Introduction
Primary malignant brain tumors have the third
highest cancer-associated mortality and morbidity rates among
individuals worldwide (1). Malignant
glioma is the most frequent intra-axial primary malignant brain
tumor (2). There are ~22,500 new
cases of primary malignant brain tumor among adults per year in the
United States, of which 70% are malignant gliomas (3). Malignant gliomas occur in all age
groups, but are most prevalent in adults aged >45 years
(4).
Gliomas can be classified as grade I to IV on the
basis histological features and genetic alterations, as defined by
the World Health Organization (WHO) (5). In general, grade I gliomas are
biologically benign and can be removed by surgical resection.
Although grade II gliomas are considered to be low-grade
malignancies, they may not be totally resectable. Grade III gliomas
are invasive and aggressive, characterized by quick progression and
poor patient outcome. Grade IV tumors, also known as glioblastoma
multiforme (GBM), are the most invasive form and are associated
with a poor prognosis (6,7). There are two types of GBM: Primary GBM
and secondary GBM. Secondary GBMs are defined as tumors that have
clinical, radiologic, or histopathological evidence of malignant
progression from a preexisting lower-grade tumor, whereas primary
GBMs have no such history and present at diagnosis as advanced
cancer (8). Despite the development
of various therapeutic strategies, including surgical resection,
radiation and adjuvant chemotherapy, the prognosis for patients
with malignant glioma remains poor; the median overall survival
time for patients with GBM is only 15 months (9). As such, the development of a more
effective therapy for malignant glioma is required.
Parsons et al (8) found that ~12% of GBM patients had a
mutation in the isocitrate dehydrogenase (IDH) 1 gene; in
>90% of these patients, this mutation was R132H. However, there
are also other IDH1 mutations at codon 132, including R132S,
R132C and R132L (10). IDH1 is
located on chromosome 2q33 and encodes the IDH1 enzyme, which
catalyzes the oxidative carboxylation of isocitrate to
α-ketoglutarate, giving rise to the generation of nicotinamide
adenine dinucleotide phosphate (NADPH). A growing body of evidence
suggests that high rates of spontaneous mutations are found in the
gene encoding cytosolic NADP+-dependent IDH1 in
glioma (11,12). Tumors without an IDH1 mutation
often exhibit a mutation at amino acid position 172 in the
mitochondrial NADP+-dependent IDH2 (R172). The
IDH2 gene is located on 15q26.1 and the most frequent
mutation is R172K (13). IDH2
mutations at R172 include R172K, R172W and R172M (14). IDH1/2 mutations are
associated with poor prognosis in glioma patients (15,16).
Mutant IDH1 may boost glioma growth and suppress glioma cell
differentiation (17). It is
therefore of considerable importance to understand the association
between IDH1/2 mutations and glioma progression.
The present study aimed to elucidate the association
between IDH1/2 mutations and glioma grades. A total
of 206 samples from patients with brain glioma and 9 samples from
patients with spinal cord glioma (as a control) were analyzed.
IDH1/2 mutations, their frequency in glioma grades
and the association between patient age and glioma grade were
examined. The data obtained here could aid in improving
understanding of the role of IDH1/2 mutations in
glioma.
Materials and methods
Tumor samples
The present study was approved by the Ethics
Committee of the Chinese People's Liberation Army No. 94 Hospital
(Nanchang, China), and all patients provided written informed
consent. Tumor tissue was obtained from human brain tumor specimens
that were diagnosed in the Neuropathology Departments of Xinqiao
Hospital of the Third Military Medical University (Chongqing,
China) and The First Affiliated Hospital of Nanchang University
(Nanchang, Jiangxi, China) between August 2011 and March 2014. The
study included 206 brain glioma samples (the mean age of the
patients was 42.06±15.21 years, 102 males and 104 females; 158 from
Xinqiao Hospital of the Third Military Medical University and 48
from The First Affiliated Hospital of Nanchang University) and 9
samples of spinal cord glioma (Department of Neurosurgery, Third
Military Medical University, Chongqing, China). The tumors were
diagnosed and graded according to the WHO classification of tumors
of the central nervous system (18).
