Introduction
Genetically defined molecular subtypes of clinical
breast cancer facilitate the accurate prediction of disease
progression and rational selection of targeted therapeutic options.
Expression of human epidermal growth factor receptor-2 (HER-2) on a
background of estrogen receptor-α (ERα) and progesterone receptor
(PR) positivity or negativity dictates distinct therapeutic
options. These options are frequently associated with systemic
toxicity, acquired tumor resistance and emergence of drug-resistant
cancer stem cells favoring progression of therapy-resistant disease
(1).
Targeted expression of HER-2, Ras and Myc oncogenes
confers tumorigenic transformation in mammary epithelial cells
(2–4).
In the clinic, amplification of HER-2 in the presence of ERα and PR
expression (luminal B subtype) or in the absence of their
expression (HER-2-enriched subtype) dictate distinct therapeutic
options, including antibody and/or small-molecule inhibitor-based
HER-2-targeted therapy with or without hormone receptor- and/or
aromatase inhibitor-based endocrine therapy (5–7).
Naturally occurring phytochemicals and herbal
extracts that exhibit minimal systemic toxicity may represent
testable alternatives to conventional chemo-endocrine therapy for
treating breast cancer in the clinic (8,9). Published
evidence on a model for HER-2-enriched breast cancer has
demonstrated potent anti-proliferative and pro-apoptotic effects of
a number of mechanistically distinct naturally occurring compounds,
including phytoalexins (10),
isoflavones (11,12), vitamin A derivatives (13) and phenolic terpenoids (14,15).
Rosemary (Rosemarinus officinalis L.) is a
herb that is frequently used as a dietary spice and also exhibits
medicinal properties. Rosemary and its constituent terpenoids are
potent anti-inflammatory agents that inhibit chronic dermal
inflammation, and skin tumor initiation and promotion (16,17);
however, there is limited knowledge regarding the effects of
rosemary and its constituent terpenoids in breast cancer, where
expression of HER-2 oncogene has a negative effect on endocrine
therapy.
The present study utilized a cellular model of human
mammary epithelial cells that were tumorigenic owing to targeted
expression of the HER-2 oncogene. These tumorigenic cells lack the
expression of ERs and PRs, thus the expression of HER-2 on ERα- and
PR-negative background provides a model for HER-2-enriched breast
cancer. Experiments in the present study were designed to: i)
Characterize the model at cellular and molecular level; ii) examine
the proliferation inhibitory effects of rosemary and its
constituent phenolic terpenoids; and iii) identify potential
molecular mechanisms responsible for proliferation inhibitory
efficacy.
Materials and methods
Cell lines
184-B5 is a triple negative human mammary epithelial
cell line that lacks the expression of ERα, PR and HER-2, and is
non-tumorigenic (18). 184-B5/HER is
a cell line derived from parental 184-B5 cells that are stably
transfected with the HER-2 oncogene. These HER-2-expressing cells
exhibit tumorigenic transformation (19). The two cell lines were obtained from
Professor Clifford W. Welsch (Michigan State University, East
Lansing, MI, USA). These cell lines were grown in Dulbecco's
modified Eagle's medium: Nutrient mixture F12 (DME-F12; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented
with 10 ng/ml epidermal growth factor, 0.5 µg/ml hydrocortisone, 10
µg/ml transferrin, 10 µg/ml insulin and 5 µg/ml gentamicin (all
from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The 184-B5/HER
cells were routinely maintained in the presence of 200 µg/ml G418
(Geneticin®; Sigma-Aldrich; Merck KGaA) to eliminate the
expression of spontaneous revertants. The cell cultures were
maintained at 37°C in humidified atmosphere containing 95% air and
5% CO2, and were sub-cultured at 80% confluency.
Test agents
Non-fractionated extract from rosemary leaves (RME)
and carnosol (CSOL; molecular mass 330.42 Da) were provided by
Nestlé Research Center (Lausanne, Switzerland). Ursolic acid (UA;
molecular mass 456.70 Da) and carnosic acid (CA; molecular mass
332.43 Da) were purchased from Sigma-Aldrich; Merck KGaA. RME
contained 20–30% UA, 15–20% CSOL and 10–15% CA. The stock solution
of RME was prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich;
Merck KGaA) at a concentration of 10 mg/ml. The stock solutions of
UA, CA and CSOL were prepared in DMSO at concentrations of 10 mM.
These stock solutions were serially diluted in DMEM-F12 culture
medium to obtain final concentrations of 2, 5 and 10 µg/ml RME; 1,
5 and 10 µM UA and CA; and 1, 2.5 and 5.0 µM CSOL. These final
concentrations were used for dose-response experiments on
184-B5/HER cells to identify the half-maximal inhibitory
concentration (IC50) and the maximum effective
inhibitory concentration (IC90).
Antibodies
The human reactive fluorescein isothiocyanate
(FITC)-conjugated antibodies anti-B-cell lymphoma-2 (Bcl-2; cat.
no. F7053) and anti-cyclin B1 (cat. no. F0169) were purchased from
Dako; Agilent Technologies, Inc. (Santa Clara, CA, USA). Anti-human
epidermal growth factor receptor-2 (HER-2; cat. no. SC7301),
anti-epidermal growth factor receptor (EGFR; cat. no. SC101),
anti-Bcl-2-associated X (Bax; cat. no. SC20067), anti-estrogen
receptor-α (ER-α; cat. no. SC787) and anti-progesterone receptor
(PR; cat. no. SC166169) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-cyclin D1 (cat. no.
BDB554109) was purchased from BD Biosciences, Inc. (San Jose, CA,
USA). These antibodies were used according to the recommended
dilutions provided in the technical protocols from the suppliers in
the present experiments to monitor the status of relevant
proteins.
