Introduction
Primary bile duct cancer usually exhibits biological
behavior; its growth is local, at the primary lesion, seldom
metastasizing to distant sites, and it requires other local therapy
at the surgically unresectable stage. Numerous previous studies of
local application using drugs for primary treatment of bile duct
cancer have been performed (1–7).
Drug delivery systems that release certain drugs
into specific sites in the body at a controlled rate include
microspheres, colloids, bioadhesives, micelles, intratumoral
injections, films, liposomes, peptide conjugates, lipid
nanoparticles, microemulsions, nanospheres, pastes and drug-eluting
stents.
Paclitaxel (PTX) is a microtubule inhibitor that has
been used in the treatment of various types of cancer, including
breast cancer, ovarian cancer and non-small cell lung cancer
(8). PTX exhibits dose-dependent
cytotoxicity, as well as antimetastatic and antiangiogenic
activities (9,10). PTX has pharmacokinetic properties,
which demonstrate a steep dose response for therapeutic effects and
toxicity, and is more adequate for localized delivery compared with
systemic delivery (11). Therefore, a
delivery system loaded with PTX at the tumor site may allow for a
high local concentration of the drug that is detrimental to cancer
cells, preventing re-growth and metastasis of the tumor (12).
PTX-eluting stents have been used for the prevention
of in-stent re-stenosis in coronary artery disease, however, a
previous in vitro study reported the efficacy of PTX-eluting
stent in malignant extrahepatic cholangiocarcinoma and serves as
the basis for development of a drug-eluting stent for malignant
biliary stricture (2). After then,
many tries are ongoing to develop PTX-eluting stent for the
treatment of malignant biliary stricture. In vitro and in
vivo animal studies have been performed to assess tissue
compatibility, and drug-releasing profiles of PTX-eluting metal
stents (5,6). In addition, a human trial for
PTX-eluting stent in malignant biliary obstruction was conducted.
In this study, the PTX-eluting stent demonstrated promising results
for prolonging the duration of stent patency (mean 429 days) and
patient survival (mean 350 days) (7).
To the best of our knowledge, there have been no reports of
antitumor activity of PTX-eluting membranes as a stent-covering
material in an animal model.
Previously, we developed a PTX-eluting membrane as a
covering material for drug-eluting stents for local control of bile
duct cancer and evaluated the release behavior of PTX in an in
vitro study of the membrane (13). In the previous study (13), the PTX-releasing profile demonstrated
that PTX was released slowly and steadily from polyurethane
membranes containing different weight percentages of PTX over 4
weeks following the initial fast-release phase in the first 4
days.
The present study evaluated the antitumor effect and
systemic toxicity of local and controlled-releasing membranes for
various amounts of PTX in a tumor model, and compared the degree of
apoptosis in the tumor according to the amount of PTX in the
membrane. The present study also assessed the biodistribution of
PTX in the tumor model in order to identify the local effects of
this releasing system.
Materials and methods
Preparation of the PTX-containing
membrane
The PTX-eluting membrane (S&G Biotech Inc.,
Seongnam, Korea) was prepared for in vivo animal study. PTX
was purchased from Samyang Genex Co., Ltd. (Daejeon, Korea) and
commercial polyurethane, Pellethane™ 2363-80 AE was purchased from
The Dow Chemical Company (Midland, MI, USA). Film casting solutions
were made by dissolving the PTX with premixed polyetherurethane in
a dimethylacetamide (PU/DMAc) solution. PT was dissolved at 0.5,
1.5, 3, and 6 mg/ml in order to make concentrations of 100, 300,
600, and 1,200 µg/disc, respectively. Subsequently, 200 µl
PTX/PU/DMAc solutions were pipetted onto 1.5 cm diameter
Teflon® wells (O&G, Hwaseong, Korea). The plate was
stored inside an oven at 50°C for 24 h to evaporate the solvent,
and then inside a vacuum oven at 70°C for an additional 24 h for
complete removal of the solvent. The films were removed from the
Teflon® plate and weighed.
The membrane was disc-shaped with a 15 mm-diameter,
176.6 mm2-area and 120 µm-thickness. The membrane, which
contained various amounts of PTX (0, 100, 300, 600 and 1,200
µg/disc) was polyurethane-based, and designed for local and
controlled release of PTX. Therefore, the amounts of PTX per unit
area of the membrane were 0, 56.5, 169.5, 339.0 and 678.0
µg/cm2 in 0, 100, 300, 600 and 1,200 µg/disc,
respectively.