The 206 brain glioma samples comprised 6 cases of grade I glioma,
66 cases of grade II glioma, 26 cases of grade II–III glioma, 61
cases of grade III glioma and 47 cases of grade IV glioma (43 cases
of primary GBM and 4 cases of secondary GBM). No patients received
preoperative radiotherapy, chemotherapy or other treatment. The
tumor tissue sections included 158 frozen specimens and 48
paraffinized specimens.
DNA extraction and polymerase chain
reaction (PCR) amplification for IDH1/IDH2 sequencing
Tumor areas in unstained histological sections were
manually micro-dissected using sterile scalpels. Using the EZNA
Tissue DNA kit (Omega Bio-Tek, Inc., Norcross, GA USA), DNA was
isolated from tumor tissue according to the manufacturer's
instructions.
For PCR, isolated DNA (1 µl) was added to 100 µl of
PCR reaction solution. The primer sequences were as follows:
IDH1 forward, 5′-CGGTCTTCAGAGAAGCCATT-3′; IDH1
reverse, 5′-GCAAAATCACATTATTGCCAAC-3′; IDH2 forward,
5′-CCACTATTATCTCTGTCCTC-3′; and IDH2 reverse,
5′GCTAGGCGAGGAGCTCCAGT3′. PCR with IDH1 primers generated a
129-bp product, whereas the IDH2 primers generated a 118-bp
product. PCR amplification was conducted using a SYBR Premix Taq™
kit (Takara Bio, Inc., Otsu, Japan). The reaction mixture underwent
an initial denaturation step at 95°C for 5 min, and then 40 cycles
of amplification, which consisted of 95°C denaturation for 30 sec,
60°C annealing for 30 sec, and 72°C extension for 30 sec.
IDH1/IDH2 were sequenced using a
semi-automated sequencer (Applied Biosystems 3100 Genetic Analyzer;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) as well as
Sequence Pilot version 3.1 software (JSI Medical Systems GmbH,
Ettenheim, Germany), as described previously (19).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistics analysis was performed using SPSS v.16 (SPSS, Inc.,
Chicago, IL, USA). The association between the disease grade
classification and patient age was examined by one-way analysis of
variance. A pairwise comparison of means was performed using the
least-significant difference test. The associations between the
disease grade classification and IDH1/IDH2 mutation
frequencies were evaluated using a Wilcoxon rank-sum test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Association between brain glioma grade
classification and patient age
The ages of the patients grouped according to brain
glioma grade were analyzed. The mean ages for patients with tumors
of grade I, II, II–III, III, IV (primary GBM) and IV (secondary
GBM) were 32.67±15.16, 38.55±14.24, 41.08±14.69, 43.82±15.31,
47.49±15.55 and 35.25±19.84 years, respectively (Table I). Increased glioma grades tended to
be association with increased age. Significant differences in mean
age were observed between grades I and IV (primary) glioma
(P=0.025), between grades II and III glioma (P=0.049), and between
grades II and IV (primary) glioma (P=0.003). Thus, patients with
grade IV (primary) were significantly older on average than
patients with grade I and II disease. Additionally, patients with
grade II glioma were significantly younger than those with grade
III disease.
 | Table I.Analysis of variance for the
associations between patient age and different grades of brain
glioma. |
Table I.
