Introduction
Human epidermal growth factor receptor type 2 (HER2
also known as ErbB2, p185), in which genes located at chromosome
17q21, is one of four members of the ErbB/HER family. The HER2
modulates its activity by a tyrosine kinase signaling pathway, and
is involved in the development of many cancers, such as non
endocrine tumors (breast, gastric, pancreatic, esophageal,
prostate, lung and colon cancers) and few endocrine tumors
(thyroid, pituitary, and pheochromocytomas) (1). The HER2 over expression in tumors is
responsible for tumor aggressiveness, poor prognosis, decreased
survival, and is also associated with enhanced invasiveness and
resistance to radiochemotherapy (2,3).
Affibody molecule (Affibody®) consists of
58 amino acids (~6.5 kDa) that contains a modified B domain of the
staphylococcal protein A, and it can be obtained via chemical
synthesis or produced in bacteria by the use of recombinant DNA
technology (4). Because of its small
molecule size and high chemical and thermal stability, there are
much interested in radiolabeling these molecules and using them for
the targeted imaging and treatment of HER2-overexpressing tumors
(4–6).
ZHER2:342 (~7 kDa) is one of basic Affibody molecule,
recently, it has been radiolabeled with 111In or
99mTc and 177Lu, which can be detected
HER2-overexpressing tumors under single photon emission computed
tomography (SPECT) (7–10). Moreover, ZHER2:342 also
could be labeled with 68Ga, 11C,
18F and could be imaged under PET/CT (9,11).
However, most of ZHER2:342 radiolabeled results
demonstrated the high abdominal accumulation in liver with prolong
renal retention, which hampers its application for the detection of
abdomen HER2 overexpression tumors (10).
The ZHER2:V2, Affibody molecule of
ZHER2:2395-C, based on the ZHER2:342 binding
sequence, with C-terminal engineered cysteine (named
ZHER2:V2) has been studied recently (12). The structure of ZHER2:V2 is
similar with ZHER2:342, only was replaced-AEN at the
N-terminal and -GGGC as a chelator at the C-terminal (13). ZHER2:V2 radiolabeled as a
probe shows good HER2 tumor target, rapid blood clearance, low
levels of stomach and salivary gland radioactivity, low levels of
renal radioactivity and low levels of hepatobiliary excretion.
Therefore, the radionuclide labeled ZHER2:V2 may be a
promising imaging agent for HER2-overexpressing tumors.
The aim of the present study was to label the
ZHER2:V2 with 99mTc directly using fast and
simple approach and evaluate its properties for HER2 positive tumor
imaging: With an eye towards clinical translation.
Materials and methods
Preparation of
99mTc-ZHER2:V2
ZHER2:V2(AEN KFN KEM RNA YWE IAL LPN LNN
QQKRAFIRSLYDDPSQSANLLAEAKKLNDAQGGGC) has been assembled by which
changing VEN- to AEN- at the N-terminal and the -GGGC as a chelator
at the C-terminal (13). ZHER2:V2 was
synthesized and purified by Skylight Biotechnology, LLC, (Beijing,
China). The labeling method referred to the paper of Tran T and
co-authors, and revised the labeling method (14). Briefly, 10 µl of 0.1 M NaOH were added
to 10 µl (10 µg) of ZHER2:V2 and the solution pH were adjusted to
about 12. Subsequently, 200 µl [111 MBq (3 mCi)] of fresh
99mTcO4- solution obtained from 99Mo/99mTc generator was
added, immediately after 0.3 µl (0.3 µg) of SnCl2was added. The
mixture solution was incubated for 20 min at 20–25°C room
temperature. Finally the pH of the mixture was about 7~8. The
labeling efficiency and radiochemical purity of the labeled
conjugate were analyzed by reversed-phase high-pressure liquid
chromatography (RP-HPLC) with a microbond C18 column (Alltech,
model 305, Deerfield IL, 4.6×250 mm) connected a ultraviolet
detector, an NaI (Tl) radioactivity monitor, and a rate meter. The
C18 column was eluted with a gradient of 5–80% buffer A (0.1%
trifluoroacetic acid-CH3CN) and buffer B (0.1% trifluoroacetic
acid-H2O) in 20 min and a flow rate of 1 ml/min at 25°C monitoring
at 260 nm.
We also analyzed the labeled probe using instant
thin layer chromatography (ITLC) method to determine the technetium
colloids, ITLC was eluted with acetonitrile. In this eluent, the
technetium colloids remained at the origin, while the radiolabeled
probe and pertechnetate migrated with solvent front.
In vitro stability analysis
To assess the stability of the radiotracer in
vitro, 99mTc-ZHER2:V2 was incubated in
physiological saline or human fresh serum at 37°C, and the
stability of the radiotracer in vitro was evaluated at 1, 2,
4, 6 and 8 h respectively. The same RP-HPLC conditions were used as
those for measuring the release of free pertechnetate.
Cell cultures
The HER2-overexpressing ovarian carcinoma cell line
SKOV3 and HER2 low expression breast carcinoma cell line MCF-7 were
purchased from the Institute of Cell Biology of the Chinese Academy
of Science (Shanghai, China). All cells were cultivated in Roswell
Park Memorial Institute 1640 (RPMI 1640) medium supplemented with
10% foetal bovine serum under standard conditions (37°C, humidified
atmosphere containing 5% CO2). Cell growth was monitored
under inverted microscope with phase contrast. When the cell
density reached 90%, the cells were harvested.
Cellular uptake, retention, binding
affinity and blocking studies
Sixtuplicate cell wells were used for each data
point. The SKOV3 cells were washed twice with phosphate-buffered
saline (PBS), then 2 ml of fresh RPMI 1640 medium containing 10%
FBS were added, followed by 37 kBq of
99mTc-ZHER2:V2 (0.0001 µg/µl) directly into
each well. Cells were harvested by the same trypsin-EDTA solution
at 1, 2, 4, 6, 8, 12, and 24 h respectively as described above.
Before cell lyses, the medium containing 10% FBS was removed, and
the wells were washed twice with PBS. The collected fractions
radioactivity was measured using an automated γ counter. Counts the
containing radioactive medium and PBS were defined as
Cup. After being lysed with 0.5 ml of trypsin-EDTA, 0.5
ml of 10% FBS was added to each well, and then cells were washed
twice with PBS. Radioactivity of the lyses solution and PBS were
designated as Cdown. The collected fractions
radioactivity was measured by an automated γ counter. The cellular
uptake ratio was calculated by the formula
Cdown/(Cdown + Cup) ×100%.
