Introduction
Acute leukemia is the most common type of pediatric
malignancy, of which 80% cases are classified as acute
lymphoblastic leukemia (ALL) and 15–25% as acute myeloblastic
leukemia (AML) (1,2). There is a significant discrepancy in the
outcomes of pediatric ALL and AML, with 5-year survival of 90% in
ALL compared with 70% in AML (1,3,4). The difficulties in AML treatment include
limited therapeutic options and multi-drug resistance exhibited by
AML myeloblasts, despite the identification of prognostic markers
(5).
One of the drugs used for the treatment of children
with AML is 6-thioguanine (6-TG), administered during maintenance
chemotherapy at a dose of 40 mg/m2/24 h for 1 year
(AML-BFM 2004 Protocol) (6). 6-TG and
6-mercaptopurine (6-MP) belong to the thiopurine drug family, which
includes purine analogs with anticancer and immunosuppressive
activities. All thiopurines are prodrugs, and must be converted
in vivo into thioguanine nucleotides (TGNs), which are
incorporated into DNA to exert a cytotoxic effect. 6-TG is
converted directly in a single-step pathway, while 6-MP requires a
three-step conversion. The major competing metabolic process is
S-methylation, catalyzed by thiopurine methyltransferase (TPMT).
6-MP is methylated to methylMP and 6-TG to methylTG. The
intermediates of the parent drugs may also be methylated:
6-thioinosine 5′-monophosphate (TIMP) to methyl-TIMP (MeTIMP) and
thioguanine monophosphate (TGMP) to methyl-TGMP (MeTGMP) (7) (Fig. 1).
MeTIMP inhibits de novo purine synthesis (DNPS) and
contributes to the cytotoxic effect of 6-MP in addition to TGN
formation. Although >1 of the thiopurine metabolites may inhibit
DNPS, MeTIMP is a more potent inhibitor compared with the others,
including MeTGMP (7–10).
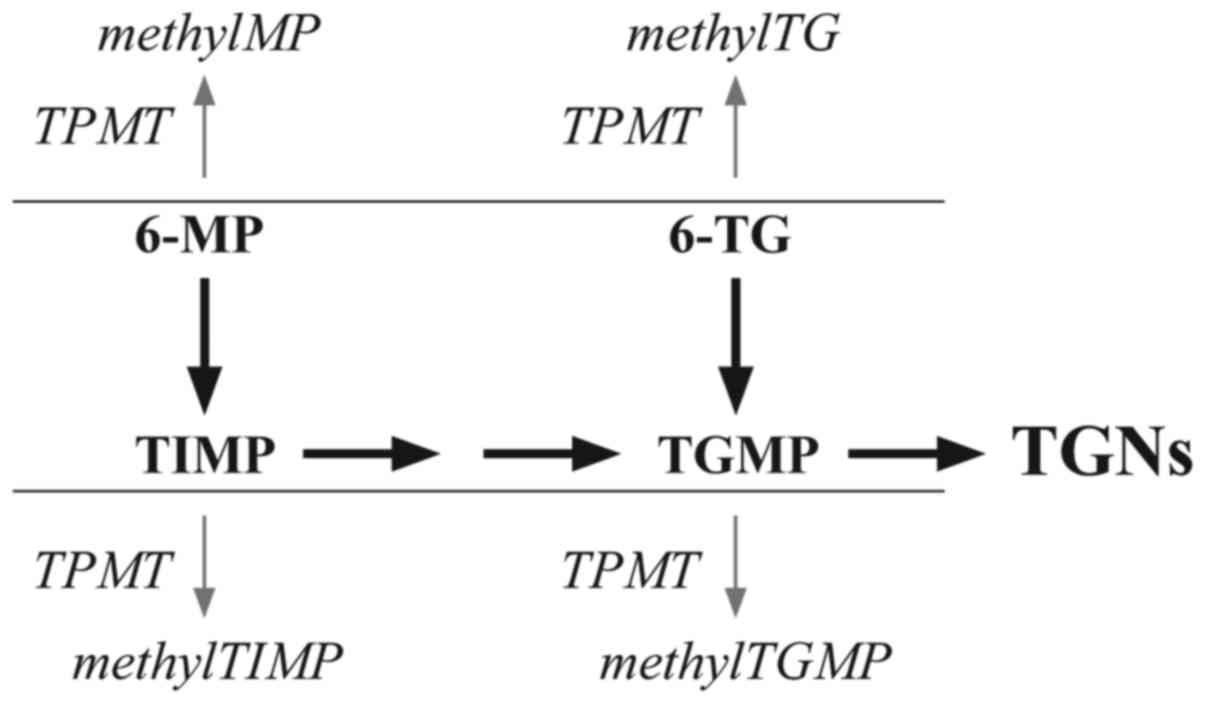 | Figure 1.Simplified schematic diagram of the
primary metabolic pathways of 6-MP and 6-TG. methylMP,
methylmercaptopurine; methylTG, methylthioguanine; methylTGMP,
methylthioguanosine monophosphate; methylTIMP, methylthioinosine
monophosphate; TGMP, thioguanosine monophosphate; TGNs, thioguanine
nucleotides; TIMP, thioinosine 5′-monophosphate; TPMT, thiopurine
methyltransferase; 6-MP, mercaptopurine; 6-TG, thioguanine. |
TPMT activity determines the availability of
thiopurines in the conversion to TGNs (8). It follows a trimodal pattern of
distribution controlled by genetic polymorphisms, with
TPMTL (low-activity variant alleles TPMT*2-*38) or
TPMTH (high-activity wild-type allele TPMT*1) at a
single genetic locus. Overall, 0.3% of the Caucasian population are
TPMT-deficient (homozygous TPMTL/TPMTL
genotype), 11% exhibit intermediate TPMT activity (heterozygous
TPMTL/TPMTH genotype) and 89% exhibit high
TPMT activity (TPMTH/TPMTH genotype)
(9,11,12). At
present, the reason for the unequal distribution of TPMT activity
in the intermediate- and high-activity groups remains unknown.
