Introduction
Ovarian cancer is the most malignant gynecological
neoplasm occurring in women (1).
According to the cancer statistic data for the United States in
2016, the mortality rate of ovarian cancer is the highest amongst
all gynecological malignancies and is substantially higher compared
with that of cervical cancer and endometrial cancer (1). Although surgery and postoperative
chemotherapy greatly contribute to the survival of the patients,
70% of them will relapse due to drug resistance (2), highlighting the need for novel
therapeutic regimens.
Accumulating evidence demonstrates that signaling
pathways including the Hippo (3,4),
Wnt/β-catenin (5,6) and Notch (7,8) signaling
pathways are involved in the pathogenesis of ovarian cancer. Among
them, the Hippo pathway serves a pivotal role in regulating cell
proliferation and apoptosis, therefore it has received substantial
attention in research (9,10). In normal tissues, the Hippo pathway
functions well in maintaining the size of an organ and tissue
homeostasis (11). However, in a
tumor, the deactivation of the Hippo pathway results in the nuclear
translocation of Yes-associated protein (YAP) (12). As the key component of the Hippo
pathway, YAP facilitates tumorigenesis (13–15).
Additionally, YAP overexpression is associated with drug resistance
(16). Conversely, the cytoplasmic
retention of YAP by cytochalasin D or blebbistatin and the
knockdown of YAP downstream transcriptional factors by short
hairpin RNA may abrogate the drug resistance mediated by YAP
(16,17). Concerning tumorigenesis and drug
resistance, YAP has been recognized as a potential therapeutic
target for the treatment of ovarian cancer.
Mangiferin, a naturally occurring glucosylxanthone,
is an effective anti-neoplastic agent in malignant cancer types
including prostate cancer (18),
nasopharyngeal cancer (19), breast
cancer (20,21) and lung cancer (22). One previous study demonstrated the
anti-neoplastic effect of mangiferin in human lung carcinomas by
inducing caspase-dependent apoptosis via the activation of nuclear
factor-κβ and cyclin B1 (23).
Additionally, the results of another previous study on
mangiferin-treated OVCAR3 cells indicated the inhibitory role of
mangiferin on cell proliferation through the regulation of Notch3
(24). Notably, Notch has been widely
acknowledged as a key downstream gene regulated by the Hippo
pathway (25). Therefore, it was
hypothesized that YAP may be involved in the antitumor effects of
mangiferin. However, until now to the best of our knowledge, no
data on the YAP-mediated neoplastic activities of mangiferin in
ovarian cancer have been reported. The present study aimed to
reveal the pivotal role of the YAP pathway in the
mangiferin-mediated antitumor effect in ovarian cancer.
Materials and methods
Reagents
Human ovarian adenocarcinoma OVCAR8 cells were
purchased from American Type Culture Collection (Manassas, VA,
USA). OVCAR8 cells are highly resistant to cisplatin (26), and respond poorly to high-dose
platinum-based chemotherapy. Additionally, OVCAR8 cells are
resistant to cadmium (26).
Mangiferin was purchased from Shanghai PureOne Bioechnology
(Shanghai, China; www.pureonebio.com), with a purity of >95%.
RPMI-1640 medium and fetal bovine serum (FBS) were purchased from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). MTT (cat.
no. M5655), paraformaldehyde (PFA; cat. no. 16005), cisplatin (cat.
no. 1134357), dimethyl sulfoxide (DMSO; cat. no. D2650), Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis detection kit (cat.
no. APOAF), HEPES (cat. no. H3375), Triton X-100 (cat. no. H9284),
2 mmol/l sodium orthovanadate (cat. no. S6508), sodium fluoride
(cat. no. S7920), 1 mmol/l edetic acid (cat. no. E9884), PMSF (cat.
no. 78830), aprotinin (cat. no. A11530) and leupeptin were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Hoechst 33342 (cat. no. C1025) and a Bradford protein assay (cat.
no. P0006) were purchased from Beyotime Institute of Biotechnology
(Suzhou, China). NuPAGE® Bis-Tris gels (cat. no.
NP0327BOX) were purchased from Thermo Fisher Scientific, Inc.
Polyvinylidene difluoride (PVDF) membranes (cat. no. ISEQ00010) and
electrochemiluminescence (ECL) reagents (cat. no. 345818) were
purchased from EMD Millipore (Billerica, MA, USA). Mouse monoclonal
β-actin antibody (cat. no. sc-47778; 1:5,000 dilution) was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Matrigel (cat. no. 356234) was purchased from BD Biosciences
(Franklin Lakes, NJ, USA). Rabbit polyclonal cleaved caspase-3
antibody (cat. no. 9661; 1:500 dilution) and rabbit polyclonal YAP
antibody (cat. no. 4912; 1:1,000 dilution) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Mouse monoclonal TEA
domain transcription factor 4 (TEAD4) antibody (cat. no. ab58310;
1:1,000 dilution) and rabbit monoclonal cleaved poly(ADP-ribose)
polymerase 1 (PARP1) antibody (cat. no. ab32138; 1:1,000 dilution)
were purchased from Abcam (Cambridge, MA, USA). Horseradish
peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG)
polyclonal antibody (cat. no. 115-035-003; 1:5,000 dilution) and
HRP-conjugated goat anti-rabbit polyclonal IgG (cat. no.
111-035-003; 1:5,000 dilution) were purchased from Jackson
ImmunoResearch Laboratories, Inc. (West Grove, PA, USA).
Cell culture and MTT colorimetric
assay
OVCAR8 cells were cultured in RPMI-1640 which
contained 20% FBS, 10 µg/ml bovine insulin, 100 µg/ml streptomycin
(Thermo Fisher Scientific, Inc.), 100 U/ml penicillin (Thermo
Fisher Scientific, Inc.) and 0.03% L-glutamine (Sigma-Aldrich;
Merck KGaA).
OVCAR8 cells at a logarithmic growth phase were
seeded in a 96-well plate (3×104 cells per well) and
incubated at 37°C for 24 h. For the cell proliferation experiments
examining the inhibitory effect of mangiferin via the YAP pathway,
OVCAR8 cells were treated with mangiferin (25 µg/ml) at 37°C for 24
h following transfection by Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) with either an empty vector (pcDNA3; Addgene,
Inc., Cambridge, MA, USA) or YAP overexpression plasmids for 12,
24, 36 and 48 h. For the experiments examining cisplatin
sensitivity, OVCAR8 cells in three groups were respectively treated
at 37°C for 6 h with cisplatin (1 µg/ml) or cisplatin (1 µg/ml) and
mangiferin (25 µg/ml) combined, following transfection by
Lipofectamine 2000 with either an empty vector (pcDNA3) or YAP
overexpression plasmids at 37°C for 48 h. Subsequently, cells in
each well were treated with 0.05 mg MTT (10 µl of 5 mg/ml) and
incubated at 37°C for 4 h. Subsequent to incubation, the
supernatant was removed. For the suspended cells, following
centrifugation at 200 × g for 5 min at 4°C, the medium was
discarded and 150 µl DMSO was added to each well. The plate was
thoroughly agitated by hand shaking for 10 min. Finally, the
absorbance was measured at a wavelength of 570 nm using a
spectrophotometer (Model 3550 Microplate Reader; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Cell viability was
determined via the following equation: Cell viability (%)=[optical
density (OD) 570 nm (drug)/OD 570 nm (control)] ×100%. Experiments
were repeated three times, and data are representative of replicate
experiments.