To determine cellular retention,
99mTc-ZHER2:V2 (37 kBq 0.0001 µg/µl) was
directly added into 6-well plates that containing 6×105
SKOV3 cells and incubated at 37°C for 4 h. Then, the medium was
removed, and all wells were washed twice with PBS solution. After 2
ml of fresh RPMI 1640 medium containing 10% FBS was added into each
well, the plates were incubated at 37°C for 1, 2, 4, 6, 8, 12, and
24 h respectively. The medium containing 10% FBS of the wells was
removed before cell lyses, and the wells were washed twice with
PBS. The collected fractions radioactivity was measured by an
automated γ counter. The cellular retention ratio was calculated by
the same formula for calculating cellular uptake kinetics given
above. In this way, the radioactivity counts of Cdown
contain 99mTc-ZHER2:V2 which have been
internalized and membrane-bound.
For internalization studies,
99mTc-ZHER2:V2 uptake was performed as
described. Several additional cell plates were used during the
binding study to separate the membrane-bound fraction of the
conjugate from externalized radioactivity. The wells were washed
twice with PBS. Then the isolated cell pellet was washed using 0.2
M acetic acid/0.5 M NaCl, pH 2.5, to remove the cell surface-bound
radioactivity at each time point. The radioactivity that was
removed from cells by an acidic buffer was defined as
membrane-bound.
For blocking studies, 1.5 µg (500 times) or 3 µg
(1,000 times) of unlabeled ZHER2:V2 and the
99mTc-ZHER2:V2 were simultaneously added to
the cell well. After incubation for 4 h, the
99mTc-ZHER2:V2 uptake of HER2-overexpression
SKOV3 cells was measured as the same method that was mentioned
above.
Biodistribution studies
All animal studies were performed in accordance with
the guidelines of local animal care and use committee.
Biodistribution studies of 99mTc-ZHER2:V2 in
BALB/c nude mice bearing SKOV3 ×enografts were performed. For
biodistribution studies, 0.1 ml 1,110 kBq dose of
99mTc-ZHER2:V2 was injected via the tail vein
into the BALB/c nude mice bearing SKOV3 cells (n=4/group), which
were sacrificed at 1, 2, 4, or 6 h after the
99mTc-ZHER2:V2 injection. The organ or tissue
samples of interest (blood, heart, liver, spleen, kidney, lung,
stomach, small intestine, brain, bone, muscle and tumor) were
harvested by dissection and weighed. A gamma well counter
(CAPRAC®; Capintec Inc., Ramsey, NJ, USA) was used to
measure radioactivity uptake in organs or tissues, which was
defined as the percentage of injected dose per gram of tissue
(%ID/g).
In vivo SPECT imaging studies
Three nude mice were used for (SPECT) imaging. Two
mice which bearing SKOV3 ×enografts and MCF-7 ×enografts were
respectively injected 37 MBq (100 µl) of
99mTc-ZHER2:V2 (0.003 µg/µl) via the tail
vein. The other SKOV3 ×enografts nude mice was injected 30 µg
unlabeled ZHER2:V2via tail vein 1 h before the
99mTc-ZHER2:V2 (0.003 µg/µl) was injected 37
MBq (100 µl). After 4 h of injection, animals were anesthetized and
placed supine near the centerfield of the view of the SPECT
detector and imaged using the e.camduet SPECT equipped
with a pinhole collimator. One static anterior image (300,000
counts) was obtained with a zoom 1.78, matrix 128×128 and energy
window 140 keV, 15%.
Statistic analysis
Variables were expressed as mean value plus or minus
standard deviation (x ± s). Analysis of variance (ANOVA) was
used to analyze the variation of cellular uptake in the blocking
experiment. P<0.05 was considered to indicate a statistically
significant difference.
Results
Radiolabeling of
ZHER2:V2
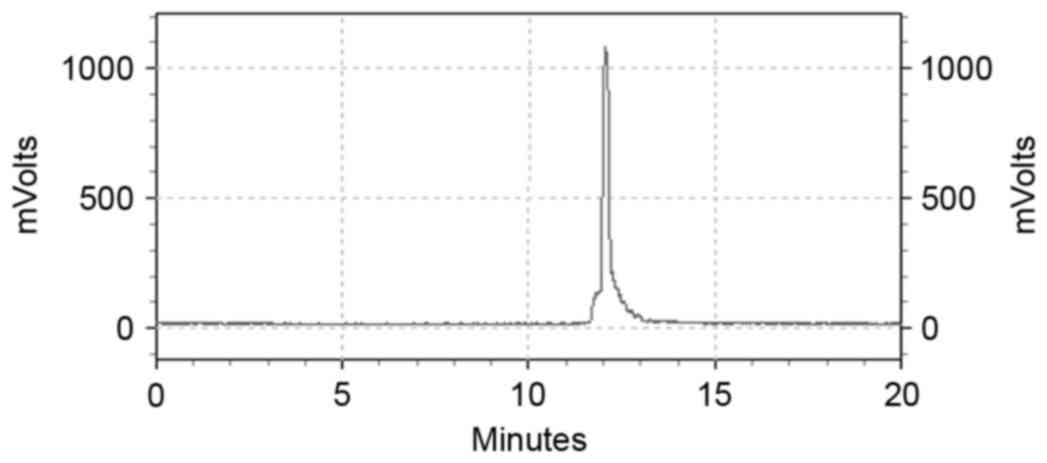
The labeling efficiency was 98.99±0.99% (n=6).
