Introduction
Conventional modern medical treatments of malignant
tumors include surgery, radiation therapy, chemotherapy and
molecular targeted therapy; however, these methods frequently
induce trauma and unwanted side effects, and are poorly tolerated
in patients with advanced disease (1,2). Cisplatin
is a widely used anti-cancer drug that may induce serious toxicity
in the organs and tissues (2). Until
now, cisplatin has been extensively used to treat many tumors,
including breast, gastric and pancreatic cancer, and cervical
carcinoma. A high dose of cisplatin may induce side-effects for
patients or in animal models, and the dosage of cisplatin must be
reduced. Traditional Chinese medicine applied to cancer therapy has
a long history, and has attracted attention (3). In recent years, in the field of cancer
therapy, especially for breast cancer and cervical carcinoma,
traditional Chinese medicine drugs have alleviated symptoms of
tumors (2,3). The traditional Chinese medicine drugs
prevented chemotherapy side effects and complications, treatment of
cancer pain, severe pleural effusion, severe ascites, cancerous
mass, cancer complications and constipation (3). Yang et al (3) reported that traditional Chinese
medicines potentially target the tumor cellular proteasome and
NF-kB pathway whose activation is dependent on the proteasome
activity. Therefore, in order to reduce the side-effects of
cisplatin, the present study firstly used traditional Chinese
medicine and cisplatin combination chemotherapy to treat tumors in
a mouse model of lung cancer, as this particular model may reflect
best the therapeutic effects of the aforementioned combination.
Yuxiao San is composed of Curcuma aromatica,
mint, rhubarb, pubescent holly root, dandelion and Glauber's salt.
In traditional Chinese medicine Yuxiao Sanis believed to treat qi
stagnation and blood stasis, eliminate phlegm and remove toxins, to
prevent swelling and relieve pain. The present study used the
established Lewis lung cancer mouse model (4) and studied the effects and underlying
mechanism of Yuxiao San and cisplatin injection in Lewis lung
cancer mice. To the best of our knowledge, there are currently no
studies published evaluating the effects of Yuxiao San on tumor
cell growth or development. The objective of the present study is
to provide basic data regarding the use of Yuxia San in the
clinical treatment of lung cancer.
Materials and methods
Animals and cell lines
A total of 45 maleC57BL/6 mice weighing 18–22 g,
aged from 6 to 8 weeks were purchased from Chongqing Medical
University Experimental Animal Center (production license:
SCXK-ChongQing-2012-0001). All of the mice were raised in the
standard cleaning environment as the following: Temperature 23±2°C,
humidity 55±10%, 12 h illumination, noise ≤60 dB and free access to
food and water. A Lewis lung cancer cell line, C57BL, was purchased
from the West China University Cancer Center (Chengdu, China). The
present study was approved by the ethics committee of College of
Traditional Chinese Medicine of Chongqing Medical University,
Chongqing, China (approval no. 2014052).
Experimental drugs, reagents and
instruments
Yuxiao San was purchased from Chongqing Tong Junge
Large Pharmacy (Chongqing, China). According to high performance
liquid chromatography, Yuxiao San primarily consisted of
hexadecenoic acid, β-sitosterol, burdock oligosaccharide, kikyo
saponin, litchi saponin, curcumin and American ginseng total
saponin. Cisplatin (cat. no. 2A1A1401002A) was purchased from Qilu
Pharmaceutical Co., Ltd. (Hainan, China). Nm-23 and K-ras antibody
(cat. nos. bs-0688R and bs-1033R, respectively) were purchased from
the Beijing Boosen Biotechnology Co., Ltd (Beijing, China).
3,3′-diaminobenzidene color developing reagent kit (cat. no.
K155922C) and Biotin-Streptavidin horseradish peroxidase Detection
System (SP test kit; cat. no. 15155A08) were purchased from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
Formaldehyde (cat. no. 2015050501), glacial acetic acid (cat. no.
2015040201) were purchased from Chengdu Kelong Chemical Co., Ltd.
