Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of cancer and primarily occurs in South Africa and
Asian countries (1,2). According to statistical analyses, the
incidence of liver cancer has increased in the majority of
countries over the past 5 years (1).
China alone accounted for ~50% of cases of newly diagnosed liver
cancer and mortality in 2012 (2).
Although curative resection is beneficial for the long-term
survival of patients with HCC, the prognosis of these patients
remains poor owing to the high rate of metastasis and recurrence
(3,4).
Therefore, identifying more accurate prognostic biomarkers of HCC
is of great clinical value for the understanding HCC and to develop
novel therapeutic strategies.
Osteopontin (OPN), a secreted glycosylated
phosphoprotein encoded by the secreted phosphoprotein 1 gene, has
been implicated as being a major mediator and potential therapeutic
target of cancer metastasis (5,6). A
previous study has demonstrated that OPN is a ligand that binds to
αvβ integrins or receptors of the cluster of differentiation 44
family to promote cell adhesion, extracellular matrix degradation,
and the prevention of apoptosis, angiogenesis and indolent tumor
growth (7). Furthermore, OPN has been
identified as a key inducer of tumor invasion and metastasis
(8,9).
The epithelial-mesenchymal transition (EMT) serves a
major role in tumor metastasis, a developmental process whereby
E-cadherin, an epithelial marker, is downregulated and vimentin and
N-cadherin, which are mesenchymal markers, are upregulated
(10–12). Additionally, the EMT results in
reduced epithelial cell intercellular adhesion and causes these
cells to acquire fibroblastoid properties, thereby improving the
ability of cells to migrate. Therefore, the EMT is important for
the development, invasion and metastasis potential of cancer.
Twist, a major EMT regulator, is essential for tumor
metastasis (13,14). In the process of metastasis, Twist
serves a crucial role in the EMT by downregulating E-cadherin and
β-catenin, and by regulating cell motility, invasiveness and
metastasis (14–17). Furthermore, Twist expression was
observed to be increased in different types of tumor, including
prostate cancer (17), melanoma
(18), pediatric osteosarcoma
(19), T-cell lymphoma (20), gastric cancer (21), breast carcinoma (22) and HCC (23,24).
Previous studies have proven that Twist and Snail, but not Slug,
are major EMT inducers in HCC, and that Twist serves a major role
in hepatitis C-associated HCC, unlike Snail (25).
A recent study demonstrated that OPN promotes EMT of
HCC (8). The present study examined
the role and clinical significance of OPN on the EMT regulator,
Twist, in HCC cell lines and tumor tissues. OPN was revealed to
regulate the expression of Twist, a major regulator of HCC
metastasis. Furthermore, interfering with the phosphoinositide
3-kinase (PI3K)/RAC serine/threonine-protein kinase (Akt) pathway
may suppress the expression of Twist enhanced by OPN. Therefore, we
hypothesize that OPN and Twist may serve as synergistic prognostic
biomarkers and therapeutic targets for HCC.
Materials and methods
Patients and follow-up
A total of 374 patients, including 306 males and 68
females (age range, 30–70 years, median age 55 years) with HCC,
underwent a hepatectomy at the Liver Cancer Institute, Zhongshan
Hospital, Fudan University (Shanghai, China) by the same surgical
team between March 2004 and December 2006. In addition, these
patients had not received any neo-adjuvant or adjuvant treatments,
but did undergo a pathological examination and complete follow-up.
Formalin-fixed, paraffin-embedded tissues, which included 374 HCC
patient tissues and 192 matched adjacent non-tumor tissues were
used to organize a tissue microarray (TMA) for immunohistochemistry
(IHC) studies. The clinicopathological characteristics of patients
whose tissues were used for the TMA are summarized in Table I. The present study was approved by
the Research Ethics Committee of Zhongshan Hospital, Fudan
University (Shanghai, China) and written informed consent was
obtained from each patient. Follow-up was completed in May 2013.
The follow-up procedures and treatment modalities following relapse
are described in previous studies (26–28). To
diagnose recurrence, α-fetoprotein (AFP) levels were analyzed and
computed tomography (CT) and/or magnetic resonance imaging (MRI)
scans were performed. Patient mortality and disease recurrence were
used as endpoints and the endpoints included the overall survival
(OS) time and the time to recurrence (TTR). The OS time was defined
as the interval between the dates of surgery and mortality. The TTR
was defined as the time between surgery and the first report of
intrahepatic or distant recurrence or the last follow-up for
patients who had not experienced recurrence at the time of
mortality (patients who had succumbed to other causes were not
included) (29). The TTR was recorded
at the date of mortality or the last follow-up (30,31).
 | Table I.Association between Twist expression
levels and clinicopathological characteristics in HCC patients. |
Table I.
Association between Twist expression
levels and clinicopathological characteristics in HCC patients.
|
| Twist expression,
n |
|
|---|
|
|
|
|
|---|
| Variable | Low (n=204) | High (n=170) | P-value |
|---|
| Sex |
|
| 0.687 |
|
Female | 39 | 29 |
|
|
Male | 165 | 141 |
|
| Age, years |
|
| 0.467 |
|
≤50 | 98 | 89 |
|
|
>50 | 106 | 81 |
|
| HBsAg |
|
| 0.543 |
| No | 16 | 10 |
|
|
Yes | 188 | 160 |
|
| ALT, U/l |
|
| 0.498 |
|
≤75 | 185 | 150 |
|
|
>75 | 19 | 20 |
|
| Liver
cirrhosis |
|
| 0.745 |
| No | 24 | 18 |
|
|
Yes | 180 | 152 |
|
| AFP, ng/ml |
|
| 0.830 |
|
≤20 | 74 | 64 |
|
|
>20 | 130 | 106 |
|
| Tumor size, cm |
|
| 0.613 |
| ≤5 | 158 | 136 |
|
|
>5 | 46 | 34 |
|
| Tumor number |
|
| 0.052a |
|
Single | 190 | 166 |
|
|
Multiple | 14 | 4 |
|
| Tumor capsule |
|
| 0.677 |
|
Complete | 112 | 89 |
|
|
None | 92 | 81 |
|
| Vascular
invasion |
|
| 0.013 |
| No | 153 | 107 |
|
|
Yes | 51 | 63 |
|
| Tumor
differentiation |
|
| 0.633 |
|
I/II | 155 | 125 |
|
|
III/IV | 49 | 45 |
|
| BCLC stage |
|
| 0.157 |
| 0 and
A | 59 | 38 |
|
| B and
C | 145 | 132 |
|
TMA and IHC
The resected specimens (2–3 mm) were fixed in 10%
formalin for four days at room temperature, and send to Pathology
department of zhongshan hospital. The construction of the TMA (in
collaboration with Shanghai Biochip Co., Ltd., Shanghai, China) and
IHC were performed as described previously (32). Immunostaining was performed on TMA
slides using a two-step process according to the manufacturer's
protocols. Following deparaffinization, 4 µm sections were
rehydrated in a descending alcohol series and subjected to antigen
retrieval by microwaving in 0.01 mol/l sodium citrate (pH 6) for 10
min. When microwaving, sodium citrate was boiled at a ~100°C and
sections placed in it, followed by the temperature ~30-40°C for 10
min, followed by the sections being allowed to cool naturally to
room temperature. Then, sections were washed using phosphate
buffered saline (PBS).
