Introduction
Poly(ADP-ribose) polymerases (PARPs) are a large
protein family involved in various cellular and molecular processes
(1–8).
PARPs transfer ADP-ribose molecules from donor NAD+ to
target proteins by post-translational modification, including
poly(ADP-ribosyl)ation (PARsylation) (1–3).
PARsylation regulates numerous cellular processes, including DNA
damage repair (4), cellular stress
signaling (5), gene transcription
(6,7)
and ageing (8). There are 17
physiological human PARPs (9).
The two tankyrase proteins, tankyrase 1 (TNKS1; also
known as PARP5A and ARTD5) and tankyrase 2 (TANK2; also known as
PARP5B and ARTD6), belong to the PARP family (3). TNKS1 consists of an amino-terminal
domain composed of homopolymeric stretches of His, Pro and Ser
residues (the HPS domain), an ankyrin domain composed of 24 ankyrin
repeats, a sterile α module (SAM) domain and a carboxy-terminal
PARP catalytic domain (10,11). TANK2 is associated with TNKS1
(10), but lacks an N-terminal HPS
domain. The TANK2 ANK domain shares 83% identity with TNKS1, and
the TANK2 SAM domain shares 74% identity with that of TNKS1
(10). The C-terminal PARP domain is
a PARP polymerase and is highly conserved, with 94% identity
(10). The ankyrin domain is
implicated in protein-protein interactions (12), and the SAM domain is implicated in
self-oligomerization (13). The HPS
domain function is currently unknown.
Tankyrases interact with a number of target proteins
and regulate cellular processes, including telomere maintenance,
via telomere repeat binding factor 1 (TRF1) (10). Tankyrase-binding partners interact
with TNKS1 using a 6-amino acid tankyrase-binding motif (RxxAxG,
RxxPxG or RxxxxG) (14–17).
Tankyrases are involved in various cellular
functions, including telomere maintenance (18), Wnt signaling (15), mitosis (19–22),
glucose metabolism (23,24) and heritable disease cherubism
(14,25). Recent studies reported novel tankyrase
binding partners, including phosphatase and tensin homolog (PTEN),
peroxiredoxin II (PrxII), adenomatous polyposis coli 2 (APC2),
angiomotins (AMOTs), abraxas brother 1 (ABRO1), cluster of
differentiation 2 associated protein (CD2AP), peroxisomal
biogenesis factor 14 (PEX14) and autophagy related 9A (ATG9A), as
detailed in Table I (16,26–31). These
data indicate novel tankyrase functionalities and provide novel
insights for further investigations in numerous cellular responses.
The present review focuses on novel tankyrase-binding partners and
discusses recent data on tankyrase roles in cancer.
 | Table I.A summary of updated
tankyrase-binding proteins. |
Table I.
A summary of updated
tankyrase-binding proteins.
| Authors, year | Novel
tankyrase-binding partners | Tankyrase-binding
motif | (Refs.) |
|---|
| Li et al,
2015 | PTEN | RXXXDG | (16) |
| Kang et al,
2017 | PrxII | N.D. | (26) |
| Croy et al,
2016 | APC2 | RXXXXG | (27) |
| Wang et al,
2015 | AMOTs | RXXPXG | (28) |
| Tripathi and Smith,
2017 | ABRO1 | RXXAXG | (29) |
| Kuusela et
al, 2016 | CD2AP | N.D. | (30) |
| Li et al,
2017 | PEX14 | RXXXXG, RXXXDG | (31) |
| Li et al,
2017 | ATG9A | RXXXXG | (31) |
Post-translational modification and
tankyrase activity
PARsylation and ubiquitination
Tankyrases catalyze post-translational modification
of target proteins, which controls their stability (15,32). TNKS1
PARsylates tankyrase target proteins, including TRF1, centrosomal
P4.1-associated protein (CPAP), AXIN, PTEN and AMOTs (15,16,21,32,33).
The PARsylated protein is then recognized by the E3 ligase, and
targeted for ubiquitination and proteasomal degradation (15,16,26).
Tankyrase activity
Tankyrase activity is controlled by a variety of
factors, including polo-like kinase-1 (PLK1) and mitogen-activated
protein kinase (MAPK). TNKS1 is phosphorylated by PLK1, glycogen
synthase kinase 3 (GSK3), and MAPK, although the precise functions
of this modification are not clear (34–36).
PLK1-mediated phosphorylation results in increased TNKS1 stability
and telomeric PARP activity (34).
GSK3-mediated phosphorylation of TNKS1 does not alter TNKS1
auto-PARsylation in vitro, and MAPK-mediated phosphorylation
of tankyrase enhances the PARsylation activity of TNKS1 in
vitro (35,36). GDP-mannose 4, 6-dehydratase binds to
TNKS1, inhibits tankyrase PARP activity in vitro and
influences TNKS1 stability in vivo (37). Kang et al (26) demonstrated that TNKS1 is involved with
the antioxidant enzyme PrxII. PrxII is essential for full TNKS1
activity, in order to maintain oncogenic β-catenin signaling in
colorectal cancer (CRC). This study demonstrated the molecular
mechanisms regulating tankyrase activity in CRC for the first
time.
Tankyrase and cancer
Different biological tankyrase functions are
relevant to cancer, including telomere maintenance, oncogenic
pathways [Wnt, yes-associated protein (YAP) and AKT], mitosis, DNA
repair and cell death, as depicted in Fig. 1.
