |
| Late |
|
|
|
|
|
|
Pneumonitis | 22 (63) | 4
(11) | 6
(17) | – | 1 (3) |
|
Esophageal
narrowing/obstruction | – | 2 (6) | – | – | – |
|
Tracheal
stenosis/obstruction | – | 2 (6) | 1 (3) | – | – |
| Pleural
effusion | 5
(14) | 4
(11) | – | – | – |
|
Hemoptysis | – | – | – | – | 1 (3) |
|
All | 27 (77) | 12 (34) | 7
(20) | – | 2 (6) |
Comparison of the patient characteristics of grade
≥3 and <3 cases of pneumonitis is shown in Table IV. Although we tried to identify
factors which showed significant differences in the two groups
using the Chi-square test, we failed to show the risk factors
associated with pneumonitis.
 | Table IV.Comparison of the patient
characteristics of grade ≥3 and <3 cases of pneumonitis. |
Table IV.
Comparison of the patient
characteristics of grade ≥3 and <3 cases of pneumonitis.
|
| Adverse events, no.
(%) |
|
|---|
|
|
|
|
|---|
| Characteristic | Grade ≥3 | Grade <3 | P-value
(univariate) |
|---|
| Total (n=35) | n=7 | n=28 |
|
| Age |
|
≥75 | 3 (42.9) | 14 (50) | 0.99 |
| Sex |
|
|
|
|
Male | 6 (85.7) | 19 (67.9) | 0.64 |
| KPS |
|
|
|
|
<90 | 3 (42.9) | 5 (17.9) | 0.31 |
| Surgical
history |
|
|
|
|
Yes | 4 (57.1) | 14 (50) | 0.99 |
| COPD |
|
|
|
|
Yes | 3 (42.9) | 8 (28.6) | 0.65 |
| KL-6 |
|
|
|
|
>500 | 1 (14.3) | 1 (3.6) | 0.36 |
| Smoking history
(Yes) |
|
|
|
|
Yes | 6 (85.7) | 17 (60.7) | 0.38 |
|
Current | 3 (42.9) | 6 (21,4) | 0.34 |
| Cancer type |
|
|
|
| Primary
NSCLC | 5 (62.5) | 7 (25) | 0.03 |
|
Recurrent NSCLC | 2 (28.6) | 12 (42.9) | 0.68 |
|
Oligo-metastases | 0 (0) | 8 (28.6) | 0.17 |
| Definition of
‘Central’ |
|
|
|
| RTOG
0236 definition | 4 (57.1) | 15 (53.6) | 0.99 |
| Maximum
diameter |
|
|
|
| ≥3
cm | 2 (28.6) | 16 (57.1) | 0.23 |
| ≥4
cm | 2 (28.6) | 7 (25) | 0.99 |
| PTV volume |
|
|
|
| ≥100
cm3 | 2 (28.6) | 6 (21.4) | 0.65 |
| Lung V5 |
|
|
|
|
≥25% | 4 (57.1) | 18 (64.3) | 0.99 |
| Lung V20 |
|
|
|
|
≥10% | 3 (42.9) | 16 (57.1) | 0.68 |
| MLD |
|
|
|
| ≥500
cGy | 5 (62.5) | 21 (75) | 0.99 |
Here we described the details of these two AEs and
esophagitis, which are the most common and can be severe.
Pneumonitis and hemoptysis are late effects of irradiation, which
occur after months to years after irradiation, and are often
irreversible changes. It is thought that the immunological
mechanism is involved, but the mechanism of development is not
clear.
On the other hand, esophagitis is a type of
mucositis caused by irradiation, and it develops and relieves in
weeks after irradiation.
Pneumonitis
Pneumonitis in seven of nine cases was grade ≥3, of
which one was grade 5. The time to onset of pneumonitis in these
cases was 6 months (range, 2–7 months) after treatment. Table IV compares characteristics in
subjects with grade ≥3 vs. <3 pneumonitis. We could not identify
risk factors significantly associated with grade >3 pneumonitis.