(Chengdu, China). Picric acid (cat. no. P9330) was purchased from
the Beijing Solarbio Science & Technology Co., Ltd. (Beijing,
China).
Establishment of the Lewis lung cancer
mouse model
Exponentially growing cells were resuspended to
1×107 cells/ml, ready for subcutaneous injection into
C57BL/6J mice (n=5). On day 14, the mice were sacrificed. A cancer
cell suspension was prepared by stripping tumor tissue using
radioimmunoprecipitation assay (RIPA) buffer at final concentration
of 1 M (cat. no. P0013B; Biyotime Biotech. Shanghai, China) and
dissolved in HuMEC Basal serum-free medium (cat. no., 12753018,
Gibco; Thermo Fisher Scientific, Inc. Waltham, MA, USA). A total of
1×107 cells/ml (total volume 0.2 ml) were subcutaneously
injected into the dorsal region of the remaining 40 C57BL/6J
mice.
Grouping and administration
After 6 days of growing subcutaneous tumors, the
mice were randomly divided into four groups (n=10 per group): Model
group, cisplatin group, traditional Chinese medicine group and
combined medication group. In the model group, a sterile gauze was
used to administer normal saline and egg white to tumors. In the
cisplatin group, cisplatin and egg white was administered to the
tumor. In the Yuxiao San group, Yuxiao San and egg white were mixed
to form a cream, which was compressed onto the skin to ~2–3 mm
thickness. In the combined medication group, Yuxiao San, cisplatin
and albumin were mixed to form a cream, which was compressed onto
the skin for ~2–3 mm thickness. Treatment for every group was
performed according to a previously published study (5). The above drugs were administered three
times a day, eight h at a time, for 21 days.
Observe the general condition and
tumor growth
Before inoculation and after vaccination mice were
weighed. General observations were noted, including their hair,
diet and activities.
For tumor growth calculations, before and after
inoculation, mice were weighed once every three days and the dose
of administration was adjusted according to weight change. After 21
days administration, the mice were sacrificed, the tumor was
removed and weighed, and then the (a) longest and (b) shortest
diameter of mice tumors was determined with a vernier caliper. The
volume of tumor was calculated according to the equation:
V=0.5ab2 (units in cm3), and the curve was
drawn. The tumor growth inhibition rate was calculated as: (Average
weight of tumors in model group-average weight of tumors in
treatment group)/average weight of tumors in model group ×100%
(6).
Pulmonary surface metastatic
nodules
On day 21, the mice were dissected, the pulmonary
tissue was removed, and fixed for 24 h with Bouin's fixative at 4°C
(picric acid, formaldehyde, glacial acetic acid in a ratio of
75:25:5, respectively), then put them into ethyl alcohol to remove
the yellow color. Metastases were observed under a microscope as
white nodules. The rate of inhibition on metastasis (%) was
calculated as: (Average metastatic nodules of pulmonary surface of
model group-average metastatic nodules of pulmonary surface of
treatment group)/average metastatic nodules of pulmonary surface of
model group ×100%.
Western blotting assay
The tumor tissue was embedded in paraffin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and cut into 4 µm
sections, and incubated with RIPA lysis buffer (Sigma-Aldrich;
Merck KGaA), and the total proteins were extracted and the
concentration was examined using bicinchoninic acid protein assay
kit (cat. no. PA115; Tiangen Biotech Co. Ltd., Beijing, China).
Equal quantities of protein (2 µg) were separated using a 10% gel
and SDS-PAGE and electro transferred onto polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Subsequently, the
membranes were blocked with 5% non-fat milk for 2 h at 4°C. The
membranes were incubated with a rabbit anti-mouse Nm23 polyclonal
antibody (1:2,000; cat. no. ab154547; Abcam, Cambridge, UK), a
rabbit anti-mouse K-ras monoclonal antibody (1:3,000; cat. no.
ab199557; Abcam) and a rabbit anti-mouse GAPDH polyclonal antibody
(1:2,000; cat. no. ab9485; Abcam) for 2 h at room temperature. The
membranes were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:2,000, cat. no.
sc-2030; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 37°C
for 1 h. Finally, the western blot bands were visualized by using
an enhanced chemiluminescent kit (Thermo Fisher Scientific, Inc.).