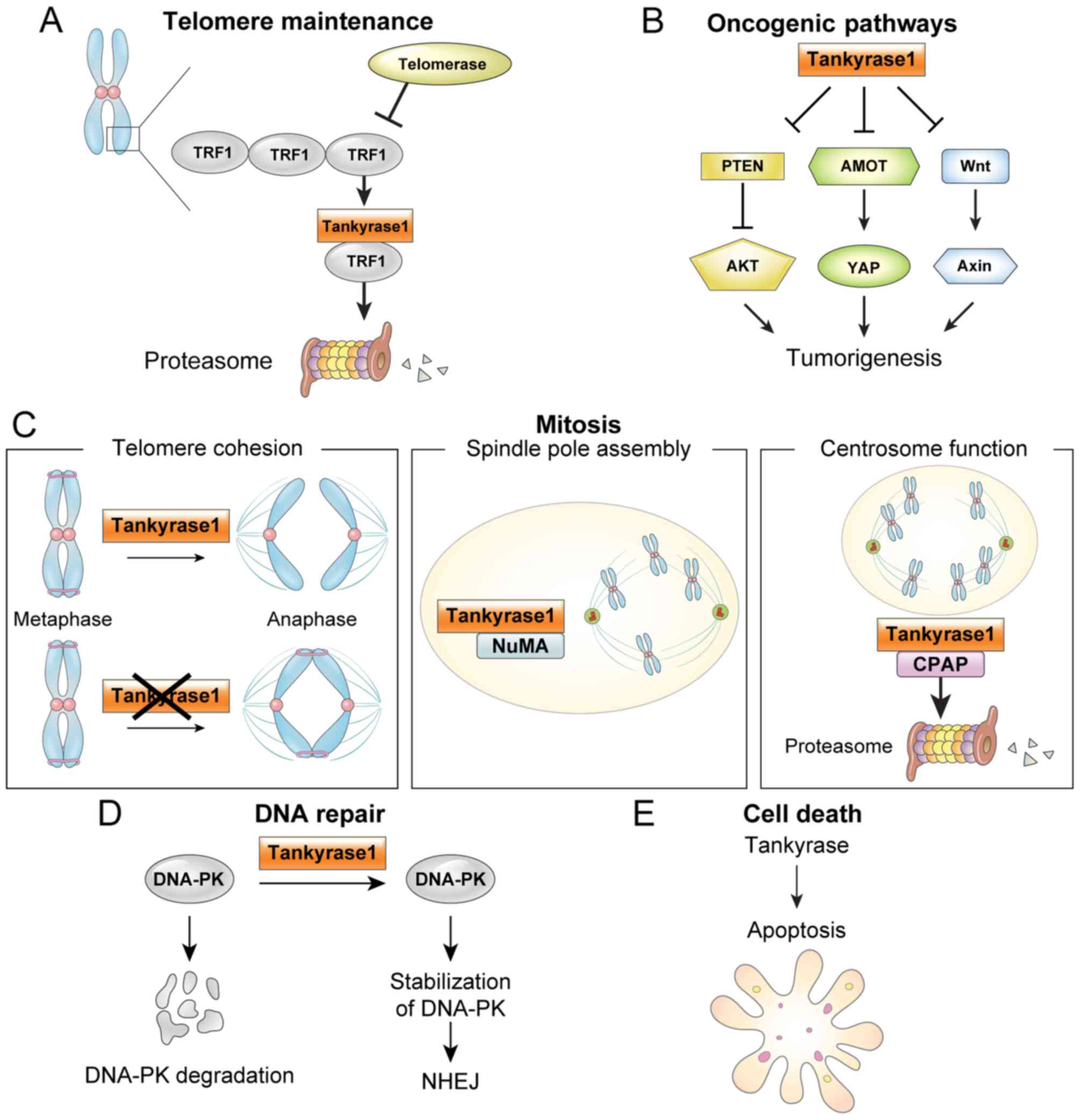 | Figure 1.Tankyrase function in cancer. (A)
ADP-ribosylation of TRF1 by tankyrase 1 releases TRF1 from
telomeres, and the released TRF1 is degraded by the
ubiquitin-proteasome pathway. Thus, telomere maintenance by
telomerase allows continued proliferation. (B) Oncogenic pathways.
Tankyrases are implicated in a number oncogenic pathways, including
Wnt, YAP and AKT. (C) Mitosis. Tankyrase 1 has multiple functions
in mitosis, including: i) required to resolve sister telomeres
during mitosis; ii) localized to mitotic spindle poles during
mitosis, where NuMA PARsylation is required for normal spindle
formation and iii) regulates CPAP protein stability and function by
its PARsylation. (D) DNA repair. Tankyrase 1 stabilizes the NHEJ
protein DNA-PK. (E) Apoptosis. Tankyrases are involved in
apoptosis, although the mechanism is unclear. TRF1, telomere repeat
binding factor 1; PTEN, phosphatase and tensin homolog; YAP,
yes-associated protein; AMOT, angiomotins; NuMA, nuclear mitotic
apparatus; CPAP, centrosomal P4.1-associated protein; DNA-PK,
DNA-dependent protein kinase; NHEJ, non-homologous end joining. |
Telomere maintenance
TNKS1 has been identified as an interaction partner
of TRF1 (18,38). TRF1 blocks the access of telomerase to
telomeres (18,32). TNKS1-mediated PARsylation of TRF1
releases TRF1 from telomeres, and the released TRF1 is degraded by
the ubiquitin-proteasome pathway (18,32).
Telomere maintenance by telomerase allows continued proliferation
of cancer cells and is considered as a promising target for
anticancer strategies. TNKS1 controls telomerase inhibition in
human cancer cells and is a potential telomere-directed anticancer
target (39,40). Telomere-directed inhibitors result in
progressive telomere shortening, with no acute cytotoxicity, and
combination with tankyrase inhibitors has been proposed (39,40). Dual
inhibition of TNKS1 and telomerase has demonstrated a synergistic
effect in lung and gastric cancer cell lines (41,42).