Analysis of variance for the
associations between patient age and different grades of brain
glioma.
|
|
| P-value for
pairwise comparison |
|---|
|
|
|
|
|---|
| Grade | Age, years (mean ±
SD), range | Grade II | Grade II–III | Grade III | Grade IV
(primary) | Grade IV
(secondary) |
|---|
| I | 32.67±15.16,
(13–48) | 0.360 | 0.218 | 0.084 | 0.025a | 0.790 |
| II | 38.55±14.24,
(7–71) | – | 0.468 | 0.049a | 0.003a | 0.671 |
| II–III | 41.08±14.69,
(26–69) | – | – | 0.437 | 0.087 | 0.471 |
| III | 43.82±15.31,
(8–86) | – | – | – | 0.221 | 0.270 |
| IV (primary) | 47.49±15.55
(13–78) | – | – | – | – | 0.121 |
| IV (secondary) | 35.25±19.84,
(25–65) | – | – | – | – | – |
IDH1/IDH2 mutation frequencies
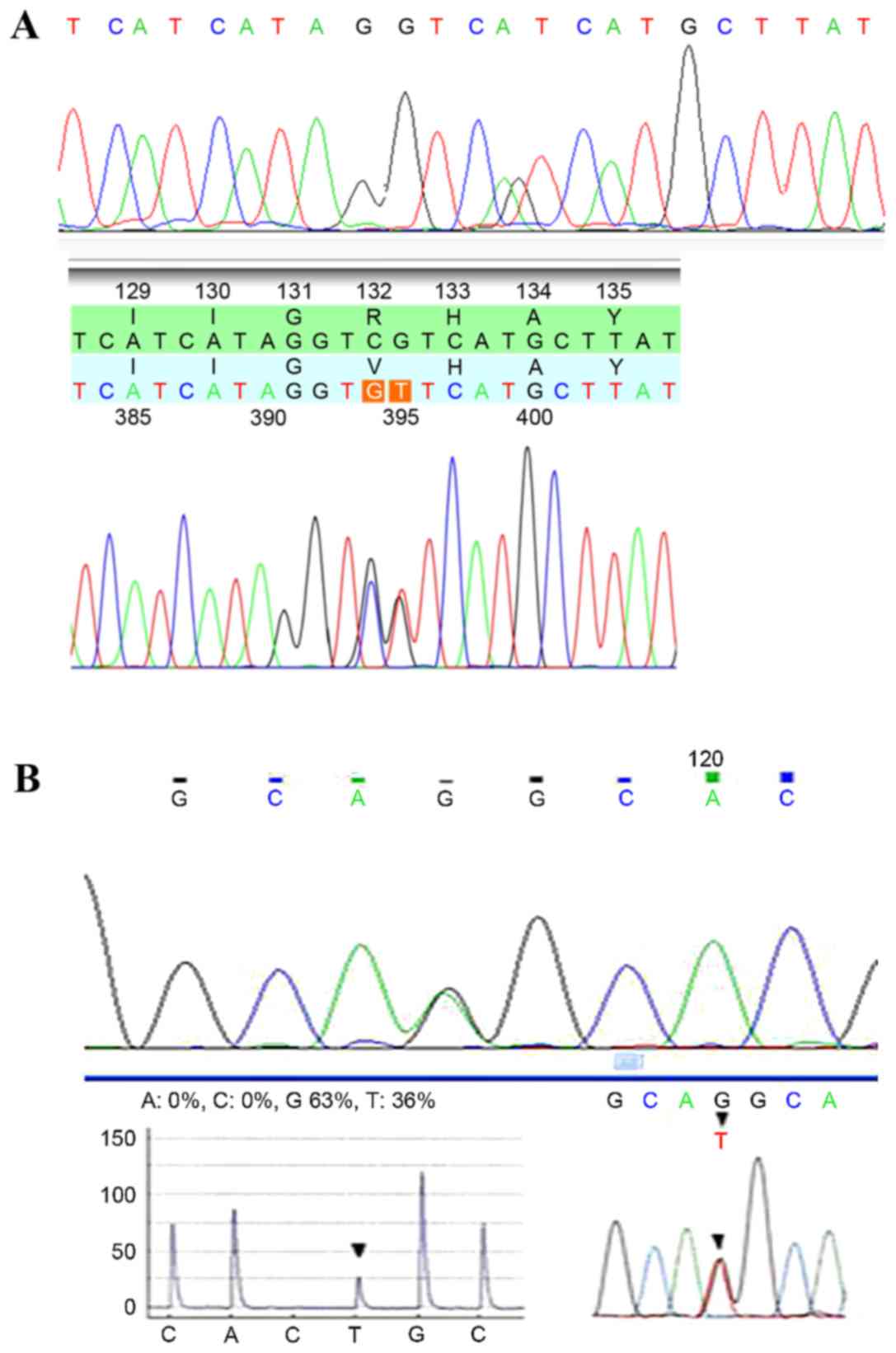
IDH1/2 mutations were detected in tissue
specimens from each patient (Fig. 1),
and the mutation frequencies were determined (Table II). No mutations in IDH1 or
IDH2 were detected in samples from patients with grade I
brain glioma (n=6). In grade II samples (n=66), IDH1
mutations were observed in 44 cases (66.67%), and IDH2
mutations were detected in 4 cases (6.06%). There were 11 instances
of IDH1 mutations (42.31%) and 4 cases of IDH2 mutations
(15.38%) among the grade II–III gliomas (n=26). Of the 61 cases of
grade III glioma, 34 and 3 cases exhibited IDH1 and
IDH2 mutations, respectively (55.74 and 4.92%). The total
frequencies of IDH1/2 mutations in grade II, II–III and III
gliomas were 72.73, 57.69 and 60.66%, respectively. The overall
IDH1/2 mutation frequency in grade II, II–III and III
gliomas was 65.36%.
 | Table II.Frequencies of IDH1 and
IDH2 mutations in brain glioma. |
Table II.
Frequencies of IDH1 and
IDH2 mutations in brain glioma.
|
| Frequency per
grade, n (%) |
|
|
|---|
| Factor | I | II | II–III | III | II, II–III and
III | IV (primary) | IV (secondary) |