Finally, the bands were scanned and analyzed by using a UVP gel
image scanning system GDS8000 software (UVP, Sacramento, CA,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from tissues using TRIzol (Tiangen
Biotech Co. Ltd.). The concentration and purity of RNA was measured
by determining the optical density by using a spectrophotometer
(Nanodrop Lite; Thermo Fisher Scientific, Inc.). cDNA was
synthesized by using a Rever Tre Ace-a-reverse transcription kit
(cat. no. FKS-101, Toyobo Life Science, Osaka, Japan). The primers
were: nm-23: Forward, 5′-CGGCAGTGATTCAGTGGAGAGT-3′ and reverse,
5′-CTGGTTTCTCCGCGTCTACTCATAC-3′; K-ras: Forward,
5′-CTGGGGAGGGCTTTCTTTGTG-3′ and reverse,
5′-CTCCTGAGCCTGTTTCGTGTCTA-3′; β-actin: Forward,
5′-ACCCCGTGCTGCTGACCGAG-3′ and reverse, 5′-TCCCGGCCAGCCAGGTCCA-3′.
The RT reaction conditions were: 42°C for 10 min, 30°C for 20 min,
99°C for 5 min and 4°C for 5 min. The PCR reaction conditions were:
94°C for 5 min, 94°C for 30 sec, 57°C for 30 sec and 72°C for 30
sec for a total of 40 cycles and termination at 72°C for 10 min.
After the reaction, the ABI StepOne Plus PCR system was used to
analyze and calculate the cycle threshold value of the target gene.
The relative mRNA expression of targeting genes was normalized to
the β-actin gene by using the comparative threshold cycle
(2−ΔΔCq) method (7).
Statistical analysis
Data in the present study were analyzed using SPSS
software version 19.0 (IBM Corp., Armonk, NY, USA). Data was
presented as the mean ± standard deviation. All data were obtained
from at least three independent experiments. Tukey's post hoc test
was used following a one-way analysis of variance between the two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
General observations
A total of 40 mice were inoculated with Lewis lung
carcinoma cells and all developed tumors. Mice experienced symptoms
of lassitude, asthenia, somnolence, lethargy and withered hair.
Compared with the model group, these symptoms were reduced in the
Traditional Chinese medicine group and combined medication group.
However, the cisplatin group not only exacerbated the above
symptoms, but also suffered side-effects of chemotherapy, such as
decreased appetite, loss of weight and loss of hair (data not
shown).
Comparison of tumor growth in each
group
The weight of tumors in the cisplatin, Yuxiao San
group and the combination group were lower than that of the model
group (P<0.01; Table I). The
weight of tumor in the Yuxiao San group and combination group were
lower than the cisplatin group (P<0.01; Table I). The combination group had a lower
tumor weight than the cisplatin group and Yuxiao San group
(P<0.01; Table I).
 | Table I.Effect of Yuxiao San on Lewis lung
cancer tumor growth and progression in C57BL/6 mice (mean ±
standard deviation, n=10 per group). |
Table I.
Effect of Yuxiao San on Lewis lung
cancer tumor growth and progression in C57BL/6 mice (mean ±
standard deviation, n=10 per group).
| Group | Weight (g) | Volume
(cm3) | Inhibition rate
(%) |
|---|
| Model group | 2.04±0.36 | 2.88±0.63 | – |
| Cisplatin group |
1.35±0.16a |
1.72±0.25a | 33.82 |
| Yuxiao San group | 1.16±0.11 |
1.27±0.23a,b | 43.14 |
| Yuxiao San +
cisplatin group |
0.94±0.17a–c |
0.90±0.13a–c | 55.88 |
The volume of tumor in the cisplatin group, Yuxiao
San group and the combination group were lower than that of the
model group (P<0.01; Table I). The
volume of tumor in the Yuxiao San group and combination group were
lower than that in the cisplatin group (P<0.01; Table I). The combination group had a lower
volume of tumor than the cisplatin group and Yuxiao San group
(P<0.01; Table I). The inhibition
rate of tumor growth in the combination group was 55.88% and was
higher than the cisplatin group (33.82%) and Yuxiao San group
(43.14%) (P<0.01; Table I).
