Introduction
Esophageal cancer is the eighth most commonly
diagnosed cancer type in the world and the sixth leading cause of
cancer-associated cases of mortality (1). Esophageal squamous cell carcinoma (ESCC)
is the most predominant pathological type of esophageal cancer,
accounting for 90% of all esophageal cancer cases in the developing
world (2,3). In addition, ESCC has been classified as
the most typical esophageal tumor type by the National Health and
Family Planning Commission of the People's Republic of China
(3). Although the current standard
treatment for patients with ESCC is an esophagectomy and adjuvant
chemoradiation, the overall 5-year survival rate is only
approximately 17% due to the risks associated with this treatment
(2). In addition, the prognosis for
patients with an equivalent stage of ESCC varies markedly (4). A selection of serum biomarkers has
previously been identified to predict the survival rate of patients
with ESCC; however, the limited sensitivity and specificity of
these markers, including squamous cell carcinoma antigen,
cytokeratin 19 fragments and carcinoembryonic antigen, have
restricted their clinical use (5–7).
Therefore, novel predictive biomarkers, which may also promote
targeted therapy for ESCC, are required.
Abdominal obesity and lipid levels are closely
associated with the occurrence of esophageal cancer and several
other tumor types (8–11), and high levels of lipids in serum and
cell membranes are associated with the prognosis of multiple tumor
types (12–14). A previous study demonstrated that
total cholesterol (TC) is a good predictive marker for grading
tumor regression in patients with locally advanced colorectal
cancers previously treated with neoadjuvant chemoradiotherapy
(15). High-density lipoprotein (HDL)
is a risk factor and a prognostic factor in prostate cancer, and a
decrease in HDL levels is associated with poor survival among
patients with non-small-cell lung carcinoma (6,13).
Additionally, apolipoprotein A1, a major component of HDL, is
considered to be a predictive biomarker of survival rate in
patients with ESCC (14). Low-density
lipoprotein (LDL) has also been implicated in ESCC (14), but a negative association has been
reported in other studies (16).
Regardless, the major receptor of LDL, lectin-like oxidized
low-density lipoprotein (LOX-1) is a verified significant
prognostic factor for multiple cancer types (17–20). To
date, a few studies have focused on the prognostic role of
triglyceride (TG) levels in breast cancer (21).
To clarify the prognostic value of lipid profiles in
ESCC, the current study retrospectively investigated preoperative
TC, HDL, LDL and TG levels in 242 patients with ESCC and analyzed
associations between these levels and overall survival (OS).
Additionally, the current study performed in vitro
experiments to preliminarily explore a possible mechanism based on
these associations.
Materials and methods
Study patients
A total of 250 eligible patients with ESCC who
underwent an esophagectomy at Shandong Cancer Hospital (Jinan,
China) between April 2012 and October 2014 were included in the
current study. The inclusion criteria were as follows: i) Patients
were previously diagnosed with ESCC and received radical surgery;
ii) TC, TG, HDL and LDL levels were examined 2–5 days prior to
surgery between 6:00 and 8:00 a.m. using a Modular p800 analyzer
(Roche Diagnostics, Basel, Switzerland); and iii) no drugs known to
affect lipids, including statins, were taken by the patients.
Exclusion criteria were as follows: i) History of another cancer
type; ii) diabetes or another endocrine or metabolic disease that
may influence serum lipid levels; and iii) cachexia [body mass
index (BMI) <20 kg/m2].
Follow-up assessment
Follow-up assessments were performed annually by
telephone interviews and review of medical records. This was
performed every 2 months for patients with evidence of distant
metastasis and local recurrence. The last follow-up was completed
in October 2016. The endpoint of the current study was OS, which
was defined as the time interval between diagnosis and mortality or
the last follow-up. Survivors were defined as alive at the time of
the last follow-up, whereas non-survivors were defined as having
died at any time during the study. Tumor and clinical
characteristics of the patients, including age, sex, Pathological
N-staging status (pT status), Pathological N-staging status (pN
status), grade and histological type, were obtained from medical
records and pathology reports.
The current study was approved by the Institutional
Review Board of Shandong Cancer Hospital. Informed consent and
survival status were verified through direct telecommunication with
the patients or their families.
Cell culture
Esophageal cancer cell lines TE-1 and ECa109 were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Institute of Shanghai Cell Biology and Chinese Type Culture
Collection, Shanghai, China). ECa109 cells were grown in RPMI-1640
and TE-1 cells were maintained in Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum (all from Hyclone;
GE Healthcare, Logan, UT, USA). All cells were grown at 37°C in
humidified air containing 5% CO2.
Cell viability detection via the Cell
Counting Kit-8 (CCK8) assay and cell cycle analysis by flow
cytometry
TE-1 and ECa109 cells were seeded in 96-well plates
at 3.0×103/well and maintained for 24 h until attachment
at 37°C in a humidified, 5% CO2, 95% air atmosphere. The
cells were then starved with serum-free medium for 4 h and
incubated with various concentrations of LDL (0, 10, 50, 100, 200
and 400 µg/ml; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
for 24, 48 and 72 h at 37°C in a humidified, 5% CO2. To
determine the effects of LDL, TE-1 and ECa109 cell viability was
measured using the CCK8 assay according to the manufacturer's
protocol (Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
and the cell cycle was detected by Propidium lodide (PI)/RNase
Staining Buffer (BD Biosciences, Franklin Lakes, NJ, USA) and
analyzed by flow cytometry with Modfit LT 4.0 (Verity Software
House, Topsham, ME, USA).