Cell culture
CT26 murine colon adenocarcinoma cells were
commercially obtained from the Korean Cell Line Bank (Seoul,
Korea). The cell line was grown in Dulbecco's modified Eagle's
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Welgen Inc.,
Worcester, MA, USA) and 1% penicillin-streptomycin. Cells were
maintained under an atmosphere of 5% CO2 at 37°C in a
humidified incubator.
Animal model
Female Balb/c mice (aged 6 weeks, mean weight 20±2
g) were purchased from Orient Bio Inc. (Seongnam, Korea). The
animals were kept in specific pathogen-free animal facilities with
controlled temperature and humidity under conventional conditions
at a temperature of 22±2°C, a relative humidity of 55±10%, and a 12
h-dark/light illumination cycle. They were fed standard diet chow
pellets and water ad libitum. All procedures performed with
the mice were approved by the Animal Care and Use Committee of the
Inha University School of Medicine (Incheon, Korea), according to
the institutional policies. Ethical approval for the experiments
with mice were obtained from the Ethics Committee of Inha
University School of Medicine (approval no. INHA 130816-226).
CT26 cell suspension containing 1×106
cells in a volume of 100 µl Dulbecco's Modified Eagle Medium was
injected subcutaneously into the backs of all animals. When tumors
reached a volume between 100 and 250 mm3, a total of 50
mice were randomly allocated into five groups for assessment of
antitumor activity of the PTX-eluting membrane; 0, 100, 300, 600
and 1,200 µg PTX/disc of the membrane.
Antitumor activity and toxicity of the
PTX-eluting membrane
Membranes containing various amounts of PTX (0, 100,
300, 600 and 1,200 µg/disc) were inserted into the subcutaneous
layer beneath the tumor mass of CT26 tumor-bearing mice 7 days
after cell inoculation. Using a caliper, tumor size was determined
repeatedly and the body weight of the tumor model was monitored
until 26 days after insertion of the membrane. Tumor volume was
evaluated according to the method suggested by Uchiyama-Kokubu and
Watanabe (14). Euthanasia was
performed with CO2 inhalation at 26 days after membrane
insertion and the tumor masses were harvested.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
The TUNEL assay was performed using the
ApopTag® Peroxidase In Situ Apoptosis Detection kit
(S7100; EMD Millipore, Billerica, MA, USA), according to the
manufacturer's protocol, following deparaffinization of 3-µm thick
tumor tissue sections for in situ detection of apoptotic
cells in the extracted tumor tissues. Hematoxylin (75 µl/5
cm2) was used for counterstaining 5 min at room
temperature.
Following TUNEL staining, brown-colored cells were
considered as apoptotic cells. To compare the degree of apoptosis,
the present study counted and summated the cells using five high
power fields (magnification, ×400) of light microscopic
examination, and calculated the mean values.
Biodistribution of PTX following
treatment of the PTX-eluting membrane
Concentrations of PTX in the tumor tissue, tissue of
various organs and serum, as well as the membrane, were determined
26 days after insertion of 1,200 µg of PTX-containing membranes
into the tumor models (n=11), according to the modified
high-performance liquid chromatography (HPLC) method reported by
Lee et al (15). The HPLC
system consisted of a Waters 1515 isocratic HPLC pump, Waters 717
plus auto sampler, Waters 2487 Dual λ absorbance detector (Waters
Corporation, Milford, MA, USA) and a computing integrator. The
ultraviolet detector was set at 227 nm. The stationary phase was a
symmetry C18 column (4.6×150 mm, 5 µm; Waters Corporation) and the
mobile phase was a ratio of acetonitrile: 99.9% methanol: 0.05 mM
phosphate buffered saline (pH 4.0) (45:10:45, v/v/v). The retention
time at a flow rate of 1 ml/min was 12.1 min for PTX.
Statistical analysis
Data are presented as mean ± standard error of the
mean (SEM). Statistical analysis was performed using Friedman test
(for tumor volume and body weight change) or Kruskal-Wallis test
(for apoptotic cell counts) at 5% significance level combined with
Dunn's multiple comparison tests provided by GraphPad Prism 6
software (GraphPad Software, Inc., La Jolla, CA, USA). *P<0.05,
was considered to indicate a statistically significant
difference.