Furthermore, the combination of tankyrase and telomerase inhibition
promotes human lung adenocarcinoma cell apoptosis and inhibits
proliferation (41). These
observations indicate that co-inhibition of telomerase and
tankyrase may be an effective strategy for the treatment of lung
cancer in humans.
Oncogenic pathways
Tankyrases have been implicated in oncogenic
pathways (Wnt, YAP and AKT) (15,16,43).
Wnt signaling
The Wnt signal transduction pathway regulates
numerous biological processes in diseases such as in cancer
(44). AXIN is the key effector in
the Wnt pathway and has been identified as a tumor suppressor.
Tankyrases target AXIN for degradation, whereas tankyrase
inhibitors generally stabilize it (44,45). The
Wnt pathway regulates proteolysis of the downstream effector
β-catenin with the β-catenin destruction complex, which includes
adenomatous polyposis coli (APC), AXIN and GSK3β (45). TNKS1-mediated AXIN PARsylation induces
AXIN degradation with the ubiquitin-proteasome pathway, and the
ensuing AXIN degradation triggers disruption of the β-catenin
destruction complex (15). Released
β-catenin translocates into the nucleus and switches on
Wnt-dependent transcription (44).
The tumor suppressor APC scaffolds the β-catenin
destruction complex (45). APC is
mutated in >80% of CRC cases (46). Therefore, due to tankyrases regulating
Wnt signaling, tankyrase inhibitors may be promising therapeutic
targets for CRC. TNKS1 inhibition suppresses Wnt signaling and
tumor growth in APC-mutant colorectal tumors (15,47,48), and
increases chemosensitivity in colon cancer cell lines (49). Due to the Wnt pathway being involved
in lung cancer (50,51), antagonizing the Wnt pathway through
tankyrase inhibition may be effective against lung cancer, and
there is evidence for tankyrases as antineoplastic targets in lung
cancer (52,53).
Croy et al (27) indicated that the fly APC homolog APC2
may be a tankyrase substrate and that tankyrases regulate
destruction complex activity, providing additional insight into
tankyrase inhibition as a potential Wnt-pathway cancer therapy.
YAP signaling
The Hippo pathway controls tissue homeostasis and
organ size (54,55). YAP has been identified as an
oncoprotein and the key effector in the Hippo pathway (54–58). YAP
signaling has also been demonstrated to be involved in human cancer
types (56–58). AMOTs are negative YAP regulators
(33), and recent studies indicated
that tankyrase inhibition suppresses YAP oncogenic activity by
stabilizing AMOTs through inhibiting their tankyrase RNF146
axis-mediated degradation (28,59). These
results indicate a potential opportunity for cancer therapy. Lin
et al (43) demonstrated that
YAP signaling is involved in drug resistance, including with RAF-
and MAPK-targeted cancer therapy. Wang et al (59) reported that tankyrase inhibition
enhances epidermal growth factor receptor (EGFR) growth inhibition
in non-small cell lung cancer (NSCLC). These data indicate that
tankyrase inhibition could be an effective approach to overcome
drug resistance for combinatorial cancer therapy.
AKT signaling
PTEN is an important tumor suppressor, and PTEN
mutations have been associated with a number of cancer types
(60,61) and Cowden syndrome (62). Li et al (16) identified PTEN as a tankyrase-binding
protein. PTEN stabilization by tankyrase inhibition induces
downregulation of AKT phosphorylation, suppressing cell
proliferation and tumor growth. These data support the therapeutic
potential of tankyrase inhibitors targeting the AKT oncogenic
pathway.
Mitosis regulation
TNKS1 is required to resolve sister telomeres during
mitosis. Sister chromatid cohesion holds sister chromatids together
from their S phase replication until their mitosis separation
(22,29). Cohesion requires a multi-protein
complex comprising structural maintenance of chromosomes protein
(Smc)1, Smc3, sister chromatid cohesion protein (Scc)1 and Scc3
(63,64). In the absence of TNKS1, cohesion is
removed from arms and centromeres, but sister telomeres remain
associated, indicating persistent cohesion, and this persistent
telomere cohesion by TNKS1 inhibition during mitosis induces a
delay in anaphase progression (22).
Tripathi and Smith (29) demonstrated
that the mechanism of cell cycle-regulated K63-ubiquitination of
tankyrase controls sister telomere resolution timing.
TNKS1 colocalizes with the nuclear mitotic apparatus
(NuMA) protein and PARsylates NuMA in mitosis (20), and TNKS1-depleted cells exhibit
defects in mitotic spindle assembly and structure (19), indicating that TNKS1 is required for
normal spindle formation. TNKS1 also localizes at the centrosomes,
promotes centrosome maturation, interacts with CPAP, PARsylates
CPAP, and regulates CPAP protein stability and function at
centrosomes across the cell cycle (21). Miki PARsylation by TNKS1 promotes
centrosome maturation (65);
therefore, CPAP and Miki may have a general role in centrosome
function. Abnormal centrosomes are involved in cancer and
contribute to chromosome missegregation and aneuploidy, thereby
promoting malignant progression (66–69).
Korzeniewski et al (70)
indicated centrosomes as a potential target for cancer therapy.
DNA repair
The DNA-dependent protein kinase (DNA-PK) is a
critical component of non-homologous end joining-mediated DNA
repair mechanisms (71). DNA-PKcs, a
catalytic subunit of DNA-PK, exists in a PARsylated state in
vitro and in vivo (72,73). TNKS1
regulates DNA repair via PARsylation-mediated stabilization of
DNA-PK and suppression of telomere-associated sister chromatid
exchange (74). Tankyrases bind to
mediator of DNA damage checkpoint protein 1 and promote homologous
recombination and checkpoint activation in response to
double-strand breaks (DSBs) (75).