99mTc-ZHER2:V2 using RP-HPLC analysis showed
that the retention time was 12 min, with a single, high and sharp
peak, which was consistent with its single absorbance peak at 260
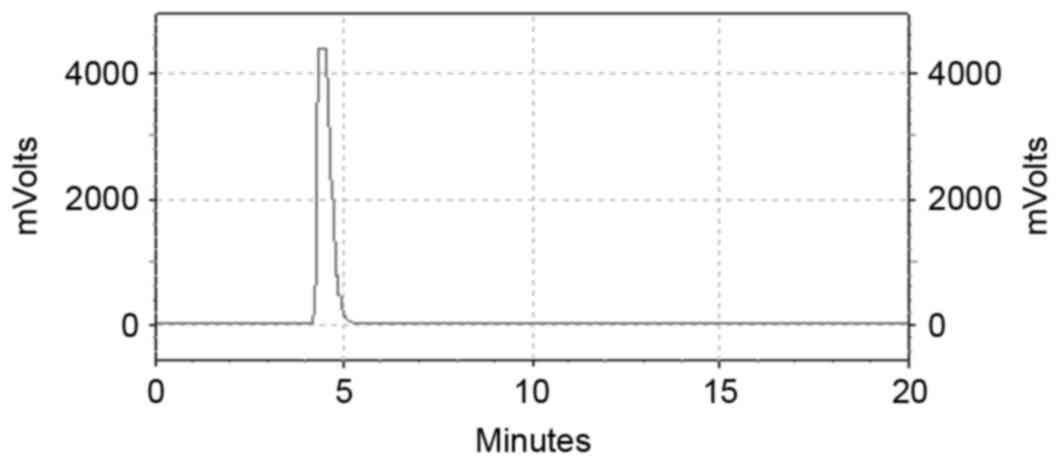
nm (Fig. 1) whereas that of the free
99mTc was about 4.5 min (Fig.
2). Even 6 h later the labeling efficiency of
99mTc-ZHER2:V2 was still 97.23%. The result
of ITLC showed that the origin has little counts, and almost
radioactivity at the front, indicating most of 99mTc
have been bound with ZHER2:V2, and almost no technetium
colloids existence.
In vitro stability analysis
The results showed that the
99mTc-ZHER2:V2 incubated in physiological
saline or human fresh serum at 37°C for 1, 2, 4, 6 and 8 h was very
stable in vitro. RP-HPLC analysis demonstrated that the
radiochemical purity of 99mTc-ZHER2:V2 was
(98.17±1.42%, 98.08±0.94%, 97.81±0.75%, 97.54±0.75%, 96.71±0.51%)
and (98.89±0.76%, 98.09±0.57%, 97.87±0.31%, 97.81±0.73%,
96.84±0.69%) respectively. The radiochemical purity of the probe
was still as high as 96% even after 6 h, indicating that no
significant degradation and off-labeling occurred in human fresh
serum or physiological saline at 37°C.
Cellular uptake, retention, binding
affinity and blocking studies
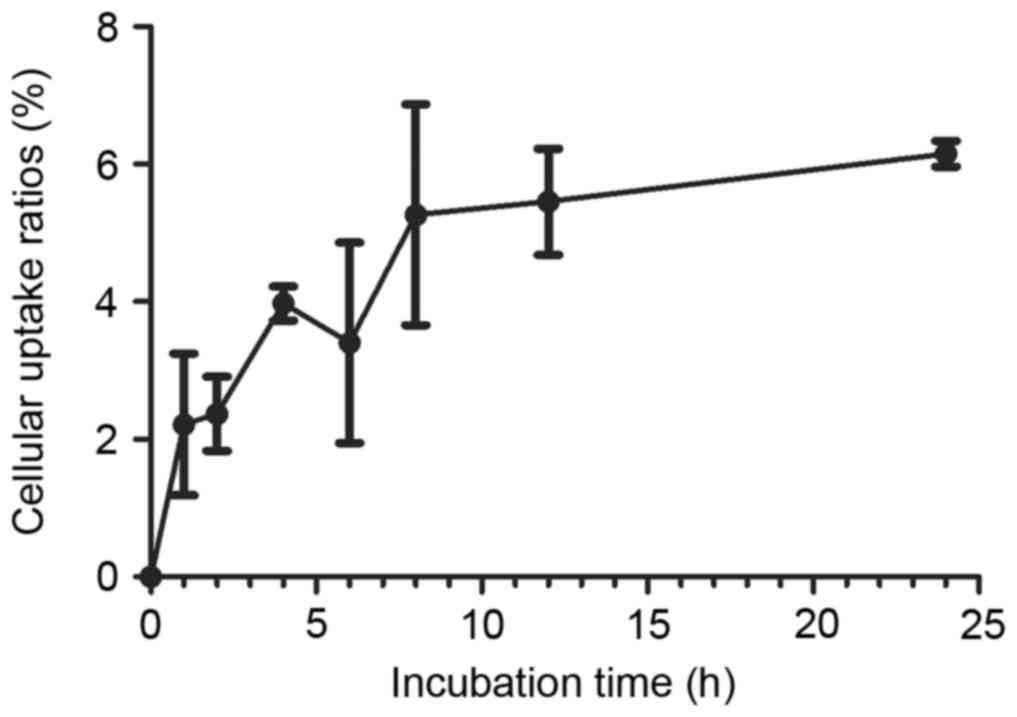
The results of cellular uptake
99mTc-ZHER2:V2 studies are presented in
Fig. 3. With the interval of time,
the cellular uptake was rising slowly, and the peak of cellular
uptake ratio appeared at 24 h was 6.15±0.18%. After the peak, the
cellular uptake ratio of 99mTc-ZHER2:V2
showed a little decline.
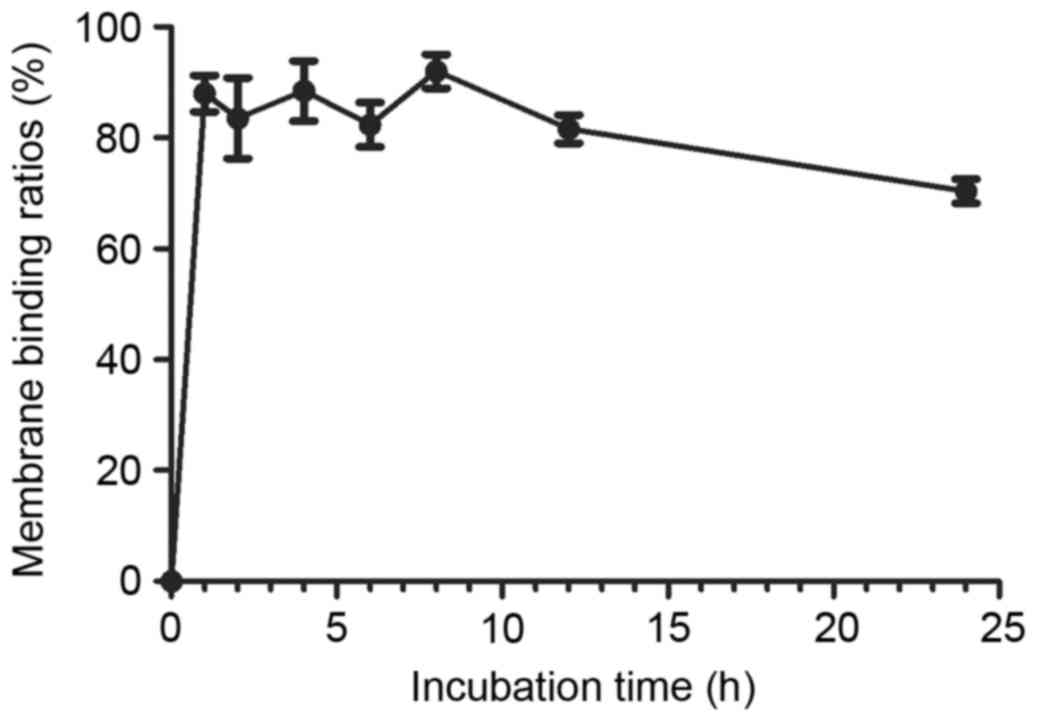
The cell membrane binding rate was showed in
Fig. 4, indicating that the cell
membrane binding is a rapid process in SKOV3 cells, greater than
85.5% of the radioactivity was bound on the cell membrane after
incubating 99mTc-ZHER2:V2 with SKOV3 cells,
and after 24 h later, there were almost 70%
99mTc-ZHER2:V2 sill binding on cell membrane.