Results
Antitumor activity of the PTX-eluting
membrane
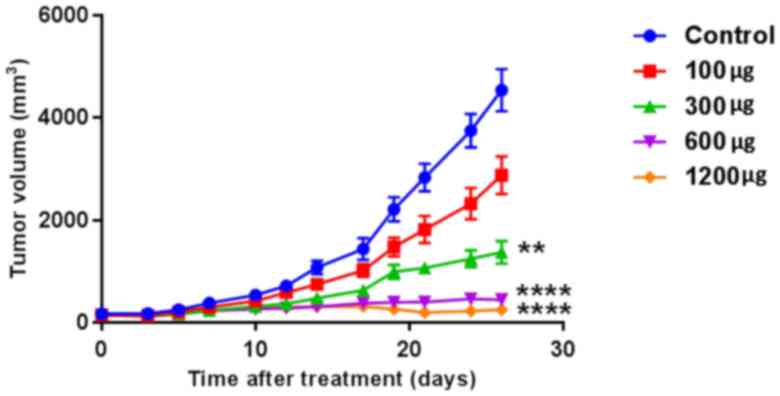
On day 26 after membrane treatment, tumor volumes
were 4,536±1,307, 2,877±1,157, 1,372±703, 449±349 and 252±325
mm3 when harvested in the 0, 100, 300, 600, and 1,200
µg-groups, respectively (n=10 per group; P<0.001; Fig. 1). Tumor growth was suppressed by a
PTX-eluting membrane in a dose-dependent manner. Tumors of the
control group revealed slow enlargement until day 12, and growth
was accelerated following that time. Tumor growth of the 100 and
300 µg-groups was inhibited, and tumors of the 600 and 1,200
µg-groups demonstrated a small level of growth from day 12 during
the observation period.
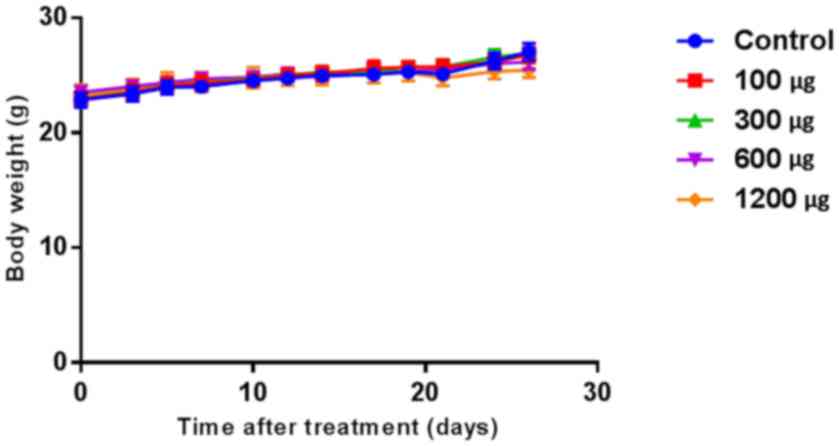
Toxicity of the PTX-eluting
membrane
Body weight changes between membrane insertion and
harvesting revealed increases of 4.2±1.4, 3.8±1.7, 3.9±1.0, 2.6±1.0
and 2.1±2.0 g in the 0, 100, 300, 600, and 1,200 µg-groups,
respectively, with no significant difference between the groups
(n=10 per group; P=0.623; Fig.
2).