TPMT activity is associated with sensitivity and
toxicity to thiopurines within individuals (11). People who are homozygous for
nonfunctional TPMT alleles (TPMTL/TPMTL
genotype) develop severe and potentially fatal 6-MP-induced
hematologic toxicity (11).
TPMT status and its implications in treatment
outcome have been studied extensively in ALL and autoimmune
disorders (13–16), but little data is available on TPMT in
AML (3,10). As a preliminary evaluation to compare
TPMT status in AML with the one in ALL, we report the results of
TPMT genotype and phenotype assessment in children treated for
AML.
Materials and methods
Patients
The present study included 33 children (18 males and
15 females), aged between 0.7–19.7 years (mean age, 10.3 years),
treated for AML according to the control arm of AML-BFM 2004
Protocol (6). Patients were recruited
between January 2007 to December 2015 from the Department of
Pediatric Oncology, Hematology and Transplantology, Karol Jonscher
Clinical Hospital, Poznań and the Department of Pediatrics,
Hematology and Oncology, Antoni Jurasz University Hospital,
Bydgoszcz. The characteristics of the children in the study group
are summarized in Table I. Based on
the protocol risk group assignments, the chemotherapy used in the
standard risk (SR) arm consisted of four cycles of intensive
chemotherapy (AIE: Cytarabine, 100 mg/m2/day 48 h
continuous-infusion on days 1–2 and 100 mg/m2 twice a
day on days 3–8, idarubicin 12 mg/m2/day on days 3, 5
and 7, etoposide 150 mg/m2/day on days 6–8; AI:
Cytarabine 500 mg/m2/day 96 h continuous-infusion days
1–4, idarubicin 7 mg/m2/day, days 3, 5; hAM: Cytarabine
1 g/m2 twice a day, on days 1, 2 and 3, mitoxantrone 10
mg/m2/day days 3 and 4; HAE: Cytarabine 3
g/m2 twice a day, on days 1, 2 and 3, etoposide 125
mg/m2/day, on days 2, 3, 4 and 5) followed by 1 year
maintenance chemotherapy with 6-TG at a dose of 40
mg/m2/24 h. Patients stratified into the high-risk (HR)
group received five cycles of intensive chemotherapy (second
induction of HAM between AIE and AI: Cytarabine 3 g/m2
twice a day on days 1, 2 and 3; mitoxantrone 10
mg/m2/day on days 3 and 4) and either proceeded to
hematopoietic stem cell transplantation (HSCT) if a well-matched
familiar donor was available, or received the same maintenance
chemotherapy as the SR group. The intensive chemotherapy treatment
did not include 6-TG. Only patients in remission were treated with
maintenance chemotherapy. None of the patients included developed
any significant infection during maintenance treatment. In total,
29 children included in the AML study group were treated with 6-TG.
Within the time-frame of the present study, the intended evaluation
of TPMT consecutive activity at different stages of treatment in
the same AML patients appeared impossible due to the following
causes: Admission to the oncology clinic shortly following red
blood cell (RBC) transfusion; transfusion-dependence during
intensive chemotherapy; transfer for HSCT; and relapses and
referrals to external clinics for follow-up subsequent to intensive
chemotherapy. TPMT evaluations were performed using blood samples
collected at diagnosis from 8 patients and during and following
maintenance chemotherapy from 10 and 17 patients. The two latter
subgroups included the only 2 patients in whom the assay was
performed at both these stages of AML. Samples were taken at least
30 days following the beginning of treatment and at least 6 weeks
following the cessation of maintenance chemotherapy. The present
study was approved by the Bioethical Committee at Poznan University
of Medical Sciences (Poznań, Poland), was performed in accordance
with the 1964 Declaration of Helsinki and its later amendments.
Written informed consent was obtained from the parents of the
patients prior to initiating the study.
 | Table I.Clinical characteristics of children
and adolescents with acute myeloblastic leukemia. |
Table I.
Clinical characteristics of children
and adolescents with acute myeloblastic leukemia.
