Introduction
Gastric cancer (GC) is one of the most common
malignant tumors in the digestive track and a leading cause of
cancer death in the world (1). In
China, according to cancer statistics, the estimated incidence rate
for GC was 679.1 per 100,000 people per year, and the mortality
rate was ~498.0 per 100,000 people per year, while over 60%
patients were in the advanced stage (2). Since the prognosis for GC patients is
very poor, many molecular markers, including HER2, E-cadherin,
EGFR, and KRAS have been evaluated as candidate prognostic factors
for GC (3,4).
RecQ helicases are a group of DNA unwinding enzymes
that participate in the process of DNA repair (5). RecQ helicases play an important role in
maintaining genome stability, replication, recombination, and
transcription (5,6). The RecQ helicase family has five members
in human cells: RECQL1, WRN, BLM, RECQL4, and RECQL5. Loss of RecQ
helicase protein expression can induce genomic instability and
predisposition to cancers (7,8). RecQ protein-like 4 (RECQL4) is a key
member of the RecQ family and plays an important role in the
initiation of DNA replication, progression of stalled replication
forks, and telomere maintenance, as well as in the repair of DNA
double-strand breaks via the homologous recombination pathway
(9,10). Mutations of the RECQL4 gene are
associated with the rare type II Rothmund-Thomson syndrome, which
has a propensity for osteosarcomas (11,12).
Recent studies have shown that RECQL4 acts as a tumor-promotor in
some cancers, such as osteosarcomas, prostate cancer, colorectal
cancer, and breast cancer (13–17).
Moreover, depletion of RECQL4 has been shown to significantly
reduce the proliferation of cancer cells, promote apoptosis, and
impair tumorgenicity in tumor-bearing mice (13,15).
However, the expression of RECQL4 in GC and its clinical and
prognostic significance have been rarely mentioned.
Therefore, in this study, we aimed to compare the
expression of RECQL4 in GC tissues with matched normal gastric
tissues and to evaluate its prognostic significance in GC patients,
by using bioinformatics prediction methodology combined with
immunohistochemical validation.
Materials and methods
Bioinformatics prediction
The expression of RECQL4 in GC was examined via the
online Oncomine database (www.onocomine.org). The following filter combination
was applied to analyze corresponding datasets to demonstrate the
differences between RECQL4 expression in GC and normal tissues. The
data type was set as mRNA, and the analysis type was set as cancer
vs. normal analysis. Each filtered dataset was analyzed separately.
Differences in RECQL4 expression between different types of GC and
normal tissues were compared by using datasets including Cho
Gastric, DErrico Gastric, Chen Gastric and Wang Gastric. The
log-transformed and normalized expression values of RECQL4 were
abstracted, analyzed, and read from a scatter plot. We performed
Kaplan-Meier survival analysis of RECQL4 by using an online tool
(http://kmplot.com/analysis/). Through
the Kaplan-Meier plotter database, we were able to assess the
effects of 54,675 genes on survival by using 10,188 cancer samples,
including breast, lung, ovarian, and gastric cancers (18). RECQL4 expression and survival data
from Affymetrix microarray, including 1,065 GC patients
(ID:213520_at), were also analyzed. To analyze the prognostic value
of the RECQL4 gene, the samples were divided into two patient
groups according to the median expression levels of RECQL4 (high
and low). Levels of expression between the two patient groups were
compared via a Kaplan-Meier survival plot. The hazard ratio (HR)
with 95% confidence intervals (CIs) and the log rank P-value were
computed.
Tissue samples and clinicopathological
data
GC samples and matched normal gastric tissues from
60 patients who had undergone initial surgical resection between
August 2008 and January 2009 were selected from the Department of
Gastrointestinal Surgery at the Sixth Affiliated Hospital of Sun
Yat-sen University (Guangzhou, China). All samples were collected
with the respective patients' informed consent after approval from
the Institute Research Medical Ethics Committee of the Sixth
Affiliated Hospital of Sun Yat-sen University.
Immunohistochemical analysis
All specimens had previously been fixed in 10%
buffered formalin and embedded in paraffin wax.