Z-valuea | P-value |
|---|
| Total patients | 6 | 66 | 26 | 61 | 153 | 43 | 4 | – | – |
| IDH1
mutation | 0 (0.00) | 44 (66.67) | 11 (42.31) | 34 (55.74) | 89 (58.17) | 3 (6.98) | 2 (50.00) | – | – |
| IDH2
mutation | 0 (0.00) | 4 (6.06) | 4 (15.38) | 3 (4.92) | 11 (7.19) | 2 (4.65) | 0 (0.00) | – | – |
| IDH1/2
mutations | 0 (0.00) | 48 (72.73) | 15 (57.69) | 37 (60.66) | 100 (65.36) | 5 (11.63) | 2 (50.00) | −4.388 | <0.001 |
In the 43 samples of grade IV primary GBM, there
were 3 and 2 cases that exhibited mutations in IDH1 (6.98%)
and IDH2 (4.65%), respectively. By contrast, in the 4
samples of grade IV secondary GBM, there were 2 IDH1
mutations (50.00%) and no IDH2 mutations observed. The total
frequencies of IDH1/2 mutations for grade IV primary
GBM and grade IV secondary GBM were 11.63 and 50.00%,
respectively.
It should be noted that IDH1 and IDH2
mutation frequencies in grade II, II–III and III brain gliomas were
higher than those in grades I and IV. By contrast, no mutations in
IDH1 or IDH2 were observed in any of the 9 spinal
cord gliomas. These data suggested that IDH1 and IDH2
mutations were more likely to occur in grade II, II–III and III
brain gliomas.
Association between IDH1/2 mutation
frequencies and brain glioma grade
Next, the association between
IDH1/IDH2 mutation frequency and brain glioma grade
was analyzed. Wilcoxon rank sum test revealed that IDH1/2
mutation frequencies differed significantly between the different
grades of brain glioma (Z=−4.388, P<0.001; Table II). The frequency of IDH1/2
mutations was higher in grade II gliomas than in grade II–III and
III gliomas, yet was higher in grade III than in grade II–III
gliomas. The data suggested that IDH1/2 mutations were
associated with grade II, II–III and III brain gliomas, and may be
involved with the progression of brain glioma from grade II to
III.