Sections were incubated at 4°C overnight with
monoclonal antibodies against OPN (dilution, 1:100; cat no. ab8448;
Abcam, Cambridge, UK) and Twist (dilution, 1:100; cat no. ab50581;
Abcam). Immunostaining was performed using ChemMate DAKO EnVision
Detection kit, Peroxidase/DAB, Rabbit/Mouse (cat no. GK500705;
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA), according
the manufacturer's protocol. Subsequently, the sections were
counterstained with hematoxylin at room temperature until the
microscopic observation of sections were discoloration, used PBS to
rinse and soaked it for 2 min, then mounted in dimethyl benzene.
Negative controls were included in all assays and were treated
identically but with the primary antibodies omitted. An optical
microscope was used at a magnification ×200.
The intensity of staining was scored manually (0, no
staining; 1, weak staining; 2, moderate; and 3, strong staining) by
two independent experienced pathologists. Tumor cells in 5 randomly
selected fields were scored based on the proportion of positively
stained cells (0–100%). The final IHC scores were determined by
multiplying the intensity scores and the proportion scores of the
positive cells. Expression levels of OPN and Twist in all 374
samples were quantified. ‘High’ vs. ‘low’ OPN and Twist expression
was defined according to the cut-off values of OPN and Twist level,
which were defined as the median of the cohort. To evaluate the
combined influence of OPN and Twist on the prognosis of patients,
the 374 patients with HCC were separated into four groups: Group I,
patients with low OPN and low Twist expression (n=117); Group II,
patients with high OPN and low Twist expression (n=65); Group III,
patients with low OPN and high Twist expression (n=87); and Group
IV, patients with high OPN and high Twist expression (n=105).
Cell lines and plasmids
Three human HCC cell lines with various metastatic
potentials, MHCC97-L, MHCC97-H and HCC-LM3, and the human
non-transformed hepatic L-02 cell line, were used in the present
study. MHCC97-L, MHCC97-H and HCC-LM3 with stepwise increasing
metastatic potential were established from the same parent human
HCC cell line at the Liver Cancer Institute, Fudan University
(Shanghai, China). They have a genetically identical background
(33,34). The L-02 cells were obtained from
American Type Culture Collection (Manassas, VA, USA). These cell
lines were routinely maintained in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS;
both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C in a humidified incubator containing 5% CO2.
Expression vectors for OPN and short hairpin RNA
targeted at OPN (shOPN), as well as methods of cell transfection,
were constructed as previously described (8). Twist small interfering RNA (siRNA)
constructs were obtained from Sigma-Aldrich; Merck KGaA. The
sequences of the primers used were as follows: Twist-siRNA forward,
5-gatccGCTGAGCAAGATTCAGACCttcaagagaGGTCTGAATCTTGCTCAGCttttta-3 and
reverse,
3-gCGACTCGTTCTAAGTCTGGaagttctctCCAGACTTAGAACGAGTCGaaaaattcga-5 and
Twist-siRNA-scramble forward,
5-gatccCGGTAACACAGACTGCAGTttcaagagaACTGCAGTCTGTGTTACCGttttta-3 and
reverse,
3-gGCCATTGTGTCTGACGTCAaagttctctGCCATTGTGTCTGACGTCAaaaaattcga-5.
Recombinant plasmids were prepared as described previously
(34). Then, 10 µg plasmids were
transfected into the MHCC-97L cells using lipofectamine 2000 (cat
no. 11668019; Thermo Fisher Scientific, Inc.). Subsequently, the
cells were collected after 24 h and cells were cleaved to extract
protein for western blot analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HCLM3, MHCC97H, MHCC97L
and L02 cell lines (that had not undergone transfection) and frozen
tumor specimens using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA (1 µg) was reverse transcribed using
PrimeScript® Reverse Transcriptase Master mix (Takara
Bio, Inc., Otsu, Japan) according to the manufacturer's protocols.
The sequences of the primers used were as follows: Twist forward,
5-GTCCGCAGTCTTACGAGGAG-3 and reverse, 5-GCTTGAGGGTCTGAATCTTGCT-3;
and β-actin forward, 5-CATGTACGTTGCTATCCAGGC-3 and reverse,
5-CTCCTTAATGTCACGCACGAT-3. An AceQ® qPCR SYBR Green
Master mix kit (Vazyme, Piscataway, NJ, USA) was used for qPCR.
β-actin was used as the reference gene. Amplification and detection
were perfor-med using the ABI PRISM® 7900HT Sequence
Detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Thermocycling conditions were as follows: 50°C for 2 min
(required for optimal AmpErase UNG activity Applied Biosystems;
Thermo Fisher Scientific, Inc.), template denaturation at 95°C for
10 min, 40 cycles of denaturation at 95°C for 15 sec, and combined
primer annealing/elongation at 60°C for 1 min. The Twist level was
normalized to β-actin to yield a 2−ΔΔCq value for
relative expression of Twist (35).
Detection of protein by western blot
analysis
Cells lysates were prepared as described previously
(8). RIPA lysis buffer (cat no.
P0013E; Beyotime Institute of Biotechnology, Haimen, China) was
used for lysis. Protein concentrations were measured using a
Bicinchoninic Acid Assay kit (Pierce; Thermo Fisher Scientific,
Inc.). A total of 40 ug/ul of protein was separated using SDS-PAGE
(5% concentration gel and 10% separation gel), according to protein
mass and transferred onto polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). 5% skimmed milk was used to block
polyvinylidene fluoride membranes at room temperature for one h.
Proteins were then incubated with primary antibodies against OPN
(cat no. sc-21742; dilution, 1:500; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and Twist (cat no. ab50581; dilution, 1:1,000;
Abcam) at 4°C overnight. A mouse anti-human monoclonal antibody
against GAPDH (cat no. 8884; dilution, 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) was used as an internal
control. In addition, primary antibodies against Akt (cat no.
ab8805; dilution, 1:1,000; Abcam), p-Akt (cat no. ab38449;
dilution, 1:1,000; Abcam), matrix metalloproteinase 2 (MMP2) (cat
no. ab37150; dilution, 1:1,000; Abcam) and urokinase (uPA) (cat no.
ab82220; dilution, 1:1,000; Abcam) were used to analyze the
mechanism of EMT. Secondary antibodies were goat anti-rabbit IgG
(dilution, 1:5,000; cat no. ab6721; Abcam) and goat anti-mouse IgG
(dilution, 1:5,000; cat no. ab6789; Abcam). We used the ECL Western
Blotting Detection Kit (Thermo Fisher Scientific, Inc) to detect
immobilized specific antigens in chemiluminescent Western blots
through horseradish peroxidase (HRP) labeled antibodies. The bands
were quantified using ImageJ v.2.0 software (National Institutes of
Health, Bethesda, MD, USA). In order to improve the accuracy of the
present study, each experiment was repeated ≥3 times.
Chemicals
LY294002 (cat no. s1105; Selleck Chemicals, Houston,
TX, USA), which is able to inhibit Akt activation to assess whether
PI3K/Akt signaling was involved in OPN-mediated metastasis. MHCC
97L cells with OPN overexpression were harvested after 1 h
incubation with 50 umol/l PI3K/Akt inhibitor LY294002 (Selleck
Chemicals) to suppress Akt activation, and collected cells to
extract protein for western blot analysis.
Statistical analysis
Statistical analyses were performed using SPSS 15.0
(SPSS, Inc., Chicago, IL, USA). The Kaplan-Meier method was used to
create survival and recurrence curves and to estimate OS and TTR.