Immunohistochemistry staining was performed according to the
manufacturer's instructions by using the rabbit polyclonal antibody
against human RECQL4 (H00009401-M09; dilution rate: 1:25, Abnova;
Taipei City, China). The protein expression level of RECQL4 was
then evaluated by microscopic examination of the stained tissue
slides. RECQL4 expression level was determined by visual
immunoreactive score (IRS), which was generated by staining
intensity (SI) × number of stained cells). The SI was scored as
follows: Negative (score 0), weak (score 1), moderate (score 2),
and strong (score 3). We scored the staining extent according to
the percentage of positively stained tumor cells in the field:
Negative (score 0), 0–25% (score 1), 26–50% (score 2), 51–75%
(score 3), and >76% (score 4). If the IRS score was >4, the
expression of RECQL4 was defined as high, and an IRS score of ≤4
was defined as low or none.
Statistical analysis
All statistical analyses were performed with SPSS
17.0 software (SPSS, Chicago, IL, USA). The association between
RECQL4 protein expression and clinicopathological features was
analyzed by using the chi square test. Survival rate was calculated
by using the Kaplan Meier method, and the difference was determined
by the log-rank test. Multivariate analysis by using the Cox
proportional hazards regression model was performed to identify the
independent prognostic factors. P<0.05 was considered to
indicate a statistically significant difference.
Results
Overexpression of RECQL4 mRNA and
protein levels in GC
By using Oncomine database mining, we examined and
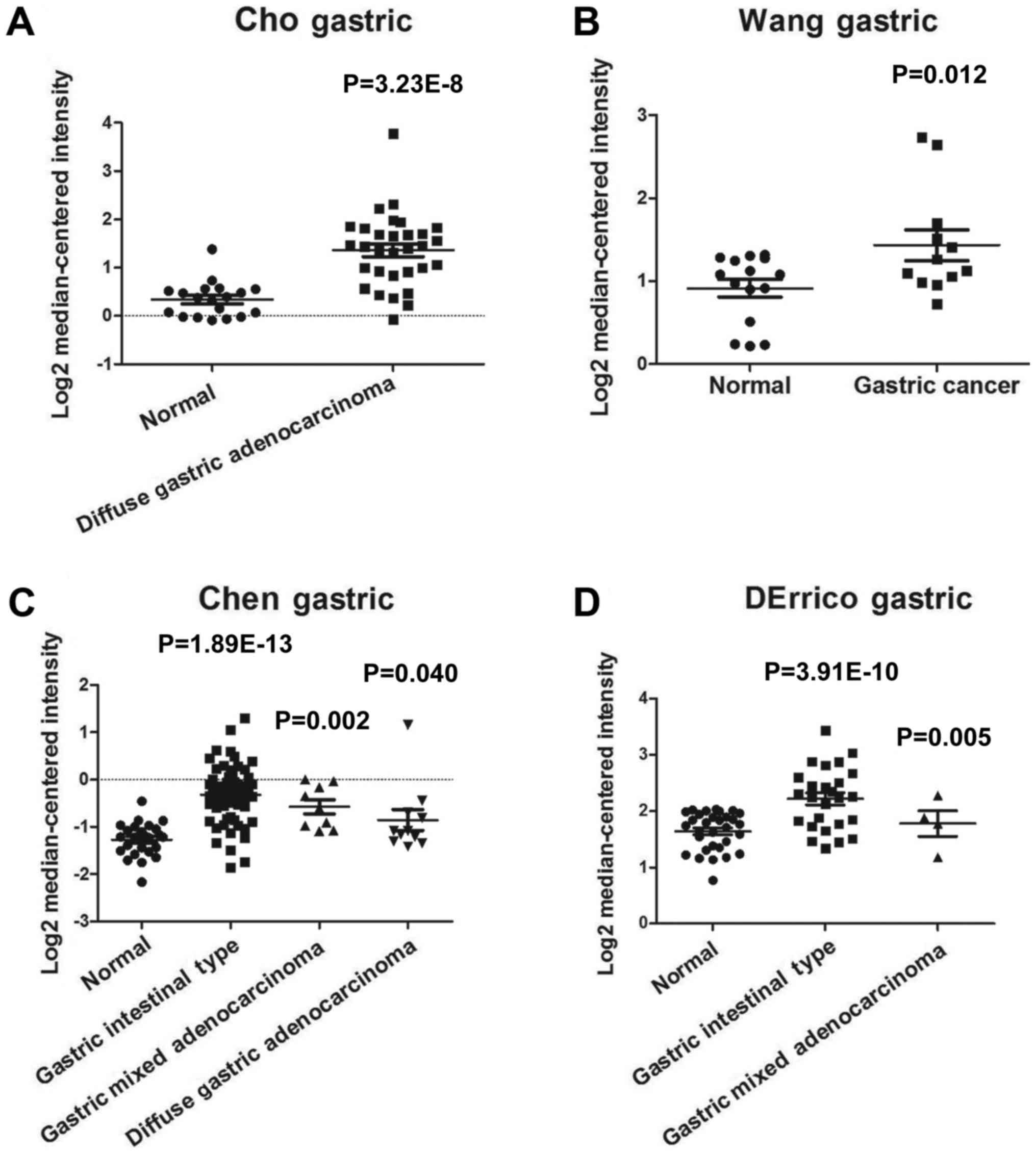
analyzed the expression levels of RECQL4 in GC tissues. As shown in
Fig. 1, expression of RECQL4 was
significantly elevated in GC tissues (n=19, P=3.23E-8) as compared
with that in normal tissues (n=31) by using the Cho Gastric dataset
(Fig. 1A). It also demonstrated that
RECQL4 expression was significantly increased in the Wang Gastric
dataset (n=27, P=0.012, Fig. 1B). The
Chen Gastric dataset showed that RECQL4 expression in gastric
intestinal type adenocarcinoma (n=63, P=1.89E-13), gastric mixed
adenocarcinoma (n=8, P=0.002), and diffuse gastric adenocarcinoma
(n=12, P=0.040) was higher than that in normal tissues (n=26,
P=0.01) (Fig. 1C). DErrico Gastric
dataset revealed that RECQL4 was upregulated in gastric intestinal
type adenocarcinoma (n=26, P=3.91E-10) and gastric mixed
adenocarcinoma (n=4, P=0.005) as compared with that in normal colon
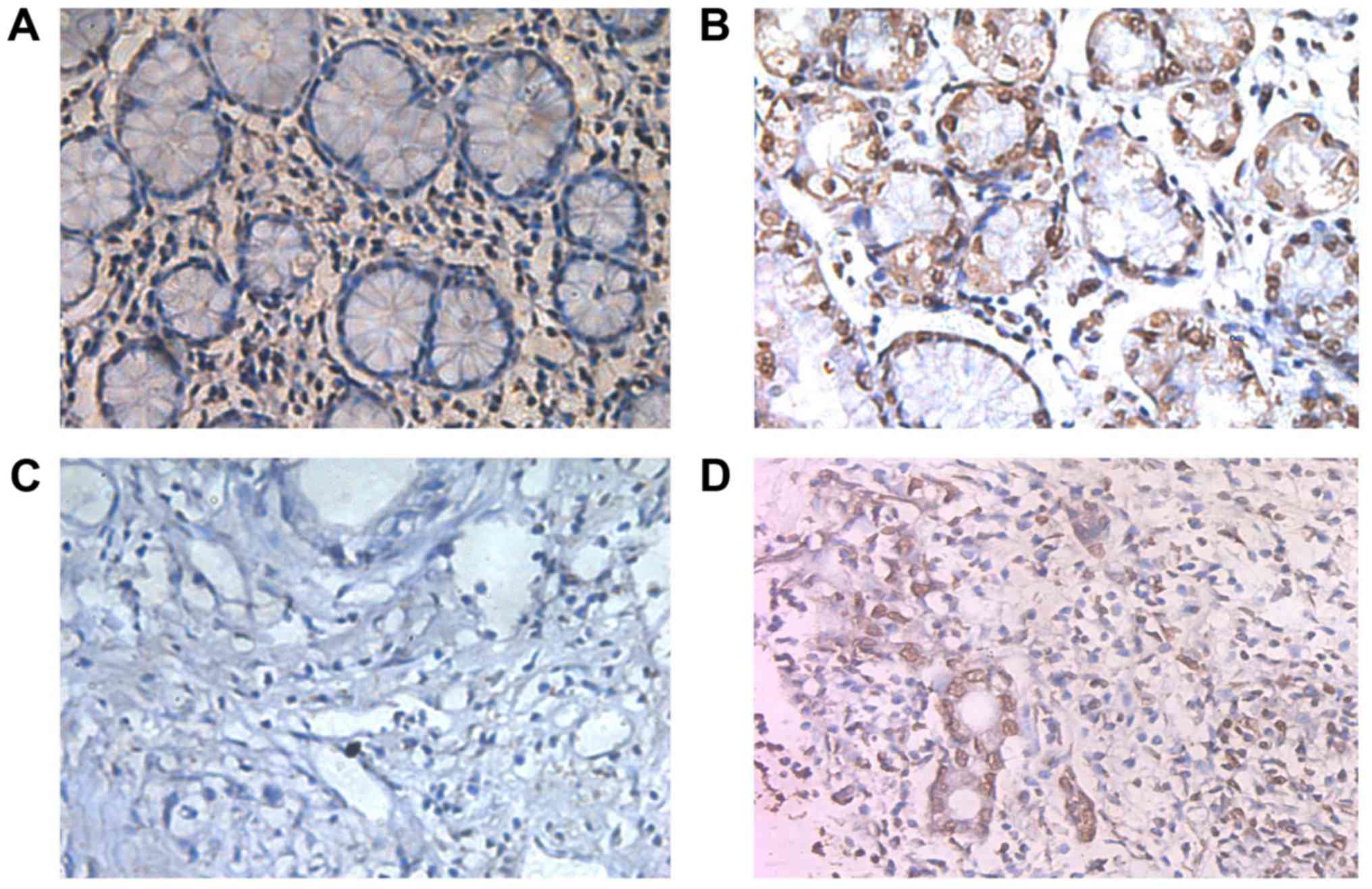
tissues (Fig. 1D). To verify the
above predictions, immunohistochemical analysis showed that RECQL4
positivity was clearly localized in the nuclei of GC cells and some
was found in the cytoplasm (Fig. 2).
The positive rate of RECQL4 in the GC samples was significantly
higher than that of the normal gastric mucosa specimens (P<0.05;
Table I).
 | Table I.Expression of RECQL4 in gastric tumor
and normal gastric tissues. |
Table I.
Expression of RECQL4 in gastric tumor
and normal gastric tissues.
| Samples | High (%) | Low or none (%) | P-value |
|---|
| Gastric cancer | 33 (55.0) | 27 (45.0) | 0.0004 |
| Normal gastric
tissue | 14 (23.3) | 46 (76.7) |
|
Association between RECQL4
differential expression and clinicopathological parameters of
patients with GC
RECQL4 expression was positively associated with
depth of invasion and TNM (P<0.05), but not with the patients'
sex or age, tumor size, tumor location, histological
differentiation, lymphatic or venous invasion, lymph node
metastasis, or distant metastasis (P>0.05; Table II).
 | Table II.Association between RECQL4 expression
and clinicopathological features of gastric carcinomas. |
Table II.
Association between RECQL4 expression
and clinicopathological features of gastric carcinomas.