Cell colony in soft agar assay
Cells were plated in triplicate wells in 6-well
plates for 14 days for a flat colony formation assay. For the soft
agar assay, 2×103 cells were plated in complete medium
(RPMI-1640 medium plus 10% FBS) in addition to 0.6% agar in
triplicate wells in 6-well plates. Subsequent to transfection with
either an empty vector (pcDNA3) or YAP overexpression plasmids,
mangiferin (25 µg/ml) was added to the medium. Medium was replaced
every 48 h and visible colonies were counted using light microscopy
after 21 days.
Cell morphology and Hoechst 33342
staining
OVCAR8 cells were seeded into a 6-well culture plate
at a density of 4×105 cells/well and cultured for 24 h.
Cells were treated with mangiferin (25 µg/ml) subsequent to the
transfection with either the empty vector (pcDNA3) or YAP
overexpression plasmids. The cell morphology was observed under
phase contrast microscopy (×400 magnification). Hoechst 33342
staining was applied to further detect viable cells. In brief,
cells were fixed with 4% PFA for 30 min at room temperature, and
then cells were washed twice with PBS. Hoechst 33342 (5 µg/ml) was
added and incubated for 15 min at 37°C, and then the cells were
washed and analyzed immediately with a fluorescence microscope
(Olympus Corporation).
Annexin V-FITC/propidium iodide (PI)
flow cytometry
OVCAR8 cells were treated with mangiferin (25 µg/ml)
for 24 h following transfection with either an empty vector
(pcDNA3) or YAP overexpression plasmids. Then, the detached and
adherent cells were collected at 300 × g at 4°C for 5 min. The
cells were labelled for 15 min at room temperature using an Annexin
V-FITC and PI apoptosis detection kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. In detail,
the cells were washed twice with PBS and centrifuged at 300 × g at
4°C for 5 min. The cells were resuspended with binding buffer and
then the Annexin V and PI staining solution was added. At least
1×105 cells were analyzed on a FACS Aria II cytometer
(BD Biosciences). Data analyses were performed using FlowJo
Software version 9.7.1 (FlowJo LLC, Ashland, OR, USA). Experiments
were repeated three times, and data are representative of replicate
experiments.
Wound healing assay
The migration of cells in the logarithmic growth was
examined through a wound healing assay. Cells were cultured in a
12-well plate at a density of 5×104 cells/well until a
confluent monolayer was formed. A 200 µl pipette tip was used to
scratch the wells and the initial with of the scratch was ~100 µm.
Then, the wells were rinsed with phosphate buffered saline (PBS)
and cultured in RPMI-1640 medium with 10% FBS at 37°C for 24 h. The
cells were treated with 25 µg/ml mangiferin at 37°C for 24 h
subsequent to transfection with either an empty vector (pcDNA3) or
YAP overexpression plasmids. Images were obtained through light
microscopy (Olympus CKX31; Olympus Corporation, Tokyo, Japan). The
cell migration was measured by Image J software (version 1.48;
National Institutes of Health, Bethesda, MD, USA) with a
contrasting quantification of pixels in the area of the scratch at
24 h. Migration inhibition rate (%)=(1-experimental group
pixels/control group pixels) ×100%.
Matrigel cell invasion assays
A Matrigel cell invasion assay was performed using
Transwell cell culture chambers (8-mm pore size; EMD Millipore).
Transwell membranes were firstly coated with 100 µl Matrigel matrix
(1 mg/ml; BD Biosciences). OVCAR8 cells (5×104
cells/well) were added to serum-free RPMI-1640 medium and placed in
the upper chamber of the Transwell insert and incubated at 37°C for
24 h with 25 µg/ml mangiferin subsequent to transfection with
either an empty vector (pcDNA3) or YAP overexpression plasmids.
RPMI-1640 containing 20% FBS was added to the lower chamber as a
chemoattractant. After 24 h incubation at 37°C, the upper surface
of the chambers was scraped using a cotton swab. The cells on the
lower surface were fixed with 4% PFA in PBS for 30 min at 37°C.
Subsequent to this, the chambers were rinsed with PBS and cells in
the lower chamber were manually counted and analyzed under a light
microscope at a ×200 magnification.
Western blot analysis
OVCAR8 cells were transfected with either an empty
vector (pcDNA3) or YAP overexpression plasmids using Lipofectamine
2000 (Thermo Fisher Scientific, Inc.) for 24 h at 37°C, and then
incubated with 25 µg/ml mangiferin for a further 24 h. Adherent and
floating cells were collected. The cell pellets were resuspended in
a lysis buffer and lysed at 4°C for 15 min. The lysis buffer
consisted of 50 mmol/l HEPES (pH 7.4), 1% Triton X-100, 2 mmol/l
sodium orthovanadate, 100 mol/l sodium fluoride, 1 mmol/l edetic
acid, 1 mmol/l PMSF, 10 mg/l aprotinin and 10 mg/l leupeptin.
Centrifugation at 12,000 × g for 15 min at 4°C was performed and
followed by the determination of supernatant protein content using
a Bradford protein assay (cat. no. P0006; Beyotime Institute of
Biotechnology). Equal quantities (10 µg) of the total protein were
separated using 4–12% NuPAGE® Bis-Tris gels (Thermo
Fisher Scientific, Inc.) and were transferred onto PVDF membranes
(EMD Millipore). The membranes were soaked in blocking buffer (5%
bovine serum albumin; Sigma-Aldrich; Merck KGaA). Then the proteins
of interest were detected using primary antibodies (incubated
overnight at 4°C) and secondary antibodies (incubated for 1 h at
room temperature), which was finally visualized using ECL (EMD
Millipore). Experiments were repeated three times. Data are
representative of replicate experiments and analyzed using ImageJ
software (version 1.44; National Institutes of Health, Bethesda,
MD, USA).
Luciferase assay
OVCAR8 cells were transfected with either an empty
vector (pcDNA3) or YAP overexpression plasmids by Lipofectamine
2000 for 24 h at 37°C, and then incubated with 25 µg/ml mangiferin
for another 24 h. Cells were grown in RPMI-1640 medium supplemented
with 20% FBS and puromycin (Thermo Fisher Scientific, Inc.) for
selection. A total of 5×104 cells in 24-well plates were
transfected at 37°C for 24 h with 0.5 µg synthetic 8×GTIIC TEAD
luciferase promoter plasmids (Addgene, Inc.) and 0.1 ug Renilla
luciferase control reporter (Promega Corporation, Madison, WI,
USA), using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.).
Single (5′-TGTGGAATGTGT-3′) and tandem
(5′-TGTGGAATGTGTGGAATGTGT-3′) TEAD4 binding sites were cloned
upstream of the CMV promoter of the pGL4-hRluc vector. A luciferase
assay was performed 24 h after transfection using a Dual-Luciferase
Reporter Assay system (Promega Corporation) according to the
manufacturer's protocol. Luminescent signaling was detected using a
GloMax-96 Microplate Luminometer (Promega Corporation) according to
the manufacturer's protocol.