As a result, the 99mTc-ZHER2:V2 has a slowly
internalizing course characterize.
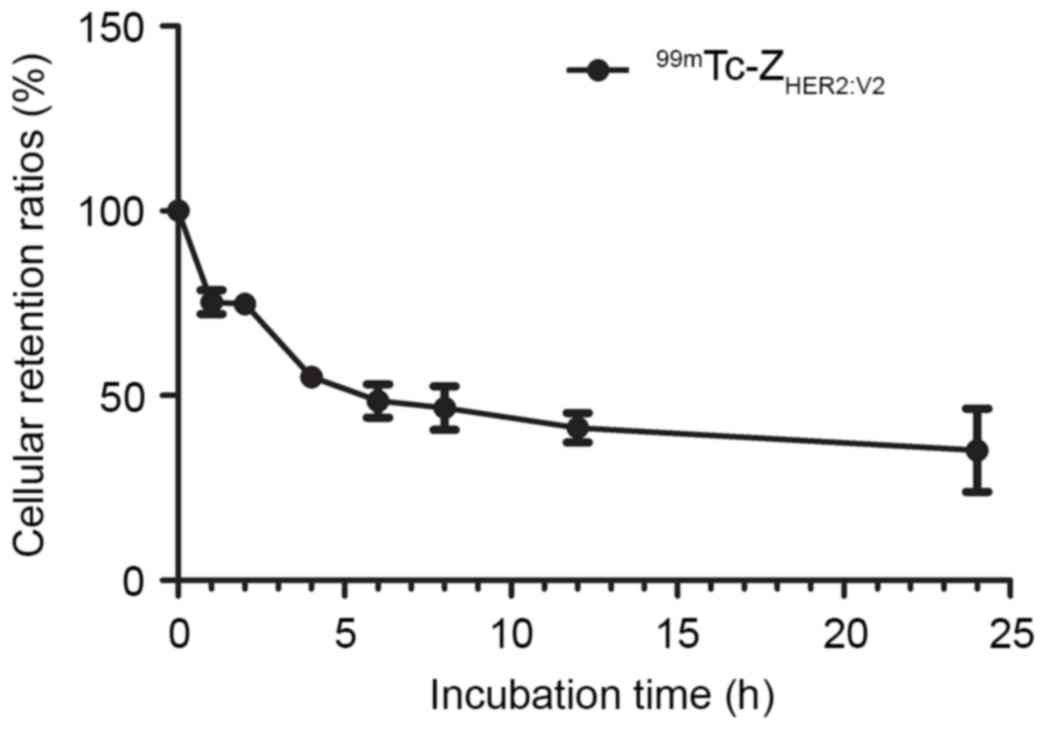
The result of retention kinetics analysis was shown
in Fig. 5. The results demonstrated
good 99mTc-ZHER2:V2 retention in SKOV3 cells,
with 48.58±4.52% at 6 h, even after 24 h, the retention ratio of
99mTc-ZHER2:V2 still reached
35.16±11.23%.
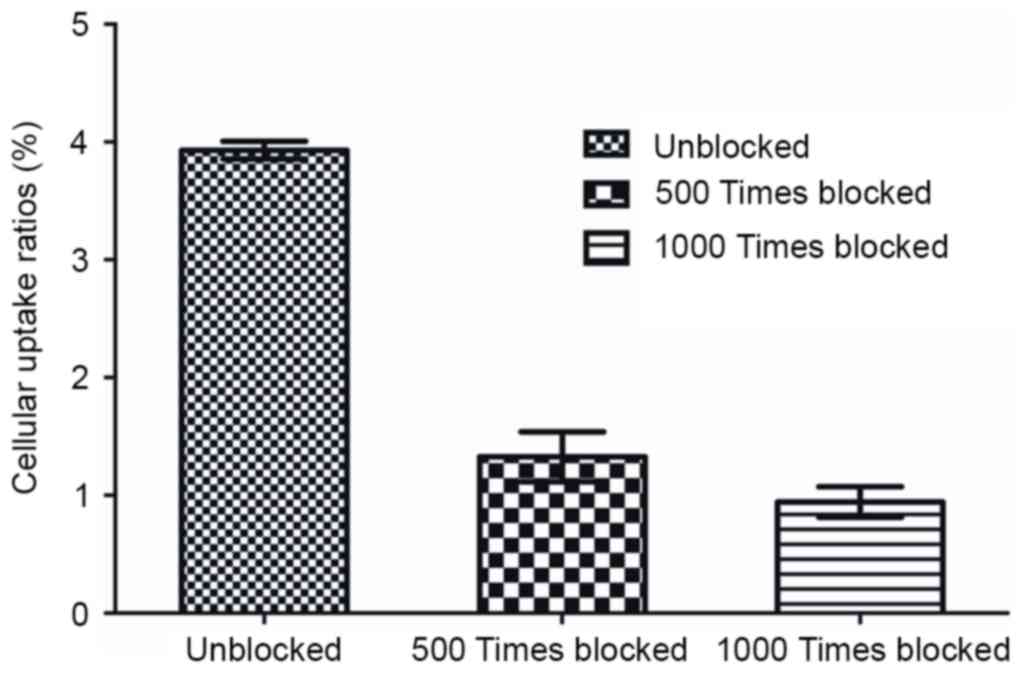
The specific binding of
99mTc-ZHER2:V2 in SKOV3 cells was shown in
Fig. 6, the
99mTc-ZHER2:V2 was blocked by an excess
amount (500 or 1,000 times) of unlabeled ZHER2:V2 in
SKOV3 cells (F=351.232, P<0.05). In addition,
99mTc-ZHER2:V2 was significantly blocked when
1,000 times of unlabeled ZHER2:V2 were added as compared
with that of 500 times of unlabeled ZHER2:V2 in SKOV3
cells, demonstrating the character of HER2-specific binding.