Statistical analysis
Statistical analyses were performed with SPSS 21.0
software (IBM Corp., Armonk, NY, USA). Serum lipid levels were
expressed as the mean ± standard deviation. Univariate and
multivariate analyses were performed using Kaplan-Meier and Cox
proportional hazards regression models to determine associations
between OS, the clinical parameters and the TC, HDL, LDL and TG
levels. The hazard ratio (HR) was reported as the relative risk
with 95% confidence intervals. Associations between the serum
lipids and clinical characteristics were determined with the
χ2 test or univariate analysis. Survival curves were
plotted on the basis of the aforementioned results and the curves
were compared using the log-rank test. All P-values were two-sided
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
In the current retrospective study, follow-up of 242
patients with ESCC was completed with a success rate of 96.8%
(242/250; 3 patients refused to provide information and
communication was lost with 5 patients). The 242 patients included
190 (78.5%) males and 52 (21.5%) females, with a median age of 61
years (range, 35–80 years). The disease characteristics were as
follows: 20 (8.3%) patients had upper thoracic esophageal cancer,
128 (52.9%) had middle thoracic esophageal cancer and 94 (38.8%)
had low thoracic esophageal cancer. Histologically
well-differentiated disease, moderately differentiated disease and
poorly differentiated disease were identified in 60 (24.8%), 122
(50.4%) and 60 (24.8%) patients, respectively. Among the patients,
19 (7.8%) had stage T1 disease, 28 (11.6%) had stage T2 disease,
158 (65.3%) had stage T3 disease and 37 (15.3%) had stage T4
disease. Regarding staging, 110 (45.5%) patients demonstrated stage
N0 disease, 94 (38.8%) stage N1 disease, 29 (12.0%) stage N2
disease and 9 (3.7%) N3 disease (Table
I).
 | Table I.Univariate and multivariate analysis
for overall survival in patients with esophageal squamous cell
carcinoma. |
Table I.
Univariate and multivariate analysis
for overall survival in patients with esophageal squamous cell
carcinoma.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | n | χ2 | P-value | β | HR | P-value |
|---|
| Age |
| 0.024 | 0.876 |
|
|
|
|
<60 | 114 |
|
|
|
|
|
|
≥60 | 128 |
|
|
|
|
|
| Sex |
| 3.617 | 0.057 |
|
|
|
|
Male | 190 |
|
|
|
|
|
|
Female | 52 |
|
|
|
|
|
| Tumor location |
| 5.745 | 0.056 |
|
|
|
|
Upper | 20 |
|
|
|
|
|
|
Middle | 128 |
|
|
|
|
|
|
Lower | 94 |
|
|
|
|
|
| Histological
differentiation |
| 12.615 | 0.002 | 1.407 | 4.083 | 0.002 |
|
Well | 60 |
|
|
|
|
|
|
Moderate | 122 |
|
|
|
|
|
|
Low | 60 |
|
|
|
|
|
| pT status |
| 27.277 | 0.001 | 0.539 | 1.714 | 0.001 |
| T1 | 19 |
|
|
|
|
|
| T2 | 28 |
|
|
|
|
|
| T3 | 158 |
|
|
|
|
|
| T4 | 37 |
|
|
|
|
|
| pN status |
| 71.184 | 0.001 | 0.676 | 1.966 | 0.001 |
| N0 | 110 |
|
|
|
|
|
| N1 | 94 |
|
|
|
|
|
| N2 | 29 |
|
|
|
|
|
| N3 | 9 |
|
|
|
|
|
| TC, mmol/l |
| 0.878 | 0.349 |
|
|
|
|
>5.2 | 72 |
|
|
|
|
|
|
≤5.2 | 170 |
|
|
|
|
|
| TG, mmol/l |
| 2.843 | 0.096 |
|
|
|
|
>1.7 | 32 |
|
|
|
|
|
|
≤1.7 | 210 |
|
|
|
|
|
| LDL, mmol/l |
| 5.454 | 0.02 | 0.722 | 2.164 | 0.005 |
|
>3.12 | 44 |
|
|
|
|
|