Plasmids and lentivirus
preparation
The polymerase chain reaction (PCR) products for YAP
with a restriction enzyme cutting site (EcoRI and XbaI) and pcDNA3
(Addgene, Inc.) with the same sticky ends were obtained and ligated
by T4 DNA ligase (Tiangen Biotech, Co., Ltd., Beijing, China). In
detail, the RNA of OVCAR8 cells were extracted using a Qiagen
RNeasy kit (Qiagen, Inc., Valencia, CA, USA), according to the
manufacturers protocol and reverse transcribed to cDNA using a
reverse transcription system (Promega Corporation), which was used
as the template. For the reverse transcription protocol, RNA was
incubated at 70°C for 10 min, centrifuged at 1,000 x g at
4°C for 20 sec and then placed on ice. Then with the random primers
provided in the kit, dNTP mixture, AMV reverse transcriptase,
MgCl2, buffer and nuclease free water were added to the
microcentrifuge tube. The reaction mixture was incubated at room
temperature for 10 min, then incubated at 42°C for 15 min. The
sample was heated at 95°C for 5 min, then incubated at 5°C for 5
min. The YAP gene was amplified using PCR from cDNA using the
following primers: Forward (containing an EcoRI restriction site as
underlined), 5′-GAATTCGAGGCAGAAGCCATGG-3′ and reverse (containing a
XbaI restriction site as underlined),
5′-TAGAGCTCTATAACCATGTAAGAAAGCT-3′. The thermocycling conditions
were as follows: 95°C for 180 sec to open the template, then 95°C
for 30 sec, 55°C for 30 sec and 72°C for 60 sec for 35 cycles.
Further extension was performed at 72°C for 10 min, and then
maintained at 4°C. Then the PCR products were electrophoresed using
1% agarose (Sigma-Aldrich; Merck KGaA), stained with ethidium
bromide (10 mg/ml; Tiangen Biotech, Co., Ltd.), visualized under
ultraviolet light, and then purified using a gel purification kit
(Tiangen Biotech, Co., Ltd.), according to the manufacturers
protocols. All restriction enzymes and the T4 DNA ligase were
purchased from Tiangen Biotech, Co., Ltd. The insertion of YAP in
pcDNA3 was performed and confirmed by sequencing.
The PCR product of YAP (as described above) was
cloned into pTY linkers. Third-generation vectors were used in this
experiment. A total of 2 µg YAP lentiviral vectors were transiently
transfected into 1×105 OVCAR8 cells using Lipofectamine
2000 at 37°C for 48 h. Briefly, OVCAR8 cells, cultured in DMEM
medium plus 10% FBS, were co-transfected with 2 µg vector plasmids,
including a helper construct, envelope plasmid, tat plasmid and pTY
linker containing YAP. Then the viral supernatant was harvested at
48 h, filtered through a 0.45-µm filter, subjected to
ultracentrifugation (113,000 × g at 4°C for 2 h) for a 100-fold
concentration and stored at −80°C. Then, the lentiviral supernatant
was thawed at 37°C and diluted in 0.9% saline (Sichuan Kelun
Pharmaceutical, Co., Ltd., Chengdu, Sichuan, China) and polybrene
(8 µg/ml final concentration; Sigma-Aldrich; Merck KGaA) to produce
a dose of 1.6×107 transducing units in a 50 µl injection
volume. The virus was injected intravenously into the BALB/c nude
mice with a 30-gauge needle on a 1-cc syringe. A total of 3
consecutive injections were administered at 3-day intervals
(n=6).
Tumor volume and survival assay in
vivo
A total of 5×106 OVCAR8 cells were
injected into BALB/c nude female mice (n=60 in total and n=20 per
group; 5–6 weeks old; 16–18 g body weight; purchased from the
Affiliated Laboratory Animal Center of Sichuan Academy of Medical
Science and Sichuan Provincial People's Hospital, Chengdu, China).
All animals were maintained at 26°C at 40–60% humidity in a 12 h
light/dark cycle with ad libitum access to water and food,
which was in accordance with the individually ventilated cages
requirements at the Sichuan Academy of Medical Science and Sichuan
Provincial People's Hospital. Prior to the initiation of daily
treatment, the tumors were allowed to grow to a size of 100–550
mm3. No mouse bearing multiple tumors was observed in
the present study. During the present study, the maximum body loss
in an animal due to cachexia was 11.3%, and the longest diameter
exhibited by a single subcutaneous tumor was 1.2 cm, which is
smaller compared with the Institutional Animal Care and Use
Committee Guidelines (2.0 cm) of the American Association for
Laboratory Animal Science (https://www.aalas.org/), therefore it was confirmed
that the tumor burden did not exceed the recommended dimensions.
Subsequent to tumor formation by OVCAR8 cells, mice were randomly
divided into three groups: i) A cisplatin group, where cisplatin
(10 mg/kg) was intraperitoneally administered; 2) A cisplatin and
mangiferin plus empty virus transfection group, where cisplatin (10
mg/kg) and mangiferin (50 mg/kg) were intraperitoneally
administered; 3) A cisplatin and mangiferin plus YAP overexpression
lentivirus transfection group, where cisplatin (10 mg/kg) and
mangiferin (50 mg/kg) were intraperitoneally administered. The
treatment lasted for a further two weeks. All experiments were
ethically approved by the ethics committee of Sichuan Academy of
Medical Science and Sichuan Provincial People's Hospital.
The body weight of the mice was measured daily prior
to and following the mangiferin treatment. Then, 5 mice in each
group were sacrificed, and 5 mice in each group were raised for a
survival assay. For the sacrificed mice, the subcutaneous tumors
were removed and weighed, while the volume of the tumors was
determined in 3 dimensions with venire calipers according to the
following formula: Tumor volume=length × width × depth ×0.5. After
20 days of treatment, mice were sacrificed by cervical dislocation,
and subcutaneous tumor masses were determined.
Statistical analysis
All data were expressed as the mean ± standard error
of the mean from at least three independent experiments. Data
analysis was performed using GraphPad Prism 5.0 software (Graphpad
Software, Inc., La Jolla, CA, USA), and P<0.05 was considered to
indicate a statistically significant difference. One-way analysis
of variance followed by Bonferroni's post-hoc test, two-way
analysis of variance followed by Bonferroni's post-hoc test and a
Student's t-test (paired) were performed to determine the
statistical significance. All experiments were performed at least
in triplicate and repeated at least three times, and the data are
representative of replicate experiments.
Results
Mangiferin inhibits cell proliferation
via the regulation of YAP
One previous study revealed that mangiferin may
inhibit the proliferation and induce the apoptosis of ovarian
cancer cells through the regulation of Notch3, which is a
downstream effector of YAP (24).