Proliferation assays
Population doubling times, saturation density, cell
cycle progression and anchorage-independent (AI) colony formation
were determined for the aforementioned cell lines following
previously published optimized protocols (11,13).
Population doubling times were determined from independent 24 h
viable cell counts during the exponential phase for 4 days.
Saturation density was determined from viable cell counts at day 7
post-seeding of 1×105 cells. The viable cell counts were
determined by the trypan blue exclusion test using a hemocytometer.
Cell cycle progression and cellular apoptosis were determined by
flow-cytometer-based fluorescence-activated cell sorting using an
EPICS 752 flow cytometer (Beckman Coulter, Inc., Miami, FL, USA).
The data on distribution of the cell population in G1,
S, G2/M and sub G0 phases of the cell cycle
was analyzed using the multi-cycle MPLUS software version 2.0
(Phoenix Flow Systems, San Diego, CA, USA), and the data are
presented as the G1:S+G2/M phase ratio and
the incidence of sub-G0 cell population. These data
indicate the status of relative proportion of quiescent compared
with proliferating cells, and the incidence of apoptotic cells. For
the AI colony formation assay, 1,000 cells were suspended in 0.33%
agar, overlaid on a basement matrix of 0.6% agar and maintained in
culture for 21 days. The AI colony formation was then quantified by
determining the number of colonies under an inverted light
microscope at magnification, ×10.
Cellular immunofluorescence assay
The cellular immunofluorescence was quantified in
184-B5 and 184-B5/HER cells stained with FITC-conjugated antibodies
following the previously published optimized protocol (11,13).
Briefly, the cell suspension was fixed in 0.25% buffered
paraformaldehyde (Polysciences, Inc., Warrington, PA, USA) made up
in PBS (pH 7.4, Sigma-Aldrich; Merck KGaA, Darmstadt Germany) for
30 min on ice. The fixed cell suspension was subsequently incubated
with 0.1% Triton X-100 (Sigma-Aldrich; Merck KGaA) on ice for 3 min
to permeabilize the cell membrane. The permeabilized cells were
washed twice with PBS (pH 7.4), and stained with the appropriate
FITC-conjugated antibodies. Antibody stained 184-B5 and 184-B5/HER
cells were monitored for antibody expression by
fluorescence-activated cell sorting using a flow cytometer. Cells
stained with isotype FITC-conjugated IgG represented the negative
controls. The experimental data were corrected for FITC IgG and are
presented as log mean fluorescence units (FU) per 1×105
fluorescence events.
Statistical analysis
Experiments for population doubling, saturation
density, cell cycle progression and antibody-based
immunofluorescence were performed in duplicate (n=6 per treatment
group). Experiments for AI colony formation were performed in
triplicate (n=18 per treatment group). The data are presented as
the mean ± standard deviation, and were analyzed for statistical
significance between control and experimental groups using an
unpaired two-sample Student's t-test using GraphPad Prism software
(version 5.0; GraphPad Software, Inc., La Jolla, CA USA). P<0.05
was considered to indicate a statistically significant difference.
Data comparing multiple treatment groups were analyzed by one-way
analysis of variance and Dunnett's multiple range test, with a
threshold of α=0.05.
Results
Proliferation characterization of the
184-B5/HER model
Experiments designed to examine the status of
proliferation and cancer risk compared selected proliferation
parameters in non-tumorigenic 184-B5 and tumorigenic 184-B5/HER
cells (Table I). In comparison with
184-B5 cells, 184-B5/HER cells exhibited a 55.4% decrease (P=0.04)
in population doubling time and a 61.2% increase (P=0.04) in
saturation density. Additionally, these cells exhibited a 55.6%
decrease (P=0.04) in the G1:S+G2/M ratio and
a 96.8% decrease (P=0.02) in the sub-G0 cell population.
Furthermore, unlike 184-B5 cells, 184-B5/HER cells exhibited a high
incidence of AI colony formation.
 | Table I.Status of homeostatic control of
proliferation and cancer risk in 184-B5/HER cells. |
Table I.
Status of homeostatic control of
proliferation and cancer risk in 184-B5/HER cells.
|
| Cell line |
|
|---|
|
|
|
|
|---|
| Biomarker | 184-B5 | 184-B5/HER | Relative to184-B5
cells (%) |
|---|
| Population
doublinga | 34.1±1.7 h | 15.2±4.1
he | −55.4 |
| Saturation
densityb |
23.7±1.3×105 |
38.2±1.7×105e | +61.2 |
|
G1:S+G2/M
ratioc | 1.8±0.3 |
0.8±0.1e | −55.6 |
| %
Sub-G0c | 18.9±2.6 |
0.6±0.2f | −96.8 |
| AI colony
formationd |
|
|
|
| Incidence | 0/18 | 18/18 |
|
| Mean colony
number | – | 25.8±4.6 |
|
The data presented in Table II compare the status of selected
cell-cycle-regulatory and apoptosis-specific gene products in
184-B5 and 184-B5/HER cells. The two cell lines lacked the
expression of ERα and PR. Tumorigenic 184-B5/HER cells exhibited a
17,830% (178.3 fold) increase (P=0.01) in HER-2 expression.
Additionally, the expression of anti-apoptotic Bcl-2 was increased
by 29.4% (P=0.04), whereas pro-apoptotic Bax was decreased by 55.5%
(P=0.03).
 | Table II.Status of molecular markers in
184-B5/HER cells. |
Table II.