The significance of OS and TTR was determined using the log-rank
test. Fisher's exact and χ2 tests were used to
demonstrate clinicopathological association. Univariate and
multivariate analyses were performed using Cox's proportional
hazards model. Values are expressed as the mean ± standard
deviation. All statistical tests were two-sided and P<0.05 was
considered to indicate a statistically significant difference. For
OPN or Twist density, the cut-off for the definition of subgroups
was the median value. Samples were separated into two groups for
each analysis. The first group was comprised of HCC with OPN and/or
Twist levels exceeding the median value, and the second group
comprised the rest. Each data set was analyzed separately.
Results
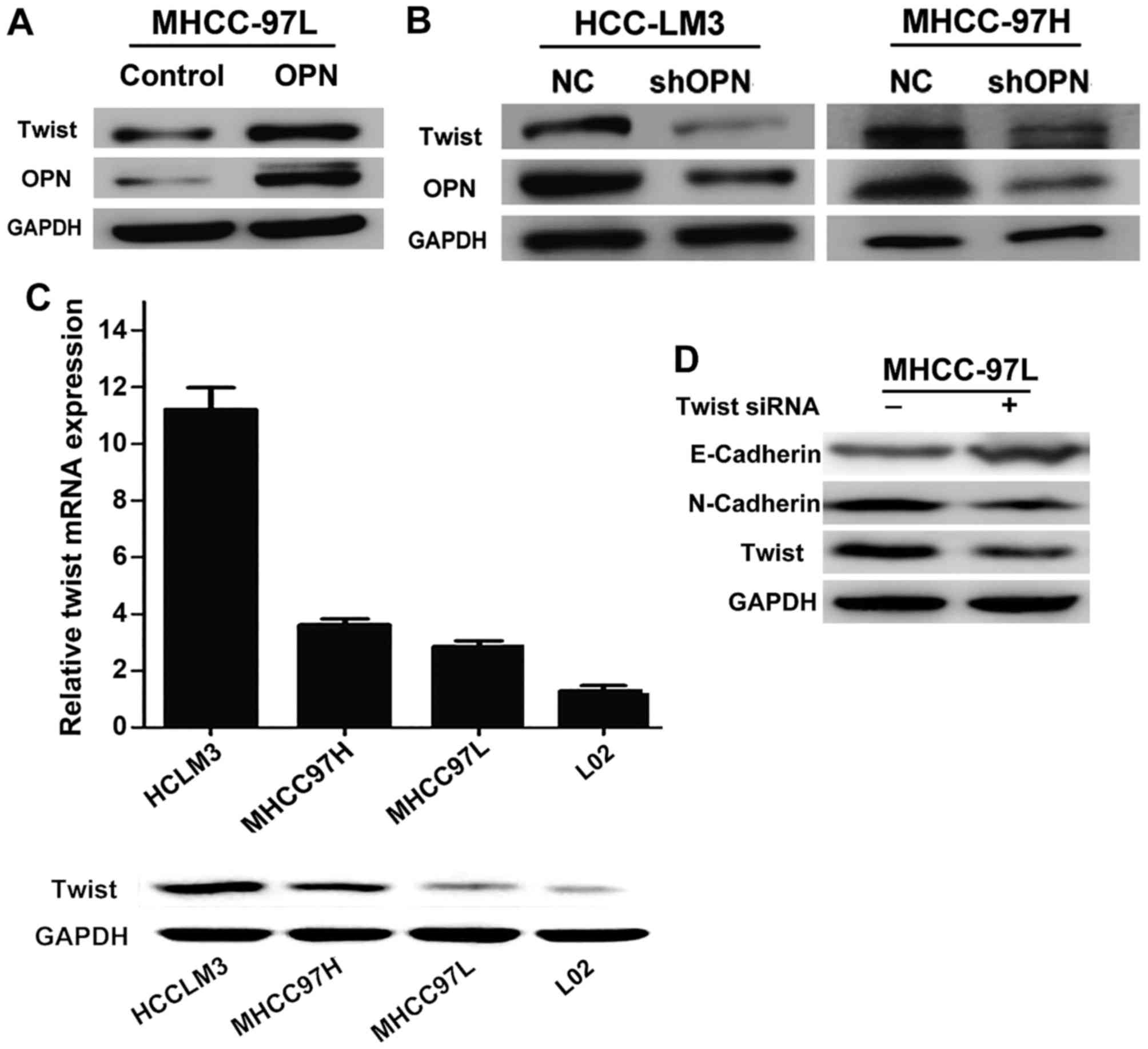
OPN enhances Twist expression in HCC
cell lines
Recent studies have demonstrated that OPN may induce
EMT in HCC (8,36). To determine the effects of OPN on the
EMT regulator Twist in HCC metastasis, the expression of Twist was
analyzed in HCC cell lines with downregulated or upregulated
expression of OPN. Twist expression was elevated when OPN was
overexpressed in the MHCC-97L cell line, which is usually not
highly metastatic and exhibits decreased levels of OPN (Fig. 1A). However, downregulation of OPN in
highly metastatic the HCC MHCC97-H and HCC-LM3 cell lines markedly
reduced Twist expression (Fig. 1B).
Therefore, we hypothesized that OPN may serve an important role in
Twist expression and therefore, may affect the metastasis of liver
cancer.
To evaluate the association between OPN, Twist and
HCC metastasis, Twist levels were detected in a panel of human HCC
cell lines with different metastatic potentials. Expression levels
of Twist protein and mRNA were substantially increased in three
established HCC cell lines compared with the non-transformed
hepatic L-02 cell line (Fig. 1C).
Additionally, the expression levels of Twist in the highly
metastatic HCC MHCC97-H and HCC-LM3 cell lines, which exhibit
increased levels of OPN, were much higher than that in the HCC
MHCC97-L cell line, which is not highly metastatic and exhibits a
low expression of OPN. These data indicated that expression of
Twist is upregulated in HCC cell lines and that this increased
expression is positively associated with the malignant phenotype of
HCC cells.
The present study also aimed to determine whether or
not Twist is involved in OPN-induced EMT. EMT markers were detected
in the lowly metastatic HCC MHCC97-L cell line, which exhibits
stable overexpression of OPN when transfected with a siRNA targeted
at Twist (siTwist) or scrambled siRNA. Knockdown of Twist
significantly reduced the expression of N-cadherin and increased
the expression of E-cadherin (Fig.
1D). These results demonstrated that Twist is required for
OPN-driven EMT.
OPN increases the expression of Twist
via the PI3K/Akt signaling pathway
Previous studies have elucidated the mechanisms of
metastasis induced by OPN, including the role of the
mitogen-activated protein kinase, nuclear factor-κB and the
PI3K/Akt pathways (9,37). The PI3K/Akt pathway is crucial in
promoting invasion and metastasis and has been documented to be
involved in the EMT of several types of human cancer (38–40). To
assess whether PI3K/Akt signaling was involved in OPN-mediated
metastasis, the present study examined the effect of OPN on the
activation of PI3K/Akt. Phosphorylation of Akt was notably enhanced
by OPN overexpression, whereas knockdown of OPN significantly
decreased the phosphorylation of Akt (Fig. 2A and B). Consistently, suppression of
Akt activation by the specific PI3K/Akt inhibitor LY294002 markedly
attenuated the expression of OPN-induced Twist (Fig. 2C). These results indicated that the
PI3K/Akt pathway is critical in the increased expression of Twist
induced by OPN.