Discussion
Malignant glioma remains a considerable threat to
human health worldwide, and the prognosis of patients with
high-grade malignant glioma is poor. Since IDH1/2
mutations have been detected in a number of GBM patients (9), the functions of IDH1/2
mutations in glioma are of interest. The present study found that
IDH1/2 mutations were frequent in grade II, II–III
and III brain gliomas. Furthermore, IDH1/IDH2
mutation frequencies were significantly different among grade II,
II–III and III, suggesting the mutations may be associated with the
progression of brain glioma from grade II to grade III.
IDH1/2-mutant tumors are reported to
primarily occur in adolescents rather than younger children or
adults (19,20). However, the present study found that
the mean ages of patients with grade II, II–III and III disease
were 38.55±14.24, 41.08±14.69 and 43.82±15.31 years, respectively,
suggesting that the majority of IDH1/2-mutated
gliomas occurred in adults. Ethnic and regional differences between
study groups may explain these paradoxical results, while limited
sample size could be another reason. Increasing glioma grade
appeared to be somewhat correlated with increasing patient age. On
average, patients with grade IV (primary) gliomas were
significantly older than those with grade I and II gliomas, and
patients with grade III gliomas were significantly older than those
with grade II gliomas. However, a larger sample size is required to
validate the results of the present study.
There is growing interest in the frequency of
IDH1/2 mutations in different types of glioma (21). A few studies have reported on
IDH1/2 mutation frequencies in different grades of
oligodendroglioma and astrocytoma (19,22,23).
Unlike these studies, the present research specifically focused on
the association between IDH1/2 mutation frequency and
different grades of brain glioma, regardless of the
histopathological type. IDH1/2 mutations occurred
more often in grade II, II–III and III gliomas (72.73, 57.69 and
60.66%, respectively) than in grade I or IV gliomas. The overall
IDH1/2 mutation frequency for grades II, II–III and
III was 65.36%. No mutations were detected in grade I gliomas. The
present results revealed that IDH1/IDH2 mutation frequencies
were significantly different between glioma grades II, II–III and
III, suggesting that the mutations may be associated with the
progression of brain gliomas from grade II to grade III.
A previous study examining 321 gliomas revealed that
IDH1 mutations were present in 82% of patients with
secondary GBM (n=34), but only 5% of patients with primary GBM
(n=59) (24). Similarly, the present
study also revealed a sharp discrepancy in IDH1 mutation
frequency between secondary and primary GBM. IDH1 mutation
was detected in 2 (50.00%) of 4 secondary GBM cases, and 3 (6.98%)
of 43 primary GBM cases. The discrepancy in IDH1 mutation
frequency in the same GBM types between the two studies may be
attributed to limited sample size or differences in ethnicity.
The IDH1 and IDH2 enzymes are localized in the
cytoplasm, peroxisomes and mitochondria. IDH1/2 play a part in the
conversion of isocitrate to α-ketoglutarate and block the reduction
of NADP+ to NADPH. These enzymes also protect the cell
against oxidative stress. There is evidence that IDH1 (R132)
mutation is an event shared by all recurrent gliomas early in
tumorigenesis (23). Certain studies
have reported that gliomas with mutations in one of the IDH
genes have a better patient outcome than those with other gene
mutations (15,16), and IDH1/2 mutations are
recognized as positive prognostic biomarkers (25,26). The
present study found that IDH1/2 mutations were most
frequent in grade II, II–III and III gliomas. It is therefore
reasonable to speculate that patients with gliomas of these grades
with IDH1/2 mutations have a better prognosis than
those without these mutations. The mutations may increase the
survival of patients by enhancing cellular oxidative stress and
reducing NADPH levels (10,27). Nevertheless, the large quantity of
2-hydroxyglutarate (2-HG) that is produced concomitantly by
IDH mutant enzymes could facilitate malignant progression
(28,29). However, the negative effect of 2-HG
may be abrogated by the beneficial effect of IDH mutations.