|
≤3.12 | 198 |
|
|
|
|
|
| HDL, mmol/l |
| 3.914 | 0.048 | 0.063 | 0.939 | 0.757 |
|
>1.42 | 74 |
|
|
|
|
|
|
≤1.42 | 168 |
|
|
|
|
|
The mean values of serum lipid levels prior to
therapy were as follows: TC=4.72±0.89 mmol/l, TG=1.10±0.57 mmol/l,
LDL=2.63±0.56 mmol/l and HDL=1.30±0.38 mmol/l (Table II).
 | Table II.Lipid levels and association with
overall survival. |
Table II.
Lipid levels and association with
overall survival.
| Serum lipid | Min, mmol/l | Max, mmol/l | Mean ± SD,
mmol/l | HR | P-value |
|---|
| TC | 2.84 | 7.12 | 4.72±0.89 | 1.096 | 0.319 |
| TG | 0.49 | 5.16 | 1.10±0.57 | 1.057 | 0.724 |
| LDL | 1.63 | 5.16 | 2.63±0.56 | 2.164 | 0.005 |
| HDL | 0.57 | 2.94 | 1.30±0.38 | 0.939 | 0.757 |
LDL, pT status, pN status and
histological differentiation are independent prognostic factors in
patients with ESCC
The χ2 test, Kaplan-Meier analysis and
Cox regression model were applied to characterize the association
between prognosis and serum lipid levels or clinical parameters,
including age, sex, tumor location, histological differentiation,
pT status and pN status, in patients with ESCC (Table I). Univariate survival analysis
revealed significant associations between poor survival and HDL
(χ2=3.914, P=0.048), LDL (χ2=5.454, P=0.020),
pT status (T1-2 vs. T3-4; χ2=27.277, P=0.001), pN status
(N0 vs. N1-3; χ2=71.184, P=0.001) and histological
differentiation (χ2=12.615, P=0.002), whereas the other
clinical features investigated in the current study demonstrated no
association with OS. Multivariate survival analysis was performed
to clarify the independent prognostic values of these five factors,
with LDL (HR=2.164, P=0.005), histological differentiation
(HR=4.083, P=0.002), pT status (HR=1.714, P=0.001) and pN status
(HR=1.966, P=0.001) demonstrating an association with ESCC
prognosis. Survival curves were then generated based on these
results. The 3-year survival rate of patients with ESCC with lower
LDL levels (≤3.12 mmol/l) was 34.7%, compared with 15.8% for
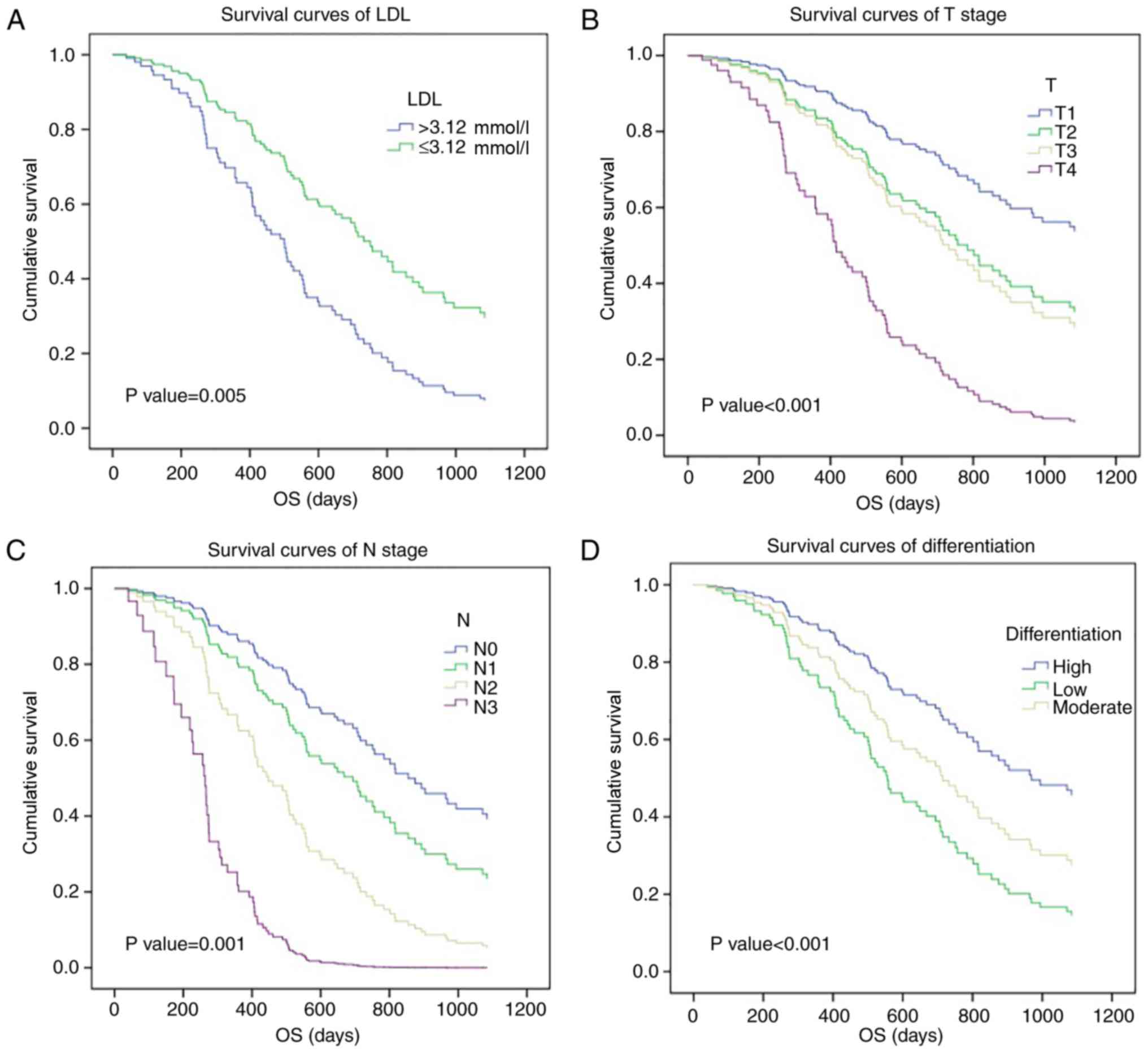
patients with higher LDL levels (>3.12 mmol/l; Fig. 1A). Additionally, a higher pT status
and pN status were associated with a poor 3-year survival rate in
patients with ESCC (T1-2 vs. T3-4, 45.5 vs. 27.1%; N0 vs. N1-3,
50.9 vs. 13.8%; Fig. 1B and C), as
was histological differentiation (high-differentiated disease vs.
low vs. moderate differentiation, 56.7% vs. 24.6% vs. 23.2%;
Fig. 1D). Therefore, the current
study identified that pre-therapy serum LDL level may be a
significant prognostic factor for patients with ESCC.
Associations between LDL levels and
other clinical characteristics
The observed associations between different levels
of LDL and other clinical features are presented in Table III. Statistical analysis identified
that the LDL level was significantly associated with sex (P=0.001),
tumor location (P=0.004) and pN status (P=0.007). However, no
significant associations were evident between the LDL level and the
other parameters investigated in the current study.
 | Table III.Clinical patient characteristics
according to the LDL level. |
Table III.
Clinical patient characteristics
according to the LDL level.