Thus, it was hypothesized that YAP may also be involved in the
antitumor efficacy of mangiferin. To verify this hypothesis and
elucidate the mechanism of mangiferin inhibiting cell
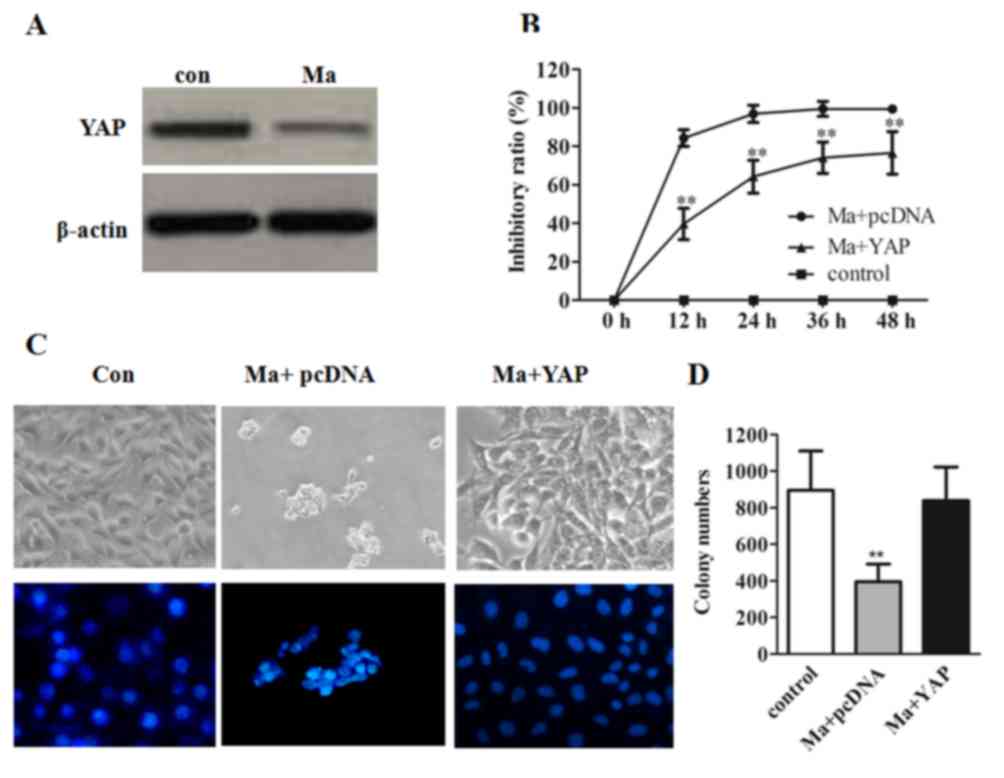
proliferation, YAP protein expression was assessed. As presented in
Fig. 1A, a comparatively decreased
expression of YAP was observed in mangiferin-treated cells. To
further validate that YAP was involved in mangiferin-inhibited cell
proliferation, a time course study for the mangiferin-treated group
and YAP-overexpressed mangiferin-treated group (abbreviated as the
YAP-overexpressed group) was performed. As predicted, compared with
the mangiferin-treated group, the inhibitory rate of the
YAP-overexpressed group was significantly decreased (P<0.01;
Fig. 1B). In addition, based on cell
fluorescent staining and cell morphology images, OVCAR8 cells
treated with mangiferin demonstrated shrinkage of the cytoplasm and
a condensed nucleus. However, a greater number of viable cells were
observed in YAP overexpressed cells, which indicated that
mangiferin may mediate cell apoptosis via the inhibition of YAP
(Fig. 1C). Additionally, the
quantified cell colony numbers revealed a significantly higher
number of colonies in the YAP-overexpressed group compared with the
mangiferin-treated group (P<0.01) further substantiated that YAP
may facilitate cell growth by counteracting the effects of
mangiferin (Fig. 1D).
Mangiferin induces apoptosis via the
regulation of YAP
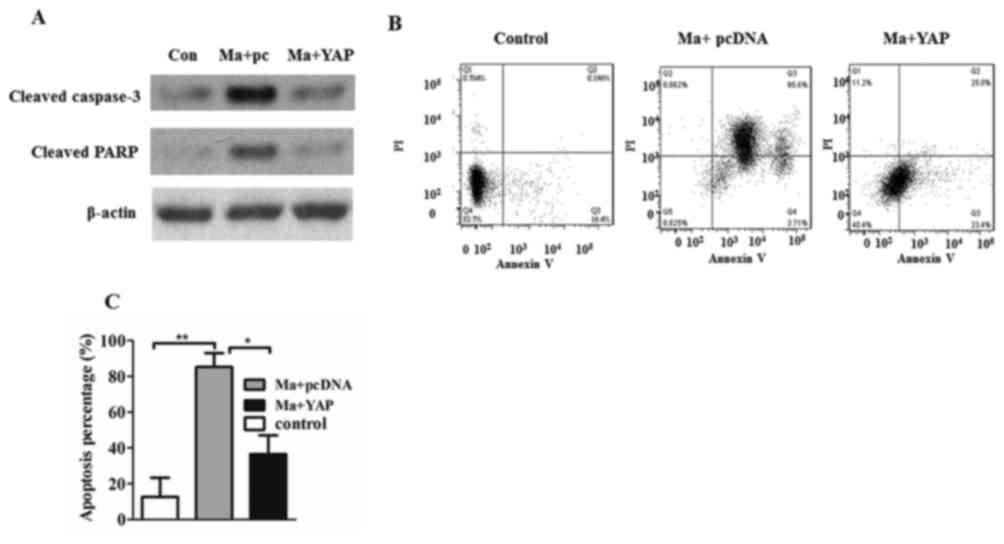
As demonstrated in Fig.
2A, increased caspase-3 cleavage and PARP cleavage were
produced in mangiferin-treated cells, compared with in the
mangiferin-treated YAP-overexpressed cells. To further demonstrate
that mangiferin may induce apoptosis through the inhibition of YAP,
cells were stained with Annexin V and PI, and the apoptotic cell
percentages were analyzed using flow cytometry. As presented in
Fig. 2B, there were more early
apoptotic cells (Annexin V+/PI−) and late
apoptotic cells (Annexin V+/PI+) in the
mangiferin-treated group compared with the YAP-overexpressed cells
(Fig. 2B). Quantified apoptotic
percentages further revealed that mangiferin-induced apoptosis was
significantly inhibited by the overexpression of YAP (P<0.05;
Fig. 2C).
Mangiferin suppresses migration and
invasion
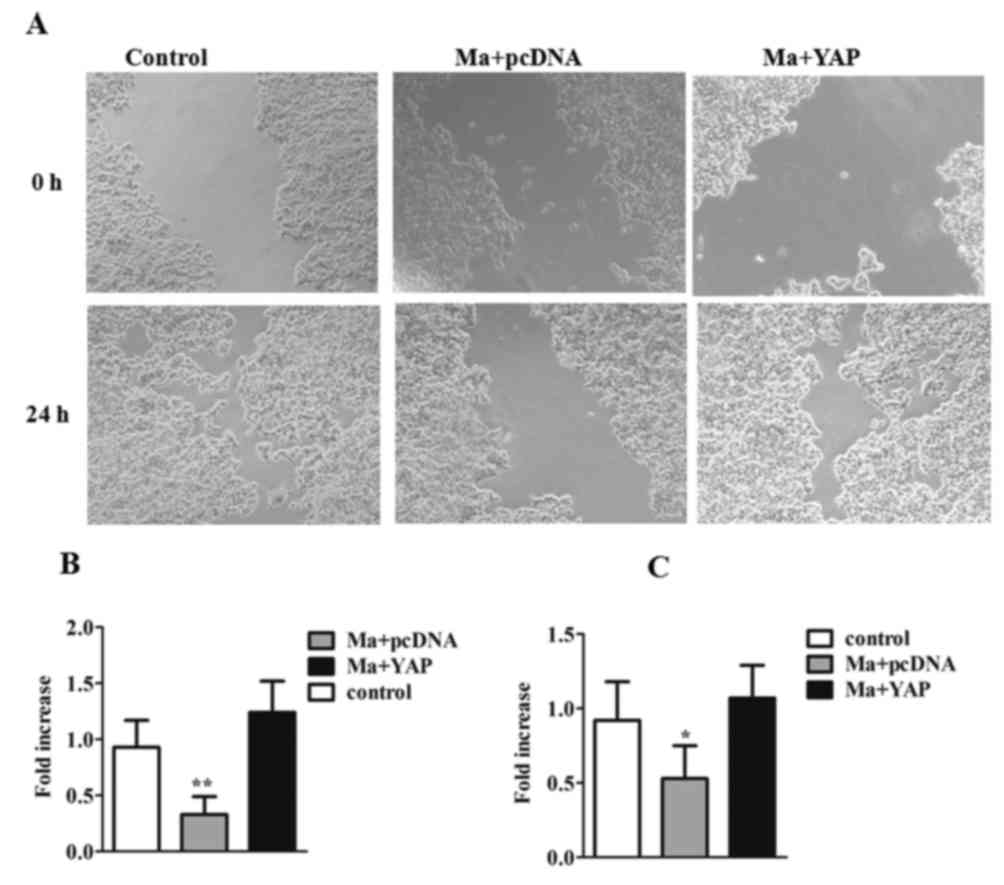
To further investigate the migration capability of
tumor cells and to address the association between
mangiferin-inhibited cell migration and YAP, a wound healing assay
was performed and the images of the cell migration were captured
through microscopy (Fig. 3A and B).