Therefore, tankyrases have a direct role in DSB repair. DNA-PK is
involved in tumor-associated processes, including genomic
stability, hypoxia, metabolism, inflammatory response and
transcription (71), which indicates
that DNA-PK may be a potential target for cancer therapy.
Cell death
Tankyrase inhibition blocks proliferation and
promotes cell apoptosis in neuroblastoma (NB) cell lines;
therefore, tankyrases are a potential target for NB (17).
Tankyrase inhibitors
Numerous studies have reported the importance and
utility of tankyrase inhibitors as cancer therapeutics (15,47,48,52,76–80).
Consequently, a number of tankyrase inhibitors with promising
therapeutic effects have been developed, including XAV939, IWR-1,
G007-LK, JW55, AZ1366, JW 74 and NVP-TNKS656 (15,47,48,52,76–80)
(Table II).
 | Table II.Tankyrase inhibitors as therapeutic
targets for cancer. |
Table II.
Tankyrase inhibitors as therapeutic
targets for cancer.
| Author, year | Tankyrase
inhibitors | Cancer type | (Refs.) |
|---|
| Stratford et
al, 2014 | JW 74 | Osteosarcoma | (79) |
| Tian et al,
2013 |
| NB | (17) |
| Huang et al,
2009 | XAV939 | CRC | (15) |
| Busch et al,
2013 |
| Lung cancer | (52) |
| Bao et al,
2012 |
| Breast cancer | (77) |
| Quackenbush et
al, 2016 | AZ1366 | CRC | (78) |
| Busch et al,
2013 | IWR-1 | Lung cancer | (52) |
| Lau et al,
2013 | G007-LK | CRC | (47) |
| Waaler et
al, 2012 | JW55 | CRC | (48) |
| Arqués et
al, 2016 | NVP-TNKS656 | NSCLC | (76) |
| Wang et al,
2016 |
|
| (59) |
Tankyrase inhibition suppresses Wnt signaling and
tumor growth in APC-mutant colorectal tumors (15,47,48). Wu
et al (49) demonstrated that
the tankyrase inhibitor XAV939 increased chemosensitivity in colon
cancer cell lines via inhibition of the Wnt signaling pathway. Lau
et al (47) indicated that the
tankyrase inhibitor G007-LK suppressed APC-mutant colorectal tumor
growth. Mashima et al (81)
reported that mechanistic target of rapamycin (mTOR) signaling
conferred resistance to tankyrase inhibitors in Wnt-driven CRC,
indicating that co-inhibition of tankyrase and mTOR may be an
effective therapeutic approach for CRC.
The Wnt pathway is also involved in lung cancer
(50,51), and therefore antagonizing the Wnt
pathway through tankyrase inhibition could be effective against
lung cancer. Casás-Selves et al (53) and Busch et al (52) demonstrated tankyrase to be an
antineoplastic target in lung cancer. Wang et al (59) indicated that the tankyrase inhibitor
NVP-TNKS656 sensitized lung cancer cells to the EGFR inhibitor
erlotinib. Busch et al (52)
screened A375 melanoma cells and identified WIKI4, a small molecule
inhibitor of Wnt/β-catenin signaling.
Co-localization of the transcription factor forkhead
box O3 and β-catenin in the nucleus mediates progression and
metastasis in CRC upon phosphoinositide 3-kinase (PI3K) or AKT
inhibition (36,82). Co-exposure to AKT or PI3K inhibitors
and the tankyrase inhibitor XAV939 impairs metastasis. Arqués et
al (76) demonstrated that the
tankyrase inhibitor NVP-TNKS656 blocked the Wnt/β-catenin pathway,
overcoming resistance to PI3K and AKT inhibitors in CRC.
Thomson et al (83) characterized a novel tankyrase
inhibitor, tetrazoloquinoxaline 41, and indicated that it inhibited
growth in tumor-derived cell lines, providing a potential cancer
therapy.
Novel tankyrase binding partners
Numerous tankyrase binding partners have been
reviewed (2), and a number of studies
have recently reported novel tankyrase binding partners (16,26–31). This
section reviews a number of these novel tankyrase binding partners,
including PTEN, PrxII, APC2, AMOTs, ABRO1, CD2AP, PEX14 and ATG9A
(Table I).
PTEN
PTEN has been characterized as a tumour suppressor,
and PTEN mutations have been reported in cancers (60,61) and
Cowden syndrome (62). Li et
al (16) identified PTEN as a
tankyrase-binding protein containing a RXXXDG tankyrase-binding
motif (RYQEDG). Tankyrases interact with and PARsylate PTEN.
PARsylated PTEN promotes PTEN degradation through E3 ligase RNF146
(16).
PTEN stabilization by tankyrase inhibition induces
downregulation of AKT phosphorylation, suppressing cell
proliferation and tumor growth (16).
These data indicate a therapeutic potential for tankyrase
inhibitors in cancer, targeting the AKT oncogenic pathway.
PrxII
Kang et al (26) demonstrated that TNKS1 is involved with
PrxII, and PrxII is essential for full TNKS1 activity to maintain
oncogenic β-catenin signaling in CRC. In addition, it was indicated
that the TNKS1 zinc-binding motif is essential for PARP activity
and is protected from oxidative inactivation by PrxII. Furthermore,
H2O2-dependent inactivation of TNKS1 PARP
activity in the absence of PrxII enhances AXIN-dependent β-catenin
degradation in APC-mutant CRC cells (26). These results indicate that PrxII
inhibition may exert therapeutic effects on APC-mutant CRC
cells.