| Characteristic | LDL >3.12
mmol/l, n (n=44) | LDL ≤3.12 mmol/l, n
(n=198) | χ2 | P-value |
|---|
| Age |
|
| 1.4114 | 0.234 |
|
<60 | 16 | 94 |
|
|
|
≥60 | 28 | 104 |
|
|
| Sex |
|
| 25.11 | 0.001 |
|
Male | 20 | 165 |
|
|
|
Female | 22 | 33 |
|
|
| Tumor location |
|
| 10.90 | 0.004 |
|
Upper | 10 | 11 |
|
|
|
Middle | 20 | 109 |
|
|
|
Lower | 14 | 78 |
|
|
| HD |
|
| 0.506 | 0.777 |
|
Well | 10 | 54 |
|
|
|
Moderate | 22 | 99 |
|
|
|
Low | 12 | 45 |
|
|
| pT status |
|
| 5.566 | 0.135 |
|
T1-2 | 11 | 36 |
|
|
|
T3-4 | 33 | 162 |
|
|
| pN status |
|
| 12.156 | 0.007 |
| N0 | 18 | 87 |
|
|
|
N1-3 | 26 | 111 |
|
|
Effect of LDL on cell
proliferation
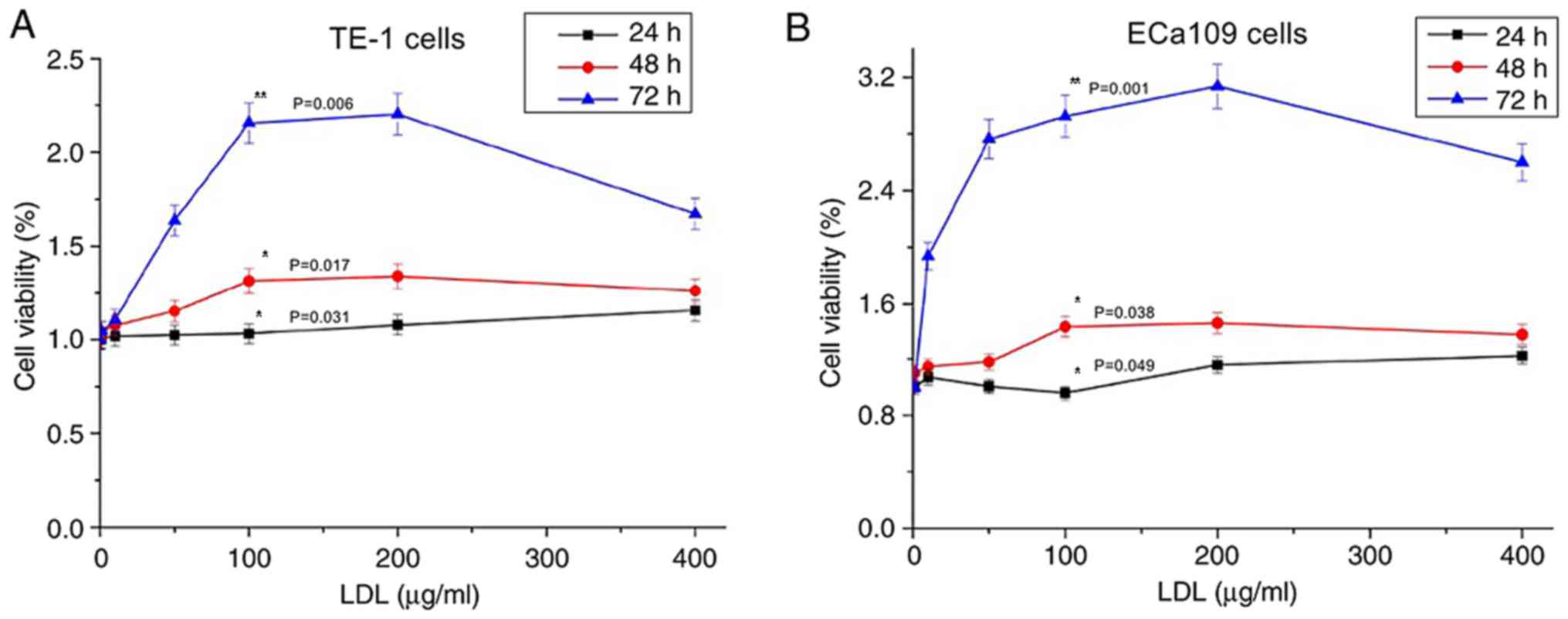
As demonstrated in Fig.
2A, TE-1 cell proliferation was promoted by LDL; the
proliferation level increased as the LDL treatment time increased
and as the LDL concentration increased (P<0.05). However, the
proliferation level declined when the LDL concentration exceeded
200 µg/ml. This finding indicated that an appropriate LDL
concentration may promote esophageal carcinoma cell growth. TE-1