|
|
| RECQL4 protein
expression (%) |
|
|---|
|
|
|
|
|
|---|
| Characteristics | No. of patients | Positive | Negative | P-value |
|---|
| Sex |
|
|
| 0.165 |
| Male | 39 | 24 (61.5) | 15 (38.5) |
|
|
Female | 21 | 9 (42.9) | 12 (57.1) |
|
| Age, years |
|
|
| 0.706 |
| ≥60 | 37 | 20 (54.05) | 17 (45.95) |
|
|
<60 | 23 | 13 (56.52) | 10 (43.48) |
|
| Location |
|
|
| 0.430 |
| Upper
third | 21 | 13 (61.9) | 8 (38.1) |
|
| Middle
and lower third | 39 | 20 (51.3) | 19 (48.7) |
|
| Tumor size, cm |
|
|
| 0.653 |
| ≥5 | 15 | 9 (60.0) | 6 (40.0) |
|
|
<5 | 45 | 24 (53.3) | 21 (46.7) |
|
| Histological
differentiated |
|
|
| 0.454 |
|
Well/moderate | 15 | 7 (46.7) | 8 (53.3) |
|
|
Poorly/other | 45 | 26 (57.8) | 19 (42.2) |
|
| Depth of
invasion |
|
|
| 0.035 |
|
T1-T2 | 18 | 6 (33.3) | 12 (66.7) |
|
|
T3-T4 | 43 | 27 (62.8) | 16 (37.2) |
|
| Vascular
invasion |
|
|
| 0.340 |
|
Yes | 24 | 15 (62.5) | 9 (37.5) |
|
| No | 36 | 18 (50.0) | 18 (50.0) |
|
| Lymphatic
invasion |
|
|
| 0.306 |
|
Yes | 22 | 14 (63.6) | 8 (36.4) |
|
| No | 38 | 19 (50.0) | 19 (50.0) |
|
| Lymph node
metastases |
|
|
| 0.172 |
| N0 | 19 | 8 (42.1) | 11 (57.9) |
|
|
N1/N2 | 41 | 25 (61.0) | 16 (39.0) |
|
| Distant
metastasis |
|
|
| 0.925 |
| M0 | 47 | 26 (55.3) | 21 (44.7) |
|
| M1 | 13 | 7 (53.8) | 6 (46.2) |
|
| TNM |
|
|
| 0.028 |
|
I–II | 20 | 7 (35.0) | 13 (65.0) |
|
|
III–IV | 40 | 26 (65.0) | 14 (35.0) |
|
Prognostic value of RECQL4 expression
in patients with GC
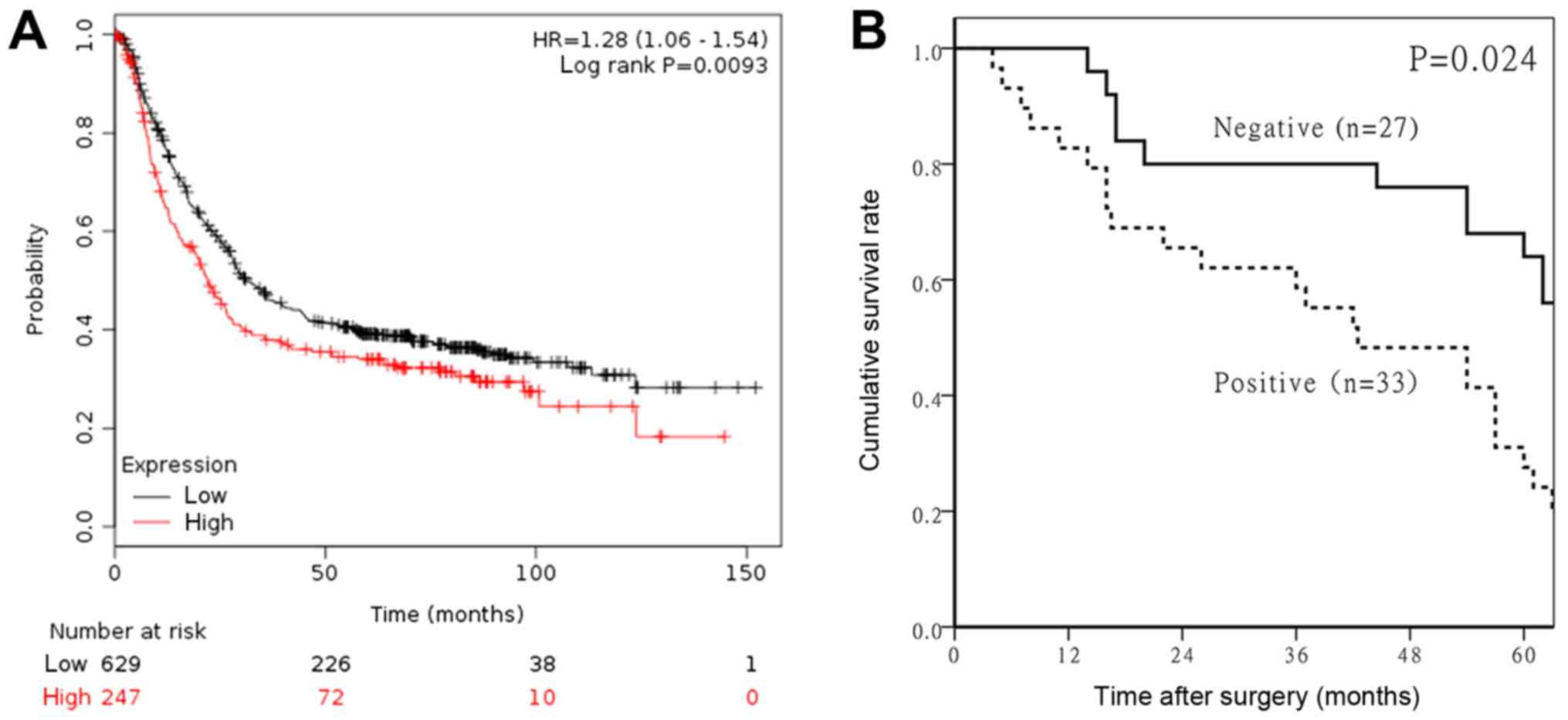
We analyzed the association between RECQL4 mRNA
expression levels and overall survival (OS) in GC patients by using
the Kaplan-Meier plotter online software (http://kmplot.com/analysis/) based on a public
database. We found that the OS of patients with low expression of
RECQL4 was remarkably longer than of patients with high expression
(HR=1.28, 95% CI=1.06–1.54, P=0.0093, Fig. 3A). To verify the above result, the
prognostic value of RECQL4 expression in patients with GC was
performed by using immunohistochemical data. Follow-up information
was available for all of the gastric carcinoma patients for periods
ranging between 0.2 months and 10.2 years (mean 65.1 months). The
overall 5-year survival rate of the study participants was 53.3%
(32/60). The 5-year survival rate of the RECQL4-negative and
RECQL4-positive groups was 66.7% (18/27) and 42.4% (14/33),