These findings indicate that mutant IDH enzymes may affect
multiple pathways in glioma, which coordinate to influence patient
prognosis.
The present study is a preliminary one. A larger
sample size is required to validate the results of this study, and
more studies to identify molecular targets and pathways that are
influenced by IDH1/2 mutations are underway. Further
information regarding the underlying mechanisms of these mutations
in glioma is therefore expected in the future.
In summary, the present study provides further
information regarding the role of IDH1/IDH2 mutations
in brain gliomas in Chinese patients. IDH1/2
mutations are associated with grade II, II–III and III brain
gliomas, and are possibly involved with the progression of brain
gliomas from grade II to grade III. The majority of
IDH1/2-mutant brain gliomas affected adults, rather
than adolescents. Further studies are required to clarify the
precise roles of these mutations in brain gliomas.
Acknowledgements
Not applicable.
Funding
This study work was supported by a grant from the
Natural Science Foundation of China (grant no. H1618).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
LD and PX were responsible for conception and design
of the research and drafting of the manuscript. YL and SL performed
data acquisition. XB and WZ were responsible tor data analysis and
interpretation. SL performed statistical analysis. SQ and SL
conducted the experiments. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cha S: Perfusion MR imaging of brain
tumors. Top Magn Reson Imaging. 15:279–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altieri R, Agnoletti A, Quattrucci F,
Garbossa D, Calamo Specchia FM, Bozzaro M, Fornaro R, Mencarani C,
Lanotte M, Spaziante R and Ducati A: Molecular biology of gliomas:
Present and future challenges. Transl Med UniSa. 10:29–37.
2014.PubMed/NCBI
|
|
3
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 15
Suppl 2:ii1–ii56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Porter KR, McCarthy BJ, Freels S, Kim Y
and Davis FG: Prevalence estimates for primary brain tumors in the
United States by age, gender, behavior, and histology. Neuro Oncol.
12:520–527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parsons DW, Jones S, Zhang X, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et
al: An integrated genomic analysis of human glioblastoma
multiforme. Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Labussiere M, Sanson M, Idbaih A and
Delattre JY: IDH1 gene mutations: A new paradigm in glioma
prognosis and therapy? Oncologist. 15:196–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohrenz IV, Antonietti P, Pusch S, Capper
D, Balss J, Voigt S, Weissert S, Mukrowsky A, Frank J, Senft C, et
al: Isocitrate dehydrogenase 1 mutant R132H sensitizes glioma cells
to BCNU-induced oxidative stress and cell death. Apoptosis.
18:1416–1425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balss J, Meyer J, Mueller W, Korshunov A,
Hartmann C and von Deimling A: Analysis of the IDH1 codon 132
mutation in brain tumors. Acta Neuropathol. 116:597–602. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ichimura K, Pearson DM, Kocialkowski S,
Bäcklund LM, Chan R, Jones DT and Collins VP: IDH1 mutations are
present in the majority of common adult gliomas but rare in primary
glioblastomas. Neuro Oncol. 11:341–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1 and IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horbinski C: What do we know about IDH1/2
mutations so far, and how do we use it? Acta Neuropathol.
125:621–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song Tao Q, Lei Y, Si G, Yan Qing D, Hui
Xia H, Xue Lin Z, LanXiao W and Fei Y: IDH mutations predict longer
survival and response to temozolomide in secondary glioblastoma.
Cancer Sci. 103:269–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zou P, Xu H, Chen P, Yan Q, Zhao L, Zhao P
and Gu A: IDH1/IDH2 mutations define the prognosis and molecular
profiles of patients with gliomas: A meta-analysis. PLoS One.