APC2
Of all colon cancer cases, >80% are initiated by
truncating mutations in the tumor suppressor APC (46). The Wnt pathway regulates proteolysis
of the downstream effector β-catenin via the β-catenin destruction
complex, including APC, AXIN and GSK3β (45). Croy et al (27) identified APC2, a fly APC homolog, as a
tankyrase binding partner and substrate. This previous study
indicated that tankyrases regulate the activity of the β-catenin
destruction complex through AXIN and APC2 ribosylation, supporting
the therapeutic value of tankyrase inhibition as a Wnt-pathway
cancer therapy.
Angiomotin family of proteins
AMOTs are negative YAP regulators (33), and tankyrase inhibition suppresses YAP
oncogenic activity by stabilizing AMOT family proteins (28,59).
Tankyrases bind to AMOTs, and tankyrase-mediated PARsylation
induces their degradation through E3 ligase RNF146 (28,59). These
observations highlight the therapeutic potential of tankyrase
inhibitors in cancer, targeting the YAP oncogenic pathway. YAP
signaling has been associated with drug resistance in cancer types,
including lung cancer, and hence YAP signaling inhibition is
important to overcome drug resistance (84). Tankyrase inhibition enhances EGFR
inhibitor growth inhibition in lung cancer cells via AMOT
stabilization and YAP signaling inhibition (59). Thus, tankyrase inhibition may be an
effective approach to overcoming drug resistance for combinatorial
cancer therapy.
ABRO1
During the S phase, DNA must not only be replicated,
but also newly synthesized DNA molecules must also be connected
with each other (85). This sister
chromatid cohesion is essential for chromosome segregation during
mitosis. Canudas and Smith (86)
demonstrated that premature resolution of telomere cohesion between
sister telomeres induced sister telomere loss. Resolution at
telomeres requires TNKS1, but TNKS1 mechanisms in timely resolution
of sister telomere cohesion are poorly understood. Tripathi and
Smith (29) identified ABRO1, the
scaffold subunit of the BRCC36 deubiquitinating enzyme (BRISC DUB)
and demonstrated that sister telomere resolution timing was ensured
through cell cycle-regulated ubiquitination of TNKS1 by RNF8 ligase
and the BRISC DUB. Perturbation of this regulation results in
persistent unresolved cohesion in mitosis or premature loss of
cohesion in the S phase, indicating that a cell cycle-regulated
post-translational modification controls sister telomere cohesion
timing to ensure genome integrity.
CD2 associated protein
The adapter protein CD2AP is essential for kidney
ultrafiltration and is expressed primarily by podocytes in the
kidney. Podocyte damage results in numerous glomerular diseases,
including nephritic syndrome and nephrotic syndrome (87). Kuusela et al (30) demonstrated that tankyrases interact
with CD2AP, and CD2AP is a negative tankyrase regulator. Tankyrase
inhibition in the absence of CD2AP increases kidney damage, which
indicates that tankyrases are essential for maintaining normal
kidney function (87).
PEX14 and ATG9A
Li et al (31)
investigated the tankyrase protein interaction network through
proteomic analysis and identified >100 high-confidence
interacting proteins for tankyrases. In particular, they
demonstrated that TNKS1 and TANK2 bind to the peroxisome protein
PEX14 and localize on peroxisomes. Overexpression of TNKS1 or TANK2
decreases peroxisome number or size, indicating that TNKS1 and
TANK2 promote pexophagy. This study also demonstrated that
tankyrases associate with the autophagy-associated protein ATG9A.
Additionally, they indicated that tankyrase may associate PEX14
with ATG9A to promote pexophagy. Further experimentation is
required to confirm the detailed mechanism. This study provides
insights into further cellular localizations and functions.
Conclusions
Tankyrases have been implicated in a variety of
cellular functions and are important therapeutic targets; however,
the details of tankyrase functions and molecular mechanisms remain
unclear. Novel tankyrase binding partners, including PTEN, AMOTs,
CD2AP, APC2, ABRO1, PrxII, PEX14 and ATG9A have been recently
reported, and these proteins will provide novel insights to
understand the functions and mechanisms of tankyrase.
A number of tankyrase substrates are tumor
suppressors, including AXIN, PTEN and AMOTs. Due to tankyrase
inhibitors targeting different oncogenic pathways, including WNT,
AKT and YAP, tankyrases may be effective targets for cancer
therapy.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Research
Foundation of Korea (grant no. NRF-2015R1C1A1A02037631).
Availability of data and materials
Not applicable.
Authors' contributions
MKK designed the present review, collected
information and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Bürkle A: Poly(ADP-ribose). The most
elaborate metabolite of NAD+. FEBS J. 272:4576–4589. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haikarainen T, Krauss S and Lehtio L:
Tankyrases: Structure, function and therapeutic implications in
cancer. Curr Pharm Des. 20:6472–6488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riffell JL, Lord CJ and Ashworth A:
Tankyrase-targeted therapeutics: Expanding opportunities in the
PARP family. Nat Rev Drug Discov. 11:923–936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malanga M and Althaus FR: The role of
poly(ADP-ribose) in the DNA damage signaling network. Biochem Cell
Biol. 83:354–364. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo X and Kraus WL: On PAR with PARP:
Cellular stress signaling through poly(ADP-ribose) and PARP-1.
Genes Dev. 26:417–432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kraus WL and Lis JT: PARP goes
transcription. Cell. 113:677–683. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeh TY, Sbodio JI, Tsun ZY, Luo B and Chi
NW: Insulin-stimulated exocytosis of GLUT4 is enhanced by IRAP and
its partner tankyrase. Biochem J. 402:279–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beneke S and Bürkle A:
Poly(ADP-ribosyl)ation in mammalian ageing. Nucleic Acids Res.