respectively. The OS of patients with GC with high expression of
RECQL4 protein was significantly less than that of patients with
low expression (P=0.000) (Fig. 3B).
Univariate analysis using Cox's proportional hazard model indicated
that the RECQL4 expression, depth of invasion, lymphatic invasion,
and TNM staging were independent prognostic factors for GCs
(P<0.05; Table III).
 | Table III.Multivariate analysis on overall
survival (Cox regression model). |
Table III.
Multivariate analysis on overall
survival (Cox regression model).
|
Characteristics | Hazard ratio | 95% CI | P-value |
|---|
| RECQL4 (+) | 1.227 | 1.062–1.522 | 0.009 |
| Depth of invasion
(T3-T4) | 2.956 | 1.035–8.443 | 0.043 |
| Lymph node
metastases (+) | 3.629 | 1.848–7.126 | 0.022 |
| TNM (III–IV) | 0.309 | 1.845–6.982 | 0.007 |
Discussion
RECQL4 has been reported to be essential for the
maintenance of genomic stability (19). As a key factor of the DNA unwinding
helicase, RECQL4 is involved in cell processes (20). A tumor-promoting function of RECQL4
has been widely described (21). For
example, Fang et al (13)
found that overexpression of RecQL4 due to gene amplification plays
a critical role in human breast tumor progression, and Arora et
al (22) demonstrated that
shRNA-mediated RECQL4 suppression in MDA-MB453 breast cancer cells
significantly inhibited in vitro clonogenic survival and
in vivo tumorigenicity. Su et al (15) found that elevation of RECQL4 level was
positively associated with the aggressiveness of prostate cancer
both in vitro and in vivo, implying that RECQL4 plays
critical roles in prostate-cancer carcinogenesis and is a valuable
biomarker for this cancer. However, the expression level of RECQL4
in GC, and its prognostic significance remains controversial.
Oncomine is the largest available cancer microarray
database. In the present study, we used data mining of the
independent microarray datasets (Cho Gastric, DErrico Gastric, Chen
Gastric, Wang Gastric, and Cui Gastric) within the Oncomine
database and demonstrated the overexpression of RECQL4 in GC. To
verify the above results, we investigated the expression of RECQL4
protein levels in GC tissues. We observed that RECQL4 was localized
mainly in the nucleus and some was found in the cytoplasm-results
similar to those of other studies (23,24).