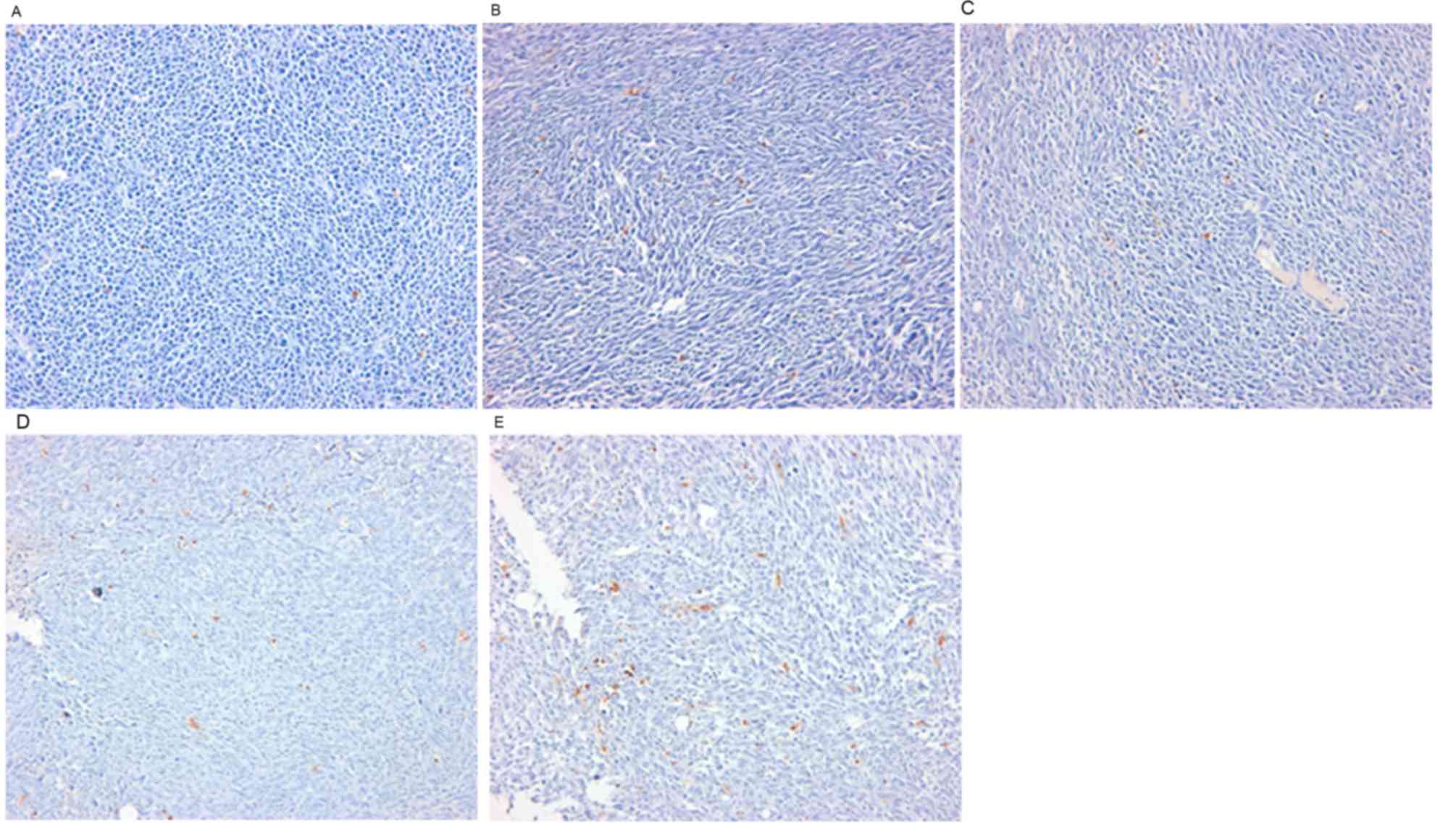
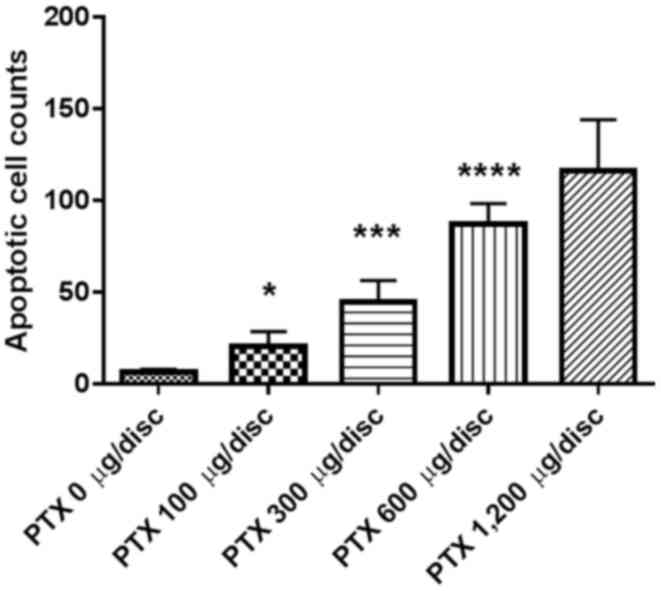
Effects of PTX-eluting membranes on
apoptosis
Results from the TUNEL assay demonstrated a
dose-dependent increase in apoptotic cells (Fig. 3). Apoptotic cell counts per high power
field (magnification, ×400) on TUNEL staining of tumor tissues were
6.0±5.0, 20.0±26.0, 45.0±38.0, 87.0±34.0 and 116.0±74.0 in the 0,
100, 300, 600, and 1,200 µg-groups, respectively (n=10 per group;
P<0.001; Fig. 4).