|
Characteristics | Valuea |
|---|
| Sex |
|
|
Male | 18 |
|
Female | 15 |
| Age, years
(mean) | 0.7–19.7
(10.3) |
| WBC at diagnosis,
median (range) | 14.0 (0.3–282.0)
[x109/l] |
|
<3×109/l | 8 |
|
3-15×109/l | 10 |
|
>15-100×109/l | 9 |
|
>100×109/l | 6 |
| Platelets at
diagnosis, median (range) | 35 (2–244)
[x109/l] |
| % of blasts in bone
marrow at diagnosis, median (range) | 66 (range
25–90) |
| Molecular
cytogenetics data |
|
| FLT3
ITD.+ | 1 |
|
t(15;17) | 3 |
|
t(8;21) | 3 |
| Other karyotype
abnormalities | 4 |
|
47,XY,+8,t(9;11)(p22;q23) | 1 |
| 90–92,
XXYY, der(4) | 1 |
| 47XX,
+8[10]/46XX[8] | 1 |
|
46,XY,t(8;3;21)(q22;?q25;q22),del(9)(q21q22) | 1 |
| Normal
karyotype | 13 |
| Data
unavailable | 9 |
| Treated with
chemotherapy alone including 6-TG | 29 |
| Treated with
chemotherapy and HSCT without 6-TG | 4 |
| WBC at the end of
chemotherapy, median (range) | 4.3 (2–8)
[x109/l] |
| Proportion of CR1,
n (%) | 33 (100) |
| Relapse, n (%) | 8 (24) |
| Succumbed in
progression of leukemia, n (%) | 6 (18) |
| EFS, years (mean ±
SEM) | 4 (75±7%), median
follow up 51.8 months |
| OS, years (mean ±
SEM) | 4 (78±7%), median
follow up 76.1 months |
Blood samples from 105 children with ALL were
obtained at: Point of diagnosis (16 children, 8 males and 8
females, aged 2–16 years, mean 6.6±3.5 years); during the
maintenance chemotherapy (55 children, 28 males and 27 females,
aged 1–17 years, mean 6.9±4.2 years); and following the cessation
of the chemotherapy (34 children, 16 males and 18 females, aged
9–28 years, mean 16±4 years). The children with ALL were treated
according to the ALL IC-BFM 2002 Chemotherapy Treatment protocol
(17), including maintenance
treatment with 6-MP (50 mg/m2/day) and methotrexate (20
mg/m2/week). The data were described previously
(18).
TPMT phenotype assays were performed at least 8
weeks after the most recent blood transfusion. RBC lysates were
obtained from the venous blood of patients with AML and healthy
adult volunteers. For the ALL study, the control group were 39
healthy children aged 2–15 years (mean 6.9±4.0 years). Among the
control group, there were 18 males aged 2–15 years (mean 7.3±3.9
years) and 21 females aged 2–14 years (mean 6.7±4.1 years). These
participants were recruited in (January-December) 2005 year in the
Outpatient Clinic for Proliferative Diseases (Karol Jonscher
Clinical Hospital, Poznań) for other than haematological
indications. For the AML study, the control group consisted of the
control group from ALL study and an additional 5 healthy adult
volunteers aged 25–60 years (mean 36±14 years; 2 men and 3 women)
who were recruited in Regional Centre of Blood Donation and Blood
Treatment in Poznań. Blood samples (1 ml) were collected into EDTA
tubes and centrifuged for 10 min at 1,700 × g at room temperature.
The plasma was removed and RBCs were washed with isotonic salt
solution (0.9% NaCl; Polfa Lublin, Poland), and re-centrifuged for
10 min at 1,700 × g at room temperature. Samples of concentrated
RBC were then stored at −80°C until analysis was performed. After
thawing, lysates were diluted using isotonic salt solution (1:1)
and centrifuged at room temperature for 10 min at 1,300 × g to
remove RBC membranes. TPMT activity and haemoglobin concentration
were determined in the same sample of RBC lysate. The activity of
TPMT was measured in RBC lysates using an enzymatic reaction,
described previously (19,20), based on the conversion of the
substrate, 6-MP, into 6-methylmercaptopurine (6-mMP), involving
S-adenozyl-L-methionine (SAM) as the methyl group donor. For the
calibration curves (generated using MS Excel 2010; Microsoft
Corporation, Redmond, WA, USA), the solutions consisted of
different final 6-mMP concentrations (3.1–75.0 ng/50 µl RBC
lysates), 0.2 M potassium dihydrogenphosphate and standard RBC
lysates were used. For determination of the TPMT activity, 50 µl
RBC lysates were mixed with 6-MP solution (500 mg/l), SAM (640
µmol/l) and 0.1 M potassium dihydrogenphosphate. Following 1 h
incubation at 37°C, 6-mMP was extracted in the non-polar phase, and
then the 6-mMP plasma concentration was determined using the
high-performance liquid chromatography (HPLC) method (20) with UV detector (at 290 nm). The flow
rate was 1 ml/min and 30 µl was injected into HPLC for analysis.
The calibration curves were expressed as the association between
the peak surface area determined in samples and 6-mMP concentration
expressed as ng/50 µl RBC lysates. In the same RBC lysate sample,
hemoglobin concentration was determined using the cyanmethemoglobin
method (Stamar; Department of Reagents for Laboratory Diagnositics;
Dąbrowa Górnicza, Poland). Hemoglobin concentration was determined
using the spectrophotometric method with Drabkin's reagent (Stamar;
Department of Reagents for Laboratory Diagnositics; Dąbrowa
Górnicza, Poland; http://stamar.pl/). According to the
manufacturers protocol, the concentration of potassium ferricyanide
and potassium cyanide were 0.6 and 0.7 nM, respectively. To
determine the concentration of hemoglobin in patient samples, 10 µl
RBC lysate was mixed with 2.5 ml Drabkin's reagent. Following after
3 min, the absorbance of the solution was determined at a
wavelength of 540 nm. Additionally, blind, control and standard
samples were prepared according to the manufacturer's protocol. A
volume of 10 µl of purified water, RBC from control groups and
cyanmethemoglobin standard solution was added into the tubes
containing 2.5 ml of Drabkin's reagent for blind, control and
standard samples, respectively. TPMT activity was expressed as nmol
of produced 6-mMP/g Hb/h. All calculations were performed using MS
Excel 2010 (Microsoft Corporation, Redmond, WA, USA). Samples from
each patient were examined twice.