Comparison of pulmonary surface
metastatic nodules in each group
The rate of inhibition on tumor metastasis in the
cisplatin group, Yuxiao San group and the combination group (35.90,
57.38 and 68.85%, respectively) was significantly increased
compared with that of the model group (24.60%) (P<0.01; Table II). The combination group also
inhibited pulmonary surface metastatic nodules to a greater extent
than the other groups (Table
II).
 | Table II.Effect of Yuxiao San on pulmonary
surface metastatic nodules of Lewis lung carcinoma in C57BL/6 mice
(mean ± standard deviation, n=10 per group). |
Table II.
Effect of Yuxiao San on pulmonary
surface metastatic nodules of Lewis lung carcinoma in C57BL/6 mice
(mean ± standard deviation, n=10 per group).
| Group | Pulmonary
metastatic nodules (n) | Metastatic
inhibition rate (%) |
|---|
| Model group | 6.10±1.59 | 24.60 |
| Cisplatin
group |
3.91±1.20a | 35.90 |
| Yuxiao San
group |
2.60±1.43a,b | 57.38 |
| Yuxiao San +
cisplatin group |
1.90±1.19c | 68.85 |
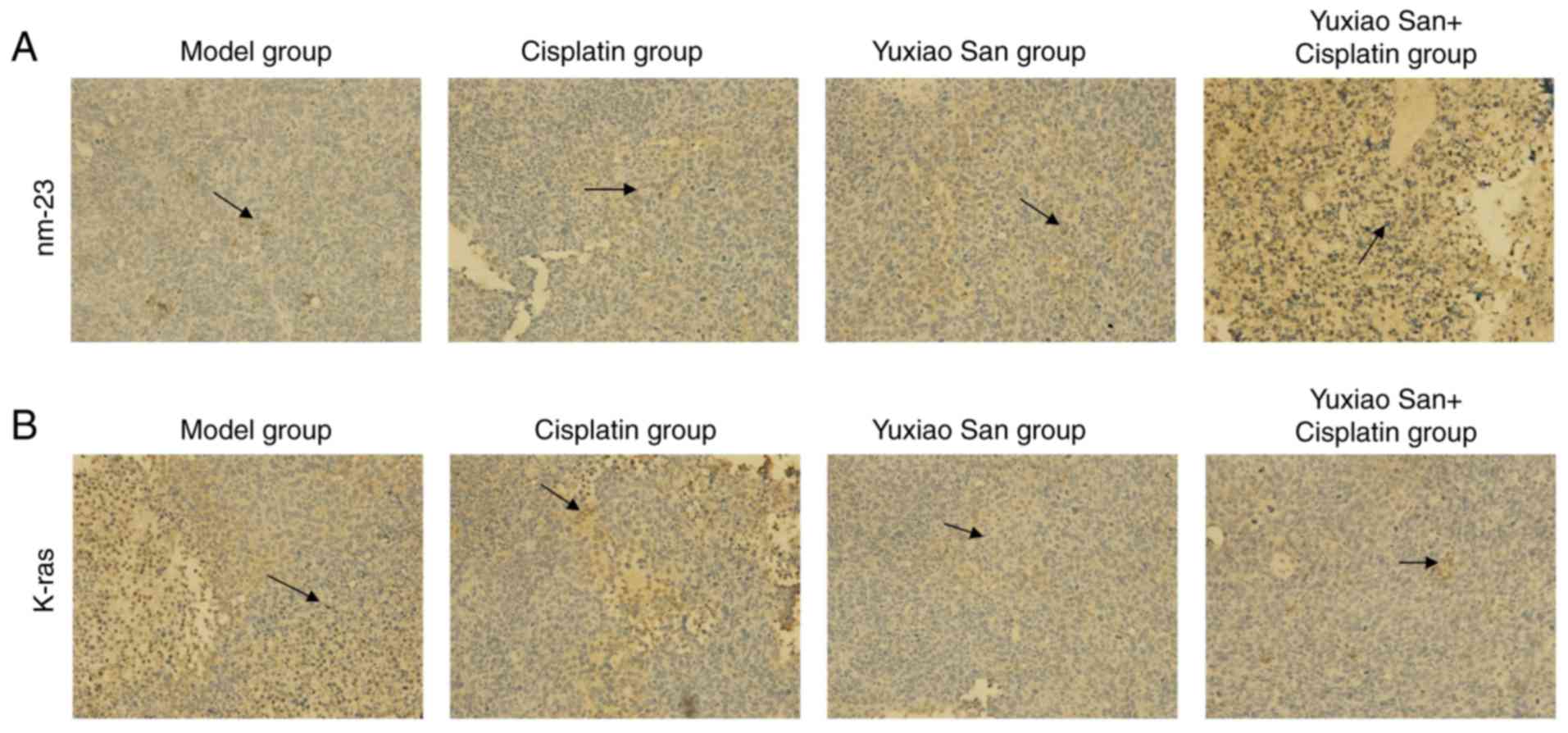
Observation for K-ras and nm-23
expression in tumor tissue by using immunohistochemistry
K-ras and nm-23 were detected via
immunohistochemical staining (Fig.
1). The mean absorbance of K-ras expression in the model group,
cisplatin group, Yuxiao San group and combination group were
1.29±0.01, 1.02±0.24, 0.87±0.17 and 0.612±0.02, respectively
(Table III). Compared with the
model group, expression of K-ras and nm-23 in the other groups were
significantly decreased (P<0.01). Compared with the model group,
the cisplatin group and the Yuxiao San group, the combination group
exhibited the most significantly decreased expression levels of
K-ras and nm-23 (P<0.01; Table
III). Compared with the cisplatin group, the Yuxiao San group