cells exhibited the largest proliferation efficiency when treated
with 200 µg/ml LDL for 72 h. The growth characteristics of ECa109
cells were equivalent to those of TE-1 cells (Fig. 2B).
Effect of LDL on cell cycle
distribution
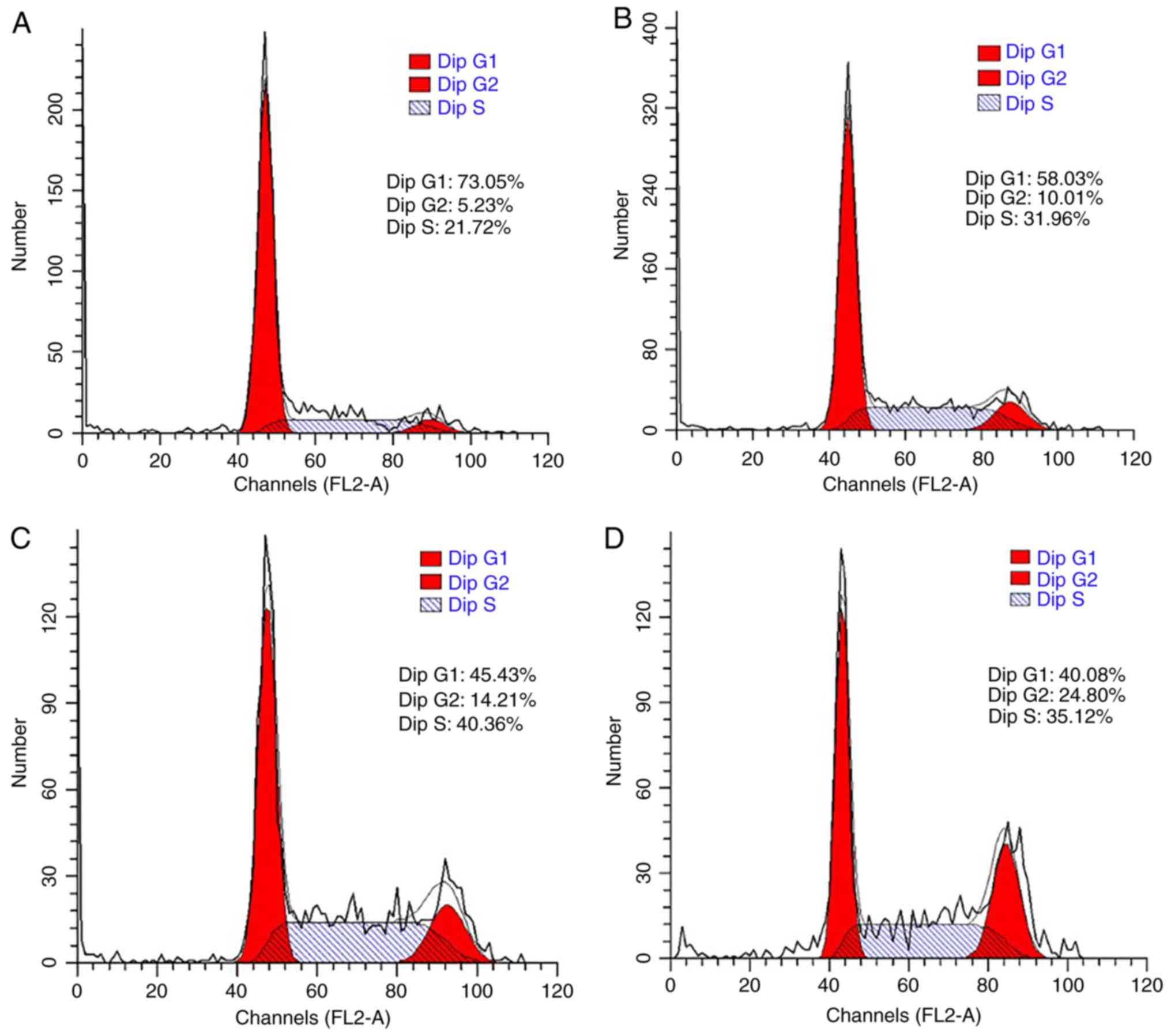
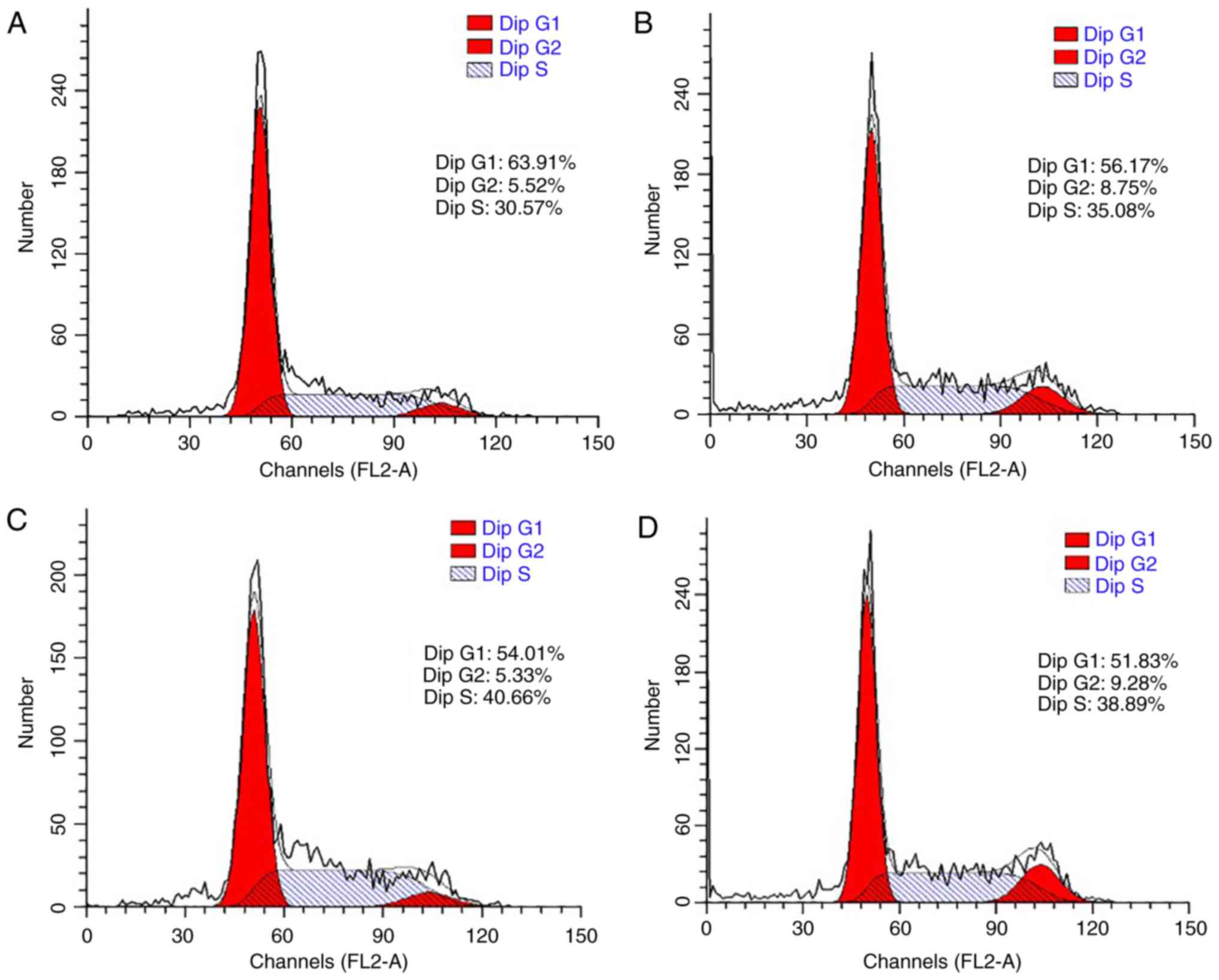
The cell cycle was also analyzed to confirm the
promoting effect of LDL on esophageal carcinoma cell proliferation.
TE-1 and ECa109 cells were incubated with different concentrations
of LDL (0, 50, 100 and 200 µg/ml) for 72 h and then collected for
cell cycle analysis. As demonstrated in Fig. 3, the percentage of G1-phase TE-1 cells
decreased from 73.05 to 40.08% in response to LDL, with a
corresponding increase in the total proportion of S and G2-phase
cells from 26.95 to 59.92%. These data indicated that proliferation
was induced by LDL in TE-1 cells. Similar results were obtained
with ECa109 cells, whereby the G1-phase proportion decreased from
63.91 to 51.83% and the total proportion of S and G2 phase cells
increased from 36.09 to 48.17% in response to LDL (Fig. 4).