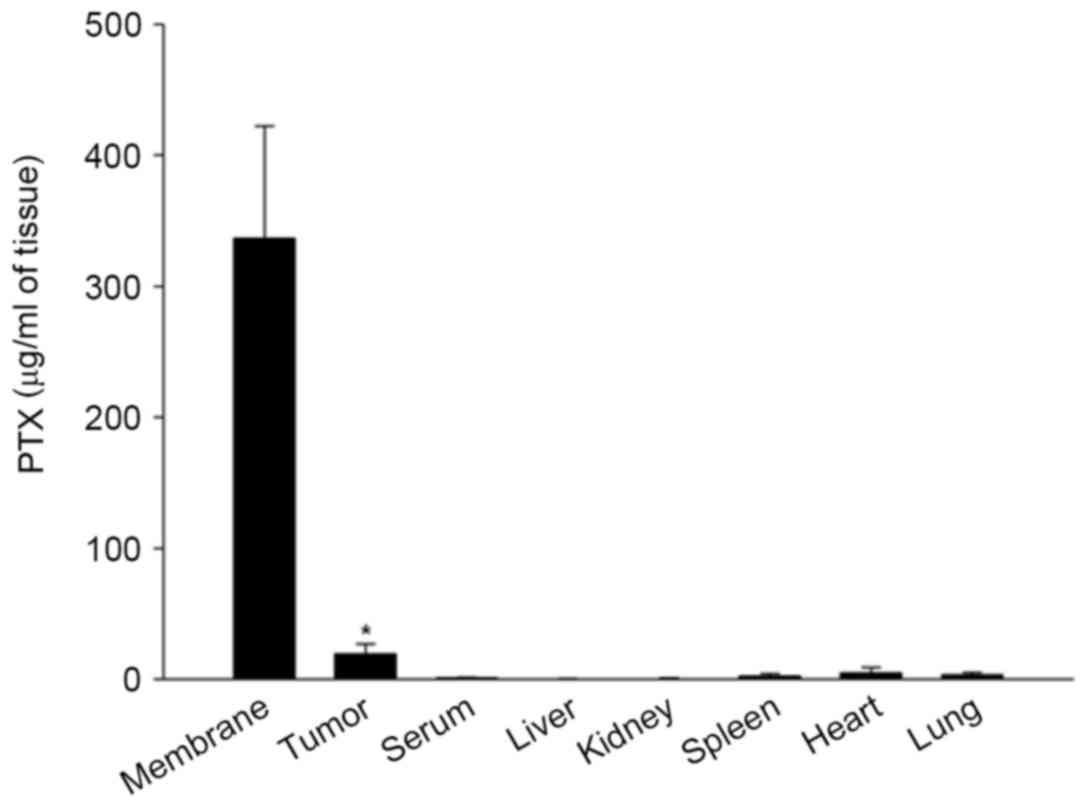
Biodistribution of PTX following
treatment of the PTX-eluting membrane
In the biodistribution analysis of PTX released from
the 1,200 µg/disc of PTX-eluting membranes, concentrations of PTX
were significantly higher in tumor tissues (19.2±2.4 µg/ml) on day
26 compared with those of other tissues and serum (serum, 0.9±0.2;
liver, 0.3±0.1; kidney, 0.7±0.1; spleen, 2.4±0.6; heart 4.9±1.3;
lung, 3.4±0.5 µg/ml; n=11; P<0.001). PTX was retained with a
high concentration on day 26 in the membranes (336.6±48.6 µg/ml;
Fig. 5).
Discussion
Bile duct cancer tends to grow locally without
distant metastasis, and uncontrolled cholestasis and/or cholangitis
caused by tumor mass obstruction of bile flow. These are important
determinants of patient survival in cases of inoperable cancer.
Mortality is usually caused by loco-regional disease rather than
distant metastasis (16,17); thus, local control of the tumor is
important in prolonging the survival time of patients. Therefore,
local treatment may be an effective option for palliative treatment
in the unresectable stage of bile duct carcinoma.