RECQL4 was expressed in 55.0% of GC samples and 23.3% of matched
normal gastric mucosal tissues. Moreover, RECQL4 expression was
positively associated with depth of invasion, TNM staging, and
survival times, but not with aggressive parameters such as lymph
node metastasis and differentiation. These results are consistent
with bioinformatics predictions and suggest that RECQL4 may be a
critical factor in promoting the development of GC.
To date, few studies have evaluated the relationship
between RECQL4 expression and prognosis of cancer patients. Online
Kaplan-Meier plotter analysis proved that RECQL4 predicts a poorer
prognosis rate in GC patients. In our present study of GC, the
5-year survival rate of the RECQL4-negative group was higher than
that of the RECQL4-positive group. Survival curves showed that
cumulative survival rate was significantly higher in the
RECQL4-negative group. Thus, RECQL4 expression was shown to be a
potential prognostic factor in survival analysis. Multivariate
analysis also showed that high expression of RECQL4 was an
independent factor predicting low OS in GC. Taken together, these
observations indicate that RECQL4 may be a predictor of poor
prognosis for GC patients.
Several limitations should be mentioned for this
study. First, a selection bias may exist due to the non-sequential
sample collections. Second, due to the limited sample size, future
studies with larger sample sizes are needed to verify these
results. Third, further investigations are needed to determine the
molecular mechanisms behind the relationship between RECQL4
expression and gastric adenocarcinoma.
In summary, we have shown that RECQL4 is
overexpressed in GC samples, and, therefore, suggest that elevated
RECQL4 protein expression is an independent factor for poor
prognosis in patients with gastric adenocarcinoma and other GCs.
The results of this study provide important implications for
improving future treatment strategies for these cancers.
Acknowledgements
Not applicable.
Funding
The present study was funded by Guangdong province
science and technology plan project (received by XW; grant no.
2017A010105004) and Guangdong province medical science and
technology research fund project (received by HC; grant no.
A2017273).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC, XBW and JP designed this study; HC and KY
analyzed and interpreted the patient data, and were major
contributors in writing the manuscript. XYW analyzed and
interpreted the patient data. HW and QW performed the histological
examination of the samples, and were major contributors in writing
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The study was approved by the Ethics Committee of the
Sixth Affiliated Hospital, Sun Yat-sen University and have been
performed in accordance with the Declaration of Helsinki. Written
informed consent was obtained from all individual participants
included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W: Cancer statistics: Updated cancer
burden in China. Chin J Cancer Res. 27:12015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen GS, Zhao JD, Zhao JH, Ma XF, Du F,
Kan J, Ji FX, Ma F, Zheng FC, Wang ZY and Xu BH: Association of
HER2 status with prognosis in gastric cancer patients undergoing R0
resection: A large-scale multicenter study in China. World J
Gastroenterol. 22:5406–5414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mahu C, Purcarea AP, Gheorghe CM and
Purcarea MR: Molecular events in gastric carcinogenesis. J Med
Life. 7:375–378. 2014.PubMed/NCBI
|
|
5
|
Bernstein KA, Gangloff S and Rothstein R:
The RecQ DNA helicases in DNA repair. Annu Rev Genet. 44:393–417.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta S, De S, Srivastava V, Hussain M,
Kumari J, Muniyappa K and Sengupta S: RECQL4 and p53 potentiate the
activity of polymerase γ and maintain the integrity of the human
mitochondrial genome. Carcinogenesis. 35:34–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Croteau DL, Singh DK, Hoh Ferrarelli L, Lu
H and Bohr VA: RECQL4 in genomic instability and aging. Trends