After 24 h of treatment, cell fusion was observed in the
mangiferin-treated YAP-overexpressed group, with a significant
increase in the fold increase of migrated cells compared with the
mangiferin-treated group (P<0.01). Conversely, a scratch was
clearly observed in the mangiferin-treated group at 24 h. These
data indicated that mangiferin may effectively suppress tumor cell
migration, and that this suppression may be reversed by YAP
overexpression. Additionally, a cell invasion assay was performed.
As revealed in Fig. 3C, the data
suggested that YAP was able to significantly reverse the
mangiferin-suppressed invasion capability (P<0.05), further
validating the involvement of YAP in mangiferin-inhibited migration
and invasion events in OVCAR8 cells.
Mangiferin results in the
downregulation of YAP
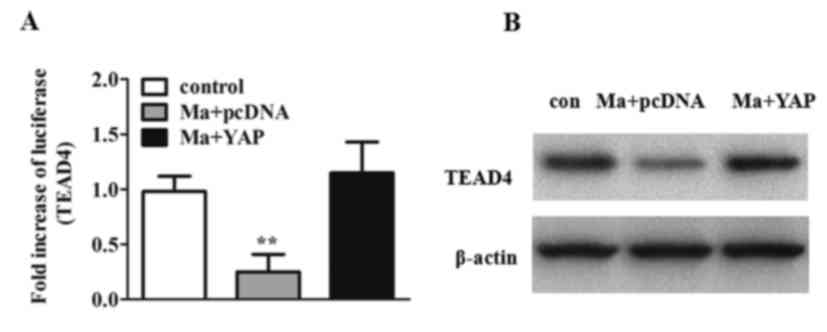
To further investigate the molecular mechanism of
mangiferin, a luciferase assay was performed. As presented in
Fig. 4A, mangiferin reduced
TEAD4-dependent luciferase activity, whereas YAP overexpression
resulted in the significant activation of TEAD4-dependent
luciferase activity (P<0.01). Furthermore, western blot analysis
of TEAD4 was performed. It was revealed that TEAD4 protein levels
were decreased upon mangiferin treatment and elevated in
YAP-overexpressed cells (Fig.
4B).
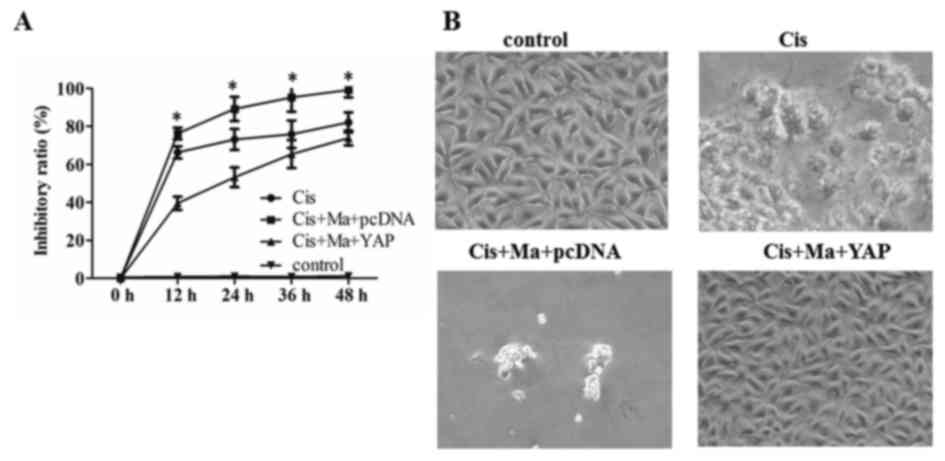
Mangiferin enhances the sensitivity of
OVCAR8 cells to cisplatin
To address the effect of YAP on the enhanced
chemotherapy sensitivity induced by mangiferin, the proliferation
of YAP-overexpressing OVCAR8 cells in the presence of mangiferin
and cisplatin was assessed. As presented in Fig. 5A, cisplatin combined with mangiferin
was able to inhibit cell proliferation, which may be abrogated by
YAP overexpression. To further validate this hypothesis, cell
morphology was observed under phase contrast microscopy. As
presented in Fig. 5B, compared with
the cisplatin-treated group, fewer viable cells were observed in
the mangiferin and cisplatin combined-treated cells, whereas a
greater number of viable cells were observed in the
YAP-overexpressed OVCAR8 cells. Altogether, these results suggested
that mangiferin enhanced the sensitivity of OVCAR8 cells to
cisplatin via the inhibition of YAP.
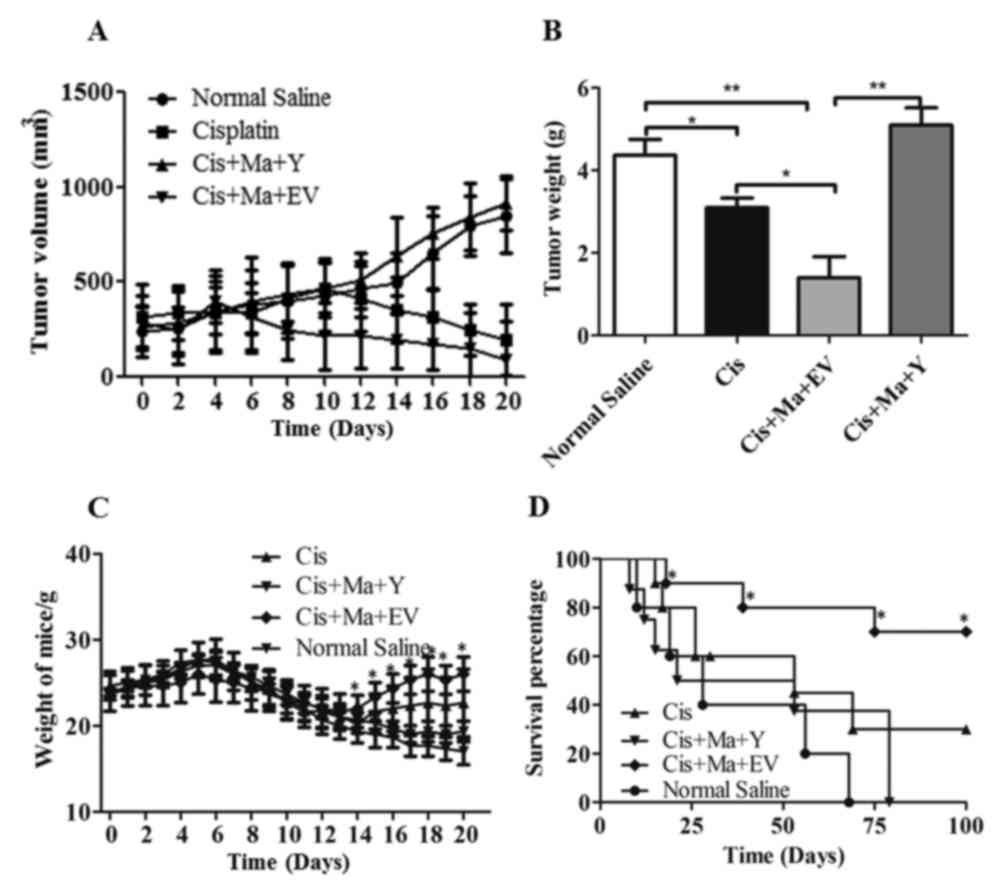
Mangiferin decreases tumor volume in
vivo
To evaluate the effect of YAP on the anti-neoplastic
properties of mangiferin and mangiferin-increased tumor sensitivity
to cisplatin in an in vivo tumor xenograft model system,
OVCAR8 cells were xenografted into nude mice. As presented in
Fig. 6A, the xenograft tumor size
decreased significantly in the empty vector-transfected mangiferin
and cisplatin combined-treated group following a 20-day treatment,
compared with that of the cisplatin monotherapy group (P<0.05).