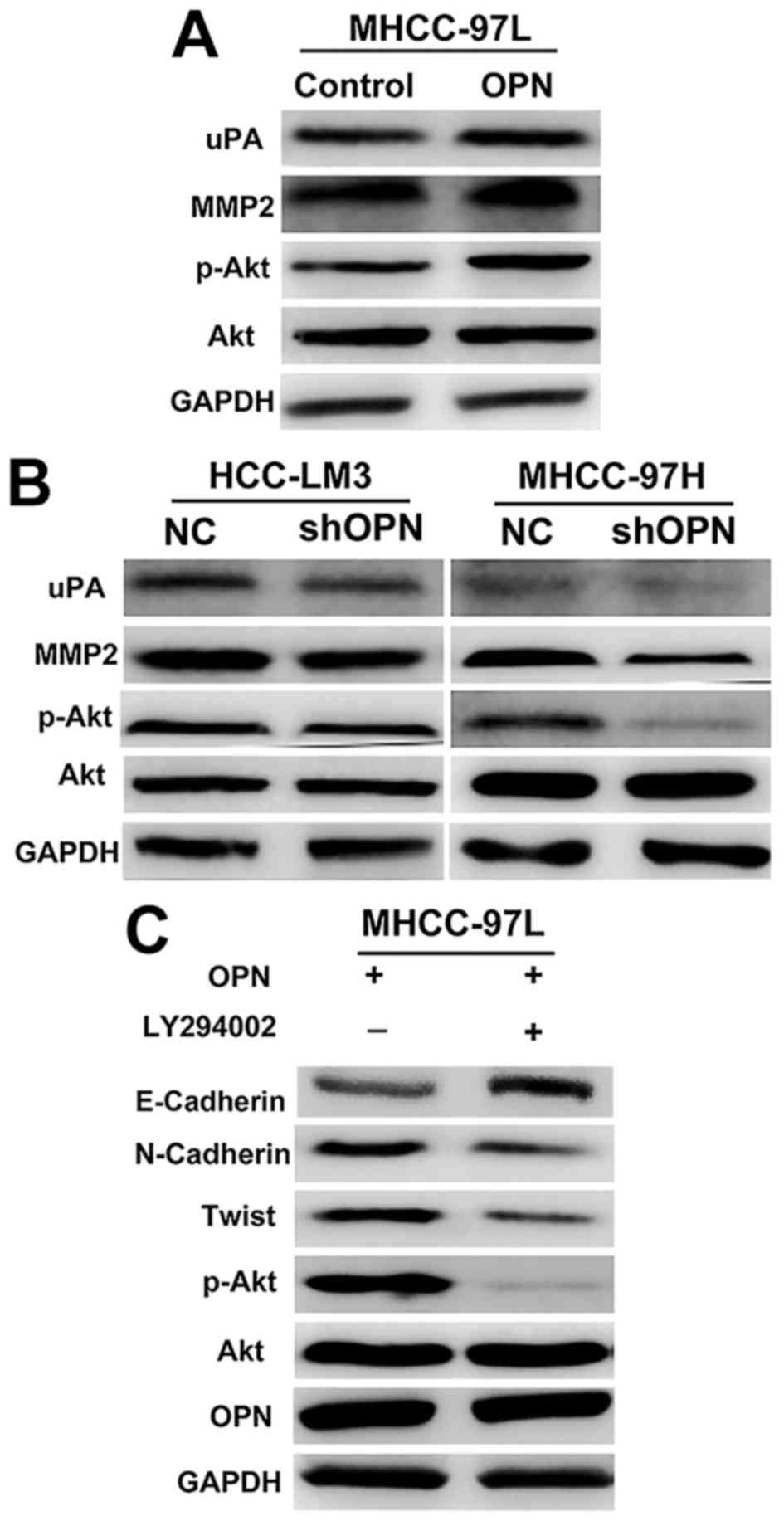 | Figure 2.OPN induced the expression of Twist
through the PI3K/Akt signaling pathway. (A) Western blot analysis
demonstrates the effect of overexpression of OPN on the protein
levels PI3K and Akt in HCC cells. (B) Western blot analysis
depicting the effect of knockdown of OPN on the protein levels of
PI3K and Akt in HCC cells. (C) Suppression of Akt activation by the
specific PI3K/Akt inhibitor LY294002 markedly attenuated
OPN-elicited EMT. OPN, osteopontin; PI3K, phosphoinositide
3-kinase; Akt, RAC serine/threonine-protein kinase; HCC,
hepatocellular carcinoma; EMT, epithelial-mesenchymal transition;
NC, negative control; uPA, urokinase; MMP2, matrix
metalloproteinase 2; p-Akt, phosphorylated Akt; shOPN, short
hairpin RNA targeted at OPN. |
To investigate whether OPN mediated metastasis via
the PI3K/Akt/Twist pathway, genes associated with metastasis,
including MMP2 and uPA, were further investigated. As demonstrated
in Fig. 2A, MMP2 and uPA were
upregulated in MHCC-97L cells stably overexpressing OPN, but were
markedly decreased in cells transfected with shOPN (Fig. 2B). Taken together, these results
suggested that OPN activates PI3K/Akt signaling, which increases
the expression of Twist and the metastasis of HCC cells.