Status of molecular markers in
184-B5/HER cells.
|
| Cell line |
|
|---|
|
|
|
|
|---|
| Molecular
marker | 184-B5a |
184-B5/HERa | Relative to 184-B5
cells (%) |
|---|
| ERα | ND | ND |
|
| PR | ND | ND |
|
| HER-2 | 0.3±0.1 |
53.8±2.5b | +17,830.0 |
| EGFR | 13.9±3.7 | 8.3±2.2 | −40.3 |
| Bcl-2 | 62.2±5.7 |
80.5±5.1c | +29.4 |
| Bax | 59.8±3.3 |
26.6±2.7d | −55.5 |
Effects of RME and constituent
terpenoids on AI colony formation
Data from experiments designed to examine the effect
of RME, CA, CSOL and UA on AI colony formation in 184-B5/HER cells
are presented in Table III. A
21-day treatment with these agents resulted in a dose-dependent
decrease in the number of AI colonies. The rank order of inhibitory
efficacy at IC50 concentration (α=0.05) for individual
agents was CSOL > CA > RME > UA.
 | Table III.Inhibition of AI colony formation in
184-B5/HER cells. |
Table III.
Inhibition of AI colony formation in
184-B5/HER cells.
| Treatment | Concentration | Number of AI
coloniesa | Inhibition (%
solvent control) |
IC50 |
|---|
| DMSO | 0.1% |
28.3±6.6b | – |
|
| RME | 2 µg/ml | 20.5±5.2 | 27.6 | 4.6 |
|
| 5 µg/ml |
12.8±3.2b | 54.7 |
|
|
| 10 µg/ml | 2.6±0.6 | 91.8 |
|
| UA | 1 Μm | 22.1±5.6 | 21.6 | 4.9 |
|
| 5 µM |
13.9±3.4c | 50.9 |
|
|
| 10 µM | 2.8±0.6 | 90.1 |
|
| CA | 1 µM | 18.4±4.6 | 34.9 | 4.2 |
|
| 5 µM |
11.5±2.9d | 59.4 |
|
|
| 10 µM | 2.3±0.5 | 93.6 |
|
| CSOL | 1 µM | 22.6±5.7 | 20.1 | 2.5 |
|
| 2.5 µM |
14.1±3.5e | 50.2 |
|
|
| 5.0 µM | 2.8±0.6 | 90.1 |
|
Inhibition of cell cycle
progression
Data from experiments designed to examine the effect
of RME and UA on the cell cycle progression of 184-B5/HER cells are
presented in Fig. 1A and B. Relative
to the G1:S+G2/M ratio of 1.2±0.3 in response
to treatment with DMSO, treatment with RME and UA demonstrated a
ratio of 2.8±0.7 (P=0.02) and 1.7±0.4 (P=0.04), respectively; thus,
a 24 h treatment with high doses of RME and UA induced a 1.3-fold
increase and a 41.7% increase in the
G1:S+G2/M ratio, respectively (Fig. 1A). Relative to cyclin D1 expression of
14.7±2.1 FU in DMSO-treated cells, treatment with RME and UA
exhibited FU values of 4.6±1.2 (P=0.03), and 2.2±0.6 (P=0.03),
