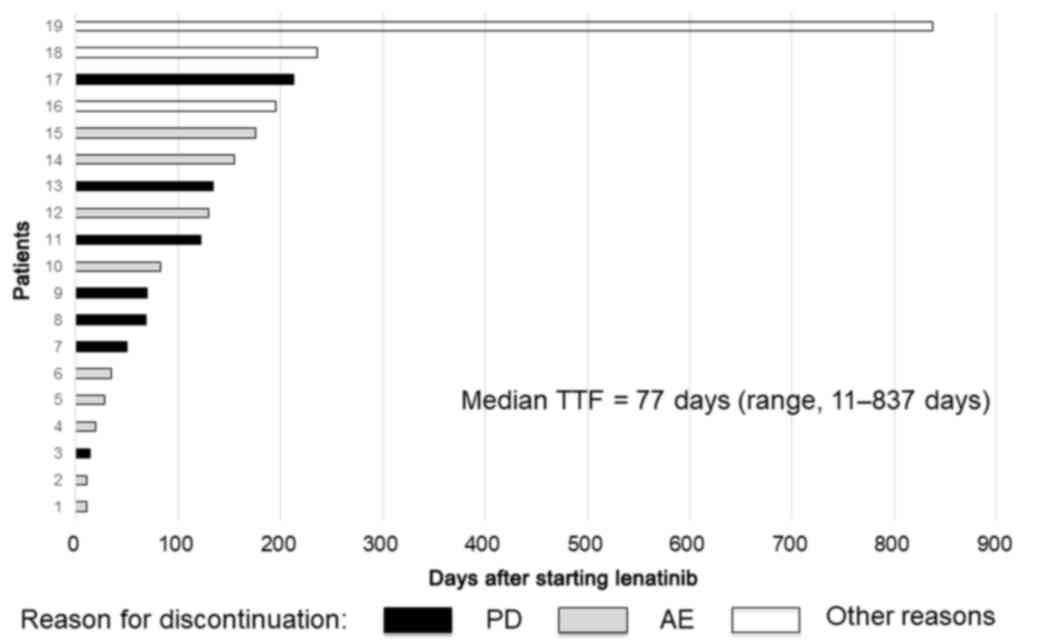
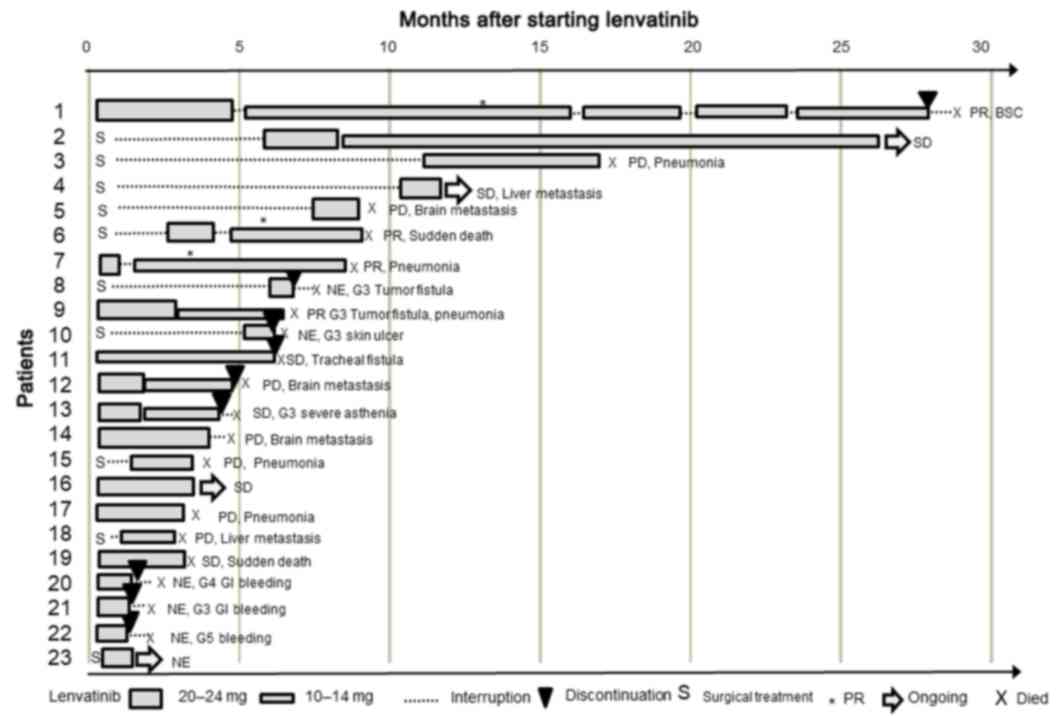
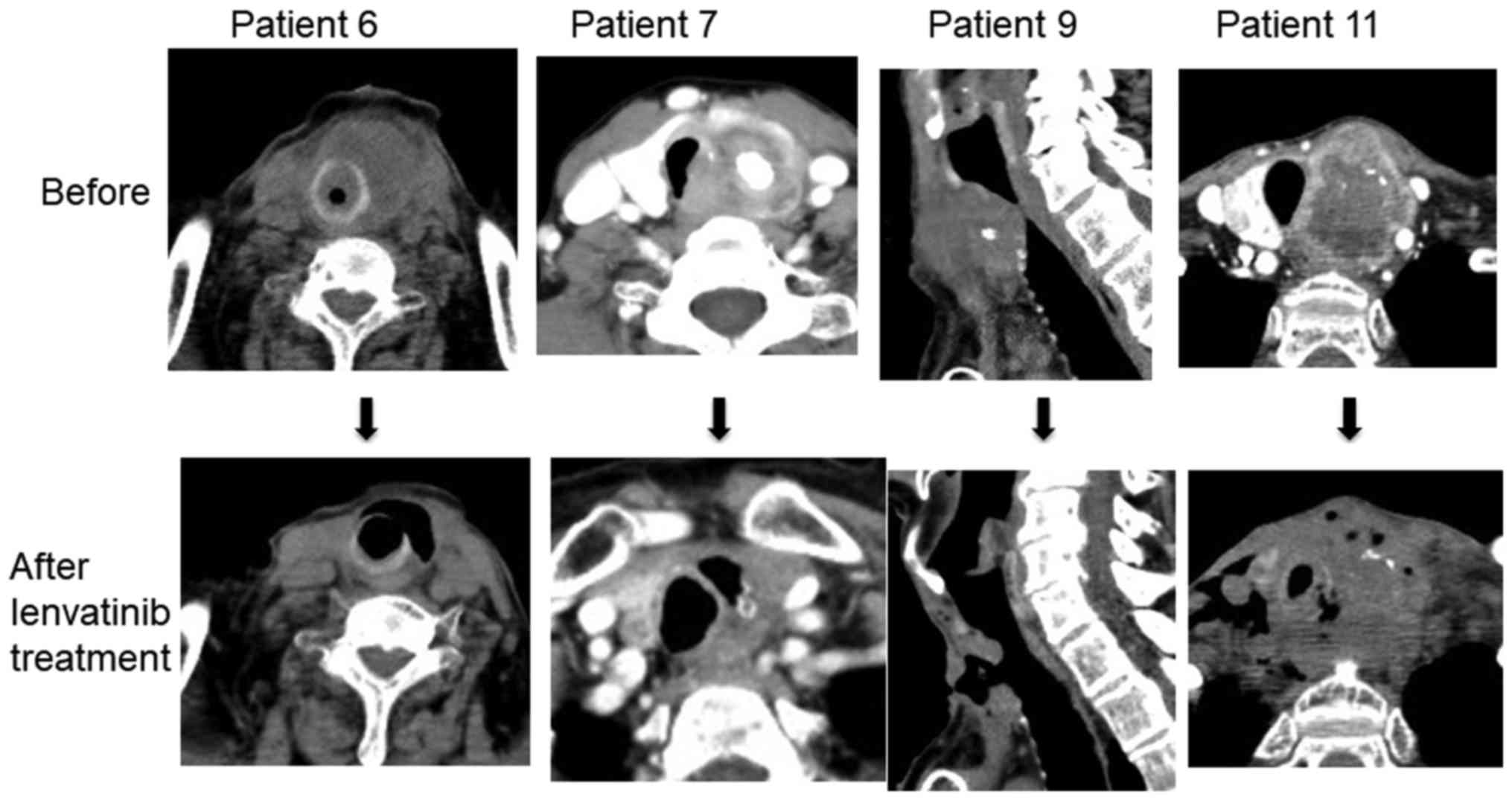
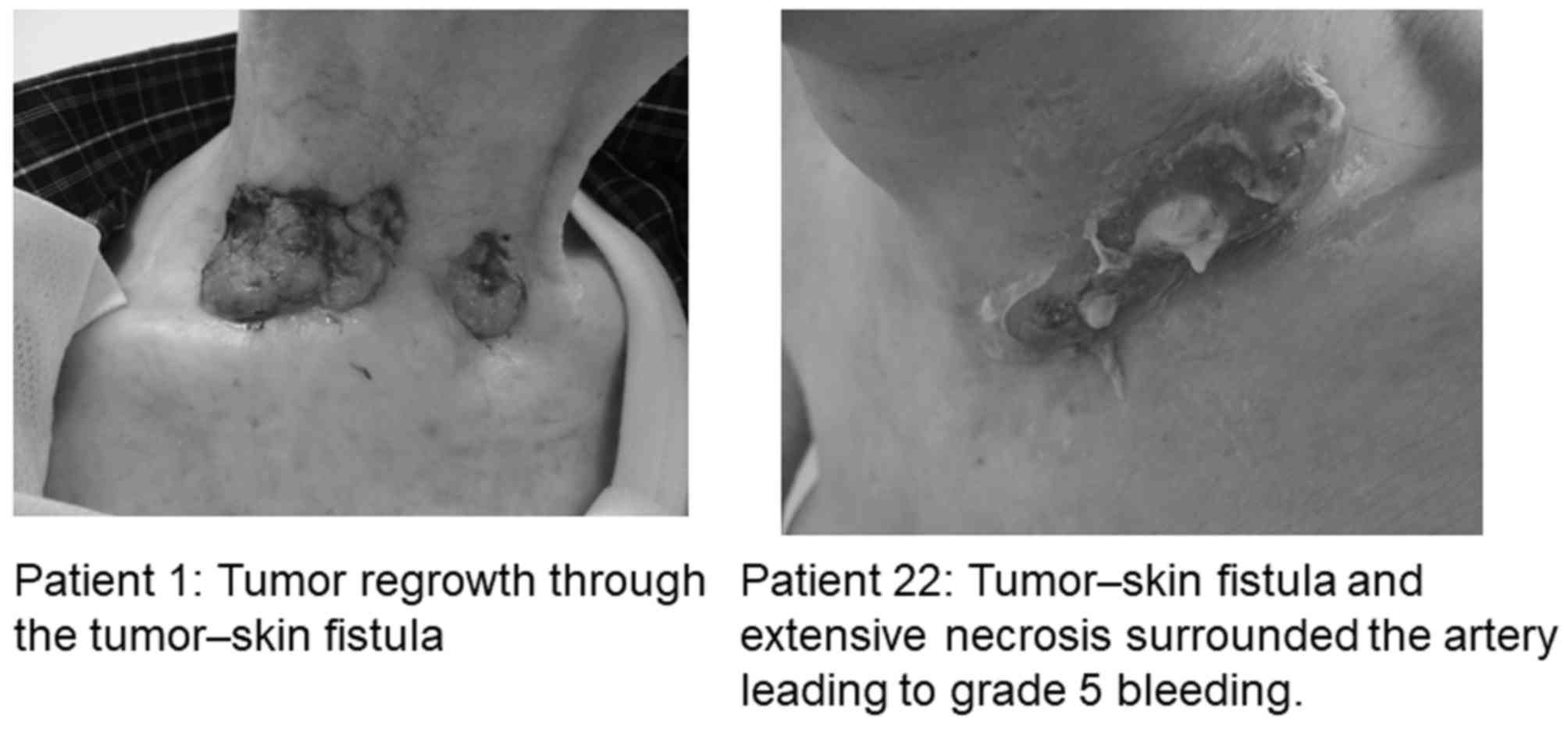
|
1
|
Kilfoy BA, Devesa SS, Ward MH, Zhang Y,
Rosenberg PS, Holford TR and Anderson WF: Gender is an age-specific
effect modifier for papillary cancers of the thyroid gland. Cancer
Epidemiol Biomarkers Prev. 18:1092–1100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lowe NM, Loughran S, Slevin NJ and Yap BK:
Anaplastic thyroid cancer: The addition of systemic chemotherapy to
radiotherapy led to an observed improvement in survival-a single
centre experience and review of the literature. In:
ScientificWorldJournal. 2014:6745832014.
|
|
3
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Venkatesh YS, Ordonez NG, Schultz PN,
Hickey RC, Goepfert H and Samaan NA: Anaplastic carcinoma of the
thyroid. A clinicopathologic study of 121 cases. Cancer.
66:321–330. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smallridge RC and Copland JA: Anaplastic
thyroid carcinoma: Pathogenesis and emerging therapies. Clin Oncol
(R Coll Radiol). 22:486–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neff RL, Farrar WB, Kloos RT and Burman
KD: Anaplastic thyroid cancer. Endocrinol Metab Clin North Am.
37:525–538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oh EM, Lee KE, Kwon H, Kim EY, Bae DS and
Youn YK: Analysis of patients with anaplastic thyroid cancer
expected to have curative surgery. J Korean Surg Soc. 83:123–129.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bisof V, Rakusic Z and Despot M: Treatment
of patients with anaplastic thyroid cancer during the last 20
years: Whether any progress has been made? Eur Arch
Otorhinolaryngol. 272:1553–1567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tahara M, Kiyota N, Yamazaki T, Chayahara
N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, et
al: Lenvatinib for anaplastic thyroid cancer. Front Oncol.