TPMT mutations (TPMT*2, TPMT*3A, TPMT*3B and
TPMT*3C) and the wild type TPMT*1 were determined with a polymerase
chain reaction/restriction fragment length polymorphism method
(21) at the Department of
Experimental and Clinical Pharmacology, Pomeranian Medical
University (Szczecin, Poland).
Statistical analysis
The data are presented as the median and range.
Although some data was normally distributed, the median was
presented to enable clear comparisons of data in subgroups.
Statistical analysis was performed using Statistica software
version 12.0 (StatSoft, Inc., Tulsa, OK, USA). P<0.05 was
considered to indicate a statistically significant difference.
Normality was determined using the Kolmogorov-Smirnov test for
groups >50 patients (all ALL patients assessed during
maintenance chemotherapy) or the Shapiro-Wilk test for the
remaining groups which were <50 patients. All differences
between groups were estimated using a Student's t-test (for
normally distributed data) and a Mann-Whitney or Kruskal-Wallis
tests for non-normally distributed data. TPMT activity was normally
distributed in males and females with AML, at the time of AML
diagnosis, during treatment of AML and in all groups with ALL.
Non-normal distributions were observed for TPMT activity in the
entire AML group and among children following AML treatment. In the
case of unequal variations, Cochran's C test was applied. The
Kaplan-Meier method was also used for the relapse risk analysis and
log rank test for the comparative analysis. The association between
TPMT activity and rank variables was assessed with Spearman's rank
correlation coefficient. The difference in the distribution of TPMT
alleles between AML and ALL groups was estimated using the z-test
for the difference of proportions.
Results
In children with AML, the levels of TPMT activity
varied between 25.9–91.6 nmol 6-mMP g−1 Hb
h−1, with median 43.6 nmol 6-mMP g−1 Hb
h−1.
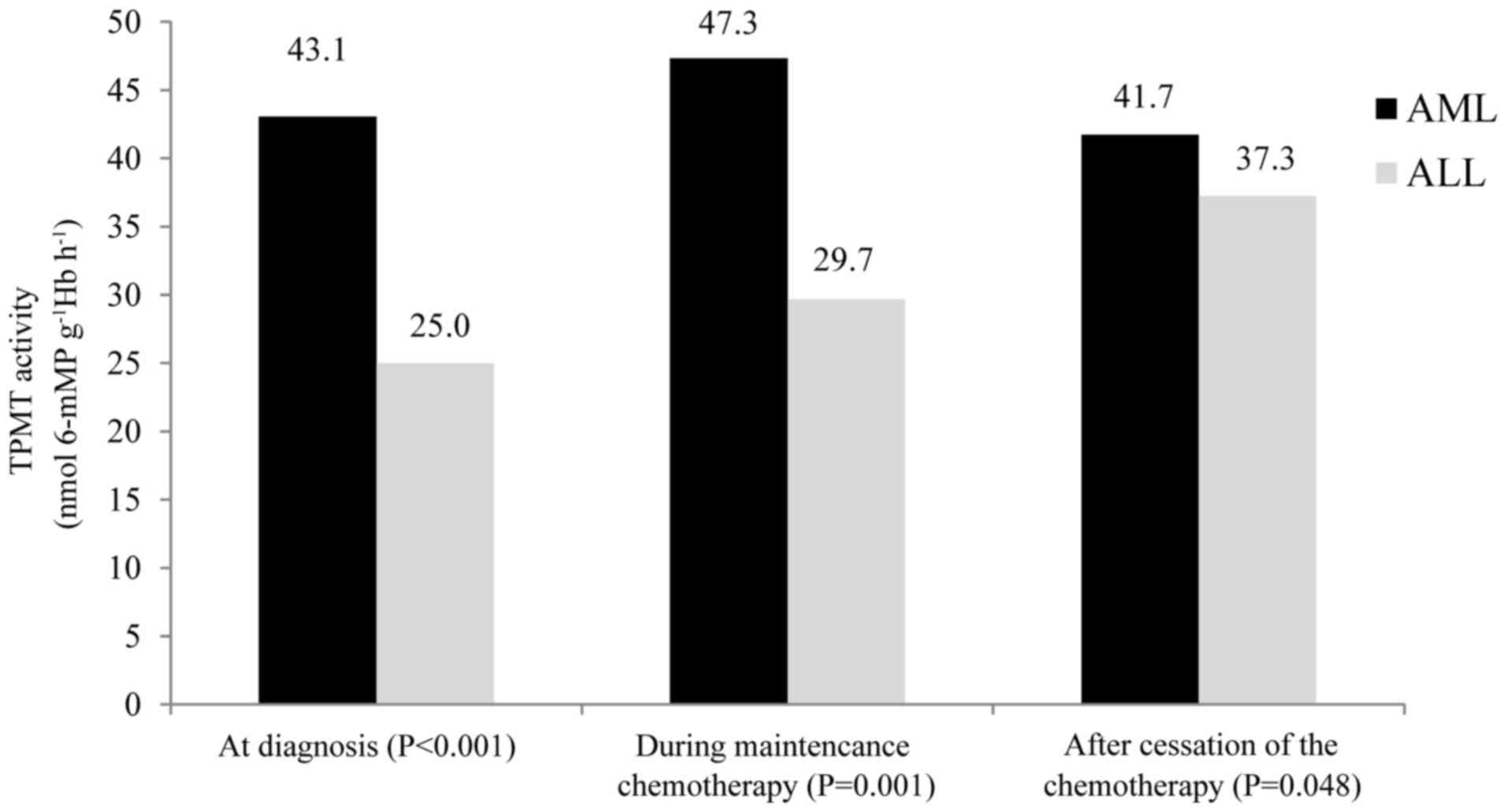
Median TPMT activity in the children with AML at the
point of diagnosis was 43.1 nmol 6-mMP g−1 Hb
h−1. During maintenance chemotherapy, median TPMT
activity was 47.3 nmol 6-mMP g−1 Hb h−1.