respectively; thus, G1 phase arrest induced by RME and
UA was associated with a 68.7% decrease and an 85% decrease in
G1-specific cyclin D1 expression, respectively (Fig. 1B).
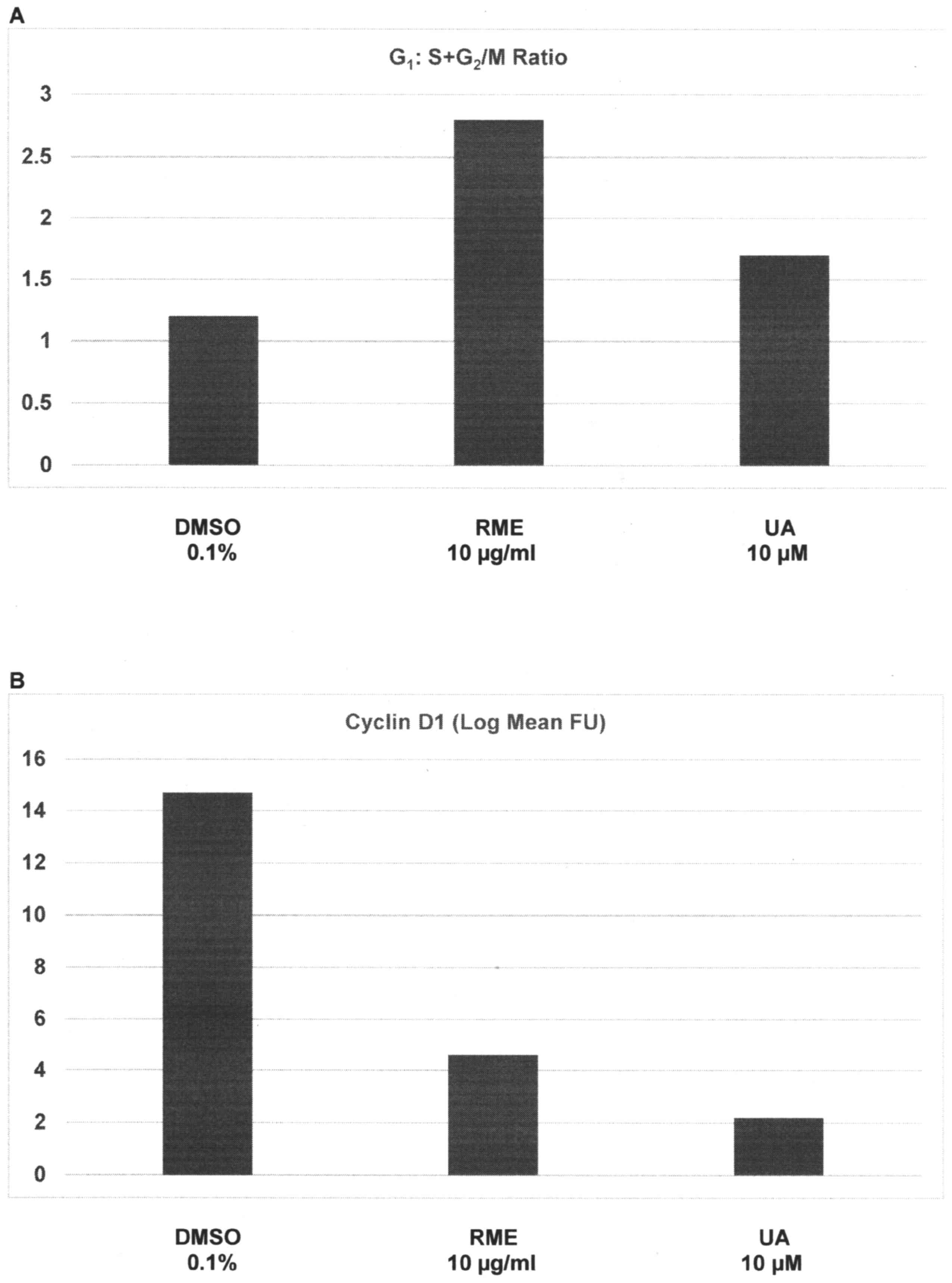 | Figure 1.Inhibition of cell cycle progression
by RME and UA. (A) Cells treated with RME and UA exhibited
increased G1:S+G2/M ratio, due to
G1 arrest. Results are presented as the mean ± SD, n=6
per treatment group. DMSO vs. RME P=0.02, DMSO vs. UA P=0.04. (B)
Treatment with RME and UA downregulates the G1
phase-specific cyclin D1. Results are presented as the log mean FU
± SD, n=6 per treatment group. DMSO vs. RME, P=0.03; DMSO vs. UA,
P=0.03. FU, fluorescence units; DMSO, dimethyl sulfoxide; RME,
rosemary extract; UA, ursolic acid; SD, standard deviation. |
Induction of cellular apoptosis
Data from experiments designed to examine the effect
of RME and UA on cellular apoptosis are presented in Fig. 2A and B. Relative to the
sub-G0 population of 0.6±0.3% in DMSO-treated cells,
treatment with RME and UA demonstrated a sub-G0
population of 6.3±1.6% (P=0.01) and 14.0±2.9% (P=0.01),
respectively; thus, a 24 h treatment with RME and UA resulted in a
9.5- and 22.3-fold increase, respectively (Fig. 2A). Relative to the Bcl-2 expression of
69.2±5.7 FU in DMSO-treated cells, treatment with RME and UA
demonstrated FU values of 41.5±1.8 (P=0.04) and 13.2±1.9 (P=0.02),
respectively; thus, induction of cellular apoptosis was associated
with a 40% and 80.9% decrease in the expression of the
anti-apoptotic Bcl-2 protein, respectively. In contrast, relative
to the Bax expression of 12.8±3.7 FU in DMSO-treated cells,
treatment with RME and UA demonstrated FU values of 26.9±1.4
(P=0.02) and 39.6±1.7 (P=0.02), respectively; thus, RME- and
UA-treated cells exhibited a 1.1-fold and 2.1-fold increase in the
expression of the pro-apoptotic Bax protein, respectively (Fig. 2B).
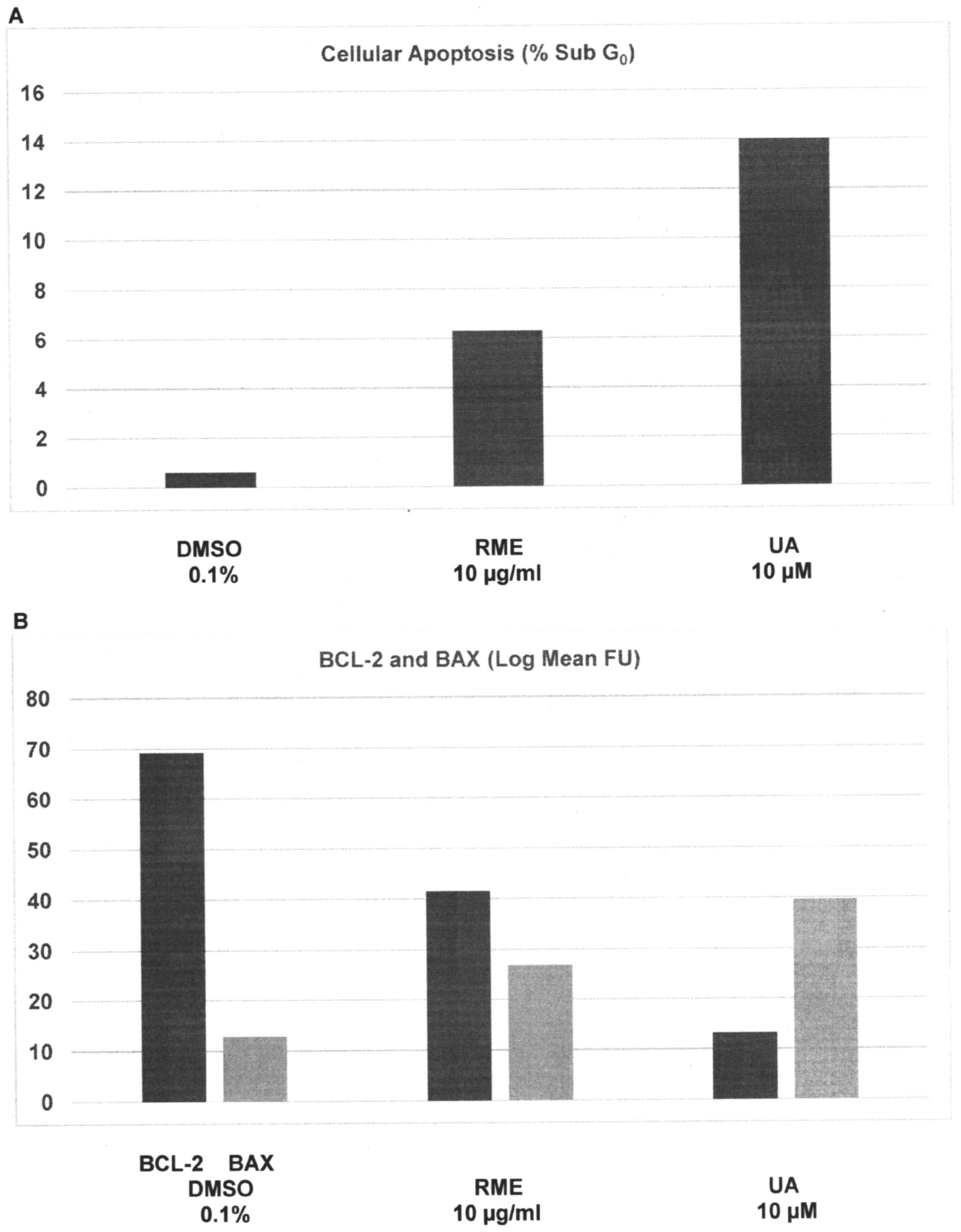 | Figure 2.Induction of cellular apoptosis by RME
and UA. (A) Treatment with RME and UA induces an increase in the
sub-G0 (apoptotic) cells. Results are presented as the
mean ± SD, n=6 per treatment group. DMSO vs. RME P=0.01, DMSO vs.