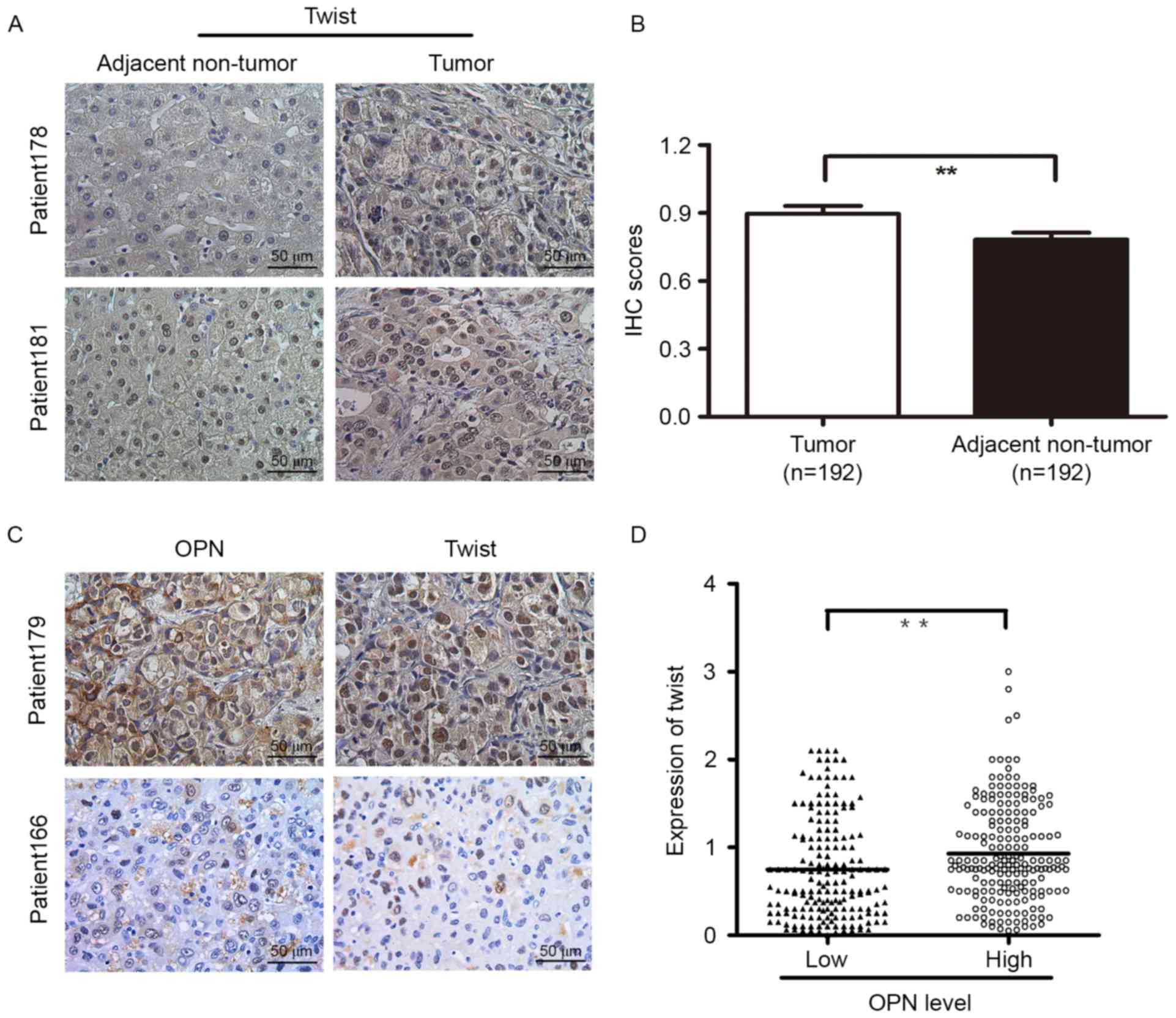
OPN and Twist expression detected by
TMA and IHC staining
To evaluate the potential role of Twist in HCC, the
expression levels of Twist in 192 human HCC tissues were identified
by IHC analyses. Higher Twist levels were detected in tumor tissues
than in their paired non-cancerous liver tissues (Fig. 3A and B). The clinical significance of
Twist was further investigated using TMAs containing HCC tissues
from 374 patients. IHC staining revealed that high expression of
Twist was significantly associated with the vascular invasion of
HCC (P=0.013; Table I), whereas no
significant association was observed between Twist density and
other clinicopathological characteristics of HCC patients. Next,
the association between OPN and Twist expression in human HCC
tissues were analyzed. On the basis of the IHC results, TMA
analysis of HCC specimens revealed that OPN expression was
positively associated with Twist expression in the HCC samples
(P<0.001; Fig. 3C and D).
Prognostic value of Twist in HCC
patients
Using the integrated optical density median value as
the cut-off value, the 374 HCC patients were divided into two
groups. The OS time of HCC patients with high Twist expression was
significantly lower than that of the patients with low Twist
expression (P=0.041; Fig. 4A),
whereas the TTR of the high-Twist-expression group was
significantly higher than that of the low-Twist-expression group
(P=0.017; Fig. 4B). The OS and TTR
times of the patients in group I were markedly longer than those
those of the patients in group IV (Fig.
4C and D).
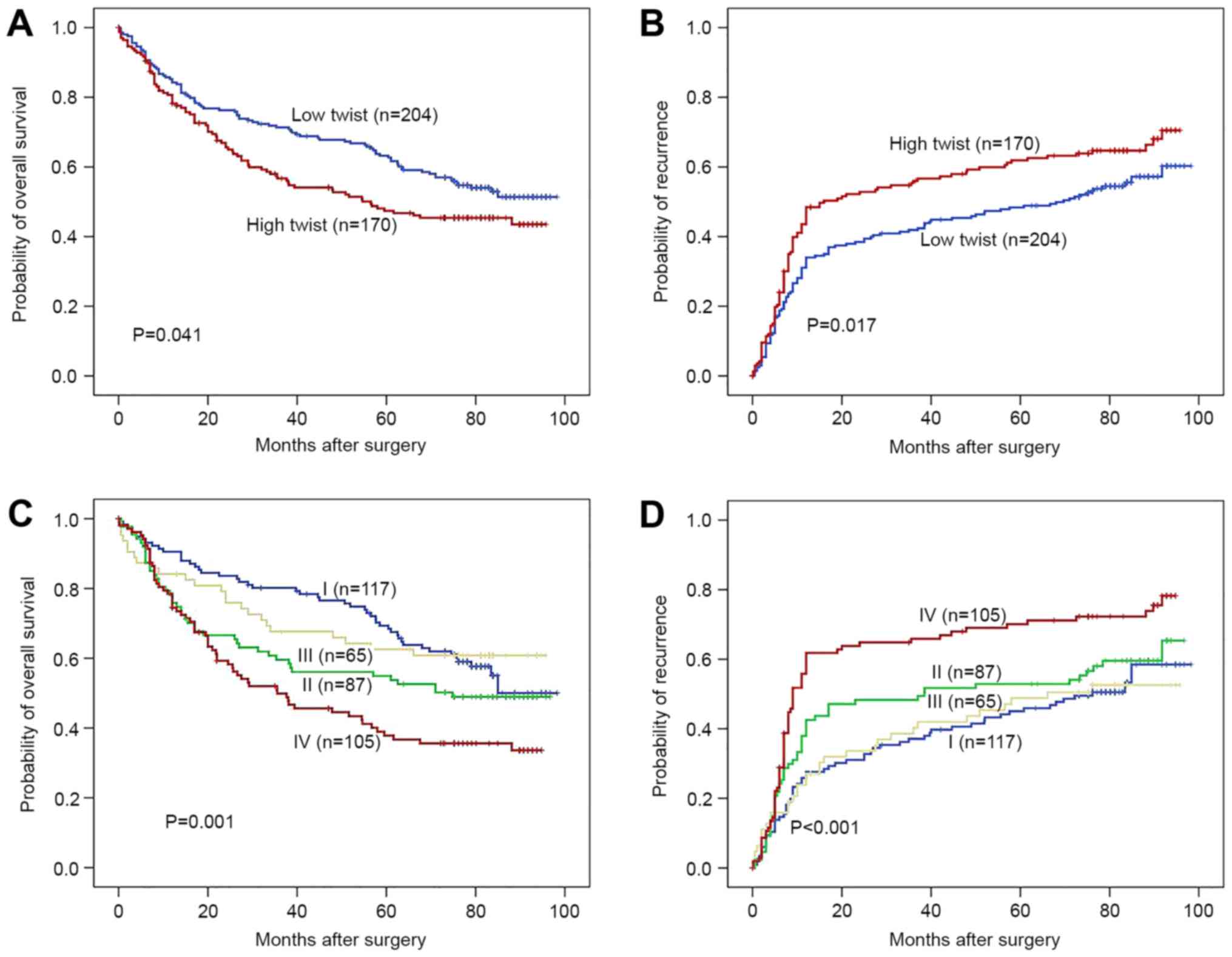 | Figure 4.Prognostic value of Twist in HCC
patients. Kaplan-Meier curves of (A) OS and (B) TTR in HCC patients
expressing different levels of Twist. The patients with higher
Twist levels exhibited significantly shorter OS and TTR times than
patients with lower Twist levels. The patients of subgroup I had
(C) the longest OS and (D) the lowest possibility of tumor
recurrence, among the four subgroups, which were divided according
to combinations of OPN and Twist expression (I, low OPN+low Twist;
II, low OPN and high Twist; III, high OPN and low Twist; IV, high
OPN and high Twist). HCC, hepatocellular carcinoma; OS, overall
survival; TTR, time to recurrence; OPN, osteopontin. |
Univariate and multivariate analyses
of the prognostic value of Twist in HCC patients
To determine the prognostic value of Twist for HCC
patients, univariate and multivariate analyses were performed on
the clinicopathological characteristics and Twist expression levels
of patients (Table II). Univariate
analysis revealed that OPN expression, Twist expression, serum AFP
level, hepatitis B surface antigen, tumor size, tumor capsulation,
vascular invasion, Barcelona Clinic Liver Cancer stage (41) and tumor differentiation were
significantly associated with the OS and TTR times of patients with
HCC (Table II). However, no
prognostic significance for OS or TTR was observed in association
with the other characteristics, including sex, age, liver
cirrhosis, ALT and tumor number. Individual characteristics that
exhibited significance by univariate analysis were adopted as
covariates in a multivariate Cox's proportional hazards model and
combined variables were further analyzed. When OPN was combined
with Twist, the combination of the two was a more potent
independent prognostic indicator for OS (P=0.001) and TTR
(P<0.001) than each factor alone.