Systemic chemotherapy has been ineffective against
hypovascular bile duct carcinoma (18–21),
meaning that an effective amount of anticancer agent was not able
to reach the target lesion. There have been a number of efforts to
increase the drug amount in cancer tissues to achieve the antitumor
effect, including additional radiation treatment (22–24).
However, an effective method to increase the amount of drug has not
yet been developed.
Previously, through in vitro and in
vivo studies, certain studies have demonstrated the possibility
of topical application of anticancer drugs for unresectable
cholangiocarcinoma (1,2,4).
Inhibition of growth of the cell line by local and
controlled-release of carboplatin, PTX or OK-432 was revealed in
these studies (1,2,4).
Furthermore, a patient with a malignant biliary obstruction
effectively treated by local injection of the bioactive drug using
endoscopy for palliation has previously been reported (3). These studies lead to development of a
drug-eluting stent for local treatment of bile duct cancer. The
PTX-eluting biliary stent was then developed and evaluated for its
tissue compatibility on the normal bile duct of an animal model,
which documented its safety in a living body (5,6). The
theoretical basis for antitumor effect of PTX in a stent is similar
with local application of antitumor agent, which is maximizing its
concentration within the bile duct while minimizing the systemic
side effects of the agent. According to the present study, the
hypothesis may be supported by the current results that
demonstrated that the higher dose of PTX in the stent revealed a
more potent antitumor effect judged by suppression of tumor growth
in a dose-dependent manner (Fig. 1),
and the concentration of PTX was significantly higher compared with
in other tissues and serum.
Preliminary clinical data on a PTX-eluting stent has
been reported in patients with malignant biliary tract obstruction
(7). The duration of patient survival
and stent patency (350 and 429 days) were revealed to be longer for
the PTX-eluting stent compared with those reported for conventional
covered metal stents. Thus, the results of this pilot human study
suggested the possibility of the PTX-eluting stent as a local
anticancer treatment option for malignant biliary obstruction
(7).
The present study developed a PTX-eluting
polyurethane membrane as a covering material for a biliary metal
stent, designed for local and controlled release of PTX (13), which is may be more suitable for local
anticancer treatment compared with systemic treatment (11). In our previous study investigating the
drug-release profile from this membrane, an initial burst of PTX
was released for the first 4 days for all different volume
percentages of the PTX-eluting membranes (13). Subsequently, the gradient of the
cumulative released-amount of PTX with time was gentle and constant
for >4 weeks. These results implied that PTX is released slowly
and regularly from the polyurethane membrane for >1 month
without reference to the volume percentage. Therefore, the
observation period of the tumor growth curve of the present study
was established in the animal study on the basis of these
results.
The slope of the tumor volume curve increased in a
time-dependent manner following inoculation of cancer cells in the
control group, indicating that tumor growth in the control group
accelerated with time. However, other results demonstrated that
tumor growth was constantly and significantly inhibited for ≥4
weeks when the amount of PTX in the membrane was ≥600 µg in the
tumor volume curve of the mice. Furthermore, the antitumor activity
was dose-dependent.
The present study analyzed body weight changes of
the models with time for evaluation of the systemic toxicity of the
PTX-eluting membrane in the experiment. Body weights were revealed
to increase slowly throughout a period of 26 days in all groups.
There was no significant difference in body weight alterations
between the control and treatment groups. Therefore, the
PTX-eluting membrane is not likely to cause any systemic
toxicity.
Cancer cell apoptosis is known to be one of the main
antitumor mechanisms of PTX. Results of the TUNEL assay
demonstrated that the degree of cancer cell apoptosis appeared to
be significantly higher in treatment groups compared with in the
control group (P<0.001). In addition, the number of apoptotic
cells increased in a dose-dependent manner among treatment groups.
These results revealed that apoptosis of cancer cells is affected
by the amount of PTX in PTX-eluting membranes.
In the biodistribution analysis of PTX released from
the drug-delivery system in the 1,200 µg-group, the concentration
of PTX was low in the blood and peripheral tissues of various
organs on day 26 after membrane insertion, whereas it was higher in
tumor tissues, and the membranes. These results demonstrated that
delivery of PTX from the membrane was controlled for >4 weeks,
and the antitumor effect of this drug-delivery system did not
result from the systemic effect of PTX, but was a local effect.