Biodistribution studies
99mTc-ZHER2:V2 biodistribution analysis was
performed in nude mice bearing SKOV3 ×enografts at 1, 2, 4, and 6 h
after intravenous injection. Data on the biodistribution of the
SKOV3 ×enografts are presented in Table
I. The results revealed low uptake of radioactivity in all
organs except kidneys (8.68±2.68% ID/g) and tumor. The other
samples were <2% ID/g except the liver (2.70±0.17% ID/g). The
radioactive uptake in SKOV3 ×enografts was higher than that for
organs other than the kidneys. The tumor accumulation was
4.14±1.61, 6.47±2.09, 5.04±2.58, 10.07±0.33% ID/g at 1, 2, 4 and 6
h, respectively. The radioactivity in all organs was reduced as
time gone by other than the tumor, demonstrating good specificity
and sensitivity of 99mTc-ZHER2:V2 in the detection of HER2
receptors.
 | Table I.Biodistribution of
99mTc-ZHER2:2891 in SKOV-3 ×enografts. |
Table I.
Biodistribution of
99mTc-ZHER2:2891 in SKOV-3 ×enografts.
| Organ | 1 h | 2 h | 4 h | 6 h |
|---|
| Blood | 2.26±1.20 | 1.55±0.59 | 1.04±0.45 | 0.75±0.10 |
| Heart | 0.40±0.15 | 0.29±0.27 | 0.43±0.36 | 0.61±0.46 |
| Liver | 2.83±2.23 | 2.72±0.00 | 2.45±1.30 | 2.80±0.16 |
| Spleen | 1.91±0.70 | 0.96±0.61 | 0.85±0.39 | 0.86±0.53 |
| Kidney | 12.17±3.26 | 9.26±2.92 | 6.04±4.69 | 7.25±1.63 |
| Lung | 0.66±0.27 | 0.38±0.05 | 0.34±0.00 | 0.60±0.23 |