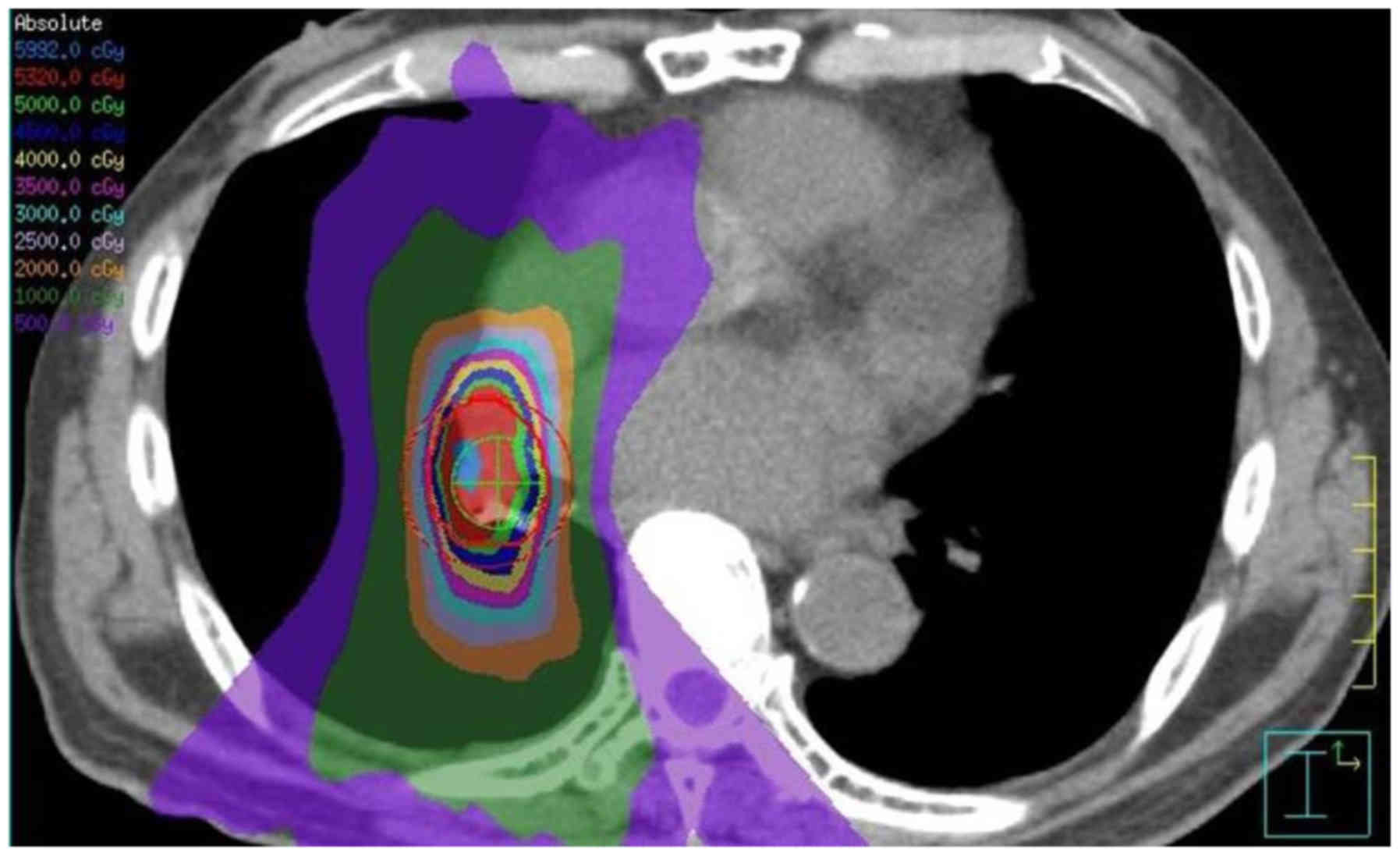
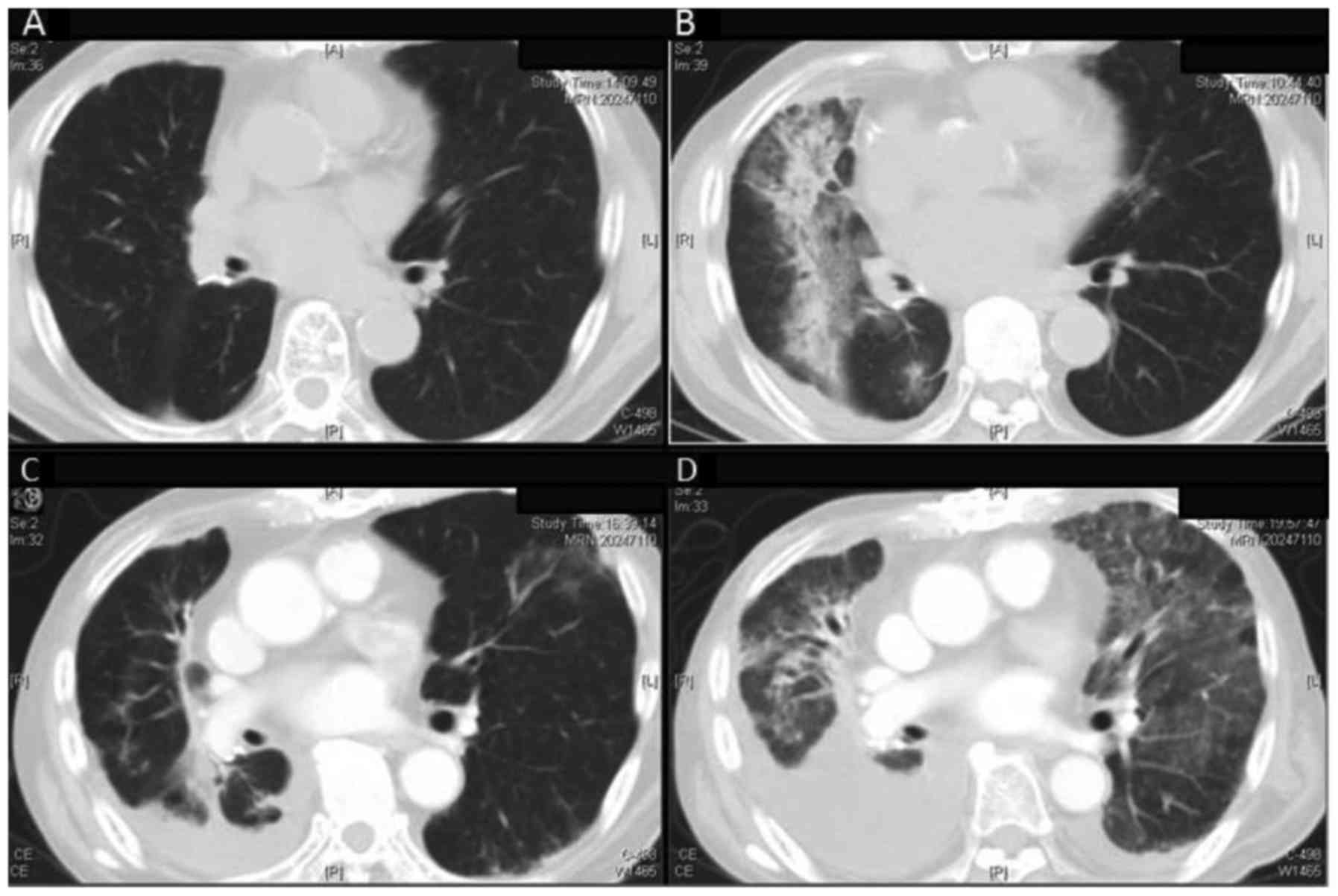
A case of grade 5 pneumonitis was seen in a 79-year-old man with a
history of video-assisted thoracoscopic surgery (VATS) lobectomy
for NSCLC in the right lower lobe. He developed an enlarged
ipsilateral hilar lymph node (26 mm) 5 months after surgery, and
received SBRT. He had stopped smoking 45 years before treatment,
and was free from COPD or other lung chronic diseases. The
pulmonary dose was as follows: V5=34.7%, V20=9.7%, MLD=6.44 Gy. He
developed pneumonitis 5 months after irradiation, and was
hospitalized and received corticosteroid pulse therapy, but died on
day 30 after onset. The image of irradiation field and a series of
follow-up CT images of this patient are shown in Figs. 2 and 3.
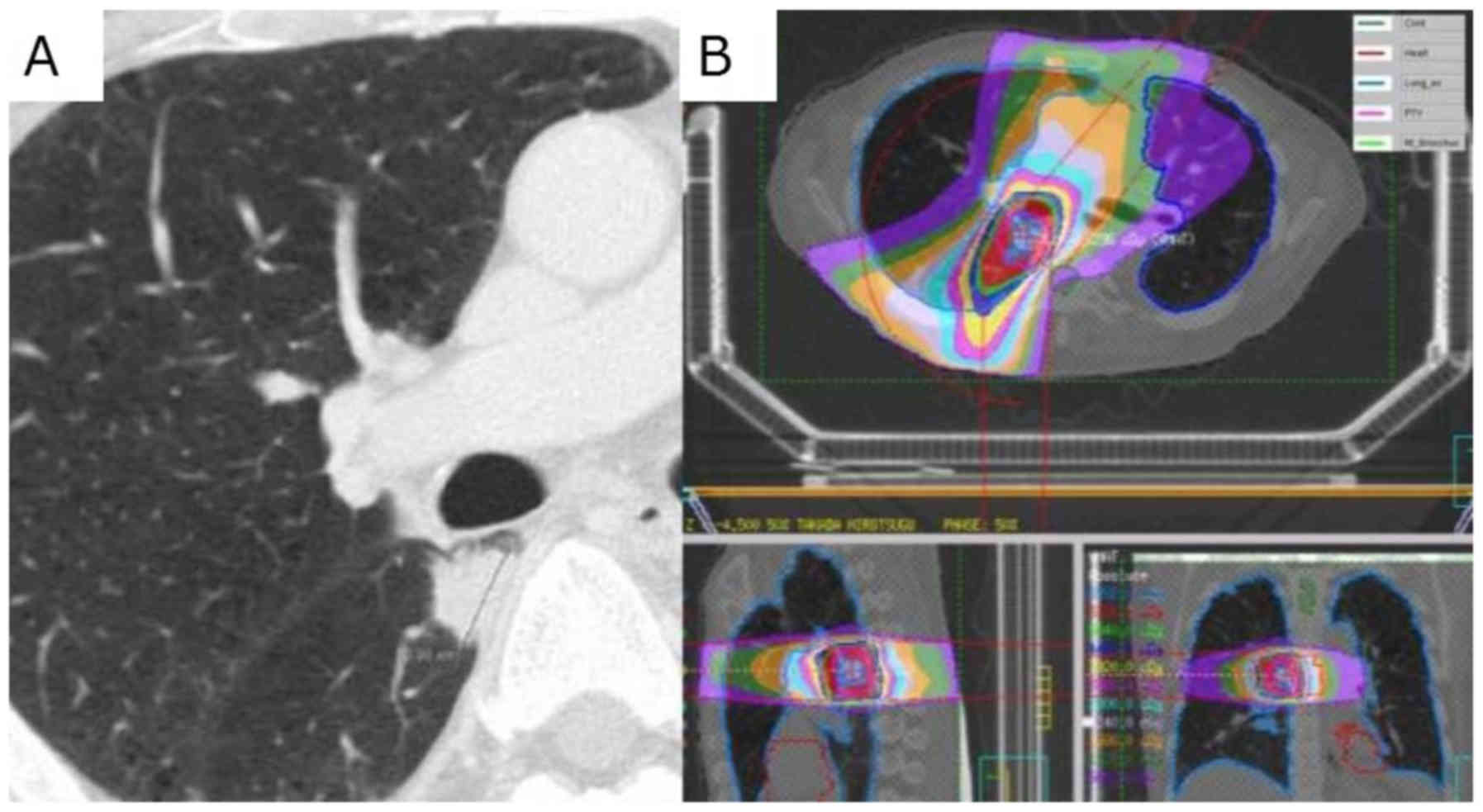
Hemoptysis
One patient in our cohort died of hemoptysis, likely
attributable to SBRT. The patient was a 64-year-old man with a
history of VATS lobectomy for primary NSCLC (squamous cell
carcinoma) pT2aN0M0 p-Stage IB. Two years later, single
oligo-recurrence (right S6) appeared and SBRT was performed as a
salvage treatment. Bloody sputum and slight fever appeared 9 months