However, in the YAP-overexpressed mangiferin and cisplatin
combined-treated group, the xenograft tumor size was increased.
Similarly, the empty vector and mangiferin and cisplatin-treated
mice also demonstrated significantly decreased xenograft tumor
weights compared with the cisplatin monotherapy group (P<0.05;
Fig. 6B), suggesting that mangiferin
may increase the sensitivity of ovarian tumor types to cisplatin.
In accordance with the decreased tumor size, the body weight of the
mice in the mangiferin and cisplatin-treated group also
demonstrated a significant increase compared with the cisplatin
monotherapy group (P<0.05; Fig.
6C). Furthermore, the survival days for the YAP-overexpressed
mangiferin and cisplatin-treated mice were 15, 17, 26, 53 and 69
days. The survival days for mice treated with mangiferin and
cisplatin were 36, 39, 75, 100 and 100. But for the control mice
treated with cisplatin alone, the survival days were 15, 33, 53, 79
and 100 days. Therefore, judging from the survival data, mangiferin
in addition to cisplatin treatment may increase the life span of
ovarian cell xenograft mice compared with any other groups
(Fig. 6D). Altogether, the in
vivo results suggested that mangiferin increased cisplatin
sensitivity via inhibition of the YAP pathway.
Discussion
As the majority of patients are diagnosed at an
advanced stage, ovarian cancer is the fifth leading cause of
mortality amongst women with gynecological malignancies (1). At the advanced stages of ovarian cancer,
extensive intraperitoneal metastases are often observed, partially
contributing to the high mortality of this disease (2,27). At the
beginning of standard treatment, the majority of patients are
sensitive to primary or interval cytoreductive surgery and
platinum-based chemotherapy. However, the majority will relapse
despite completing surgery and chemotherapy, and develop drug
resistance (28). Therefore, it is of
great urgency to develop alternative therapeutic agents for the
treatment of ovarian cancer. Abundantly isolated from different
parts of Mangifera indica L (mango tree), mangiferin has
been identified to be a valuable compound with manifold uses in
immunomodulatory, antidiabetic, hepatoprotective, analgesic,
antioxidative, antiaging, antitumor, anti-bacterial and antiviral
effects (29–33). A previous study demonstrated that
mangiferin reduced the Notch3 protein levels in ovarian cancer
(24). Additionally, YAP is an
ovarian cancer oncogene and promotes ovarian cancer cell
tumorigenesis (4,34,35), and
the Notch signaling pathway is generally acknowledged as the
downstream target of YAP (25). The
Notch signaling pathway is a key determinant of embryogenesis, and
comparative upregulations of several elements of the Notch
signaling pathway and a number of Notch targeted genes were
observed with the activation of YAP (25,36,37). Due
to the robust binding of TEAD4 and YAP to the promoter regions of
Notch2 and SRY-Box 9 (Sox9), C2 and Sox9 were validated to be the
direct transcriptional targets of YAP and TEAD complexes (25,38). In
other words, Hippo/YAP is able to directly regulate the Notch
pathway genes to control Notch signaling. Therefore, based on these
studies, it was hypothesized that mangiferin may induce apoptosis
through the regulation of YAP in ovarian cancer. In the present
study, it was elucidated that mangiferin is able to suppress the
expression of YAP, inhibit cell proliferation, limit migration and
invasion capabilities and induce apoptosis in ovarian cancer cells.
However, these antitumor effects of mangiferin may be abrogated
through the overexpression of YAP. Additionally, luciferase assay
data further verified that mangiferin served an antitumor function
via the downregulation of YAP. Furthermore, data on cell
proliferation, cell morphology, xenografted tumor volume and
weight, mice body weight and mice survival collectively suggested
that mangiferin is able to increase chemotherapy sensitivity in
vitro and in vivo through the inhibition of YAP.
Nevertheless, the present study only investigated the YAP-mediated
antitumor effect of mangiferin in ovarian cancer OVCAR8 cells,
which was a defect of the present study. Thus, follow-up studies
using other cell lines are required to further validate the
mediation effect of YAP on mangiferin-treated ovarian cancer. Aside
from the expression of YAP and Notch signaling in ovarian cancer
cells, there are previous studies reporting on their expression in
clinical samples of ovarian cancer (4,7,8,34,35,39).
Specifically, in the ovarian carcinoma samples from patients with
ovarian cancer, YAP was upregulated and associated with patient
prognosis. The present study aimed to provide solid evidence for
the therapeutic effect of mangiferin on ovarian cancer, and expound
the involved molecular mechanisms.
The Hippo/YAP pathway is an evolutionarily and
functionally conserved signaling network, serving a pivotal
function in controlling organ size by stimulating cell
proliferation and reducing apoptosis (40,41). As an
important protein in the Hippo pathway, YAP may be translocated
into the nucleus where it binds with TEAD. The binding of YAP with
TEAD consequently contributes to cell proliferation, apoptosis,
evasion and the amplification of stem cells (13,15).
Previous studies on the Hippo/YAP pathway demonstrate that the
overexpression of YAP is present in malignant cancer types
including liver cancer, breast cancer, colorectal cancer and
prostate carcinomas with advanced proliferation, metastasis and
poor survival rate (42–45). According to previous evidence, YAP
served a critical role in the cell growth and tumorigenesis of
ovarian cancer in vitro and in vivo (34). In the present study, although tumor
cell proliferation was greatly inhibited and apoptosis was
substantially induced by mangiferin treatment, the antitumor effect
of mangiferin was significantly abrogated by YAP overexpression
(P<0.05). Therefore, mangiferin served an inhibitory role in
cell proliferation and an inductive role in apoptosis through the
suppression of YAP. In addition, as the most important nuclear
transcription factor downstream of YAP gene, the activity and
regulation of TEAD are crucial to YAP function (25,38).
Therefore, to ascertain the influence of YAP activity on
mangiferin, a luciferase assay was performed in mangiferin-treated
cells and mangiferin-treated YAP overexpression cells. As expected,
compared with YAP-overexpressing cells treated with mangiferin, a
significant lower activity of TEAD4-dependent luciferase was
observed in the mangiferin-treated cells. The inactivation of
TEAD4-dependent luciferase activity demonstrated that mangiferin
treatment suppressed the expression of YAP.
Mangiferin may influence not only tumor cell
proliferation and apoptosis, but also the migration and invasion
capability of ovarian cancer cells. A study on breast cancer cells
indicated that mangiferin may significantly weaken cell invasion
and inhibit cell migration in a dose-dependent manner (20). As reported by Gayani et al
(18), mangiferin was also determined
to be a potential anti-invasive agent in prostate cancer cells
through an invasion assay. In addition, a study by Takeda et
al (46) revealed that mangiferin
inhibited spontaneous metastasis and tumor growth through in
vivo experiments on a mouse metastatic melanoma model. Based on
the potential suppressive effect of mangiferin on the migration and
invasion capabilities of breast cancer, prostate cancer and
melanoma, it was hypothesized that mangiferin may possess a similar
function in ovarian cancer. Thus, cell migration and invasion
studies were performed. As expected, the results of the present
study revealed a substantial gap in the cells treated with
mangiferin. To further study the mechanism of mangiferin-mediated
inhibition of metastasis, the present study focused on the YAP
pathway. As predicted, cell fusion was observed in YAP
overexpressed cells treated with mangiferin, indicating that
mangiferin mediated cell migration through the YAP pathway. A cell
invasion assay using Matrigel also proved the inhibitory function
of mangiferin on ovarian cancer cells through the downregulation of
YAP. However, the absence of single-cell movement analyses is a
limitation of the present study.