Following the cessation of chemotherapy, the median TPMT activity
was 41.72 nmol 6-mMP g−1 Hb h−1.
All children with AML exhibited the TPMT*1/*1
genotype, with the exception of 1, who was a heterozygote with the
genotype TPMT*1/*3C. TPMT activity in this child was determined at
diagnosis, and measured 42.5 nmol 6-mMP g−1 Hb
h−1.
No statistical differences in TPMT activity were
observed with respect to age (rs=0.001, P=0.997 in the
whole group; rs=−0.733, P=0.695 among 29 children
treated with 6-TG) or sex (P=0.677). The TPMT activity was similar
regardless of whether the assay was performed at diagnosis, during
or following maintenance chemotherapy (P=0.919). The TPMT activity
results are detailed in Table II.
Overall, two consecutive TPMT evaluations during and following
chemotherapy for 2 children also revealed convergent results (50.9
and 52.5 nmol 6-mMP g−1 Hb h−1 for the first
child, and 34.6 and 34.9 nmol 6-mMP g−1 Hb
h−1 for the second child).
 | Table II.TPMT activity in association with sex
and stage of therapy in all children with acute myeloblastic
leukemia, and in the subgroup treated with 6-TG. |
Table II.
TPMT activity in association with sex
and stage of therapy in all children with acute myeloblastic
leukemia, and in the subgroup treated with 6-TG.
| A, All studied
children (n=33) |
|---|
|
|---|
|
Characteristics | TPMT activity (nmol
6-mMP g−1 Hb h−1) | P-value |
|---|
| Total, median
(range) | 43.7
(25.9–91.6) |
|
| Sex, median
(range) |
| 0.677 |
|
Male | 43.5
(25.8–84.5) |
|
|
Female | 43.6
(27.0–91.6) |
|
| Stage of therapy,
median (range) |
| 0.919 |
| At
diagnosis | 43.1
(30.6–62.7) |
|
| During
maintenance | 47.3
(25.9–84.5) |
|
|
Following cessation of
chemotherapy | 41.7
(28.2–91.6) |
|
|
| B, Children
treated with 6-TG (n=29) |
|
|
Characteristics | TPMT activity
(nmol 6-mMP g−1 Hb h−1) | P-value |
|
| Total, median
(range) | 43.3
(25.9–91.6) |
|
| Sex, median
(range) |
| 0.397 |
|
Male | 42.5
(25.8–84.5) |
|
|
Female | 45.2
(27.2–91.6) |
|
| Stage of therapy,
median (range) |
| 0.964 |
| At
diagnosis | 42.5
(30.6–55.8) |
|
| During
maintenance | 43.8
(25.9–84.5) |
|
|
Following cessation of
chemotherapy | 41.7
(28.2–91.6) |
|
Subsequent to a median follow-up period of 4.3 years
(range, 0.3–17.0 years), 8/33 patients (24%) relapsed 0.3–3.2 years
following diagnosis (median, 1.3 years), with a cumulative risk of
relapse=0.240. The risk of relapse was not significantly correlated
with TPMT activity (rs=−0.002, P=0.912 in the whole
group; rs=−0.026, P=0.887 among 29 children treated with
6-TG). The only patient with the heterozygous TPMT genotype
relapsed 1.1 year following the first remission; however, the
patient's TPMT activity of 42 nmol 6-mMP g−1 Hb
h−1 assessed at diagnosis did not deviate from the
median of the cohort.
In the children with ALL, the median TPMT activity
was 30.9 nmol 6-mMP g−1Hb h−1 (range,
6.0–49.5 nmol 6-mMP g−1Hb h−1); at diagnosis,
25.0 nmol 6-mMP g−1Hb h−1 (range, 6.0–44.0
nmol 6-mMP g−1Hb h−1); during maintenance
chemotherapy, 29.7 nmol 6-mMP g−1Hb h−1
(range, 6.6–47.1 nmol 6-mMP g−1Hb h−1); and
following cessation of chemotherapy, 37.3 nmol 6-mMP
g−1Hb h−1 (range, 16.3–37.3 nmol 6-mMP
g−1Hb h−1).
Overall, 1 child with ALL included in the group
measured at the point of diagnosis exhibited a heterozygous
TPMT*1/*3A genotype. Among the patients with ALL measured during
maintenance chemotherapy, 4 were heterozygous (3 TPMT*1/*3A and 1
TMPT*1/*2). The remaining 100 children with ALL were TMPT*1/*1
homozygous.
The proportion of heterozygous patients in the AML
group (3.0%, n=1/33) did not differ significantly from that in the
ALL group (4.7%, n=5/105; P=0.674).
Median TPMT activities in children with AML at
diagnosis, during maintenance chemotherapy and following the
cessation of the chemotherapy were increased compared with the
median TPMT activities in children with ALL, and the differences
were statistically significant (P<0.001, P=0.001 and P=0.048,
respectively) (Fig. 2).
Discussion
Variations in TPMT activity according to types of
medical condition have been demonstrated in ALL in comparison with
inflammatory bowel disease (IBD) and cystic fibrosis (22,23).
In the present study, the median level of TPMT
activity in children with AML was significantly increased compared
with that in ALL. This did not likely result from the distribution
of TPMT alleles, as the proportion of heterozygous patients in the
AML group did not differ significantly from that in the ALL group.