35:7456–7465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otto H, Reche PA, Bazan F, Dittmar K, Haag
F and Koch-Nolte F: In silico characterization of the family of
PARP-like poly(ADP-ribosyl)transferases (pARTs). BMC Genomics.
6:1392005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsiao SJ and Smith S: Tankyrase function
at telomeres, spindle poles, and beyond. Biochimie. 90:83–92. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith S, Giriat I, Schmitt A and de Lange
T: Tankyrase, a poly(ADP-ribose) polymerase at human telomeres.
Science. 282:1484–1487. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seimiya H and Smith S: The telomeric
poly(ADP-ribose) polymerase, tankyrase 1, contains multiple binding
sites for telomeric repeat binding factor 1 (TRF1) and a novel
acceptor, 182-kDa tankyrase-binding protein (TAB182). J Biol Chem.
277:14116–14126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Rycker M and Price CM: Tankyrase
polymerization is controlled by its sterile alpha motif and
poly(ADP-ribose) polymerase domains. Mol Cell Biol. 24:9802–9812.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guettler S, LaRose J, Petsalaki E, Gish G,
Scotter A, Pawson T, Rottapel R and Sicheri F: Structural basis and
sequence rules for substrate recognition by Tankyrase explain the
basis for cherubism disease. Cell. 147:1340–1354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang SM, Mishina YM, Liu S, Cheung A,
Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner
S, et al: Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li N, Zhang Y, Han X, Liang K, Wang J,
Feng L, Wang W, Songyang Z, Lin C, Yang L, et al: Poly-ADP
ribosylation of PTEN by tankyrases promotes PTEN degradation and
tumor growth. Genes Dev. 29:157–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian XH, Hou WJ, Fang Y, Fan J, Tong H,
Bai SL, Chen Q, Xu H and Li Y: XAV939, a tankyrase 1 inhibitior,
promotes cell apoptosis in neuroblastoma cell lines by inhibiting
Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res. 32:1002013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith S and de Lange T: Tankyrase promotes
telomere elongation in human cells. Curr Biol. 10:1299–1302. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang P, Coughlin M and Mitchison TJ:
Tankyrase-1 polymerization of poly(ADP-ribose) is required for
spindle structure and function. Nat Cell Biol. 7:1133–1139. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang W, Dynek JN and Smith S: NuMA is a
major acceptor of poly(ADP-ribosyl)ation by tankyrase 1 in mitosis.
Biochem J. 391:177–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim MK, Dudognon C and Smith S: Tankyrase
1 regulates centrosome function by controlling CPAP stability. EMBO
Rep. 13:724–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim MK and Smith S: Persistent telomere
cohesion triggers a prolonged anaphase. Mol Biol Cell. 25:30–40.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo HL, Zhang C, Liu Q, Li Q, Lian G, Wu
D, Li X, Zhang W, Shen Y, Ye Z, et al: The Axin/TNKS complex
interacts with KIF3A and is required for insulin-stimulated GLUT4
translocation. Cell Res. 22:1246–1257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh TY, Sbodio JI and Chi NW: Mitotic
phosphorylation of tankyrase, a PARP that promotes spindle
assembly, by GSK3. Biochem Biophys Res Commun. 350:574–579. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levaot N, Voytyuk O, Dimitriou I,
Sircoulomb F, Chandrakumar A, Deckert M, Krzyzanowski PM, Scotter
A, Gu S, Janmohamed S, et al: Loss of Tankyrase-mediated
destruction of 3BP2 is the underlying pathogenic mechanism of
cherubism. Cell. 147:1324–1339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang DH, Lee DJ, Lee S, Lee SY, Jun Y, Kim
Y, Kim Y, Lee JS, Lee DK, Lee S, et al: Interaction of tankyrase
and peroxiredoxin II is indispensable for the survival of
colorectal cancer cells. Nat Commun. 8:402017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Croy HE, Fuller CN, Giannotti J, Robinson
P, Foley AV, Yamulla RJ, Cosgriff S, Greaves BD, von Kleeck RA, An
HH, et al: The poly(ADP-ribose) polymerase enzyme tankyrase
antagonizes activity of the β-catenin destruction complex through
ADP-ribosylation of axin and APC2. J Biol Chem. 291:12747–12760.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang W, Li N, Li X, Tran MK, Han X and
Chen J: Tankyrase inhibitors target YAP by stabilizing angiomotin
family proteins. Cell Rep. 13:524–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tripathi E and Smith S: Cell
cycle-regulated ubiquitination of tankyrase 1 by RNF8 and
ABRO1/BRCC36 controls the timing of sister telomere resolution.
EMBO J. 36:503–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuusela S, Wang H, Wasik AA, Suleiman H
and Lehtonen S: Tankyrase inhibition aggravates kidney injury in
the absence of CD2AP. Cell Death Dis. 7:e23022016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Han H, Zhou MT, Yang B, Ta AP, Li N,
Chen J and Wang W: Proteomic analysis of the human tankyrase
protein interaction network reveals its role in pexophagy. Cell
Rep. 20:737–749. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang W, Dynek JN and Smith S: TRF1 is
degraded by ubiquitin-mediated proteolysis after release from
telomeres. Genes Dev. 17:1328–1333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang W, Huang J and Chen J:
Angiomotin-like proteins associate with and negatively regulate
YAP1. J Biol Chem. 286:4364–4370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ha GH, Kim HS, Go H, Lee H, Seimiya H,
Chung DH and Lee CW: Tankyrase-1 function at telomeres and during
mitosis is regulated by Polo-like kinase-1-mediated
phosphorylation. Cell Death Differ. 19:321–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chi NW and Lodish HF: Tankyrase is a
golgi-associated mitogen-activated protein kinase substrate that
interacts with IRAP in GLUT4 vesicles. J Biol Chem.