after treatment. Because there was no deterioration of pneumonia or
tumor recurrence in CT imaging, he was followed up without
treatment. One month later, massive hemoptysis occurred and he
died. The diameter of the target lesion was 20 mm, the distance
from the right main bronchus was 4 mm, the maximum tracheal dose
was 34.02 Gy (point), and the maximum bronchial dose was 63.33 Gy
(point) (Fig. 4).
Esophagitis
In this group, no patient developed grade ≥3
esophageal toxicity, including three patients (8.6%) in whom the
PTV overlapped the esophagus. Only one patient (2.9%) developed
grade 2 esophagitis. In this case, the tumor touched the esophagus,
and the maximum esophageal dose at that point was 59.62 Gy/7fr. The
mean maximum esophageal dose was 31.25 Gy for the point dose and
12.88 Gy for the 5 cc dose (17,24–26).
Discussion
SBRT provides excellent LC for peripheral lung
tumors, with >90–95% 2-year LCR (5,16,23,27,28). In
reports on SBRT of centrally located tumors, on the other hand,
2-year LCR ranges from 60 (29) to
94% (30). This is thought to be
related to the lower BED10 used to avoid severe AEs.
The impact of BED on LC is widely known, and
BED10 ≥100 has been established as a significant
predictor of LC (31). In reports
with BED10 <100 Gy, LC is relatively poor.
Andratschke et al reported a 3-year LCR of 64%, and OS of
29% (32). Oshiro et al
reported a 2-year LCR of 60% with BED10=80 Gy (29), and Bradley et al reported an
86% 2-year LCR, and 75% 2-year OS with BED10=86 Gy
(33). As for reports of ≥100 Gy,
Timmerman et al reported a 2-year LCR of 95% using a regimen
of 60–66 Gy in 3 fr (13), and Rowe
et al reported a 2-year LCR of 94% with a BED10
≥100 Gy, and 80% when BED10 <100 Gy, (P=0.02)
(34). Milano et al reported
relatively poor results with the high dose: The 2-year LCR was 73%
and the 2-year OS was 72% with BED10=100 Gy (35). As they stated in their paper, the
poorer OS and LC of their series likely reflected their patient
population, which included stage1 NSCLC, non-stage 1 NSCLC (NSCLC
Stage 1:2:3=7:4:6), and oligo-recurrences. The 2-year survival of
each group was 72, 12 and 49% respectively, suggesting very
different patient populations. In our results, six of eight
oligo-recurrences re-relapsed (75%), while only four of 28 (14%)
NSCLC cases recurred, suggesting that the results are better in
NSCLC.