UA P=0.01. (B) Treatment with RME and UA demonstrated a decrease in
anti-apoptotic Bcl-2 expression, and an increase in pro-apoptotic
Bax expression. Results are presented as the log mean FU ± SD, n=6
per treatment group. Bcl-2: DMSO vs. RME, P=0.04; DMSO vs. UA,
P=0.02. Bax: DMSO vs. RME, P=0.02; DMSO vs. UA, P=0.02. FU,
fluorescence units; DMSO, dimethyl sulfoxide; RME, rosemary
extract; UA, ursolic acid; Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2-associated X protein; SD, standard deviation. |
Regulation of cell cycle
progression
The effects of CA and CSOL on cell cycle progression
are presented in Fig. 3A and B.
Relative to the G2/M population of 17.5±2.6% in
DMSO-treated cells, treatment with CA and CSOL demonstrated a
G2/M population of 40.0±1.7% (P=0.03) and 36.0±1.7%
(P=0.03), respectively; thus, these treatments resulted in a
1.3-fold and a 1.0-fold increase of cell population in the
G2/M phase of the cell cycle (Fig. 3A). Relative to cyclin B1 expression of
13.0±3.7 FU in DMSO-treated cells, treatment with CA and CSOL
demonstrated FU values of 27.0±1.5 (P=0.02) and 24.0±1.3 (P=0.02),
respectively; thus, consistent with arrest of cells in the
G2/M phase of the cell cycle, CA- and CSOL-treated cells
exhibited a 1.1-fold and 84.6% increase in the expression of the
G2-specific cyclin B1, respectively (Fig. 3B).
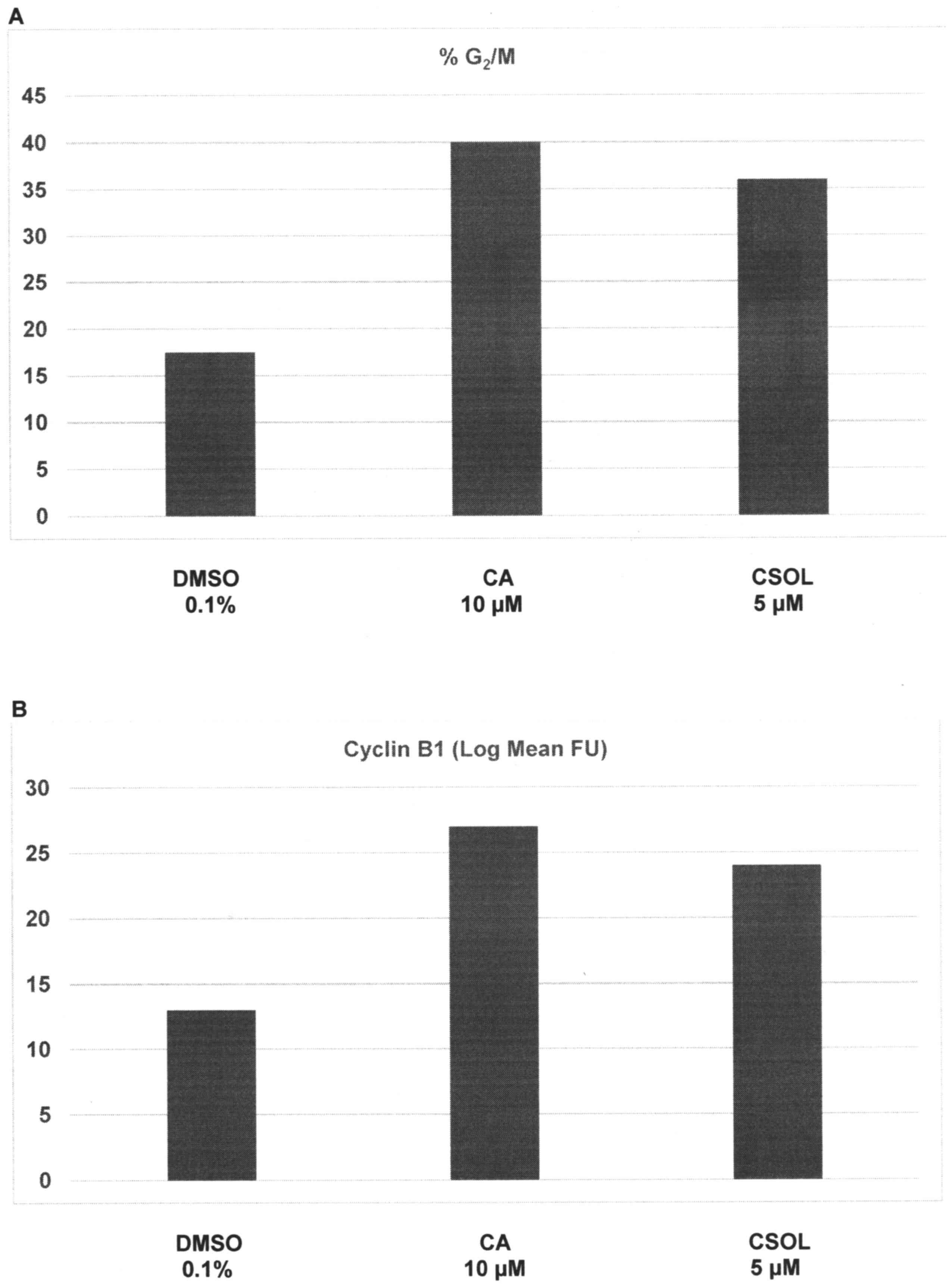 | Figure 3.Regulation of cell cycle progression
by CA and CSOL. (A) Treatment with CA and CSOL elicited an increase
in G2/M arrest. Results are presented as the mean ± SD,
n=6 per treatment group. DMSO vs. CA, P=0.03; DMSO vs. CSOL,
P=0.03. (B) Treatment with CA and CSOL resulted in an increase in
the G2 phase-specific cyclin B1 expression. Results are
presented as the log mean FU ± SD, n=6 per treatment group. DMSO
vs. CA, P=0.02; DMSO vs. CSOL, P=0.02. FU, fluorescence units;
DMSO, dimethyl sulfoxide; CA, carnosic acid; CSOL, carnosol; SD,
standard deviation. |
Discussion
The hormone receptor-positive, HER-2-expressing
breast cancer (luminal B) molecular subtype is primarily treated
using HER-2-targeted therapy and conventional endocrine therapy,
including selective estrogen receptor modulators, selective
estrogen receptor degraders and aromatase inhibitors. Hormone
receptor-negative HER-2-expressing breast cancer is primarily
treated with HER-2-targeted therapy and conventional chemotherapy,
including anthracyclines and taxanes. These long-term treatment
options are associated with systemic toxicity and acquired tumor
resistance that compromise treatment efficacy and favor
drug-resistant disease progression (5–7,20). These limitations emphasize the
importance of identifying novel, less toxic treatment options for
chemo-endocrine therapy-resistant breast cancer. The present study
utilized a cellular model for HER-2-enriched breast cancer to
examine the proliferation inhibitory effects of RME and its
constituent naturally occurring terpenoids, and to identify
potential mechanisms of action for their efficacy.