The results of the present study are in agreement with those of
Coulthard et al (10), who
also observed increased mean TPMT activity in adult patients with
AML, even though TPMT activity was measured in leukemic blasts,
whereas it was determined in RBC lysates in the present study.
According to Coulthard et al (10), measurements of TPMT activity in RBC
may more accurately reflect its systemic expression in comparison
with leukemic blasts. No other studies comparing TPMT activity
between patients with AML and ALL were identified during the
literature search of the present study.
Anasari et al (24) described the phenomenon of very high
TPMT activity was identified in the study of 106 patients with IBD;
~15% of those who represented high activity demonstrated a value
above the reference interval (>15 units/ml RBC), which was
classified as very high. The proportion of ultra-high activity in
this group was estimated at 1–2% (24,25). The
same proportion of individuals with ultra-high TPMT activity was
determined in a cohort of healthy Caucasian people (25–28).
Several candidate factors, including variable number tandem repeats
(VNTRs) identified on the TPMT gene promotor, protein
kinase C, casein kinase substrate in neurons 2 (PACSIN2) and
SAM, responsible for the upregulation of TPMT activity were
identified by the previous studies (25,26,29,30).
Ultra-high TPMT activity is negatively associated
with VNTRs (25). VNTRs modulate
TPMT transcription, potentially by altering the spacing
between transcription factor binding sites. In particular, the
presence of the GCC trinucleotide repeat polymorphism was
associated with ultra-high TPMT activity (25,27). The
structure of the VNTR region also contributes to the increase in
TPMT gene expression observed during maintenance
chemotherapy for childhood ALL, presumably due to an interaction
between the VNTR genotype and factors associated with the therapy
(22). One potential hypothesis is
that TPMT gene expression may be high in AML due to the
modulation of the VNTR region by an, as of yet, unidentified
AML-specific factor.
PACSIN2, a protein subject to genetic polymorphism,
is a member of the PACSIN family that regulates cell cycle,
endocytosis and autophagy. It has been demonstrated in vitro
that individuals with the PACSIN2 rs2413739 CC genotype exhibited
higher TPMT activities compared with carriers of other PACSIN2
variants (29). It was also confirmed
that PACSIN2 rs2413739 CC-positive patients with ALL exhibited a
lower incidence of gastrointestinal and hematological
mercaptopurine toxicities in contrast to the ones with 25 CT and TT
allele (29,30).
SAM, a methyl donor in reactions catalyzed by TPMT
and a number of other methyltransferases, was demonstrated in
vitro to be associated with TPMT activity by post-translational
stabilization of the TPMT molecule. This effect was confirmed in
vivo in >1,000 healthy individuals (31). It was realized that the level of SAM
was increased in human lung epithelial-like cells exposed to
cigarette smoke extract, and that smokers exhibited significantly
higher TPMT activity compared with nonsmokers (26,31).
Factors affecting SAM biosynthesis exhibited an association with
TPMT activity. Methionine, a direct precursor of SAM, is an
essential dietary amino acid and is regenerated from homocysteine
and 5-methyltetrahydrofolate (5-Me-THF) (26). 5-Me-THF is a final product of the
folate cycle and its activity depends on folate intake and the
activity of folate-metabolizing enzymes including methylene
tetrahydrofolate reductase (MTHFR). A positive association between
folate and SAM levels was indicated in a mathematical model of the
methionine cycle, which predicted a rise in SAM levels due to
increase of 5-Me-THF. MTHFR activity is modulated by MTHFR
gene polymorphisms, with a 50% reduction in homozygosity for its
677C>T variant exhibited by 25–43% of population (26). Individuals with decreased MTHFR
activity exhibit lower serum folate level, reduced
homocysteine-to-methionine regeneration and lower SAM synthesis,
but adequate folate intake may reduce this effect (26). The expression of factors, including
PACSIN2, MTHFR, folates, methionine and SAM may contribute to
different TPMT activity in ALL as compared with AML. It was
demonstrated that the inhibition of DNPS by MeTIMP resulted in a
decreased concentration of ATP, which impairs SAM biosynthesis
(32). Therefore, the lack of MeTIMP
formed exclusively with 6-MP leads to increased SAM (32). This may be in concordance with the
hypothesis of Coulthard et al (10), which suggested that higher TPMT
activity was observed in patients with AML as they were not treated
with 6-MP (10). However, Lennard
et al (33) did not identify a
difference in TPMT activity in children treated for ALL with 6-MP
compared with those treated with 6-TG.
Lower TPMT activity in ALL in comparison with AML
may be associated with co-administration of methotrexate, which was
identified to bind to TPMT and inhibit its activity (31). Variety in TPMT activity due to
ethnicity, RBC lifespan and kidney function has also been
demonstrated (13). The effects of
other untested or unknown TPMT gene allelic variants in the
results of the present study cannot be completely excluded;
however, they occur very rarely (13).
The results of the present study may have clinical
implications. Previous studies have indicated that TPMT activity in
ALL is inversely associated with TGNs concentration and overall
cytotoxic effect of thiopurines, while the risk of relapse was
demonstrated to be lower for patients with low TPMT activity levels
(3,9,15). High
TPMT activity in cell lines and in patients with IBD was suggested
to be associated with thiopurine resistance and a high
methylMP/TGNs ratio (25). Escalation
of thiopurine dose for these patients is ineffective due to a
preferential methylMP production (25,34).