In 2006, Timmerman et al reported treating 70
patients with T ≤2 N0M0 NSCLC using SBRT with 60–66 Gy in 3 fr, and
Grade 3–5 toxicity occurred in a total of 14 patients (20%),
including six grade 5 cases (13).
They stated that four of the six deaths from toxicity were in
patients with central tumors, and that the risk of severe toxicity
increased 11-fold in central lesions compared with peripheral ones
(13). Thereafter, many researchers
have published on the increased risk of SBRT for central lesions,
and the central location is considered to be an independent risk
factor (36,37). Recently, several groups have reported
their experience with central SBRT using a larger number of
fractions (≥5 fr) and smaller doses per fraction, and suggested
that their regimen would be safer and more appropriate. Haasbeek
et al achieved a 3-year LCR of 90.2% and 3-year OS of 51.1%
with no grade 4/5 AEs administering 60 Gy/8 fr, finding no
significant differences between central and peripheral tumors
(38). Chang et al (39) and Li et al (40) reported the results of their regimen
using 70 Gy/10 fr; the median OS was 55.6 months and the 3-year OS
rate was 70.5%, with only 1% grade ≥3 pneumonitis and no grade 4 or
5 toxicity (39,40).
On the other hand, in our study, all treated with 56
Gy/7 fr, 9 of 35 cases (25.7%) had grade ≥3 AEs (of which 7 cases
were pneumonia), This is somewhat higher than the above reports
with equivalent dose prescriptions (5,10,39,40). In
attempt to clarify risk factors related to severe pneumonia, we
examined the difference between cases with and without AEs of grade
3 or higher among our subjects. Although we were unable to identify
factors which showed significant differences in the two groups
(Table IV), Roesch et al have
written an interesting report on this matter. They classified
‘central tumors’ by risk based on three criteria: tumor size, OAR
infiltration, and distance from the carina (10). They argued that the most prominent
contraindications for SBRT (so-called ‘high-risk’ cases) were
proximity to the carina, possible infiltration of the central
airways (tumor immediately adjacent to the main bronchus) and tumor
size >4 cm. According to their questionnaire survey, SBRT for
high-risk cases was rejected by almost all radiation oncologists.
If this classification were applied to our 35 cases, 17 (48.6%)
would be classified as ‘high-risk’ as defined by Roesch et
al Certainly, among our cases experiencing grade ≥3 AEs, 5
cases (55.6%) were classifiable as ‘high-risk’ (10).
In addition, half of our patients had a history of
thoracic surgery. The treatment of tumors arising
post-pneumonectomy is often difficult, as subsequent surgery is
often not feasible due to the higher risk of re-operation and lower
lung function (41,42). Data on cure for patients who develop a
second tumor after pneumonectomy are scarce, and historic outcomes
with conventional radiotherapy have been poor with a narrow
therapeutic ratio (43,44). Diagnosis of tumor localization is
often difficult in these cases. The use of luminescent probes could
be helpful for it. The information about peer efforts for analysis
of detection platforms in the introduction has been reported
(45–50). We have actively treated post-surgery
patients with oligo-recurrent/secondary cancers using SBRT, and
achieved good LC.
Previous reports on the efficacy and safety of
central SBRT mainly focused on early stage NSCLC (38–40). In
clinical practice, in contrast, it is often necessary to perform
radiation therapy for high-risk cases where other treatments are
more difficult. In the present paper, we also reported on SBRT for
high risk cases such as postoperative recurrence and large tumors.
Such an examination appears to be useful for selection and
expansion of the target of central SBRT.
Limitations of this study are the small number of
cases and short observation period. Although this paper focused on
AEs, it is not sufficient to discuss survival period, and further
observation is necessary in the future. Retrospective observational
studies will inevitably have missing data, such as loss of
pathological diagnosis and laboratory findings.
We reported the result of SBRT for central lesions
with 56 Gy/7 fr. Considering the background of the subject, tumor
control of our central SBRT is promising, especially in primary
NSCLC. However, the safety of SBRT to central lung cancer is still
controversial.
Acknowledgements
The authors would like to thank Mrs. Libby Cone for
editing the draft of this manuscript.