| Stomach | 2.03±0.59 | 1.16±0.84 | 0.87±0.76 | 0.34±0.26 |
| Intestine | 1.17±0.44 | 0.71±0.53 | 0.59±0.44 | 0.51±0.38 |
| Brain | 0.32±0.06 | 0.08±0.02 | 0.11±0.01 | 0.13±0.01 |
| Bone | 1.02±0.75 | 0.69±0.27 | 0.47±0.37 | 0.21±0.18 |
| Muscle | 0.85±0.35 | 0.37±0.10 | 0.10±0.07 | 0.56±0.22 |
| Tumor | 4.14±1.42 | 6.47±2.09 | 5.04±2.58 | 10.07±0.33 |
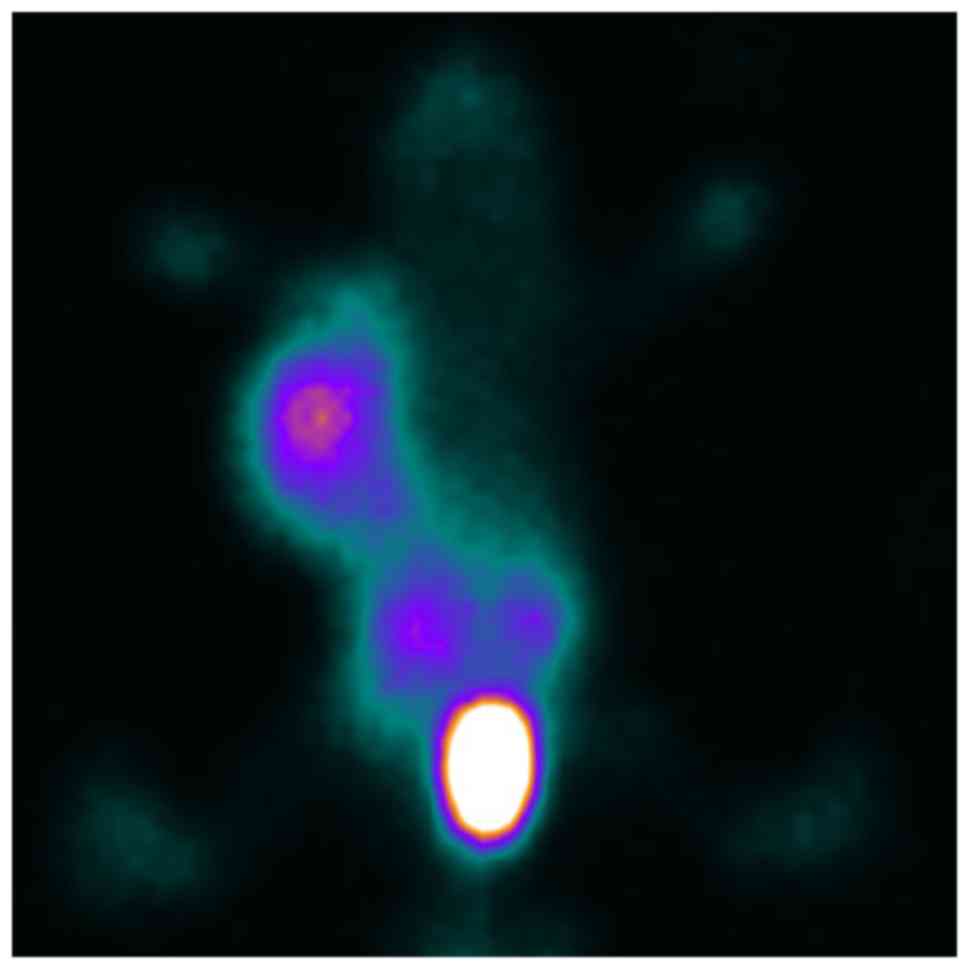
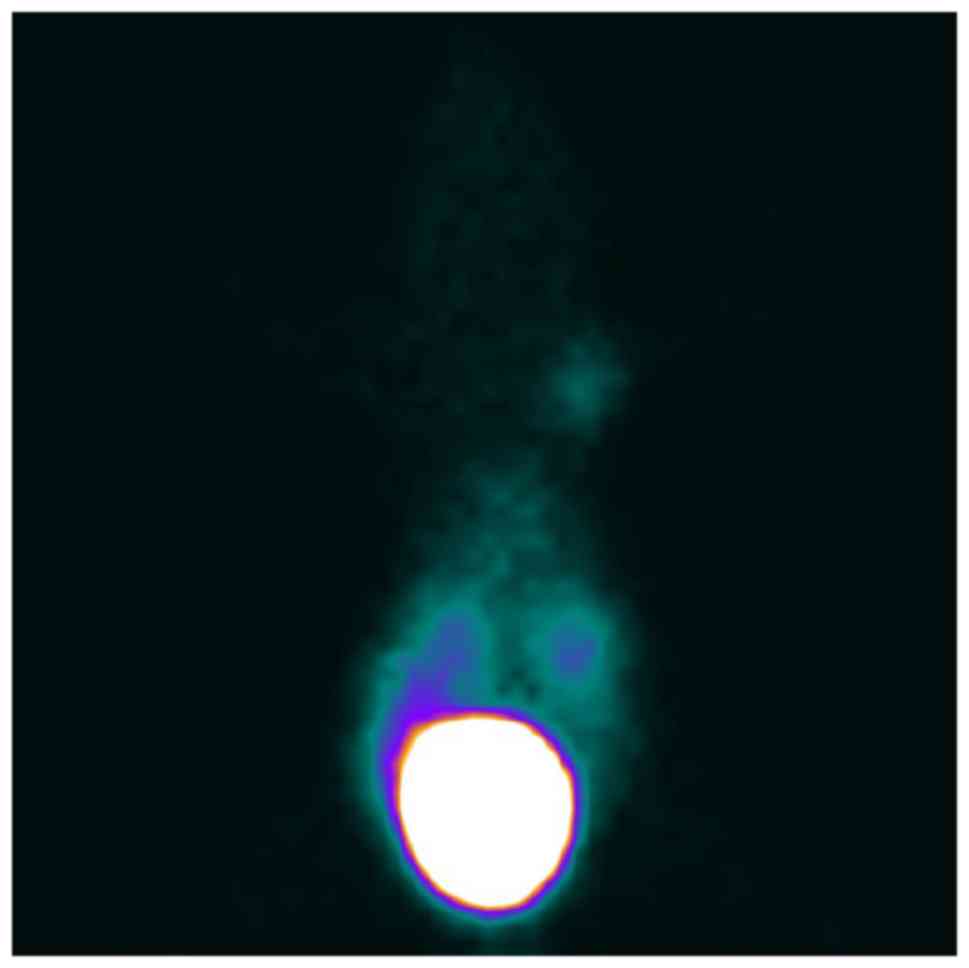
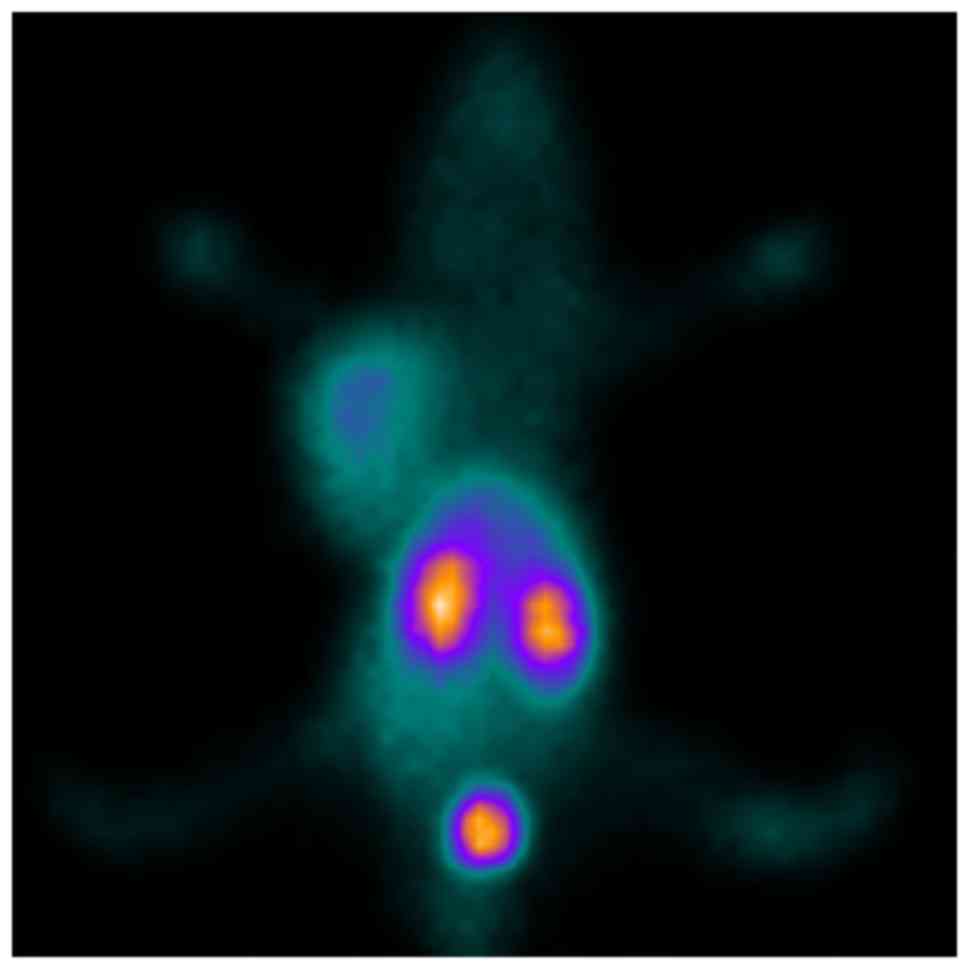
In vivo SPECT imaging studies
After injection of
99mTc-ZHER2:V2 in nude mice bearing SKOV3
×enograft via the tail vein, the images revealed intense tracer
uptake in the tumor (Fig. 7) and the
T/NT ratios was 9.98 at 4 h after injection, while the MCF-7
×enograft has low tracer accumulated (Fig. 8) and the T/NT ratios was 3.79,
indicating 99mTc-ZHER2:V2 could be highly
uptake by HER2-overexpression tumors, and further proved the probe
HER2-specific binding. The kidneys also accumulated a lot of
99mTc-ZHER2:V2 radioactivity, however, little
radioactivity stay in the liver, indicating that the
99mTc-ZHER2:V2 radioactivity was removed
mainly through the renal routes and decreased gradually throughout
the body. And the SKOV3 ×enografts imaging can be blocked by excess
amount unlabelled ZHER2:V2 at 2 h after injection of the
tracer (Fig. 9).