Discussion
The preoperative serum lipid levels of patients with
ESCC were evaluated in the current study. Operable patients were
selected because they had well-defined disease stages and good
nutrition statuses, which may avoid abnormal blood lipid levels
caused by dystrophia. In addition, BMI and serum albumin levels
were comparable between the higher LDL group and lower LDL group
(data not shown). Therefore, in the current study, preoperative
serum lipid levels were considered to be independent of nutritional
status. The number of male patients was more than three times that
of female patients (190 vs. 52). In China, there is a significant
sex difference in the incidence of esophageal cancer and the
proportion of men and women included in the current study is
consistent with the incidence (22).
This predominance may be because abdominal adiposity is more common
in males; abdominal adiposity increases intragastric pressure and
relaxes the lower esophageal sphincter, which leads to acid reflux
(23). Furthermore, males are more
likely to consume alcohol and smoke cigarettes, and these factors
have been suggested as the underlying reason for the higher number
of males with ESCC relative to females (24).
The results of the current study demonstrate that
high LDL (>3.12 mmol/l) is positively associated with a short OS
time, as are other parameters, including pT status, pN status and
histological differentiation. This association may be caused by
increased lymphatic metastasis. However no significant association
was identified between serum LDL level and pN status in a similar
study (25). There may be several
reasons for this; firstly, retrospective studies are prone to bias
and may explain why the current study and the previous study draw
different conclusions. Secondly, the research data used in the
current study and the previous study were collected between
2012–2014 and 2007–2008, respectively, but both datasets are
limited. In addition, the number of patients in both studies was
more than 200, this facilitates preliminary results to be obtained
but a larger sample size is required for further confirmation. The
authors of both studies are currently preparing to collaborate and
analyze a larger sample with joint multi-centric data in the hope
of obtaining more convincing results. Lastly, different patient
groups were selected in the two different studies; the proportion
of patients with T3 and T4 stage disease in the current study was
as high as 80.4%, whereas this number was 61.7% in the previous
study. Differences in the stage of ESCC may affect the nutritional
status of patients and cause a decrease in blood lipid levels.
Several previous studies have also suggested there
is a significant association between LDL levels and cancer
prognosis. Zhou et al (26)
demonstrated that LDL is a prognostic index for the survival of
patients with small-cell lung cancer and Rodrigues et al
(27) indicated that the LDL level is
associated with disease-free survival in patients with breast
cancer. In patients with prostate cancer, preoperative LDL
cholesterol is an independent predictor of recurrence (28) and LDL is also an independent
prognostic factor for patients with colorectal cancer (29). Additionally, in the current study, the
LDL level demonstrated an independent association with the pN
status of ESCC, which was consistent with the research conclusion
of the current study (Table III).
Sako et al (30) also
identified that hyperlipidemia is a risk factor for lymphatic
metastasis in superficial esophageal carcinoma, which supports the
conclusion made by the current study.
Several years ago, multiple studies supported the
view that exogenous LDL promoted the proliferation of breast cancer
and colon adenocarcinoma cells (31,32). The
results of the current study, which are based on cell viability
assays and cell cycle analysis, support a similar conclusion in
esophageal cancer cells. To the best of our knowledge, the current
study is the first to demonstrate that LDL enhances the growth rate
of esophageal cancer cells in vitro. This observation may be
attributed to the possible involvement of the LDL receptor-related
protein 1 (LRP1) in regulating cancer cell survival and metastatic
potential, which occurs through the ability of LDL to promote
cancer cell proliferation and differentiation (30,33). This
hypothesis requires a more detailed investigation.
Although the findings of the current study provide
evidence of a preliminary mechanism through our in vitro
analysis, the intrinsic mechanism by which LDL promotes cell
development is unclear. Several interesting studies have suggested
that many factors may explain the findings to date. Firstly,
migration and invasion of tumor cells is partially dependent on
exogenous LDL cholesterol and is possibly driven through
LDL-related receptors (34,35). LOX-1 may facilitate the proliferation
of gastric cancer cells by driving the epithelial-mesenchymal
transition and phosphoinositide 3-kinase/Akt/glycogen synthase
kinase β activation (34).
Additionally, low-density lipoprotein receptor serves an important
role in tumor cancer growth and invasion by regulating nuclear
factor κ-light-chain enhancer of activated B cells signaling, and
this serves as a prognostic index in patients with small cell lung
cancer (26,36). Furthermore, LRP1 may contribute to the
ability of cancer cells to form large metastases via increased
expression of vascular endothelial cell growth factor (VEGF) and
reduced cell death in response to hypoxia (33); tumor invasion is promoted via
stimulation of the extracellular signal-regulated kinase pathway
and inhibition of the c-Jun N-terminal kinase pathway (35).
Similar to the study by Montel et al
(33), the addition of LDL
cholesterol may induce activation of microvascular endothelial
cells, which facilitates lymph node metastases of colon cancer
cells (31). Additionally, Lu et
al (37) demonstrated that L5, a
cytokine through which LDL induces endothelial apoptosis, increases
secretion of an angiogenic factor, amphiregulin, by breast cancer
cells and promotes progression and metastasis (37). Other inflammatory cytokines, including
L1 and tumor necrosis factor, can also promote progression and
metastasis, which may induce hyper-adhesion to vascular endothelial
cells and augment tumor arrest and metastasis (38,39).
Furthermore, suppressive effects on the function of
immune cells may be another mechanism through which a high LDL
level enhances tumor metastasis. LRP1-deficient myeloid cells may
allow tumor-associated macrophages to provide increased amounts of
VEGF to a tumor (40). McCarthy et
al (41) reported that a high LDL
level inhibits T cell proliferation and macrophage tumoricidal
activity may be decreased by a high-fat diet in mice (42).