7:252017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsui J and Funahashi Y: Preclinical
biomarker research and patient stratification of molecular target
agents: The anti-angiogenic inhibitor Lenvatinib mesylate (E7080)
(Japanese). Nihon Yakurigaku Zasshi. 142:162–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamazaki H, Shimizu S, Iwasaki H, Yoshida
T, Suganuma N, Yamanaka T, Kojima I, Masudo K, Toda S, Nakayama H
and Masuda M: Efficacy and safety of lenvatinib for unresectable
anaplastic thyroid cancer. Gan To Kagaku Ryoho. 44:695–697.
2017.PubMed/NCBI
|
|
13
|
Tuttle RM, Haugen B and Perrier ND:
Updated american joint committee on cancer/tumor-node-metastasis
staging system for differentiated and anaplastic thyroid cancer
(Eighth edition): What changed and why? Thyroid. 27:751–756. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Japanese translation of common terminology
criteria for adverse events (CTCAE), and instructions and
guidelines (Japanese). Int J Clin Oncol. 9 (Suppl):1–82. 2004.
|
|
16
|
Derbel O, Limem S, Ségura-Ferlay C,
Lifante JC, Carrie C, Peix JL, Borson-Chazot F, Bournaud C, Droz JP
and de la Fouchardière C: Results of combined treatment of
anaplastic thyroid carcinoma (ATC). BMC Cancer. 11:4692011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ain KB, Egorin MJ and DeSimone PA:
Treatment of anaplastic thyroid carcinoma with paclitaxel: Phase 2
trial using ninety-six-hour infusion. Collaborative anaplastic
thyroid cancer health intervention trials (CATCHIT) group. Thyroid.
10:587–594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sosa JA, Elisei R, Jarzab B, Balkissoon J,
Lu SP, Bal C, Marur S, Gramza A, Yosef RB, Gitlitz B, et al:
Randomized safety and efficacy study of fosbretabulin with
paclitaxel/carboplatin against anaplastic thyroid carcinoma.
Thyroid. 24:232–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goffredo P, Thomas SM, Adam MA, Sosa JA
and Roman SA: Impact of timeliness of resection and thyroidectomy
margin status on survival for patients with anaplastic thyroid
cancer: An analysis of 335 cases. Ann Surg Oncol. 22:4166–4174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al: American thyroid association guidelines for management
of patients with anaplastic thyroid cancer. Thyroid. 22:1104–1139.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perrier ND, Brierley JD and Tuttle RM:
Differentiated and anaplastic thyroid carcinoma: Major changes in
the American Joint Committee on cancer eighth edition cancer
staging manual. CA Cancer J Clin. 68:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haigh PI, Ituarte PH, Wu HS, Treseler PA,
Posner MD, Quivey JM, Duh QY and Clark OH: Completely resected
anaplastic thyroid carcinoma combined with adjuvant chemotherapy
and irradiation is associated with prolonged survival. Cancer.
91:2335–2342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haymart MR, Banerjee M, Yin H, Worden F
and Griggs JJ: Marginal treatment benefit in anaplastic thyroid
cancer. Cancer. 119:3133–3139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hui EP, Ma BB, King AD, Mo F, Chan SL, Kam
MK, Loong HH, Ahuja AT, Zee BC and Chan AT: Hemorrhagic
complications in a phase II study of sunitinib in patients of
nasopharyngeal carcinoma who has previously received high-dose
radiation. Ann Oncol. 22:1280–1287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Machiels JP, Henry S, Zanetta S, Kaminsky
MC, Michoux N, Rommel D, Schmitz S, Bompas E, Dillies AF, Faivre S,
et al: Phase II study of sunitinib in recurrent ormetastatic
squamous cell carcinoma of the head and neck: GORTEC 2006-01. J
Clin Oncol. 28:21–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Savvides P, Nagaiah G, Lavertu P, Fu P,
Wright JJ, Chapman R, Wasman J, Dowlati A and Remick SC: Phase II
trial of sorafenib in patients with advanced anaplastic carcinoma
of the thyroid. Thyroid. 23:600–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weitzman SP and Cabanillas ME: The
treatment landscape in thyroid cancer: A focus on cabozantinib.
Cancer Manag Res. 7:265–278. 2015.PubMed/NCBI
|
|
28
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME,
Urbanowitz G, et al: Dabrafenib and trametinib treatment in
patients with locally advanced or metastatic BRAF V600-mutant
anaplastic thyroid cancer. J Clin Oncol. 36:7–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blevins DP, Dadu R, Hu M, Baik C,
Balachandran D, Ross W, Gunn B and Cabanillas ME: Aerodigestive
fistula formation as a rare side effect of antiangiogenic tyrosine
kinase inhibitor therapy for thyroid cancer. Thyroid. 24:918–922.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mani N, McNamara K, Lowe N, Loughran S and
Yap BK: Management of the compromised airway and role of
tracheotomy in anaplastic thyroid carcinoma. Head Neck. 38:85–88.
2016. View Article : Google Scholar : PubMed/NCBI
|