Aside from the confirmed therapeutic effect of
mangiferin monotherapy in malignant tumor types, the question
arises on whether mangiferin may contribute to tackling the high
rates of chemoresistance in tumor chemotherapy. Platinum-based
chemotherapeutic agents are widely deemed to be primary anticancer
drugs and are clinically used for ovarian cancer treatment
(47). Although high-grade serious
ovarian cancer types initially respond well to platinum-based
chemotherapy, these patients ultimately suffer from relapse and
progression to chemotherapy resistance (48). Drug resistance is a serious and
frequent problem in ovarian cancer which requires a more
comprehensive understanding of the resistance mechanism and a
better solution. In the present study, it was demonstrated that the
dysregulation of the Toll-like receptor 4 (TLR4)-interleukin 6
(IL6)-Janus kinase (JAK)/signal transducer and activator of
transcription 3 (STAT3) pathway was closely associated with the
development of a diverse range of human solid tumor types (49–51),
highlighting the importance of this pathway as a therapeutic target
to treat the persistent disease of high-grade serious ovarian
cancer. Interestingly, IL6 is able to trigger the activation of
downstream YAP through glycoprotein 130, a co-receptor of IL6,
which ultimately resulted in the nuclear translocation of YAP
(52). Therefore, as a participative
effector of the TLR4-IL6-JAK/STAT3 pathway, YAP expression may also
be involved in chemotherapy resistance-associated relapse. A study
by Jeong et al (53)
demonstrated that the activation of YAP was tightly associated with
drug resistance to paclitaxel in the treatment of ovarian cancer.
Additionally, increased chemoresistance was associated with the
elevated expression of YAP. A study by Xia et al (34) proved that YAP increased the resistance
of ovarian cancer cell lines to cisplatin and taxol. However,
another previous study sufficiently demonstrated that mangiferin
was able to increase the chemotherapeutic sensitivity of a tumor,
the mechanism of which remains obscure (24). Hence, it was proposed that mangiferin
may mediate improved drug sensitivity by the inhibition of YAP. As
predicted, subsequent to genetic engineering studies on ovarian
cancer cells, it was clearly identified that mangiferin was able to
inhibit the expression of YAP, thereby increasing the sensitivity
of a tumor to cisplatin. Therefore, YAP served as an important
factor counteracting mangiferin in chemotherapy resistance. This
conclusion was further confirmed by the cell inhibitory curve and
cell morphology. In an OVCAR8 ×enografted murine model, the in
vivo data further demonstrated that mangiferin substantially
improved the chemosensitivity of ovarian tumor types to cisplatin
and inhibited tumor growth. Additionally, migratory tumor types did
not occur in other parts of the murine body during the 100-day
observation period. Thus, it was further indicated that not only
the tumor growth was influenced by mangiferin, but also tumor
migration and invasion were suppressed following mangiferin
treatment.
In summary, as a molecular targeted therapeutic
agent of YAP, mangiferin may be a valuable potential novel drug for
the treatment of human ovarian cancer. Further investigations on
the molecular mechanism of mangiferin in the near future will
ensure that the present study will result in mangiferin being used
as a novel therapeutic drug by inhibiting cell growth, metastasis
and enhancing the tumor response to cisplatin treatment for ovarian
cancer treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Funding of China (grant no. 81802504), Sichuan Health and
Family Planning Commission Funding (grant no. 16ZD0253), the
Sichuan National Science Research Funding (grant no. 2018JY0645),
the Sichuan Provincial People's Hospital and a Sichuan Scientific
Research Grant for Returned Overseas Chinese Scholars for Dr. Yi
Wang. The present study was supported by the National Science
Funding of China (grant no. 81503589) and Sichuan Education Bureau
Funding (grant no. 14ZB0089) for Dr. Yaodong You. The study was
also supported by the National Key Specialty Construction Project
of Clinical Pharmacy (grant no. 30305030698).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW and RT designed the study. SD, YY and WH
performed the cytological experiments. TL, HW and XL performed the
animal experiments. XH performed the statistical analysis. SD and
TL were major contributors to the figures and writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal handling was in performed accordance with the
Ethics Committee of Sichuan Academy of Medical Science and Sichuan
Provincial People's Hospital (Sichuan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. Ca Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hennessy BT, Coleman RL and Markman M:
Ovarian cancer. Lancet. 374:13712009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cai H and Xu Y: The role of LPA and YAP
signaling in long-term migration of human ovarian cancer cells.
Cell Commun Signal. 11:312013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hall CA, Wang R, Miao J, Oliva E, Shen X,
Wheeler T, Hilsenbeck SG, Orsulic S and Goode S: Hippo pathway
effector yap is an ovarian cancer oncogene. Cancer Res.
70:8517–8525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting wnt, notch, and hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JT, Li M, Nakayama K, Mao TL,
Davidson B, Zhang Z, Kurman RJ, Eberhart CG, Shih IeM and Wang TL:
Notch3 gene amplification in ovarian cancer. Cancer Res.
66:6312–6318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rose SL, Kunnimalaiyaan M, Drenzek J and
Seiler N: Notch 1 signaling is active in ovarian cancer. Gynecol
Oncol. 117:130–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Halder G and Johnson RL: Hippo signaling:
Growth control and beyond. Development. 138:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu FX and Guan KL: The hippo pathway:
Regulators and regulations. Genes Dev. 27:355–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santucci M, Vignudelli T, Ferrari S, Mor
M, Scalvini L, Bolognesi ML, Uliassi E and Costi MP: The hippo
pathway and YAP/TAZ-TEAD protein-protein interaction as targets for
regenerative medicine and cancer treatment. J Med Chem.
58:4857–4873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zaidi SK, Sullivan AJ, Medina R, Ito Y,
van Wijnen AJ, Stein JL, Lian JB and Stein GS: Tyrosine
phosphorylation controls Runx2-mediated subnuclear targeting of YAP
to repress transcription. EMBO J. 23:790–799. 2014. View Article : Google Scholar
|
|
13
|
Barry ER, Morikawa T, Butler BL, Shrestha
K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et
al: Restriction of intestinal stem cell expansion and the
regenerative response by YAP. Nature. 493:106–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai J, Zhang N, Zheng Y, de Wilde RF,
Maitra A and Pan D: The Hippo signaling pathway restricts the
oncogenic potential of an intestinal regeneration program. Genes
Dev. 24:2383–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong W and Guan KL: The YAP and TAZ
transcription co-activators: Key downstream effectors of the
mammalian Hippo pathway. Semin Cell Dev Biol. 23:785–793. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Escoll M, Gargini R, Cuadrado A, Anton IM
and Wandosell F: Mutant p53 oncogenic functions in cancer stem
cells are regulated by WIP through YAP/TAZ. Oncogene. 36:3515–3527.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferrarelli LK: Actin against BRAF
inhibitors. 9:ec51–ec. 2016.