Funding
This study was partially supported by a Grant-in-Aid
from JSPS (Japan Society for the Promotion of Science) KAKENHI JP
Scientific Research (C) grant number 15K08692.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SA collected and assembled the data, drafted the
manuscript and critically revised the article for important
intellectual content. HY and WT supervised all the above work, and
conceived the study design. AH and TO interpreted the collected
data. SO, KaN and TI contributed to acquisition and analysis of the
data. OA and KeN analyzed the data and helped to draft the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee, University of Tokyo Hospital.
Patient consent for publication
Patients provided written consent for data
collection and analysis.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BED10
|
biologically effective dose α/β=10
Gy
|
|
fr
|
fraction
|
|
LC
|
local control
|
|
LCR
|
local control rate
|
|
NSCLC
|
non-small cell lung cancer
|
|
OAR
|
organ at risk
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
SBRT
|
stereotactic body radiation
therapy
|
|
VATS
|
video-assisted thoracoscopic
surgery
|
|
VMAT
|
volumetric modulated arc therapy
|
References
|
1
|
de Perrot M, Licker M, Reymond MA, Robert
J and Spiliopoulos A: Influence of age on operative mortality and
long-term survival after lung resection for bronchogenic carcinoma.
Eur Respir J. 14:419–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang JY, Senan S, Paul MA, Mehran RJ,
Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, et al:
Stereotactic ablative radiotherapy versus lobectomy for operable
stage I non-small-cell lung cancer: A pooled analysis of two
randomised trials. Lancet Oncol. 16:630–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dahele M, Hatton M, Slotman B and
Guckenberger M: Stereotactic body radiotherapy: A survey of
contemporary practice in six selected European countries. Acta
Oncol. 54:1237–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daly ME, Perks JR and Chen AM:
Patterns-of-care for thoracic stereotactic body radiotherapy among
practicing radiation oncologists in the United States. J Thorac
Oncol. 8:202–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang JY, Bezjak A and Mornex F: IASLC
Advanced radiation technology committee: Stereotactic ablative
radiotherapy for centrally located early stage non-small-cell lung
cancer: What we have learned. J Thorac Oncol. 10:577–585. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tree AC, Khoo VS, Eeles RA, Ahmed M,
Dearnaley DP, Hawkins MA, Huddart RA, Nutting CM, Ostler PJ and van
As NJ: Stereotactic body radiotherapy for oligometastases. Lancet
Oncol. 14:e28–e37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palma DA, Salama JK, Lo SS, Senan S,
Treasure T, Govindan R and Weichselbaum R: The oligometastatic
state - separating truth from wishful thinking. Nat Rev Clin Oncol.
11:549–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niibe Y and Hayakawa K: Oligometastases
and oligo-recurrence: The new era of cancer therapy. Jpn J Clin
Oncol. 40:107–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niibe Y and Chang JY: Novel insights of
oligometastases and oligo-recurrence and review of the literature.
Pulm Med. 2012:2610962012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roesch J, Panje C, Sterzing F, Mantel F,
Nestle U, Andratschke N and Guckenberger M: SBRT for centrally
localized NSCLC- What is too central? Radiat Oncol. 11:1572016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegenthaler MP, Pisters KM, Merriman KW,
Roth JA, Swisher SG, Walsh GL, Vaporciyan AA, Smythe WR and Putnam
JB Jr: Preoperative chemotherapy for lung cancer does not increase
surgical morbidity. Ann Thorac Surg. 71:1105–1112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito M, Furukawa K, Miura T and Kato H:
Evaluation of T factor, surgical method, and prognostic factors in
central type lung cancer. Jpn J Thorac Cardiovasc Surg. 50:413–417.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Timmerman R, McGarry R, Yiannoutsos C,
Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C,
Williams M and Fletcher J: Excessive toxicity when treating central
tumors in a phase II study of stereotactic body radiation therapy
for medically inoperable early-stage lung cancer. J Clin Oncol.
24:4833–4839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaudhuri AA, Tang C, Binkley MS, Jin M,
Wynne JF, von Eyben R, Hara WY, Trakul N, Loo BW Jr and Diehn M:
Stereotactic ablative radiotherapy (SABR) for treatment of central
and ultra-central lung tumors. Lung Cancer. 89:50–56. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stanic S, Paulus R, Timmerman RD,
Michalski JM, Barriger RB, Bezjak A, Videtic GM and Bradley J: No
clinically significant changes in pulmonary function following
stereotactic body radiation therapy for early- stage peripheral
non-small cell lung cancer: An analysis of RTOG 0236. Int J Radiat
Oncol Biol Phys. 88:1092–1099. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Timmerman R, Paulus R, Galvin J, Michalski
J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone
D, et al: Stereotactic body radiation therapy for inoperable early
stage lung cancer. JAMA. 303:1070–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Modh A, Rimner A, Williams E, Foster A,
Shah M, Shi W, Zhang Z, Gelblum DY, Rosenzweig KE, Yorke ED, et al:
Local control and toxicity in a large cohort of central lung tumors
treated with stereotactic body radiotherapy. Int J Radiat Oncol
Biol Phys. 90:1168–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wheldon TE, Deehan C, Wheldon EG and
Barrett A: The linear-quadratic transformation of dose-volume
histograms in fractionated radiotherapy. Radiother Oncol.