Genet. 28:624–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Croteau DL, Popuri V, Opresko PL and Bohr
VA: Human RecQ helicases in DNA repair, recombination, and
replication. Annu Rev Biochem. 83:519–552. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chi Z, Nie L, Peng Z, Yang Q, Yang K, Tao
J, Mi Y, Fang X, Balajee AS and Zhao Y: RecQL4 cytoplasmic
localization: Implications in mitochondrial DNA oxidative damage
repair. Int J Biochem Cell Biol. 44:1942–1951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh DK, Popuri V, Kulikowicz T, Shevelev
I, Ghosh AK, Ramamoorthy M, Rossi ML, Janscak P, Croteau DL and
Bohr VA: The human RecQ helicases BLM and RECQL4 cooperate to
preserve genome stability. Nucleic Acids Res. 40:6632–6648. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Capp C, Wu J and Hsieh TS: RecQ4: The
second replicative helicase? Crit Rev Biochem Mol Biol. 45:233–242.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin J, Kwon YT, Varshavsky A and Wang W:
RECQL4, mutated in the Rothmund-Thomson and RAPADILINO syndromes,
interacts with ubiquitin ligases UBR1 and UBR2 of the N-end rule
pathway. Hum Mol Genet. 13:2421–2430. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang H, Nie L, Chi Z, Liu J, Guo D, Lu X,
Hei TK, Balajee AS and Zhao Y: RecQL4 helicase amplification is
involved in human breast tumorigenesis. PLoS One. 8:e696002013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lao VV, Welcsh P, Luo Y, Carter KT,
Dzieciatkowski S, Dintzis S, Meza J, Sarvetnick NE, Monnat RJ Jr,
Loeb LA and Grady WM: Altered RECQ helicase expression in sporadic
primary colorectal cancers. Transl Oncol. 6:458–469. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su Y, Meador JA, Calaf GM, Proietti
De-Santis L, Zhao Y, Bohr VA and Balajee AS: Human RecQL4 helicase
plays critical roles in prostate carcinogenesis. Cancer Res.
70:9207–9217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin JW, Chilton-Mac Neill S, Koti M,
van Wijnen AJ, Squire JA and Zielenska M: Digital expression
profiling identifies RUNX2, CDC5L, MDM2, RECQL4, and CDK4 as
potential predictive biomarkers for neo-adjuvant chemotherapy
response in paediatric osteosarcoma. PLoS One. 9:e958432014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maire G, Yoshimoto M, Chilton-Mac Neill S,
Thorner PS, Zielenska M and Squire JA: Recurrent RECQL4 imbalance
and increased gene expression levels are associated with structural
chromosomal instability in sporadic osteosarcoma. Neoplasia.
11:260–268, 3p following 268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szász AM, Lánczky A, Nagy Á, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh DK, Ahn B and Bohr VA: Roles of RECQ
helicases in recombination based DNA repair, genomic stability and
aging. Biogerontology. 10:235–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thangavel S, Mendoza-Maldonado R, Tissino
E, Sidorova JM, Yin J, Wang W, Monnat RJ Jr, Falaschi A and
Vindigni A: Human RECQ1 and RECQ4 helicases play distinct roles in
DNA replication initiation. Mol Cell Biol. 30:1382–1396. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu L, Harutyunyan K, Jin W, Wu J, Yang T,
Chen Y, Joeng KS, Bae Y, Tao J, Dawson BC, et al: RECQL4 regulates
p53 function in vivo during skeletogenesis. J Bone Miner Res.
30:1077–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arora A, Agarwal D, Abdel-Fatah TM, Lu H,
Croteau DL, Moseley P, Aleskandarany MA, Green AR, Ball G, Rakha
EA, et al: RECQL4 helicase has oncogenic potential in sporadic
breast cancers. J Pathol. 238:495–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mo D, Fang H, Niu K, Liu J, Wu M, Li S,
Zhu T, Aleskandarany MA, Arora A, Lobo DN, et al: Human helicase
RECQL4 drives cisplatin resistance in gastric cancer by activating
an AKT-YB1-MDR1 signaling pathway. Cancer Res. 76:3057–3066. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Croteau DL, Rossi ML, Canugovi C, Tian J,
Sykora P, Ramamoorthy M, Wang ZM, Singh DK, Akbari M,
Kasiviswanathan R, et al: RECQL4 localizes to mitochondria and
preserves mitochondrial DNA integrity. Aging Cell. 11:456–466.
2012. View Article : Google Scholar : PubMed/NCBI
|