|
|
18
|
Gayani DM, Kang CH, Hyun CY and Gi-Young
K: Mangiferin inhibits tumor necrosis factor-α-induced matrix
metalloproteinase-9 expression and cellular invasion by suppressing
nuclear factor-κB activity. BMB Rep. 48:559–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan LL, Wang AY, Huang YQ, Luo Y and Ling
M: Mangiferin induces apoptosis by regulating Bcl-2 and Bax
expression in the CNE2 nasopharyngeal carcinoma cell line. Asian
Pac J Cancer Prev. 15:7065–7068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Huang J, Yang B, Xiang T, Yin X,
Peng W, Cheng W, Wan J, Luo F, Li H and Ren G: Mangiferin exerts
antitumor activity in breast cancer cells by regulating matrix
metalloproteinases, epithelial to mesenchymal transition, and
β-catenin signaling pathway. Toxicol Appl Pharmacol. 272:180–190.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Louisa M, Soediro TM and Suyatna FD: In
vitro modulation of P-glycoprotein, MRP-1 and BCRP expression by
mangiferin in doxorubicin-treated MCF-7 cells. Asian Pac J Cancer
Prev. 15:1639–1642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rajendran P, Rengarajan T, Nishigaki I,
Ekambaram G and Sakthisekaran D: Potent chemopreventive effect of
mangiferin on lung carcinogenesis in experimental Swiss albino
mice. J Cancer Res Ther. 10:1033–1039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi W, Deng J, Tong R, Yang Y, He X, Lv J,
Wang H, Deng S, Qi P, Zhang D and Wang Y: Molecular mechanisms
underlying mangiferin-induced apoptosis and cell cycle arrest in
A549 human lung carcinoma cells. Mol Med Rep. 13:3423–3432. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou B, Wang H, Liu Y, Qi P, Lei T, Sun M
and Wang Y: Mangiferin induces apoptosis in human ovarian
adenocarcinoma OVCAR3 cells via the regulation of Notch3. Oncol
Rep. 38:1431–1441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yimlamai D, Christodoulou C, Galli GG,
Yanger K, Pepe-mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ
and Camargo FD: Hippo pathway activity influences liver cell fate.
Cell. 157:1324–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schilder RJ, Hall L, Monks A, Handel LM,
Fornace AJ Jr, Ozols RF, Fojo AT and Hamilton TC: Metallothionein
gene expression and resistance to cisplatin in human ovarian
cancer. Int J Cancer. 45:416–422. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Driel WJ, Lok CA, Verwaal V and Sonke
GS: The role of hyperthermic intraperitoneal intraoperative
chemotherapy in ovarian cancer. Curr Treat Options Oncol.
16:142015.Analgesic and antioxidant activity. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dar A, Faizi S, Naqvi S, Roome T,
Zikr-ur-Rehman S, Ali M, Firdous S and Moin ST: Analgesic and
antioxidant activity of mangiferin and its derivatives: The
structure activity relationship. Biol Pharm Bull. 28:596–600. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ajila CM, Rao LJ and Rao UJ:
Characterization of bioactive compounds from raw and ripe mangifera
indica L. Peel extracts. Food Chem Toxicol. 48:3406–3411. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duang XY, Wang Q, Zhou XD and Huang DM:
Mangiferin: A possible strategy for periodontal disease to therapy.
Med Hypotheses. 76:486–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guha S, Ghosal S and Chattopadhyay U:
Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a
naturally occurring glucosylxanthone. Chemotherapy. 42:443–451.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iseda S: On Mangiferin, the Coloring
Matter of Mango (Mangifera indica Linn.). V. Identification of
Sugar Component and the Structure of Mangiferin. Bulletin Chem Soc
Japan. 30:629–633. 2006. View Article : Google Scholar
|
|
34
|
Xia Y, Chang T, Wang Y, Liu Y, Li W, Li M
and Fan HY: YAP promotes ovarian cancer cell tumorigenesis and is
indicative of a poor prognosis for ovarian cancer patients. PLoS
One. 9:e917702014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, George J, Deb S, Degoutin JL,
Takano EA, Fox SB; AOCS Study group, ; Bowtell DD and Harvey KF:
The Hippo pathway transcriptional co-activator, YAP, is an ovarian
cancer oncogene. Oncogene. 30:2810–2822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hofmann JJ, Zovein AC, Koh H, Radtke F,
Weinmaster G and Iruela-Arispe ML: Jagged1 in the portal vein
mesenchyme regulates intrahepatic bile duct development: Insights
into alagille syndrome. Development. 137:4061–4072. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zong Y, Panikkar A, Xu J, Antoniou A,
Raynaud P, Lemaigre F and Stanger BZ: Notch signaling controls
liver development by regulating biliary differentiation.
Development. 136:1727–1739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Home P, Saha B, Ray S, Dutta D,
Gunewardena S, Yoo B, Pal A, Vivian JL, Larson M, Petroff M, et al:
Altered subcellular localization of transcription factor TEAD4
regulates first mammalian cell lineage commitment. Proc Natl Acad
Sci USA. 109:7362–7367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rose SL: Notch signaling pathway in
ovarian cancer. Int J Gynecol Cancer. 19:564–566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Justice RW, Zilian O, Woods DF, Noll M and
Bryant PJ: The Drosophila tumor suppressor gene warts encodes a
homolog of human myotonic dystrophy kinase and is required for the
control of cell shape and proliferation. Genes Dev. 9:534–546.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pantalacci S, Tapon N and Léopold P: The
salvador partner hippo promotes apoptosis and cell-cycle exit in
drosophila. Nat Cell Biol. 5:921–927. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dong J, Feldmann G, Huang J, Wu S, Zhang
N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D:
Elucidation of a universal size-control mechanism in drosophila and
mammals. Cell. 130:1120–1133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci USA. 103:12405–12410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zender L, Spector MS, Xue W, Flemming P,
Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et
al: Identification and validation of oncogenes in liver cancer
using an integrative oncogenomic approach. Cell. 125:1253–1267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takeda T, Tsubaki M, Sakamoto K, Ichimura
E, Enomoto A, Suzuki Y, Itoh T, Imano M, Tanabe G, Muraoka O, et
al: Mangiferin, a novel nuclear factor kappa B-inducing kinase
inhibitor, suppresses metastasis and tumor growth in a mouse
metastatic melanoma model. Toxicol Appl Pharmacol. 306:105–112.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Gynecologic Oncology Group: Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
gynecologic oncology group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Giaccone G: Clinical perspectives on
platinum resistance. Drugs. 4 Suppl 59:S9–S17. 2000. View Article : Google Scholar
|
|
49
|
Chang Q, Bournazou E, Sansone P, Berishaj
M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al:
The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and
metastasis. Neoplasia. 15:848–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu W, Xie S, Chen X, Rao X, Ren H, Hu B,
Yin T, Xiang Y and Ren J: Activation of the IL-6/JAK/STAT3
signaling pathway in human middle ear cholesteatoma epithelium. Int
J Clin Exp Pathol. 7:709–715. 2014.PubMed/NCBI
|
|
51
|
Wang SW and Sun YM: The IL-6/JAK/STAT3
pathway: Potential therapeutic strategies in treating colorectal
cancer (Review). Int J Oncol. 44:1032–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Taniguchi K, Wu LW, Grivennikov SI, de
Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, et al:
A gp130-Src-YAP module links inflammation to epithelial
regeneration. Nature. 519:57–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jeong W, Kim SB, Sohn BH, Park YY, Park
ES, Kim SC, Kim SS, Johnson RL, Birrer M, Bowtell DSL, et al:
Activation of YAP1 is associated with poor prognosis and response
to taxanes in ovarian cancer. Anticancer Res. 34:811–817.
2014.PubMed/NCBI
|