 | Table II.Univariate and multivariate analyses
of factors associated with OS and TTR of HCC (n=374). |
Table II.
Univariate and multivariate analyses
of factors associated with OS and TTR of HCC (n=374).
|
| OS | TTR |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Univariate
analysis |
|
|
|
|
|
|
| OPN
(high vs. low) | 1.52 | 1.13–2.04 | 0.005 | 1.42 | 1.09–1.86 | 0.009 |
| Twist
(high vs. low) | 1.35 | 1.01–1.81 | 0.043 | 1.38 | 1.06–1.79 | 0.018 |
| Sex
(female vs. male) | 1.06 | 0.72–1.55 | 0.779 | 1.13 | 0.76–1.70 | 0.548 |
| Age,
years (>50 vs. ≤50) | 1.11 | 0.83–1.48 | 0.478 | 1.09 | 0.81–1.47 | 0.555 |
| ALT,
U/l (≥75 vs. <75) | 1.09 | 0.68–1.73 | 0.722 | 1.04 | 0.65–1.68 | 0.874 |
| AFP,
ng/ml (>20 vs. ≤20) | 1.66 | 1.21–2.28 | 0.002 | 1.47 | 1.08–2.02 | 0.016 |
| Liver
cirrhosis (yes vs. no) | 1.42 | 0.85–2.38 | 0.179 | 1.58 | 0.93–2.68 | 0.092 |
| HBsAg
(positive vs. negative) | 1.91 | 0.94–3.87 | 0.075 | 2.50 | 1.23–5.06 | 0.011 |
| Tumor
size, cm (>5 vs. ≤5) | 1.64 | 1.18–2.29 | 0.003 | 1.55 | 1.09–2.21 | 0.015 |
| Tumor
number (multiple vs. single) | 1.59 | 0.84–3.00 | 0.157 | 1.18 | 0.58–2.41 | 0.641 |
| Tumor
capsule (none vs. complete) | 1.53 | 1.15–2.05 | 0.004 | 1.39 | 1.03–1.87 | 0.031 |
|
Vascular invasion (yes vs.
no) | 2.03 | 1.50–2.73 | <0.001 | 1.23 | 1.05–1.45 | 0.010 |
| BCLC
stage (B and C vs. 0 and A) | 1.79 | 1.24–2.58 | 0.002 | 1.53 | 1.07–2.18 | 0.020 |
| Tumor
differentiation (III–IV vs. I–II) | 1.57 | 1.14–2.16 | 0.006 | 1.43 | 1.02–2.00 | 0.037 |
|
Combination of OPN and
Twist |
|
| 0.006 |
|
| 0.004 |
|
II vs. I | 1.40 | 0.93–2.10 | 0.193 | 1.28 | 0.88–1.86 | 0.193 |
|
III vs. I | 1.15 | 0.73–1.81 | 0.543 | 1.16 | 0.78–1.74 | 0.459 |
|
IV vs. I | 1.92 | 1.31–2.82 | 0.001 | 1.87 | 1.32–2.65 | <0.001 |
| Multivariate
analysis |
|
|
|
|
|
|
| AFP,
ng/ml (>20 vs. ≤20) | 1.47 | 1.06–2.04 | 0.023 | 1.37 | 1.02–1.84 | 0.035 |
| Tumor
size, cm (>5 vs. ≤5) | 1.43 | 1.00–2.04 | 0.051 | 1.47 | 1.02–2.10 | 0.088 |
| Tumor
capsule (none vs. complete) | 1.45 | 1.08–1.95 | 0.013 | 1.38 | 1.06–1.80 | 0.018 |
|
Vascular invasion (yes vs.
no) | 1.43 | 1.01–2.02 | 0.042 | 1.30 | 0.95–1.79 | 0.104 |
| BCLC
stage (B and C vs. 0 and A) | 1.38 | 0.90–2.09 | 0.138 | 1.46 | 1.00–2.11 | 0.048 |
| Tumor
differentiation (III–IV vs. I–II) | 1.19 | 0.85–1.67 | 0.320 | 1.14 | 0.83–1.56 | 0.419 |
|
Combination OPN and Twist |
|
| 0.038 |
|
| 0.019 |
|
II vs. I | 1.40 | 0.93–2.13 | 0.111 | 1.27 | 0.87–1.86 | 0.223 |
|
III vs. I | 1.16 | 0.74–1.83 | 0.525 | 1.18 | 0.79–1.76 | 0.430 |
|
IV vs. I | 1.77 | 1.19–2.65 | 0.005 | 1.77 | 1.23–2.54 | 0.002 |
Discussion
It is well-known that liver cancer is associated
with a high mortality and primarily occurs in less developed
countries (1,2). Although patients with HCC exhibit
significantly improved survival times following curative resection,
the prognosis of these patients remains poor owing to tumor
invasiveness and metastasis (42).
Therefore, identifying more accurate prognostic biomarkers is of
great clinical value, allowing for further understanding of HCC and
the development of novel therapeutic strategies.
EMT is a major step in tumor metastasis (10,43). This
process is regulated by major EMT regulators, including Twist,
Snail and Slug, and is initiated by suppression of E-cadherin
expression (10). Twist serves an
important role through its regulation of E-cadherin expression in
human cancer. The role of Twist in cancer metastasis was first
reported in study on a breast cancer model, the results of which
indicating that Twist induced EMT, resulting in the promotion of
tumor invasion (13). Twist has been
revealed to be associated with metastasis in various types of
cancer, including HCC, through the induction of EMT changes and
cancer invasiveness (23). The
present study detected an association between Twist expression and
OPN expression in HCC cell lines and in patients with HCC. The
results of the present study demonstrated that Twist expression was
induced by OPN in HCC. This finding was further confirmed using
TMA, which revealed that OPN overexpression in HCC tumor tissues
was associated with Twist expression. Additionally, Twist
expression was observed in various HCC cell lines with different
metastatic potentials. Twist was overexpressed in metastatic cell
lines compared with non-metastatic primary cell lines. The TMA
immunohistochemical assay performed in the present study also
supported the hypothesis that Twist expression was positively
associated with metastasis in HCC tumor tissues. Therefore, these
data indicated that OPN serves a crucial role in the induction of
HCC progression through the regulation of Twist expression.