Discussion
Recently, HER2-receptor imaging has been developed
as a method to detect tumor HER2-overexpression, and it can
revealed important molecular clues for cancer patient
management.
Molecular imaging is an advance imaging that can
visually characterize, represent, and quantify biologic processes
at the cellular or subcellular level. In previous studies,
researchers used 64Cu-MM-302 (15) and 18F-ZHER2:342
(11) proved to be potential for PET
HER2 imaging. But these radiotracers have some limitations:
18F labeling peptide requires an in-house cyclotron
system, a complex infrastructure, a time-consuming synthesis
procedure and tedious purification; 64Cu-DOTA conjugates
generally display high liver accumulation because of the possible
off-labeling of 64Cu from the chelator (15).
99mTc has been successfully labeled on
Affibody molecule and its derivatives. As is widely known, it is a
complicated process that 99mTc was labeled on peptides,
‘incubate in boiling water’ was used in most of previous studies
(10,16,17). In
this study, direct labeling method was performing: NaOH was added
to adjust the PH and SnCl2 as the reluctant of
99mTc, not incubated in boiling water but in room
temperature. As a result, the labeling efficiency was exceeding
98%. This labeling approach is similar with Tran and co-authors
(14), and the labeling efficiency
has no difference (98.99% vs. 98.1%). These results indicated that
this revised labeling method is very good.
Direct labeling has a disadvantage of yielding
technetium colloids, to avoid this situation, how many
SnCl2 were added is the important. In this study,
99mTc were almost labeled on ZHER2:V2 and
technetium colloids were yielded hardly. And from the ITLC test,
outcome showed that no technetium colloids were yielded.
At present, lots of studies on the 99mTc
labeled HER2 molecular probes to image the HER2-overexpression
tumors in vivo have been performed. But from the
biodistribution studies, the results demonstrated that there were a
high level hepatic accumulation of radioactivity and a prolong
renal retention (16–19). A high level of hepatic accumulation
reduces the imaging sensitivity and may obstruct the detection of
liver metastases, and an elevated renal uptake was hindering the
detection of metastases in the lumbar and abdomen area. Thus, it is
very important to improve the probe tumor uptake and decrease liver
and kidneys uptake as much as possible.
Some of previous studies demonstrated that the amino
acid consists of the N-terminal was significant for the
biodistribution in terms of liver accumulation and the extent of
hepatobiliary excretion. Scholars agreed on that N-terminal
chelator contains amino acids with hydrophilic (polar or charged)
side chains could reduced liver accumulation and had low
hepatobiliary excretion (18,20–23),
whereas the consists of the C-terminal was shown to be less
influential in that aspect (24).
Furthermore, validation experiments showed that Ala, Glu at the
N-terminal was associated with low hepatic uptake and low
hepatobiliary excretion (16,22,23,25). In
the present study of biodistribution, the results showed the low
hepatic uptake of 99mTc-ZHER2:V2, and the
liver hardly seen from molecular image.
Our intriguing findings were further confirmed by
the vivo research outcome. The images of nude mice bearing
HER2-overexpression SKOV3 ×enograft was seen clearly and the tumor
showed a significant contrast compared to other organs, on the
contrary, the images of nude mice bearing HER2 low expression MCF-7
×enograft showed few of 99mTc-ZHER2:V2
uptake. These demonstrated that the
99mTc-ZHER2:V2 was significantly uptake by
HER2-overexpression tumors. The vitro and vivo
blocking study showed that HER2-overexpression SKOV3 cells uptake
99mTc-ZHER2:V2 can be successfully blocked
using excess amount unlabeled ZHER2:V2. These results
further revealed binding specificity of HER2-recptors of
99mTc-ZHER2:V2. Moreover, radioactivity
accumulation in liver was limited which illustrates that using-GGGC
as a chelator at C-terminal could be reduced the hepatic
uptake.
In conclusion, 99mTc-ZHER2:V2
can be labeled simply and quickly in directly labeling method, with
good labeling yield and radiochemical purity. It is easy for
99mTc-ZHER2:V2 kit formulation.
99mTc-ZHER2:V2 can specifically and
efficiently target HER2-overexpressing tumors with promising
sensitivity and specificity. We strongly anticipate that it may be
a promising probe for clinical translation to detect
HER2-overexpression tumors.
Acknowledgements
The authors would like to acknowledge the assistance
of all coworkers involved in the study. The present study was
supported by the National Natural Science Foundation of China
(NSFC) project (81571702) and Hebei Province Government Founding
for Clinical Excellent Talent Project (2016).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goebel SU, Iwamoto M, Raffeld M, Gibril F,
Hou W, Serrano J and Jensen RT: HER-2/neu expression and gene
amplification in gastrinomas: Correlations with tumor biology,
growth and aggressiveness. J Cance Res. 62:3702–3710. 2002.