8:e687822013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rohle D, Popovici-Muller J, Palaskas N,
Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B,
Komisopoulou E, et al: An inhibitor of mutant IDH1 delays growth
and promotes differentiation of glioma cells. Science. 340:626–630.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cohen N and Weller RO: Who classification
of tumours of the central nervous system. Wiley Online Library;
2007
|
|
19
|
Hartmann C, Meyer J, Balss J, Capper D,
Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, et
al: Type and frequency of IDH1IDH2 mutations are related to
astrocytic and oligodendroglial differentiation and age: A study of
1,010 diffuse gliomas. Acta Neuropathol. 118:469–474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pollack IF, Hamilton RL, Sobol RW,
Nikiforova MN, Lyons-Weiler MA, LaFramboise WA, Burger PC, Brat DJ,
Rosenblum MK, Holmes EJ, et al: IDH1 mutations are common in
malignant gliomas arising in adolescents: A report from the
children's oncology group. Childs Nerv Syst. 27:87–94. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Megova M, Drabek J, Koudelakova V,
Trojanec R, Kalita O and Hajduch M: Isocitrate dehydrogenase 1 and
2 mutations in gliomas. J Neurosci Res. 92:1611–1620. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hartmann C, Hentschel B, Wick W, Capper D,
Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch
T, et al: Patients with IDH1 wild type anaplastic astrocytomas
exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1
mutation status accounts for the unfavorable prognostic effect of
higher age: Implications for classification of gliomas. Acta
Neuropathol. 120:707–718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lass U, Nümann A, von Eckardstein K, Kiwit
J, Stockhammer F, Horaczek JA, Veelken J, Herold-Mende C, Jeuken J,
von Deimling A and Mueller W: Clonal analysis in recurrent
astrocytic, oligoastrocytic and oligodendroglial tumors implicates
IDH1-mutation as common tumor initiating event. PLoS One.
7:e412982012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe T, Nobusawa S, Kleihues P and
Ohgaki H: IDH1 mutations are early events in the development of
astrocytomas and oligodendrogliomas. Am J Pathol. 174:1149–1153.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Metellus P, Coulibaly B, Colin C, de Paula
AM, Vasiljevic A, Taieb D, Barlier A, Boisselier B, Mokhtari K,
Wang XW, et al: Absence of IDH mutation identifies a novel
radiologic and molecular subtype of WHO grade II gliomas with
dismal prognosis. Acta Neuropathol. 120:719–729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gorlia T, Delattre JY, Brandes AA, Kros
JM, Taphoorn MJ, Kouwenhoven MC, Bernsen H, Frénay M, Tijssen CC,
Lacombe D and van den Bent MJ: New clinical, pathological and
molecular prognostic models and calculators in patients with
locally diagnosed anaplastic oligodendroglioma or oligoastrocytoma.
A prognostic factor analysis of European organisation for research
and treatment of cancer brain tumour group study 26951. Eur J
Cancer. 49:3477–3485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li S, Chou AP, Chen W, Chen R, Deng Y,
Phillips HS, Selfridge J, Zurayk M, Lou JJ, Everson RG, et al:
Overexpression of isocitrate dehydrogenase mutant proteins renders
glioma cells more sensitive to radiation. Neuro Oncol. 15:57–68.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dang L, White DW, Gross S, Bennett BD,
Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et
al: Cancer-associated IDH1 mutations produce 2-hydroxyglutarate.
Nature. 462:739–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin G, Reitman ZJ, Spasojevic I,
Batinic-Haberle I, Yang J, Schmidt-Kittler O, Bigner DD and Yan H:
2-hydroxyglutarate production, but not dominant negative function,
is conferred by glioma-derived NADP+-dependent
isocitrate dehydrogenase mutations. PLoS One. 6:e168122011.
View Article : Google Scholar : PubMed/NCBI
|