Comparative experiments on non-tumorigenic 184-B5
cells and tumorigenic 184-B5/HER cells provided evidence that
relative to 184-B5 cells, 184-B5/HER cells exhibited
hyper-proliferation, accelerated cell cycle progression,
downregulated cellular apoptosis and a high incidence of AI colony
formation, the latter representing an in vitro surrogate
endpoint marker for in vivo tumorigenic transformation.
Notably, tumorigenic potential and AI colony formation have been
indicated to have a positive correlation for experimentally induced
tumorigenic transformation in human mammary epithelial cells
(2,19,21).
Additionally, AI colony formation is detectable in cellular models
for luminal A and triple negative molecular subtypes for clinical
breast cancer (22,23). Collectively, these data provide
evidence that AI colony formation represents an in vitro
surrogate endpoint for tumorigenic transformation and an indicator
for cancer risk; thus, these data indicate the loss of homeostatic
control of proliferation and persistence of cancer risk. At the
molecular level, 184-B5/HER cells exhibited modulated expression of
HER-2 and EGFR. Furthermore, HER-2-expressing cells exhibited
upregulation of anti-apoptotic Bcl-2 and downregulation of
pro-apoptotic Bax. These molecular data may facilitate
identification of potential targets for the proliferation
inhibitory efficacy of novel anticancer compounds.
Experiments designed to examine the effects of RME
and its constituent terpenoids CA, CSOL and UA on AI colony
formation in 184-B5/HER cells indicated that these agents decreased
the number of AI colonies in a dose-dependent manner. This
dose-response experiment identified individual IC50
concentrations of the compounds and identified a rank order for the
inhibitory efficacy of CSOL > CA > RME > UA.
The IC90 non-toxic concentration of RME
was identified to be 10 µg/ml. The maximum effective non-toxic
concentrations for individual terpenoids were determined as 10 µM
for UA, 10 µM for CA and 5 µM for CSOL. These concentrations
contain 4.57 µg/ml UA, 3.32 µg/ml CA and 1.65 µg/ml CSOL,
respectively; thus, a comparison of the concentrations (µg/ml) of
RME and the three terpenoids indicates that the proliferation
inhibitory efficacy of RME may be partially due to combined effects
of these mechanistically distinct terpenoids, which are present in
differing concentrations in RME.
Experiments designed to examine the effects RME and
UA on cell cycle progression of 184-B5/HER cells demonstrated that
RME, as well as UA, increased the G1:S+G2/M
ratio and decreased cyclin D1 expression. These data indicated
inhibition of cell cycle progression via inhibition of cyclin
D1-dependent G1 to S phase transition and resultant
G1 phase arrest. The pro-apoptotic effects of RME and UA
in the present study were demonstrated by an increase of cell
population in the sub-G0 phase of the cell cycle,
decreased expression of anti-apoptotic Bcl-2 and increased
expression of pro-apoptotic Bax proteins.