Maintenance chemotherapy in pediatric AML has been demonstrated to
be a subject of controversy: No benefit was indicated in several
studies (35); however, it is assumed
that it may have positive effect in subgroups of patients currently
emerging from new risk-stratification AML therapy (35). 6-TG-based maintenance chemotherapy is
being used in the AML-BFM 2012 Protocol (36) for children and adolescents assigned to
standard and intermediate risk groups according to molecular
genetic factors.
A small sample size with a heterogeneous structure
in terms of AML subtypes and other risk factors, including
molecular and cytogenetic data, were not always available, making
it difficult to draw comprehensive conclusions from the data of the
present study. An additional limitation is that different children
with AML were included in each of the groups: At the moment of
diagnosis; during the maintenance therapy; and following the
cessation of the chemotherapy. A 19.7-year old patient was included
to the study group as he was diagnosed with AML in the pediatric
center at the age of 18, and the TPMT evaluation took place
following chemotherapy. According to previous studies, children
demonstrate almost equivalent levels of TPMT activity compared with
adults (37).
The preliminary results of the present study suggest
that TPMT activity in patients with AML may be increased compared
with those in patients with ALL. Additional studies on thiopurine
metabolism in AML are required to confirm this, and to clarify the
clinical implications of the associations with treatment outcome,
and with regard to the cytogenetic and molecular factors currently
used for AML risk stratification.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW designed the present study; JS and JSS wrote the
manuscript; and MC and JW revised the manuscript. JS, JSS, MC, MR,
SK, MW and JW contributed to the acquisition, analysis and
interpretation of the data and have seen and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Bioethical Committee
at Poznan University of Medical Sciences (Poznań, Poland), was
performed in accordance with the 1964 Declaration of Helsinki and
its later amendments. Written informed consent was obtained from
the parents of the patients prior to initiating the study.
Consent for publication
Patients consent for the publication their data was
received.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Rooij JD, Zwaan CM and van den
Heuvel-Eibrink M: Pediatric AML: From biology to clinical
management. J Clin Med. 4:127–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tarlock K and Meshinchi S: Pediatric acute
myeloid leukemia: Biology and therapeutic implications of genomic
variants. Pediatr Clin North Am. 62:75–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stensman LM, Kjeldsen E, Nersting J,
Schmiegelow K and Hasle H: Treatment-related myelodysplastic
syndrome in a child with acute myeloid leukemia and TPMT
heterozygosity. J Pediatr Hematol Oncol. 37:e242–e244. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pui CH, Carroll WL, Meshinchi S and Arceci
RJ: Biology, risk stratification, and therapy of pediatric acute
leukemias: An update. J Clin Oncol. 29:551–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mussai FJ, Yap C, Mitchell C and Kearns P:
Challenges of clinical trial design for targeted agents against
pediatric leukemias. Front Oncol. 4:3742015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Creutzig U, Zimmermann M, Bourquin JP,
Dworzak MN, Fleischhack G, Graf N, Klingebiel T, Kremens B,
Lehrnbecher T, von Neuhoff C, et al: Randomized trial comparing
liposomal daunorubicin with idarubicin as induction for pediatric
acute myeloid leukemia: Results from study AML-BFM 2004. Blood.
122:37–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Munshi PN, Lubin M and Bertino JR:
6-thioguanine: A drug with unrealized potential for cancer therapy.
Oncologist. 19:760–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pizzo PA and Poplack DG: Principles and
practice of pediatric oncology. Philadelphia: Lippincott Williams
and Wilkins; pp. 3092011
|
|
9
|
Coulthard S and Hogarth L: The
thiopurines: An update. Invest New Drugs. 23:523–532. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coulthard SA, Howell C, Robson J and Hall
AG: The relationship between thiopurine methyltransferase activity
and genotype in blasts from patients with acute leukemia. Blood.
92:2856–2862. 1998.PubMed/NCBI
|
|
11
|
Katara P and Kuntal H: TPMT polymorphism:
When shield becomes weakness. Interdiscip Sci. 8:150–155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lennard L and Lilleyman JS:
Individualizing therapy with 6-mercaptopurine and 6-thioguanine
related to the thiopurine methyltransferase genetic polymorphism.
Ther Drug Monit. 18:328–334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abaji R and Krajinovic M: Thiopurine
S-methyltransferase polymorphisms in acute lymphoblastic leukemia,
inflammatory bowel disease and autoimmune disorders: Influence on
treatment response. Pharmgenomics Pers Med. 10:143–156.
2017.PubMed/NCBI
|
|
14
|
Schmiegelow K, Forestier E, Kristinsson J,
Söderhäll S, Vettenranta K, Weinshilboum R and Wesenberg F: Nordic
Society of Paediatric Haematology and Oncology: Thiopurine
methyltransferase activity is related to the risk of relapse of
childhood acute lymphoblastic leukemia: Results from the NOPHO
ALL-92 study. Leukemia. 23:557–564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lennard L, Cartwright CS, Wade R and Vora
A: Thiopurine methyltransferase and treatment outcome in the UK
acute lymphoblastic leukaemia trial ALL2003. Br J Haematol.
170:550–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tamm R, Mägi R, Tremmel R, Winter S,
Mihailov E, Smid A, Möricke A, Klein K, Schrappe M, Stanulla M, et
al: Polymorphic variation in TPMT is the principal determinant of
TPMT phenotype: A meta-analysis of three genome-wide association
studies. Clin Pharmacol Ther. 101:684–695. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stary J, Zimmermann M, Campbell M,
Castillo L, Dibar E, Donska S, Gonzalez A, Izraeli S, Janic D,
Jazbec J, et al: Intensive chemotherapy for childhood acute
lymphoblastic leukemia: Results of the randomized intercontinental
trial ALL IC-BFM 2002. J Clin Oncol. 32:174–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chrzanowska M, Kuehn M,
Januszkiewicz-Lewandowska D, Kurzawski M and Droździk M: Thiopurine
S-methyltransferase phenotype-genotype correlation in children with
acute lymphoblastic leukemia. Acta Pol Pharm. 69:405–410.