275:38437–38444. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan Y and Lackner MR: FOXO3a and β-catenin
co-localization: Double trouble in colon cancer? Nat Med.
18:854–856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bisht KK, Dudognon C, Chang WG, Sokol ES,
Ramirez A and Smith S: GDP-mannose-4,6-dehydratase is a cytosolic
partner of tankyrase 1 that inhibits its poly(ADP-ribose)
polymerase activity. Mol Cell Biol. 32:3044–3053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaminker PG, Kim SH, Taylor RD,
Zebarjadian Y, Funk WD, Morin GB, Yaswen P and Campisi J: TANK2, a
new TRF1-associated poly(ADP-ribose) polymerase, causes rapid
induction of cell death upon overexpression. J Biol Chem.
276:35891–35899. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cerone MA, Burgess DJ, Naceur-Lombardelli
C, Lord CJ and Ashworth A: High-throughput RNAi screening reveals
novel regulators of telomerase. Cancer Res. 71:3328–3340. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seimiya H, Muramatsu Y, Ohishi T and
Tsuruo T: Tankyrase 1 as a target for telomere-directed molecular
cancer therapeutics. Cancer Cell. 7:25–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu H, Lei Z, Lu Z, Lu Q, Lu C, Chen W,
Wang C, Tang Q and Kong Q: Silencing tankyrase and telomerase
promotes A549 human lung adenocarcinoma cell apoptosis and inhibits
proliferation. Oncol Rep. 30:1745–1752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang H, Yang MH, Zhao JJ, Chen L, Yu ST,
Tang XD, Fang DC and Yang SM: Inhibition of tankyrase 1 in human
gastric cancer cells enhances telomere shortening by telomerase
inhibitors. Oncol Rep. 24:1059–1065. 2010.PubMed/NCBI
|
|
43
|
Lin L, Sabnis AJ, Chan E, Olivas V, Cade
L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al: The Hippo
effector YAP promotes resistance to RAF- and MEK-targeted cancer
therapies. Nat Genet. 47:250–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rubinfeld B, Albert I, Porfiri E, Fiol C,
Munemitsu S and Polakis P: Binding of GSK3beta to the
APC-beta-catenin complex and regulation of complex assembly.
Science. 272:1023–1026. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cancer Genome Atlas Network, .
Comprehensive molecular characterization of human colon and rectal
cancer. Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lau T, Chan E, Callow M, Waaler J, Boggs
J, Blake RA, Magnuson S, Sambrone A, Schutten M, Firestein R, et
al: A novel tankyrase small-molecule inhibitor suppresses APC
mutation-driven colorectal tumor growth. Cancer Res. 73:3132–3144.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Waaler J, Machon O, Tumova L, Dinh H,
Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova
O, et al: A novel tankyrase inhibitor decreases canonical Wnt
signaling in colon carcinoma cells and reduces tumor growth in
conditional APC mutant mice. Cancer Res. 72:2822–2832. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu X, Luo F, Li J, Zhong X and Liu K:
Tankyrase 1 inhibitior XAV939 increases chemosensitivity in colon
cancer cell lines via inhibition of the Wnt signaling pathway. Int
J Oncol. 48:1333–1340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nguyen DX, Chiang AC, Zhang XH, Kim JY,
Kris MG, Ladanyi M, Gerald WL and Massagué J: WNT/TCF signaling
through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis.
Cell. 138:51–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pacheco-Pinedo EC, Durham AC, Stewart KM,
Goss AM, Lu MM, Demayo FJ and Morrisey EE: Wnt/β-catenin signaling
accelerates mouse lung tumorigenesis by imposing an embryonic
distal progenitor phenotype on lung epithelium. J Clin Invest.
121:1935–1945. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Busch AM, Johnson KC, Stan RV, Sanglikar
A, Ahmed Y, Dmitrovsky E and Freemantle SJ: Evidence for tankyrases
as antineoplastic targets in lung cancer. BMC Cancer. 13:2112013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Casás-Selves M, Kim J, Zhang Z, Helfrich
BA, Gao D, Porter CC, Scarborough HA, Bunn PA Jr, Chan DC, Tan AC
and DeGregori J: Tankyrase and the canonical Wnt pathway protect
lung cancer cells from EGFR inhibition. Cancer Res. 72:4154–4164.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Halder G and Johnson RL: Hippo signaling:
Growth control and beyond. Development. 138:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: An
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dong J, Feldmann G, Huang J, Wu S, Zhang
N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D:
Elucidation of a universal size-control mechanism in Drosophila and
mammals. Cell. 130:1120–1133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mo JS, Park HW and Guan KL: The Hippo
signaling pathway in stem cell biology and cancer. EMBO Rep.
15:642–656. 2014.PubMed/NCBI
|
|
59
|
Wang H, Lu B, Castillo J, Zhang Y, Yang Z,
McAllister G, Lindeman A, Reece-Hoyes J, Tallarico J, Russ C, et
al: Tankyrase inhibitor sensitizes lung cancer cells to Endothelial
Growth Factor Receptor (EGFR) inhibition via stabilizing
angiomotins and inhibiting YAP signaling. J Biol Chem.
291:15256–15266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Steck PA, Pershouse MA, Jasser SA, Yung
WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T,
et al: Identification of a candidate tumour suppressor gene, MMAC1,
at chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI,
Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, et al: Germline
mutations of the PTEN gene in Cowden disease, an inherited breast
and thyroid cancer syndrome. Nat Genet. 16:64–67. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Losada A and Hirano T: Dynamic molecular
linkers of the genome: The first decade of SMC proteins. Genes Dev.