A further important finding from the present study
is that the hyper-activation of the PI3K/Akt signaling pathway is
responsible for the progression of HCC cells induced by OPN. Twist
is the most important transcription factor in the negative
regulation of E-cadherin expression and the EMT of epithelial
cells. Evidence indicates that Twist phosphorylation is
predominantly regulated by the PI3K/Akt pathway (44). An increase in Akt signaling is a key
tumor survival mechanism that promotes tumor metastatic processes.
A previous study (45) have
demonstrated that activated Akt serves a critical role in
hematogenous intrahepatic metastasis in an orthotopic implantation
model of HCC. The present study revealed that, through the
activation of PI3K/Akt signaling induced by OPN, the level of Twist
increased correspondingly, which led to the downregulation of
E-cadherin. This concept was further supported by the observation
that knockdown of OPN markedly reduced OPN-induced Akt activation.
When HCC cell lines stably overexpressing OPN were incubated with
LY294002, a PI3K inhibitor, the expression of Twist and N-cadherin
was markedly reduced. Taken together, these results indicated that
OPN activates PI3K/Akt/Twist signaling, which promotes the
metastasis of HCC cells. To the best of our knowledge, the present
study is the first to document a link among OPN, PI3K/Akt and Twist
in human HCC.
Previous studies identified that OPN was associated
with aggressive and metastatic HCC phenotypes, and with poor
patient prognosis (46–48). The results of the present study
indicated that patients with high Twist expression exhibit
significantly shorter OS and TTR times than patients with low Twist
expression. Additionally, according to univariate and multivariate
Cox proportional hazards regression analyses, the predictive range
of OPN expression levels combined with those of Twist was more
sensitive than that of OPN alone for OS and TTR. Taken together,
the results of the present study clearly demonstrate that a
combination of OPN and Twist expression levels may serve as a
powerful prognostic indicator of HCC.
In conclusion, the data presented in the current
study revealed that Twist was markedly upregulated in HCC patients
and that high expression of Twist was associated with poor patient
prognosis. Additionally, the expression of Twist, as a major
regulator of EMT, was regulated by OPN though the PI3K/Akt
signaling pathway. These findings indicate that OPN and Twist may
serve as synergistic indicators for HCC patients following curative
resection. Therefore, Twist may be a potential therapeutic target
to inhibit HCC metastasis in patients with high OPN expression.
Acknowledgements
Not applicable.
Funding
The present study was supported by the China
National Natural Science Foundation (grant nos. 81772563, 81372647
and 81472672), the National Key Basic Research Program of China
(grant no. 2013CB910500) and the China National Key Projects for
Infectious Diseases (grant no. 2012ZX10002-012).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XXY, YZ, XCZ and XMG designed and performed the
experiments, analyzed data. CQW, YYS and WC participated in
collecting the samples and correcting patient sample's following-up
investigation data. XXY and YZ performed bioinformatics analyses.
NR and LXQ participated in data interpretation and provided
valuable discussions with regard to clinical correlates. QZD and
HLJ designed and supervised the entire project, designed the
experiments, and prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Zhongshan Hospital, Fudan University (Shanghai,
China). Clinical samples were collected from these patients after
obtaining informed consent according to an established protocol
approved by committee's regulations. The data did not contain any
information that could lead to patient identification.
Patient consent for publication
Written informed consent for publication was
obtained from all participants.
Competing interests
The authors have declared that no competing
interests exist.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Nagano H, Ota H, Morimoto O,
Nakamura M, Wada H, Noda T, Damdinsuren B, Marubashi S, Miyamoto A,
Miyamoto A, et al: Patterns and clinicopathologic features of
extrahepatic recurrence of hepatocellular carcinoma after curative
resection. Surgery. 141:196–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye
QH, Wang L, Zhou J, Qiu SJ, Li Y, et al: A decade's studies on
metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol.
130:187–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wai PY and Kuo PC: Osteopontin: Regulation
in tumor metastasis. Cancer Metastasis Rev. 27:103–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tuck AB, Chambers AF and Allan AL:
Osteopontin overexpression in breast cancer: Knowledge gained and
possible implications for clinical management. J Cell Biochem.
102:859–868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McAllister SS, Gifford AM, Greiner AL,
Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander
BL, Repasky EA, et al: Systemic endocrine instigation of indolent
tumor growth requires osteopontin. Cell. 133:994–1005. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong QZ, Zhang XF, Zhao Y, Jia HL, Zhou
HJ, Dai C, Sun HJ, Qin Y, Zhang WD, Ren N, et al: Osteopontin
promoter polymorphisms at locus-443 significantly affect the
metastasis and prognosis of human hepatocellular carcinoma.
Hepatology. 57:1024–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhattacharya SD, Mi Z, Kim VM, Guo H,
Talbot LJ and Kuo PC: Osteopontin regulates epithelial mesenchymal
transition-associated growth of hepatocellular cancer in a mouse
xenograft model. Ann Surg. 255:319–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang SH, Wu XC, Zhang MD, Weng MZ, Zhou D
and Quan ZW: Upregulation of H19 indicates a poor prognosis in
gallbladder carcinoma and promotes epithelial-mesenchymal
transition. Am J Cancer Res. 6:15–26. 2015.PubMed/NCBI
|
|
12
|
Huang Q, Han J, Fan J, Duan L, Guo M, Lv
Z, Hu G, Chen L, Wu F, Tao X, et al: IL-17 induces EMT via Stat3 in
lung adenocarcinoma. Am J Cancer Res. 6:440–451. 2016.PubMed/NCBI
|
|
13
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang MH and Wu KJ: TWIST activation by
hypoxia inducible factor-1 (HIF-1): Implications in metastasis and
development. Cell Cycle. 7:2090–2096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng GZ, Zhang W and Wang LH: Regulation
of cancer cell survival, migration, and invasion by Twist: AKT2
comes to interplay. Cancer Res. 68:957–960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C,
Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, et al: Up-regulation
of TWIST in prostate cancer and its implication as a therapeutic
target. Cancer Res. 65:5153–5162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoek K, Rimm DL, Williams KR, Zhao H,
Ariyan S, Lin A, Kluger HM, Berger AJ, Cheng E, Trombetta ES, et
al: Expression profiling reveals novel pathways in the
transformation of melanocytes to melanomas. Cancer Res.
64:5270–5282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Entz-Werle N, Stoetzel C, Berard-Marec P,
Kalifa C, Brugiere L, Pacquement H, Schmitt C, Tabone MD, Gentet
JC, Quillet R, et al: Frequent genomic abnormalities at TWIST in
human pediatric osteosarcomas. Int J Cancer. 117:349–355. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Doorn R, Dijkman R, Vermeer MH,
Out-Luiting JJ, van der Raaij-Helmer EM, Willemze R and Tensen CP:
Aberrant expression of the tyrosine kinase receptor EphA4 and the
transcription factor twist in Sezary syndrome identified by gene
expression analysis. Cancer Res. 64:5578–5586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosivatz E, Becker I, Specht K, Fricke E,
Luber B, Busch R, Höfler H and Becker KF: Differential expression
of the epithelial-mesenchymal transition regulators Snail, SIP1,
and twist in gastric cancer. Am J Pathol. 161:1881–1891. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Hou Y, Zhou M, Wen S, Zhou J, Xu
L, Tang X, Du YE, Hu P and Liu M: Twist induces
epithelial-mesenchymal transition and cell motility in breast
cancer via ITGB1-FAK/ILK signaling axis and its associated
downstream network. Int J Biochem Cell Biol. 71:62–71. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok
WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL and Fan ST: Twist
overexpression correlates with hepatocellular carcinoma metastasis
through induction of epithelial-mesenchymal transition. Clin Cancer
Res. 12:5369–5376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY,
Xiao YS, Xu Y, Li YW and Tang ZY: Intratumoral balance of
regulatory and cytotoxic T cells is associated with prognosis of
hepatocellular carcinoma after resection. J Clin Oncol.