46:285–295. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamashita H, Takahashi W, Haga A, Kida S,
Saotome N and Nakagawa K: Stereotactic body radiotherapy for small
lung tumors in the university of Tokyo hospital. Biomed Res Int.
2014:1365132014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakagawa K, Haga A, Sakumi A, Yamashita H,
Igaki H, Shiraki T, Ohtomo K, Iwai Y and Yoda K: Impact of
flattening-filter-free techniques on delivery time for lung
stereotactic volumetric modulated arc therapy and image quality of
concurrent kilovoltage cone-beam computed tomography: A preliminary
phantom study. J Radiat Res. 55:200–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamashita H, Takahashi W, Haga A and
Nakagawa K: Radiation pneumonitis after stereotactic radiation
therapy for lung cancer. World J Radiol. 6:708–715. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakagawa K, Haga A, Kida S, Masutani Y,
Yamashita H, Takahashi W, Sakumi A, Saotome N, Shiraki T, Ohtomo K,
et al: 4D registration and 4D verification of lung tumor position
for stereotactic volumetric modulated arc therapy using
respiratory-correlated cone-beam CT. J Radiat Res. 54:152–156.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lagerwaard FJ, Haasbeek CJ, Smit EF,
Slotman BJ and Senan S: Outcomes of risk-adapted fractionated
stereotactic radiotherapy for stage I non-small-cell lung cancer.
Int J Radiat Oncol Biol Phys. 70:685–692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas TO, Hasan S, Small W Jr, Herman JM,
Lock M, Kim EY, Mayr NA, Teh BS and Lo SS: The tolerance of
gastrointestinal organs to stereotactic body radiation therapy:
What do we know so far? J Gastrointest Oncol. 5:236–246.
2014.PubMed/NCBI
|
|
25
|
Stephans KL, Djemil T, Diaconu C, Reddy
CA, Xia P, Woody NM, Greskovich J, Makkar V and Videtic GM:
Esophageal dose tolerance to hypofractionated stereotactic body
radiation therapy: Risk factors for late toxicity. Int J Radiat
Oncol Biol Phys. 90:197–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pham AH, Yorke E, Rimner A and Wu AJ:
Potential for interfraction motion to increase esophageal toxicity
in lung SBRT. Technol Cancer Res Treat: 1533034617711353. 2017.
View Article : Google Scholar
|
|
27
|
Chang JY, Liu H, Balter P, Komaki R, Liao
Z, Welsh J, Mehran RJ, Roth JA and Swisher SG: Clinical outcome and
predictors of survival and pneumonitis after stereotactic ablative
radiotherapy for stage I non-small cell lung cancer. Radiat Oncol.
7:1522012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taremi M, Hope A, Dahele M, Pearson S,
Fung S, Purdie T, Brade A, Cho J, Sun A, Bissonnette JP and Bezjak
A: Stereotactic body radiotherapy for medically inoperable lung
cancer: Prospective, single-center study of 108 consecutive
patients. Int J Radiat Oncol Biol Phys. 82:967–973. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oshiro Y, Aruga T, Tsuboi K, Marino K,
Hara R, Sanayama Y and Itami J: Stereotactic body radiotherapy for
lung tumors at the pulmonary hilum. Strahlenther Onkol.
186:274–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bral S, Gevaert T, Linthout N, Versmessen
H, Collen C, Engels B, Verdries D, Everaert H, Christian N, De
Ridder M and Storme G: Prospective, risk-adapted strategy of
stereotactic body radiotherapy for early-stage non-small-cell lung
cancer: Results of a Phase II trial. Int J Radiat Oncol Biol Phys.
80:1343–1349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Onishi H, Araki T, Shirato H, Nagata Y,
Hiraoka M, Gomi K, Yamashita T, Niibe Y, Karasawa K, Hayakawa K, et
al: Stereotactic hypofractionated high-dose irradiation for stage I
nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in
a Japanese multiinstitutional study. Cancer. 101:1623–1631. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Andratschke N, Zimmermann F, Boehm E,
Schill S, Schoenknecht C, Thamm R, Molls M, Nieder C and Geinitz H:
Stereotactic radiotherapy of histologically proven inoperable stage
I non-small cell lung cancer: Patterns of failure. Radiother Oncol.