Based on these speculations and the results of the
current study, it can be proposed that LDL is a prognostic factor
in patients with ESCC because it promotes lymphatic metastasis.
Although the intrinsic mechanism is unclear, a high level of LDL
has an apparent role in promoting growth of TE-1 and ECa109
esophageal cancer cells. However, the current study has several
limitations. Firstly, this was a retrospective study and not a
prospective study. Additionally, patients from only one institution
were recruited and only 242 patients were included. Future studies
may involve more patients from multiple centers and the detailed
mechanism of the effect of LDL on esophageal carcinoma cells may be
investigated further
In summary, the LDL level is an adverse prognostic
factor for ESCC. Additionally, LDL is an economical and convenient
biomarker that may support clinical needs. Furthermore, to the best
of our knowledge, the current study is the first to hypothesize
that a high LDL level is associated with poor OS because LDL
promotes lymphatic metastasis and this hypothesis has been
partially confirmed in vitro. A more detailed mechanism is
required to be investigated in future studies.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81301936 and
81472811) and the Science Technology Development Project of
Shandong Province (grant nos. 2014GGC03038 and ZR2013HM080).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL and TZ participated in study design. HD, YY and
CL carried out the experiments and the data analysis. XM was
responsible for the implementation of cell experiments. TZ and YY
participated in data discussion and revision of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the
Institutional Review Board of Shandong Cancer Hospital. Informed
consentwas verified through direct telecommunication with the
patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rustgi AK and El-Serag HB: Esophageal
carcinoma. N Engl J Med. 371:2499–2509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey
SM, Dong ZW, Mark SD, Qiao YL and Taylor PR: Prospective study of
risk factors for esophageal and gastric cancers in the Linxian
general population trial cohort in China. Int J Cancer.
113:456–463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemoradiotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng X, Xing S, Liu XM, Liu W, Liu D, Chi
PD, Chen H, Dai SQ, Zhong Q, Zeng MS, et al: Establishment of using
serum YKL-40 and SCCA in combination for the diagnosis of patients
with esophageal squamous cell carcinoma. BMC Cancer. 14:4902014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao HH, Zhang SY, Shen JH, Wu ZY, Wu JY,
Wang SH, Li EM and Xu LY: A three-protein signature and clinical
outcome in esophageal squamous cell carcinoma. Oncotarget.
6:5435–5448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen GQ, Tian H, Yue WM, Li L, Li SH, Qi
L, Gao C, Si LB and Lu M: NCOA5 low expression correlates with
survival in esophageal squamous cell carcinoma. Med Oncol.
31:3762014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta A, Das A, Majumder K, Arora N, Mayo
HG, Singh PP, Beg MS and Singh S: Obesity is independently
associated with increased risk of hepatocellular cancer-related
mortality: A systematic review and meta-analysis. Am J Clin Oncol.
49:874–881. 2018. View Article : Google Scholar
|
|
9
|
Chang SJ, Hou MF, Tsai SM, Wu SH, Hou LA,
Ma H, Shann TY, Wu SH and Tsai LY: The association between lipid
profiles and breast cancer among Taiwanese women. Clin Chem Lab
Med. 45:1219–1223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Zhao XW, Liu DB, Han CZ, Du LL,
Jing JX and Wang Y: Lipid levels in serum and cancerous tissues of
colorectal cancer patients. World J Gastroenterol. 20:8646–8652.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duggan C, Onstad L, Hardikar S, Blount PL,
Reid BJ and Vaughan TL: Association between markers of obesity and
progression from Barrett's esophagus to esophageal adenocarcinoma.
Clin Gastroenterol Hepatol. 11:934–943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pelton K, Freeman MR and Solomon KR:
Cholesterol and prostate cancer. Curr Opin Pharmacol. 12:751–759.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chi PD, Liu W, Chen H, Zhang JP, Lin Y,
Zheng X, Liu W and Dai S: High-density lipoprotein cholesterol is a
favorable prognostic factor and negatively correlated with
C-reactive protein level in non-small cell lung carcinoma. PLoS
One. 9:e910802014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XP, Li XH, Zhang L, Lin JH, Huang H,
Kang T, Mao MJ, Chen H and Zheng X: High level of serum
apolipoprotein A-I is a favorable prognostic factor for overall
survival in esophageal squamous cell carcinoma. BMC Cancer.
16:5162016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Liu C, Zhang J, Liu Y, Gong G, Mo
X, Liu P, Li B and Yin Y: Predictive value of blood lipid
association with response to neoadjuvant chemoradiotherapy in
colorectal cancer. Tumour Biol. 37:4955–4961. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Li H, Diao Y, Li H, Zhang Y, Yin
C, Cui Y, Ma Q, Fang X, Zhou Y and Yang Y: Relationship between
oxidized LDL antibodies and different stages of esophageal
carcinoma. Arch Med Res. 39:760–777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
González-Chavarría I, Cerro RP, Parra NP,
Sandoval FA, Zuñiga FA, Omazábal VA, Lamperti LI, Jiménez SP,
Fernandez EA, Gutiérrez NA, et al: Lectin-like oxidized LDL
receptor-1 is an enhancer of tumor angiogenesis in human prostate
cancer cells. PLoS One. 9:e1062192014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan F, Qin X, Zhang G, Lu X, Zhu Y, Zhang
H, Dai B, Shi G and Ye D: Oxidized low-density lipoprotein is
associated with advanced-stage prostate cancer. Tumour Biol.
36:3573–3582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murdocca M, Mango R, Pucci S, Biocca S,
Testa B, Capuano R, Paolesse R, Sanchez M, Orlandi A, di Natale C,
et al: The lectin-like oxidized LDL receptor-1: A new potential
molecular target in colorectal cancer. Oncotarget. 7:14765–14780.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang L, Jiang S, Lin Y, Yang H, Zhao Z,
Xie Z, Lin Y and Long H: Combination of body mass index and
oxidized low density lipoprotein receptor 1 in prognosis prediction
of patients with squamous non-small cell lung cancer. Oncotarget.