The results of present study demonstrated the
antitumor effect of PTX membrane objectively by revealing the
inhibition of tumor cell growth and apoptosis of tumor cells. In
addition, the results also demonstrated that the antitumor activity
of PTX in the membrane was dose-dependent. Furthermore, the present
study evaluated the potential side effect of the PTX eluting
membrane by assessing the biodistribution of PTX in tumor a model
and weight changes of the mice. Prior to clinical application,
further animal studies are required to prevent the errors in
clinical application of novel medical devices in humans. The
present study estimated that these results would be the
cornerstones of clinical application of PTX-eluting membrane stents
in patients with malignant biliary stricture.
The main limitation of the present study was that a
colon adenocarcinoma cell line was selected that was syngeneic to
murine rather than human cholangiocarcinoma cell lines, in which
response to PTX and biological behavior may differ. The present
study could not establish the cholangiocarcinoma model because an
appropriate cholangiocarcinoma cell line could not be obtained.
Therefore, for future clinical applications, long-term antitumor
activity and safety of the PTX-eluting membrane should be
documented using a cholangiocarcinoma xenograft model.
In summary, the PTX-eluting membrane demonstrated a
significant, dose-dependent antitumor effect. No local or systemic
toxicity was observed in all groups inserted with membranes
containing various amounts of PTX. These results appeared to occur
by a local effect of this system, and the system may be safe, at
least within the range of the amount of PTX per membrane.
Therefore, the PTX-releasing membrane may be applied in the future
for development of the drug-eluting stent for local therapy of bile
duct cancer. However, further studies are required in order to
confirm whether the PTX-delivery system has significant antitumor
activity in a human cholangiocarcinoma xenograft model, and to
elucidate the mechanisms underlying a local antitumor effect of
this system prior to advancement of clinical applications.
Acknowledgements
Not applicable.
Funding
The present study was supported by Inha University
Hospital Research Grant.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JSP was responsible for study conception and design,
collection and assembly of data, analysis and interpretation of
data, drafting of the article, provision of study materials, and
administrative and technical or logistic support. SJ was
responsible for study conception and design, provision of study
materials, critical revision of the article for important
intellectual content and final approval of the article. DHL and JHM
were responsible for administrative and technical support of the
animal experiments. DHL made substantial contributions to the
conception and design of the work. JHM made substantial
contributions to the acquisition of data and analysis for the work.
ISP made substantial contributions to the design of the work and
final approval of the version to be published. SP made substantial
contributions to the acquisition of data for the work and drafting
it critically for important intellectual content.
Ethics approval and consent to
participate
Ethical approval for the experiments with mice was
obtained from the Ethics Committee of Inha University School of
Medicine (approval no. INHA 130816-226).
Consent for publication
Not applicable.
Competing interests
All authors state that there are no conflicts of
interest in this study.
Glossary
Abbreviations
Abbreviations:
|
PTX
|
paclitaxel
|
|
PU/DMAc
|
polyetherurethane in a
dimethylacetamide
|
|
TUNEL
|
terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
|
|
HPLC
|
high-performance liquid
chromatography
|
References
|
1
|
Mezawa S, Homma H, Sato T, Doi T,
Miyanishi K, Takada K, Kukitsu T, Murase K, Yoshizaki N, Takahashi
M, et al: A study of carboplatin-coated tube for the unresectable
cholangiocarcinoma. Hepatology. 32:916–923. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalinowski M, Alfke H, Kleb B, Dürfeld F
and Joachim Wagner H: Paclitaxel inhibits proliferation of cell
lines responsible for metal stent obstruction: Possible topical
application in malignant bile duct obstructions. Invest Radiol.
37:399–404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park SW, Lee DH, Park YS, Chung JB, Kang
JK and Song SY: Percutaneous transhepatic choledochoscopic
injection of ethanol with OK-432 mixture for palliation of
malignant biliary obstruction. Gastrointest Endosc. 57:769–773.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee DH, Kang SG, Jeong S, Yoon CJ, Choi
JA, Byun JN, Park JH and Lee KB: Local delivery system of immune
modulating drug for unresectable adenocarcinoma: In vitro
experimental study and in vivo animal study. Cardiovasc Intervent
Radiol. 29:832–837. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee DK, Kim HS, Kim KS, Lee WJ, Kim HK,
Won YH, Byun YR, Kim MY, Baik SK and Kwon SO: The effect on porcine
bile duct of a metallic stent covered with a
paclitaxel-incorporated membrane. Gastrointest Endosc. 61:296–301.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SS, Shin JH, Han JM, Cho CH, Kim MH,
Lee SK, Kim JH, Kim KR, Shin KM, Won YH and Song HY: Histologic
influence of paclitaxel-eluting covered metallic stents in a canine
biliary model. Gastrointest Endosc. 69:1140–1147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suk KT, Kim JW, Kim HS, Baik SK, Oh SJ,
Lee SJ, Kim HG, Lee DH, Won YH and Lee DK: Human application of a
metallic stent covered with a paclitaxel-incorporated membrane for
malignant biliary obstruction: Multicenter pilot study.