2012.PubMed/NCBI
|
|
19
|
Kröplin T, Weyer N, Gutsche S and Iven H:
Thiopurine S-methyltransferase activity in human erytrocytes: A new
HPLC method using 6-thioguanine as substrate. Eur J Clin Pharmacol.
54:265–271. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kröplin T and Iven H: Methylation of
6-mercaptopurine and 6-thioguanine by thiopurine
S-methyltransferase. A comparison of activity in red blood cell
samples of 199 donors. Eur J Clin Pharmacol. 56:343–345. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurzawski M, Gawrońska-Szklarz B and
Droździk M: Frequency distribution of thiopurine
S-methyltransferase alleles in a polish population. Ther Drug
Monit. 26:541–545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kotur N, Dokmanovic L, Janic D, Stankovic
B, Krstovski N, Tosic N, Katsila T, Patrinos GP, Zukic B and
Pavlovic S: TPMT gene expression is increased during maintenance
therapy in childhood acute lymphoblastic leukemia patients in a
TPMT gene promoter variable number of tandem repeat-dependent
manner. Pharmacogenomics. 16:1701–1712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chouchana L, Narjoz C, Roche D, Golmard
JL, Pineau B, Chatellier G, Beaune P and Loriot MA: Interindividual
variability in TPMT enzyme activity: 10 years of experience with
thiopurine pharmacogenetics and therapeutic drug monitoring.
Pharmacogenomics. 15:745–757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anasari A, Hassan C, Duley J, Marinaki A,
Shobowale-Bakre EM, Seed P, Meenan J, Yim A and Sanderson J:
Thiopurine methyltransferase activity and the use of azathioprine
in inflammatory bowel disease. Aliment Pharmacol Ther.
16:1743–1750. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chouchana L, Narjoz C, Beaune P, Loriot MA
and Roblin X: Review article: The benefits of pharmacogenetics for
improving thiopurine therapy in inflammatory bowel disease. Aliment
Pharmacol Ther. 35:15–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karas-Kuzelicki N and Mlinaric-Rascan I:
Individualization of thiopurine therapy: Thiopurine
S-methyltransferase and beyond. Pharmacogenomics. 10:1309–1322.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roberts RL, Gearry RB, Bland MV, Sies CW,
George PM, Burt M, Marinaki AM, Arenas M, Barclay ML and Kennedy
MA: Trinucleotide repeat variants in the promoter of the thiopurine
S-methyltransferase gene of patients exhibiting ultra-high enzyme
activity. Pharmacogenet Genomics. 18:434–438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chouchana L, Roche D, Jian R, Beaune P and
Loriot MA: Poor response to thiopurine in inflammatory bowel
disease: How to overcome therapeutic resistance? Clin Chem.
59:1023–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stocco G, Yang W, Crews KR, Thierfelder
WE, Decorti G, Londero M, Franca R, Rabusin M, Valsecchi MG, Pei D,
et al: PACSIN2 polymorphism influences TPMT activity and
mercaptopurine-related gastrointestinal toxicity. Hum Mol Genet.
21:4793–4804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smid A, Karas-Kuzelicki N, Jazbec J and
Mlinaric-Rascan I: PACSIN2 polymorphism is associated with
thiopurine-induced hematological toxicity in children with acute
lymphoblastic leukaemia undergoing maintenance therapy. Sci Rep.
6:302442016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karas-Kuželički N, Šmid A, Tamm R,
Metspalu A and Mlinarič-Raščan I: From pharmacogenetics to
pharmacometabolomics: SAM modulates TPMT activity.
Pharmacogenomics. 15:1437–1449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Milek M, Kuzelicki Karas N, Smid A and
Mlinaric-Rascan I: S-adenosylmethionine regulates thiopurine
methyltransferase activity and decreases 6-mercaptopurine
cytotoxicity in MOLT lymphoblasts. Biochem Pharmacol. 77:1845–1853.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lennard L, Cartwright CS, Wade R, Richards
SM and Vora A: Thiopurine methyltransferase genotype-phenotype
discordance and thiopurine active metabolite formation in childhood
acute lymphoblastic leukaemia. Br J Clin Pharmacol. 76:125–136.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chouchana L, Fernández-Ramos AA, Dumont F,
Marchetti C, Ceballos-Picot I, Beaune P, Gurwitz D and Loriot MA:
Molecular insight into thiopurine resistance: Transcriptomic
signature in lymphoblastoid cell lines. Genome Med. 7:372015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gibson B, Perentesis J, Alonzo TA and
Kaspers GL: Treatment of Acute Myeloid LeukemiaChildhood Leukemia.
Springer-Verlag; Berlin Heidelberg: pp. 136–137. 2011
|
|
36
|
https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-000018-39/DE(entry
date 20 April 2018.).
|
|
37
|
Asadov C, Aliyeva G and Mustafayeva K:
Thiopurine S-methyltransferase as a pharmacogenetic biomarker:
Significance of testing and review of major methods. Cardiovasc
Hematol Agents Med Chem. 15:23–30. 2017. View Article : Google Scholar : PubMed/NCBI
|