Notably, RME terpenoids are effective in
proliferation inhibition of cancer cells via multiple mechanistic
pathways; thus, RME inhibits P-glycoprotein activity and reverses
multi-drug resistance in hormone receptor-positive MCF-7 cells
(24). Rosemary terpenoid UA has
documented inhibitory efficacy against transcriptional activity of
tumor promoter-inducible cyclooxygenase-2 (COX-2) via
extracellular-signal-regulated kinases 1/2 (ERK1/2), c-Jun
N-terminal kinase (JNK) and p38 mitogen-activated protein kinase
(MAPK) pathways in 184-B5/HER cells (14). In ERα-/PR-positive MCF-7 cells,
proliferation inhibitory activity of UA is due to induction of
apoptosis via the intrinsic mitochondrial pathway, involving the
downregulation of Bcl-2 (25).
Additionally, UA promotes the induction of autophagy, apoptosis,
and anti-inflammatory responses via the suppression of
phosphoinositide 3-kinase/protein kinase B and nuclear factor-κB
pathways in a number of cellular models for breast cancer (26).
Experiments designed to examine the effects of CA
and CSOL demonstrated that in comparison with RME and UA, these
terpenoids induced G2/M phase arrest in 184-B5/HER cells
and upregulated the expression of the G2 phase-specific
cyclin B1. Consistent with the present results, published evidence
has demonstrated that hydrophobic herbal flavonoids induce
G2/M phase arrest and upregulated the expression of
cyclin B1 in colorectal adenocarcinoma-derived HCT-116 and HT-29
cell lines (27). In the colorectal
adenocarcinoma-derived Caco-2 cell line, CSOL and CA induce
G2/M phase arrest via distinct modulation of increased
cyclin B1 or decreased cyclin A levels (28). Furthermore, treatment of
HER-2-expressing breast cancer cells with trastuzumab-emtansine
(T-DM1) conjugate upregulates cyclin B1 expression in
T-DM1-sensitive, but not resistant, phenotypes (29); thus, high levels of cyclin B1 in
G2/M arrested cells raise the possibility that
proteasome-mediated degradation of the G2-specific
cyclin may be impaired resultant to treatment with CA and CSOL.
Furthermore, CA has been documented to synergize the antitumor
activity of trastuzumab in HER-2-positive breast cancer cells
(30), and CSOL has been identified
to function as a potent inhibitor of transcriptional activation of
inducible COX-2 and of prostaglandin production in 184-B5/HER
cells. The mechanisms for efficacy of CSOL in this model involve
protein kinase C, ERK1/2, JNK and p38-associated MAPK pathways
(15). Collectively, the results of
the present study provide evidence that the proliferation
inhibitory efficacy of RME, UA, CA and CSOL is due to their
selective effects on distinct phases of cell cycle progression
and/or induction of cellular apoptosis via multiple
context-dependent molecular mechanisms.
The results of the present study validate an
experimental approach to identify clinically relevant mechanistic
leads for efficacy of naturally occurring phytochemicals that may
represent testable alternatives for treatment of HER-2-enriched
breast cancer.
Acknowledgements
The author wishes to acknowledge active
participation of Dr Hiromitsu Jinno from the Keio University School
of Medicine, (Tokyo, Japan), Dr Melissa Steiner from the
Weill-Cornell Medical College, (New York, NY, USA) and Dr Elizabeth
Offord from the Nestlé Research Center, (Lausanne, Switzerland) in
the research program entitled ‘Cellular models for molecular
subtypes of clinical breast cancer: Mechanistic approaches for lead
compound efficacy’.
Funding
The present study was supported by the US National
Cancer Institute (NCI) FIRST Award (grant no. CA 44741), Program
Project Grant (grant no. PO1-CA 29502), NCI Contract Research
Master Agreement (grant no. CN 75029-63), Department of Defense
Breast Cancer Research Program IDEA Award (grant no.
DAMD-17-94-J-4208), and by philanthropic funds to Strang Cancer
Prevention Center.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The author contributed towards study conception,
experimental design, data analysis, data interpretation, and
prepared the manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that there are no competing
interests.
References
|
1
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, vande Rijn M, Jefrrey SS,
et al: Gene expression patterns in breast carcinomas distinguish
tumor subclasses with clinical implications. Proc Natl Acad Sci
USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pierce JH, Arnstein P, DiMarco E, Artrip
J, Kraus MH, Lonardo F, DiFiore PP and Aaronson SA: Oncogenic
potential of erB-2 in human mammary epithelial cells. Oncogene.
6:1189–1194. 1991.PubMed/NCBI
|
|
3
|
Telang NT, Narayanan R, Bradlow HL and
Osborne MP: Coordinated expression of intermediate markers for
tumorigenic transformation in Ras transfected mouse mammary
epithelial cells. Breast Cancer Res Treat. 18:155–163. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Telang NT, Osborne MP, Sweterlitsch L and
Narayanan R: Neoplastic transformation of mouse mammary epithelial
cells by deregulated myc expression. Cell Regul. 1:863–872. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnston SRD and Dowsett M: Aromatase
inhibitors for breast cancer: Lessons from the laboratory. Nat Rev
Cancer. 3:821–831. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER-2 positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baselga J and Swain SM: Novel anti-cancer
targets: Revisiting ERB2 and discovering ERB3. Nat Rev Cancer.
9:463–475. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee KW, Bode AM and Dong Z: Molecular
targets of phytochemicals for cancer prevention. Nat Rev Cancer.
11:211–218. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye I, Jia Y, JI KE, Sanders AJ, Xue K, Ji
J, Mason MD and Jiang WG: Traditional Chinese medicine in the
prevention and treatment of breast cancer and cancer metastasis.