101:245–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bradley JD, El Naqa I, Drzymala RE, Trovo
M, Jones G and Denning MD: Stereotactic body radiation therapy for
early-stage non-small-cell lung cancer: The pattern of failure is
distant. Int J Radiat Oncol Biol Phys. 77:1146–1150. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rowe BP, Boffa DJ, Wilson LD, Kim AW,
Detterbeck FC and Decker RH: Stereotactic body radiotherapy for
central lung tumors. J Thorac Oncol. 7:1394–1399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Milano MT, Chen Y, Katz AW, Philip A,
Schell MC and Okunieff P: Central thoracic lesions treated with
hypofractionated stereotactic body radiotherapy. Radiother Oncol.
91:301–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oskan F: The quality of toxicity reporting
and the story of the lung SBRT ‘NO-FLY ZONe’. Int J Radiat Oncol
Biol Phys. 92:514–515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Timmerman RD: The quality of toxicity
reporting and the story of the lung SBRT ‘No-Fly Zone’. In Regard
to Oskan. Int J Radiat Oncol Biol Phys. 93:726–727. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haasbeek CJ, Lagerwaard FJ, Slotman BJ and
Senan S: Outcomes of stereotactic ablative radiotherapy for
centrally located early-stage lung cancer. J Thorac Oncol.
6:2036–2043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang JY, Li QQ, Xu QY, Allen PK, Rebueno
N, Gomez DR, Balter P, Komaki R, Mehran R, Swisher SG and Roth JA:
Stereotactic ablative radiation therapy for centrally located early
stage or isolated parenchymal recurrences of non-small cell lung
cancer: How to fly in a ‘no fly zone’. Int J Radiat Oncol Biol
Phys. 88:1120–1128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Q, Swanick CW, Allen PK, Gomez DR,
Welsh JW, Liao Z, Balter PA and Chang JY: Stereotactic ablative
radiotherapy (SABR) using 70 Gy in 10 fractions for non-small cell
lung cancer: Exploration of clinical indications. Radiother Oncol.
112:256–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Senthi S, Haasbeek CJ, Lagerwaard FJ,
Verbakel WF, de Haan PF, Slotman BJ and Senan S: Radiotherapy for a
second primary lung cancer arising post-pneumonectomy: Planning
considerations and clinical outcomes. J Thorac Dis. 5:116–122.
2013.PubMed/NCBI
|
|
42
|
Donington JS, Miller DL, Rowland CC,
Deschamps C, Allen MS, Trastek VF and Pairolero PC: Subsequent
pulmonary resection for bronchogenic carcinoma after pneumonectomy.
Ann Thorac Surg. 74:154–159. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rowell NP and Williams CJ: Radical
radiotherapy for stage I/II non-small cell lung cancer in patients
not sufficiently fit for or declining surgery (medically
inoperable): A systematic review. Thorax. 56:628–638. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lagerwaard FJ, Voet PW, van Meerbeeck JP,
Burgers SA and Senan S: Rotterdam oncological thoracic study group:
Curative radiotherapy for a second primary lung cancer arising
after pneumonectomy-techniques and results. Radiother Oncol.
62:21–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou J, Amrane S, Korkut DN, Bourdoncle A,
He HZ, Ma DL and Mergny JL: Combination of i-motif and G-quadruplex
structures within the same strand: Formation and application. Angew
Chem Int Ed Engl. 52:7742–7746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Salmon H, Franciszkiewicz K, Damotte D,
Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F and
Donnadieu E: Matrix architecture defines the preferential
localization and migration of T cells into the stroma of human lung
tumors. J Clin Invest. 122:899–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
He HZ, Chan DS, Leung CH and Ma DL:
G-quadruplexes for luminescent sensing and logic gates. Nucleic
Acids Res. 41:4345–4359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma DL, He HZ, Leung KH, Chan DS and Leung
CH: Bioactive luminescent transition-metal complexes for biomedical
applications. Angew Chem Int Ed Engl. 52:7666–7682. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Carretero J, Shimamura T, Rikova K,
Jackson AL, Wilkerson MD, Borgman CL, Buttarazzi MS, Sanofsky BA,
McNamara KL, Brandstetter KA, et al: Integrative genomic and
proteomic analyses identify targets for Lkb1-deficient metastatic
lung tumors. Cancer Cell. 17:547–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Leung CH, Zhong HJ, Yang H, Cheng Z, Chan
DS, Ma VP, Abagyan R, Wong CY and Ma DL: A metal-based inhibitor of
tumor necrosis factor-α. Angew Chem Int Ed Engl. 51:9010–9015.
2012. View Article : Google Scholar : PubMed/NCBI
|