19:1269–1287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nasmyth K and Haering CH: The structure
and function of SMC and kleisin complexes. Annu Rev Biochem.
74:595–648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ozaki Y, Matsui H, Asou H, Nagamachi A,
Aki D, Honda H, Yasunaga S, Takihara Y, Yamamoto T, Izumi S, et al:
Poly-ADP ribosylation of Miki by tankyrase-1 promotes centrosome
maturation. Mol Cell. 47:694–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Boveri T: Concerning the origin of
malignant tumours by Theodor Boveri. Translated and annotated by
Henry Harris. J Cell Sci. 121 Suppl 1:S1–S84. 2008. View Article : Google Scholar
|
|
67
|
Duensing S and Münger K: Centrosome
abnormalities, genomic instability and carcinogenic progression.
Biochim Biophys Acta. 1471:M81–M88. 2001.PubMed/NCBI
|
|
68
|
Ganem NJ, Godinho SA and Pellman D: A
mechanism linking extra centrosomes to chromosomal instability.
Nature. 460:278–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Guerrero AA, Martínez-A C and van Wely KH:
Merotelic attachments and non-homologous end joining are the basis
of chromosomal instability. Cell Div. 5:132010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Korzeniewski N, Hohenfellner M and
Duensing S: The centrosome as potential target for cancer therapy
and prevention. Expert Opin Ther Targets. 17:43–52. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Goodwin JF and Knudsen KE: Beyond DNA
repair: DNA-PK function in cancer. Cancer Discov. 4:1126–1139.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gagné JP, Isabelle M, Lo KS, Bourassa S,
Hendzel MJ, Dawson VL, Dawson TM and Poirier GG: Proteome-wide
identification of poly(ADP-ribose) binding proteins and
poly(ADP-ribose)-associated protein complexes. Nucleic Acids Res.
36:6959–6976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ruscetti T, Lehnert BE, Halbrook J, Le
Trong H, Hoekstra MF, Chen DJ and Peterson SR: Stimulation of the
DNA-dependent protein kinase by poly(ADP-ribose) polymerase. J Biol
Chem. 273:14461–14467. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dregalla RC, Zhou J, Idate RR, Battaglia
CL, Liber HL and Bailey SM: Regulatory roles of tankyrase 1 at
telomeres and in DNA repair: Suppression of T-SCE and stabilization
of DNA-PKcs. Aging (Albany NY). 2:691–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nagy Z, Kalousi A, Furst A, Koch M,
Fischer B and Soutoglou E: Tankyrase promote homologous
recombination and check point activation in response to DSBs. PLoS
Genet. 12:e10057912016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Arqués O, Chicote I, Puig I, Tenbaum SP,
Argilés G, Dienstmann R, Fernández N, Caratù G, Matito J,
Silberschmidt D, et al: Tankyrase inhibition blocks Wnt/β-catenin
pathway and reverts resistance to PI3K and AKT inhibitors in the
treatment of colorectal cancer. Clin Cancer Res. 22:644–656. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bao R, Christova T, Song S, Angers S, Yan
X and Attisano L: Inhibition of tankyrases induces Axin
stabilization and blocks Wnt signalling in breast cancer cells.
PLoS One. 7:e486702012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Quackenbush KS, Bagby S, Tai WM,
Messersmith WA, Schreiber A, Greene J, Kim J, Wang G, Purkey A,
Pitts TM, et al: The novel tankyrase inhibitor (AZ1366) enhances
irinotecan activity in tumors that exhibit elevated tankyrase and
irinotecan resistance. Oncotarget. 7:28273–28285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Stratford EW, Daffinrud J, Munthe E,
Castro R, Waaler J, Krauss S and Myklebost O: The
tankyrase-specific inhibitor JW74 affects cell cycle progression
and induces apoptosis and differentiation in osteosarcoma cell
lines. Cancer Med. 3:36–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tian X, Hou W, Bai S, Fan J, Tong H and Xu
H: XAV939 inhibits the stemness and migration of neuroblastoma
cancer stem cells via repression of tankyrase 1. Int J Oncol.
45:121–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mashima T, Taneda Y, Jang MK, Mizutani A,
Muramatsu Y, Yoshida H, Sato A, Tanaka N, Sugimoto Y and Seimiya H:
mTOR signaling mediates resistance to tankyrase inhibitors in
Wnt-driven colorectal cancer. Oncotarget. 8:47902–47915. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tenbaum SP, Ordóñez-Morán P, Puig I,
Chicote I, Arqués O, Landolfi S, Fernández Y, Herance JR, Gispert
JD, Mendizabal L, et al: β-catenin confers resistance to PI3K and
AKT inhibitors and subverts FOXO3a to promote metastasis in colon
cancer. Nat Med. 18:892–901. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Thomson DW, Wagner AJ, Bantscheff M,
Benson RE, Dittus L, Duempelfeld B, Drewes G, Krause J, Moore JT,
Mueller K, et al: Discovery of a highly selective tankyrase
inhibitor displaying growth inhibition effects against a diverse
range of tumor derived cell lines. J Med Chem. 60:5455–5471. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Keren-Paz A, Emmanuel R and Samuels Y: YAP
and the drug resistance highway. Nat Genet. 47:193–194. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Peters JM and Nishiyama T: Sister
chromatid cohesion. Cold Spring Harb Perspect Biol. 4(pii):
a0111302012.PubMed/NCBI
|
|
86
|
Canudas S and Smith S: Differential
regulation of telomere and centromere cohesion by the Scc3
homologues SA1 and SA2, respectively, in human cells. J Cell Biol.
187:165–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Matovinović MS: Podocyte injury in
glomerular diseases. EJIFCC. 20:21–27. 2009.PubMed/NCBI
|