Oncol Lett. 10:1240–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Subbaramaiah K, Chung WJ, Michaluart P,
Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM and Dannenberg AJ:
Resveratrol inhibits cyclo-oxygenase-2 transcription and activity
in phorbol ester treated human mammary epithelial cells. J Biol
Chem. 273:21875–21882. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katdare M, Osborne MP and Telang NT: Soy
isoflavone genestein modulates cell cycle progression and induces
apoptosis in HER2/neu oncogene expressing human breast epithelial
cells. Int J Oncol. 21:809–815. 2002.PubMed/NCBI
|
|
12
|
Katdare M, Osborne MP and Telang NT: Novel
cell culture models for prevention of human breast cancer (Review).
Int J Oncol. 22:509–515. 2003.PubMed/NCBI
|
|
13
|
Jinno H, Steiner MG, Nason-Burchenal K,
Osborne MP and Telang NT: Preventive efficacy of receptor class
selective retinoids on HER-2/neu oncogene expressing pre-neoplastic
human mammary epithelial cells. Int J Oncol. 21:127–134.
2002.PubMed/NCBI
|
|
14
|
Subbaramaiah K, Michaluart P, Sporn MB and
Dannenberg AJ: Ursolic acid inhibits cyclo-oxygenase-2
transcription in human mammary epithelial cells. Cancer Res.
60:2399–2404. 2000.PubMed/NCBI
|
|
15
|
Subbaramaiah K, Cole PA and Dannenberg AJ:
Retinoids and carnosol suppress cyclo-oxygenase-2 transcription by
CREB-binding protein/p300-dependent and -independent mechanisms.
Cancer Res. 62:2522–2530. 2002.PubMed/NCBI
|
|
16
|
Manez S, Recio MC, Giner RM and Ríos JL:
Effect of selected triterpenoids on chronic dermal inflammation.
Eur J Pharmacol. 334:103–105. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang MT, Ho CT, Wang ZY, Ferraro T, Lou
YR, Stauber K, Ma W, Georgiadis C, Laskin JD and Conney AH:
Inhibition of skin tumorigenesis by rosemary and its constituents
carnosol and ursolic acid. Cancer Res. 54:701–708. 1994.PubMed/NCBI
|
|
18
|
Stampfer MR and Bartley JC: Induction of
transformation and continuous cell lines from normal human mammary
epithelial cells after exposure to Benzo (α) pyrene. Proc Natl Acad
Sci USA. 82:2394–2398. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhai YF, Biettenmiller H, Wang B, Gould
MN, Oakley C, Esselman WL and Welsch CW: Increased expression of
specific tyrosine phosphatases in human breast epithelial cells
neoplastically transformed by the neu oncogene. Cancer Res.
53:2272–2278. 1993.PubMed/NCBI
|
|
20
|
Musgrove EA and Sutherland RL: Biological
determinants of endocrine resistance in breast cancer. Nat Rev
Cancer. 9:631–643. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Gildea JJ and Yue W: Aromatase
over-expression induces malignant changes in estrogen receptor-α
negative MCF-10A cells. Oncogene. 32:5233–5240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Telang N: Putative cancer initiating stem
cells in cell culture models for molecular subtypes of clinical
breast cancer. Oncol Lett. 10:3840–3846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Telang N: Growth inhibitory efficacy of
natural products in a model for triple negative molecular subtype
of clinical breast cancer. Biomed Rep. 7:199–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Plouzek CA, Ciolino HP, Clarke R and Yeh
GC: Inhibition of P-glycoprotein activity and reversal of multidrug
resistance in vitro by rosemary extract. Eur J Cancer.
35:1541–1545. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kassi E, Sourlingas TG, Spiliotaki M,
Papoutsi Z, Prastinis H, Aligiannis N and Moutsatsou P: Ursolic
acid triggers apoptosis and BCL-2 down-regulation in MCF-7 breast
cancer cells. Cancer Investig. 27:723–733. 2009. View Article : Google Scholar
|
|
26
|
Lou J, Hu YL and Wang H: Ursolic acid
inhibits breast cancer growth by inhibiting proliferation, inducing
autophagy and apoptosis, and suppressing inflammatory responses via
the PI3K/AKT and NFkB signaling pathways in vitro. Exp Ther
Med. 14:3623–3631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang CZ, Calway TD, Wen XD, Smith J, Yu C,
Wang Y, Mehendale SR and Yuan CS: Hydrophobic flavonoids from
Scutellaria baicelensis induce colorectal cancer cell apoptosis
through a mitochondrial-mediated pathway. Int J Oncol.
42:1018–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vissanji JM, Thompson DG and Padfield PJ:
Induction of G2/M phase cell cycle arrest by carnosol and carnosic
acid is associated with alteration of cyclin A and cyclin B1
levels. Cancer Lett. 237:130–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sabbaghi MA, Gil-Gomez G, Guardia C,
Servitja S, Arpi O, Garcia-Alonso S, Menedez S, Arumi-Uria M,
Serrano L, Salido M, et al: Defective cyclin B1 induction in
trastuzumab-emtansine (T-DM1) acquired resistance in HER-2 positive
breast cancer. Clin Cancer Res. 23:7006–7019. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
D'Alesio C, Bellese G, Gagliani MC, Aiello
C, Grasselli E, Marcocci G, Bisio A, Tavella S, Daniele T, Cortese
K and Castagnola P: Cooperative anti-tumor activities of carnosic
acid and Trastuzumab in ERBB2+ breast cancer cells. J
Exp Clin Cancer Res. 36:1542017. View Article : Google Scholar : PubMed/NCBI
|

