|
|
2
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen JS, Lan K and Hung MC: Strategies to
target HER2/neu overexpression for cancer therapy. Drug Resist
Updat. 6:129–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feldwisch J, Tolmachev V, Lendel C, Herne
N, Sjöberg A, Larsson B, Rosik D, Lindqvist E, Fant G,
Höidén-Guthenberg I, et al: Design of an optimized scaffold for
affibody molecules. J Mol Biol. 398:232–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Löfblom J, Feldwisch J, Tolmachev V,
Carlsson J, Ståhl S and Frejd FY: Affibody molecules: Engineered
proteins for therapeutic, diagnostic and biotechnological
applications. FEBS Lett. 584:2670–2680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tolmachev V and Orlova A: Influence of
labeling methods on biodistribution and imaging properties of
radiolabeled peptides for visualisation of molecular therapeutic
targets. Curr Med Chem. 17:2636–2655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Orlova A, Feldwisch J, Abrahmsen L and
Tolmachev V: Update: Affibody molecules for molecular imaging and
therapy for cancer. Cancer Biother Radiopharm. 22:573–584. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Price EW, Zeglis BM, Cawthray JF, Ramogida
CF, Ramos N, Lewis JS, Adam MJ and Orvig C: H(4)octapa-trastuzumab:
Versatile acyclic chelate system for 111In and 177Lu imaging and
therapy. J Am Chem Soc. 135:12707–12721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wållberg H, Grafström J, Cheng Q, Lu L,
Martinsson Ahlzén HS, Samén E, Thorell JO, Johansson K, Dunås F,
Olofsson MH, et al: HER2-positive tumors imaged within 1 hour using
a site-specifically 11C-labeled Sel-tagged affibody molecule. J
Nucl Med. 53:1446–1453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JM, Zhao XM, Wang SJ, Ren XC, Wang
N, Han JY and Jia LZ: Evaluation of 99mTc-peptide-ZHER2:342
Affibody® molecule for in vivo molecular imaging. Br J
Radiol. 87:201304842014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kramer-Marek G, Bernardo M, Kiesewetter
DO, Bagci U, Kuban M, Aras O, Zielinski R, Seidel J, Choyke P and
Capala J: PET of HER2-positive pulmonary metastases with
18F-ZHER2:342 affibody in a murine model of breast cancer:
Comparison with 18F-FDG. J Nucl Med. 53:939–946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Engfeldt T, Orlova A, Tran T, Bruskin A,
Widström C, Karlström AE and Tolmachev V: Imaging of
HER2-expressing tumours using a synthetic Affibody molecule
containing the 99mTc-chelating mercaptoacetyl-glycyl-glycyl-glycyl
(MAG3) sequence. Eur J Nucl Med Mol Imaging. 34:722–733. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wållberg H, Orlova A, Altai M,
Hosseinimehr SJ, Widström C, Malmberg J, Ståhl S and Tolmachev V:
Molecular design and optimization of 99mTc-labeled recombinant
affibody molecules improves their biodistribution and imaging
properties. J Nucl Med. 52:461–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tran T, Engfeldt T, Orlova A, Widström C,
Bruskin A, Tolmachev V and Karlström AE: In vivo evaluation of
cysteine-based chelators for attachment of 99mTc to tumor-targeting
Affibody molecules. Bioconjug Chem. 18:549–558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee H, Zheng J, Gaddy D, Orcutt KD,
Leonard S, Geretti E, Hesterman J, Harwell C, Hoppin J, Jaffray DA,
et al: A gradient-loadable (64)Cu-chelator for quantifying tumor
deposition kinetics of nanoliposomal therapeutics by positron
emission tomography. Nanomedicine. 11:155–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahlgren S, Wållberg H, Tran TA, Widström
C, Hjertman M, Abrahmsén L, Berndorff D, Dinkelborg LM, Cyr JE,
Feldwisch J, et al: Targeting of HER2-expressing tumors using a
site-specifically 99mTc-labeled recombinant Affibody molecule,
ZHER2:2395, with C-terminal engineered cysteine. J Nucl Med.
50:781–789. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orlova A, Nilsson FY, Wikman M, Widström
C, Ståhl S, Carlsson J and Tolmachev V: Comparative in vivo
evaluation of iodine and technetium labels on anti-HER2 Affibody
for single-photon imaging of HER2 expression in tumors. J Nucl Med.
47:512–519. 2006.PubMed/NCBI
|
|
18
|
Tolmachev V, Hofström C, Malmberg J,
Ahlgren S, Hosseinimehr SJ, Sandström M, Abrahmsén L, Orlova A and
Gräslund T: HEHEHE-tagged affibody molecules may be purified by
IMAC, are conveniently labeled with
[99(m)Tc(CO)3](+), and show improved
biodistribution with reduced hepatic radioactivity accumulation.
Bioconjug Chem. 21:2013–2022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahlgren S, Orlova A, Rosik D, Sandström M,
Sjöberg A, Baastrup B, Widmark O, Fant G, Feldwisch J and Tolmachev
V: Evaluation of maleimide derivative of DOTA for site-specific
labeling of recombinant Affibody molecules. Bioconjug Chem.
19:235–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tran T, Engfeldt T, Orlova A, Sandström M,
Feldwisch J, Abrahmsén L, Wennborg A, Tolmachev V and Karlström AE:
(99m)Tc-maEEE-Z(HER2:342), an Affibody molecule-based tracer for
detection of HER2 expression in malignant tumors. Bioconjug Chem.
18:1956–1964. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ekblad T, Tran T, Orlova A, Widström C,
Feldwisch J, Abrahmsén L, Wennborg A, Karlström AE and Tolmachev V:
Development and preclinical characterization of 99mTc-labeled
Affibody molecules with reduced renal uptake. Eur J Nucl Med Mol
Imaging. 35:2245–2255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tran TA, Ekblad T, Orlova A, Sandström M,
Feldwisch J, Wennborg A, Abrahmsén L, Tolmachev V and Eriksson
Karlström A: Effects of lysine-containing mercaptoacetyl-based
chelators on the biodistribution of 99mTc-labeled anti-HER2
Affibody molecules. Bioconjug Chem. 19:2568–2576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Engfeldt T, Tran T, Orlova A, Widström C,
Feldwisch J, Abrahmsen L, Wennborg A, Karlström AE and Tolmachev V:
99mTc-chelator engineering to improve tumour targeting properties
of a HER2-specific Affibody molecule. Eur J Nucl Med Mol Imaging.
34:1843–1853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tran T, Engfeldt T, Orlova A, Sandström M,
Feldwisch J, Abrahmsén L, Wennborg A, Tolmachev V and Karlström AE:
(99m)Tc-maEEE-Z(HER2:342), an Affibody molecule-based tracer for
detection of HER2-expression in malignant tumors. Bioconjug Chem.
18:1956–1964. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tran TA, Rosik D, Abrahmsén L, Sandström
M, Sjöberg A, Wållberg H, Ahlgren S, Orlova A and Tolmachev V:
Design, synthesis and biological evaluation of a HER2-specific
affibody molecule for molecular imaging. Eur J Nucl Med Mol
Imaging. 36:1864–1873. 2009. View Article : Google Scholar : PubMed/NCBI
|