6:22072–80. 2015.PubMed/NCBI
|
|
21
|
Li X, Tang H, Wang J and Xie X, Liu P,
Kong Y, Ye F, Shuang Z, Xie Z and Xie X: The effect of preoperative
serum triglycerides and high-density lipoprotein-cholesterol levels
on the prognosis of breast cancer. Breast. 32:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Zheng R, Zhang S, Zeng H, Fan Y,
Qiao Y and Zhou Q: Esophageal cancer incidence and mortality in
China, 2010. Thorac Cancer. 5:343–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh S, Sharma AN, Murad MH, Buttar NS,
El-Serag HB, Katzka DA and Iyer PG: Central adiposity is associated
with increased risk of esophageal inflammation, metaplasia, and
adenocarcinoma: A systematic review and meta-analysis. Clin
Gastroenterol Hepatol. 11(1399–1412): e72013.
|
|
24
|
Corrao G, Bagnardi V, Zambon A and La
Vecchia C: A meta-analysis of alcohol consumption and the risk of
15 diseases. Prev Med. 38:613–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen P, Han L, Wang C, Jia Y, Song Q, Wang
J, Guan S, Tan B, Liu B, Jia W, et al: Preoperative serum lipids as
prognostic predictors in esophageal squamous cell carcinoma
patients with esophagectomy. Oncotarget. 8:41605–41619.
2017.PubMed/NCBI
|
|
26
|
Zhou T, Zhan J, Fang W, Zhao Y, Yang Y,
Hou X, Zhang Z, He X, Zhang Y, Huang Y and Zhang L: Serum
low-density lipoprotein and low-density lipoprotein expression
level at diagnosis are favorable prognostic factors in patients
with small-cell lung cancer (SCLC). BMC Cancer. 17:2692017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dos Rodrigues Santos C, Fonseca I, Dias S
and de Almeida Mendes JC: Plasma level of LDL-cholesterol at
diagnosis is a predictor factor of breast tumor progression. BMC
Cancer. 14:1322014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wettstein MS, Saba K, Umbehr MH, Murtola
TJ, Fankhauser CD, Adank JP, Hofmann M, Sulser T, Hermanns T, Moch
H, et al: Prognostic role of preoperative serum lipid levels in
patients undergoing radical prostatectomy for clinically localized
prostate cancer. Prostate. 77:549–556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu YL, Qian HX, Qin L, Zhou XJ and Zhang
B: Serum LDL-C and LDL-C/HDL-C ratio are positively correlated to
lymph node stages in males with colorectal cancer.
Hepatogastroenterology. 58:383–387. 2011.PubMed/NCBI
|
|
30
|
Sako A, Kitayama J, Kaisaki S and Nagawa
H: Hyperlipidemia is a risk factor for lymphatic metastasis in
superficial esophageal carcinoma. Cancer Lett. 208:43–49. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mehta N, Hordines J, Sykes D, Doerr RJ and
Cohen SA: Low density lipoproteins and Lovastatin modulate the
organ-specific transendothelial migration of primary and metastatic
human colon adenocarcinoma cell lines in vitro. Clin Exp
Metastasis. 16:587–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Wood N, Grimsley P, Yellowlees D and
Donnelly PK: In vitro invasiveness of human breast cancer cells is
promoted by low density lipoprotein receptor-related protein.
Invasion Metastasis. 18:240–251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Montel V, Gaultier A, Lester RD, Campana
WM and Gonias SL: The low-density lipoprotein receptor-related
protein regulates cancer cell survival and metastasis development.
Cancer Res. 67:9817–9824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li C, Zhang J, Wu H, Li L, Yang C, Song S,
Peng P, Shao M, Zhang M, Zhao J, et al: Lectin-like oxidized
low-density lipoprotein receptor-1 facilitates metastasis of
gastric cancer through driving epithelial-mesenchymal transition
and PI3K/Akt/GSK3β activation. Sci Rep. 7:452752017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Langlois B, Perrot G, Schneider C, Henriet
P, Emonard H, Martiny L and Dedieu S: LRP-1 promotes cancer cell
invasion by supporting ERK and inhibiting JNK signaling pathways.
PLoS One. 5:e115842010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gopal U, Bohonowych JE, Lema-Tome C, Liu
A, Garrett-Mayer E, Wang B and Isaacs JS: A novel extracellular
Hsp90 mediated co-receptor function for LRP1 regulates EphA2
dependent glioblastoma cell invasion. PLoS One. 6:e176492011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu CW, Lo YH, Chen CH, Lin CY, Tsai CH,
Chen PJ, Yang YF, Wang CH, Tan CH, Hou MF, et al: VLDL and LDL, but
not HDL, promote breast cancer cell proliferation, metastasis and
angiogenesis. Cancer Lett. 388:130–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Holland JA, Pritchard KA, Rogers NJ and
Stemerman MB: Perturbation of cultured human endothelial cells by
atherogenic levels of low density lipoprotein. Am J Pathol.
132:474–478. 1988.PubMed/NCBI
|
|
39
|
Redl H, Schlag G, Kneidinger R, Ohlinger W
and Davies J: Response of the endothelium to trauma and sepsis.
Adherence, cytokine effects and procoagulatory response.
Arzneimittelforschung. 44:443–446. 1994.PubMed/NCBI
|
|
40
|
Staudt ND, Jo M, Hu J, Bristow JM, Pizzo
DP, Gaultier A, VandenBerg SR and Gonias SL: Myeloid cell receptor
LRP1/CD91 regulates monocyte recruitment and angiogenesis in
tumors. Cancer Res. 73:3902–3912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McCarthy BM, Okano Y, Nakayasu T, Macy M,
Watson SR and Harmony JA: Plasma lipoproteins and transferrin
regulate the proliferation of a continuous T lymphocyte cell line.
J Lipid Res. 28:1067–1077. 1987.PubMed/NCBI
|
|
42
|
Stewart-Phillips JL, Lough J and Phillips
NC: The effect of a high-fat diet on murine macrophage activity.
Int J Immunopharmacol. 13:325–332. 1991. View Article : Google Scholar : PubMed/NCBI
|