25:2586–2593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang MH, Chen CL, Chau GY, Chiou SH, Su
CW, Chou TY, Peng WL and Wu JC: Comprehensive analysis of the
independent effect of twist and snail in promoting metastasis of
hepatocellular carcinoma. Hepatology. 50:1464–1474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marrero JA, Fontana RJ, Barrat A, Askari
F, Conjeevaram HS, Su GL and Lok AS: Prognosis of hepatocellular
carcinoma: Comparison of 7 staging systems in an American cohort.
Hepatology. 41:707–716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Llovet JM, Fuster J and Bruix J;
Barcelona-Clinic Liver Cancer Group, : The Barcelona approach:
Diagnosis, staging, and treatment of hepatocellular carcinoma.
Liver Transpl. 10 2 Suppl 1:S115–S120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun HC, Zhang W, Qin LX, Zhang BH, Ye QH,
Wang L, Ren N, Zhuang PY, Zhu XD, Fan J and Tang ZY: Positive serum
hepatitis B e antigen is associated with higher risk of early
recurrence and poorer survival in patients after curative resection
of hepatitis B-related hepatocellular carcinoma. J Hepatol.
47:684–690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian YB, Zhang JB, Wu WZ, Fang HB, Jia WD,
Zhuang PY, Zhang BH, Pan Q, Xu Y, Wang L, et al: P48 is a
predictive marker for outcome of postoperative interferon-alpha
treatment in patients with hepatitis B virus infection-related
hepatocellular carcinoma. Cancer. 107:1562–1569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Llovet JM and Bruix J: Molecular targeted
therapies in hepatocellular carcinoma. Hepatology. 48:1312–1327.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh PP, Shi Q, Foster NR, Grothey A,
Nair SG, Chan E, Shields AF, Goldberg RM, Gill S, Kahlenberg MS, et
al: Relationship between metformin use and recurrence and survival
in patients with resected Stage III colon cancer receiving adjuvant
chemotherapy: Results from north central cancer treatment group
N0147 (Alliance). Oncologist. 21:1509–1521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang
W, Xiong YQ, Wu WZ, Wang L, Tang ZY and Sun HC: High expression of
macrophage colony-stimulating factor in peritumoral liver tissue is
associated with poor survival after curative resection of
hepatocellular carcinoma. J Clin Oncol. 26:2707–2716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Tian B, Yang J, Zhao L, Wu X, Ye SL,
Liu YK and Tang ZY: Stepwise metastatic human hepatocellular
carcinoma cell model system with multiple metastatic potentials
established through consecutive in vivo selection and studies on
metastatic characteristics. J Cancer Res Clin Oncol. 130:460–468.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY,
Chen J and Xue Q: New human hepatocellular carcinoma (HCC) cell
line with highly metastatic potential (MHCC97) and its expressions
of the factors associated with metastasis. Br J Cancer. 81:814–821.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong Q, Zhu X, Dai C, Zhang X, Gao X, Wei
J, Sheng Y, Zheng Y, Yu J, Xie L, et al: Osteopontin promotes
epithelial-mesenchymal transition of hepatocellular carcinoma
through regulating vimentin. Oncotarget. 7:12997–13012. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun BS, Dong QZ, Ye QH, Sun HJ, Jia HL,
Zhu XQ, Liu DY, Chen J, Xue Q, Zhou HJ, et al: Lentiviral-mediated
miRNA against osteopontin suppresses tumor growth and metastasis of
human hepatocellular carcinoma. Hepatology. 48:1834–1842. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fu J, Chen Y, Cao J, Luo T, Qian YW, Yang
W, Ren YB, Su B, Cao GW, Yang Y, et al: p28GANK overexpression
accelerates hepatocellular carcinoma invasiveness and metastasis
via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1α
pathways. Hepatology. 53:181–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hua Z, Gu X, Dong Y, Tan F, Liu Z, Thiele
CJ and Li Z: PI3K and MAPK pathways mediate the BDNF/TrkB-increased
metastasis in neuroblastoma. Tumour Biol. 37:16227–16236. 2016.
View Article : Google Scholar
|
|
40
|
Zhang C, Wang Y, Feng Y, Zhang Y, Ji B,
Wang S and Sun Y, Zhu C, Zhang D and Sun Y: Gli1 promotes
colorectal cancer metastasis in a Foxm1-dependent manner by
activating EMT and PI3K-AKT signaling. Oncotarget. 7:86134–86147.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kikuchi L, Chagas AL, Alencar RSSM, Tani
C, Diniz MA, D'Albuquerque LAC and Carrilho FJ: Adherence to BCLC
recommendations for the treatment of hepatocellular carcinoma:
Impact on survival according to stage. Clinics (Sao Paulo).
72:454–460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huo TI, Lin HC, Huang YH, Wu JC, Chiang
JH, Lee PC and Lee SD: The model for end-stage liver disease-based
Japan Integrated Scoring system may have a better predictive
ability for patients with hepatocellular carcinoma undergoing
locoregional therapy. Cancer. 107:141–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li CW, Xia W, Lim SO, Hsu JL, Huo L, Wu Y,
Li LY, Lai CC, Chang SS, Hsu YH, et al: AKT1 Inhibits
Epithelial-to-Mesenchymal transition in breast cancer through
phosphorylation-dependent Twist1 degradation. Cancer Res.
76:1451–1462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nakanishi K, Sakamoto M, Yasuda J,
Takamura M, Fujita N, Tsuruo T, Todo S and Hirohashi S: Critical
involvement of the phosphatidylinositol 3-kinase/Akt pathway in
anchorage-independent growth and hematogeneous intrahepatic
metastasis of liver cancer. Cancer Res. 62:2971–2975. 2016.
|
|
46
|
Huang H, Zhang XF, Zhou HJ, Xue YH, Dong
QZ, Ye QH and Qin LX: Expression and prognostic significance of
osteopontin and caspase-3 in hepatocellular carcinoma patients
after curative resection. Cancer Sci. 101:1314–1319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ye QH, Qin LX, Forgues M, He P, Kim JW,
Peng AC, Simon R, Li Y, Robles AI, Chen Y, et al: Predicting
hepatitis B virus-positive metastatic hepatocellular carcinomas
using gene expression profiling and supervised machine learning.
Nat Med. 9:416–423. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang H, Ye QH, Ren N, Zhao L, Wang YF, Wu
X, Sun HC, Wang L, Zhang BH, Liu YK, et al: The prognostic
significance of preoperative plasma levels of osteopontin in
patients with hepatocellular carcinoma. J Cancer Res Clin Oncol.
132:709–717. 2006. View Article : Google Scholar : PubMed/NCBI
|