Gastrointest Endosc. 66:798–803. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mekhail TM and Markman M: Paclitaxel in
cancer therapy. Expert Opin Pharmacother. 3:755–766. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Markman M, Rowinsky E, Hakes T, Reichman
B, Jones W, Lewis JL Jr, Rubin S, Curtin J, Barakat R, Phillips M,
et al: Phase I trial of intraperitoneal taxol: A gynecoloic
oncology group study. J Clin Oncol. 10:1485–1491. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burt HM, Jackson JK, Bains SK, Liggins RT,
Oktaba AM, Arsenault AL and Hunter WL: Controlled delivery of taxol
from microspheres composed of a blend of ethylene-vinyl acetate
copolymer and poly (d,l-lactic acid). Cancer Lett. 88:73–79. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dhanikula AB and Panchagnula R: Localized
paclitaxel delivery. Int J Pharm. 183:85–100. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pawar R, Shikanov A, Vaisman B and Domb
AJ: Intravenous and regional paclitaxel formulations. Curr Med
Chem. 11:397–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang SG, Lee SC, Choi SH, Park S, Jeong S,
Lee HD and Kim M: Paclitaxel-polyurethane film for anti-cancer drug
delivery: Film characterization and preliminary in vivo study.
Macromol Res. 18:680–685. 2010. View Article : Google Scholar
|
|
14
|
Uchiyama-Kokubu N and Watanabe T:
Establishment and characterization of adriamycin-resistant human
colorectal adenocarcinoma HCT-15 cell lines with multidrug
resistance. Anticancer Drugs. 12:769–779. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SH, Yoo SD and Lee KH: Rapid and
sensitive determination of paclitaxel in mouse plasma by
high-performance liquid chromatography. J Chromatogr B Biomed Sci
Appl. 724:357–363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mittal B, Deutsch M and Iwatsuki S:
Primary cancers of extrahepatic biliary passages. Int J Radiat
Oncol Biol Phys. 11:849–854. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klatskin G: Adenocarcinoma of the hepatic
duct at its bifurcation within the porta hepatis. an unusual tumor
with distinctive clinical and pathological features. Am J Med.
38:241–256. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harvey JH, Smith FP and Schein PS:
5-fluorouracil, mitomycin, and doxorubicin (FAM) in carcinoma of
the biliary tract. J Clin Oncol. 2:1245–1248. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Falkson G, MacIntyre JM and Moertel CG:
Eastern cooperative oncology group experience with chemotherapy for
inoperable gallbladder and bile duct cancer. Cancer. 54:965–969.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Altaee MY, Johnson PJ, Farrant JM and
Williams R: Etiologic and clinical characteristics of peripheral
and hilar cholangiocarcinoma. Cancer. 68:2051–2055. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones DV Jr, Lozano R, Hoque A, Markowitz
A and Patt YZ: Phase II study of paclitaxel therapy for
unresectable biliary tree carcinomas. J Clin Oncol. 14:2306–2310.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buskirk SJ, Gunderson LL, Adson MA,
Martinez A, May GR, McIlrath DC, Nagorney DM, Edmundson GK, Bender
CE and Martin JK Jr: Analysis of failure following curative
irradiation of gallbladder and extrahepatic bile duct carcinoma.
Int J Radiat Oncol Biol Phys. 10:2013–2023. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flickinger JC, Epstein AH, Iwatsuki S,
Carr BI and Starzl TE: Radiation therapy for primary carcinoma of
the extrahepatic biliary system. An analysis of 63 cases. Cancer.
68:289–294. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsujino K, Landry JC, Smith RG, Keller JW,
Williams WH and Davis LW: Definitive radiation therapy for
extrahepatic bile duct carcinoma. Radiology. 196:275–280. 1995.
View Article : Google Scholar : PubMed/NCBI
|