and combination group exhibited significantly decreased expression
of the two proteins (P<0.01). Compared with the Yuxiao San
group, the combination group exhibited the most significantly
decreased expression levels. For the decreased expression of K-ras
and nm-23, the combination of cisplatin and Yuxiao San was superior
to single drug treatment (P<0.01; Table III).
 | Table III.Comparison of groups of the mean
absorbance of K-ras and nm23 (mean ± standard deviation, n=10 per
group). |
Table III.
Comparison of groups of the mean
absorbance of K-ras and nm23 (mean ± standard deviation, n=10 per
group).
| Group | Dose (g/kg) | K-ras | nm-23 |
|---|
| Model group | – | 1.29±0.01 | 0.58±0.02 |
| Cisplatin
group | 0.02 |
1.02±0.24a |
0.65±0.01a |
| Yuxiao San
group | 10 |
0.87±0.17a,b |
0.89±0.01a,b |
| Yuxiao San +
cisplatin group | 10/0.02 |
0.61±0.02a–c |
1.08±0.02a–c |
The average absorbance values of nm-23 in the model
group, cisplatin group, Yuxiao San group and combination group were
0.58±0.02, 0.65±0.01, 0.89±0.01 and 1.08±0.02, respectively
(Table III). Compared with the
model group, the other groups exhibited significantly greater
expression levels of nm-23 (P<0.01; Table III). Compared with the model group,
cisplatin group and Yuxiao San group, the combination group
exhibitedthe most significantly increased expression of nm-23
(P<0.01; Table III). Compared
with the cisplatin group, the Yuxiao San group and combination
group exhibited significantly increased nm-23 expression levels
(P<0.01; Table III). Compared
with Yuxiao San group, the combination group demonstrated the most
significantly increased nm-23 expression levels (P<0.01;
Table III). For the decreased
expression of K-ras and nm-23, the combination of cisplatin and
Yuxiao San is superior to single drug treatment (P<0.01;
Table III).
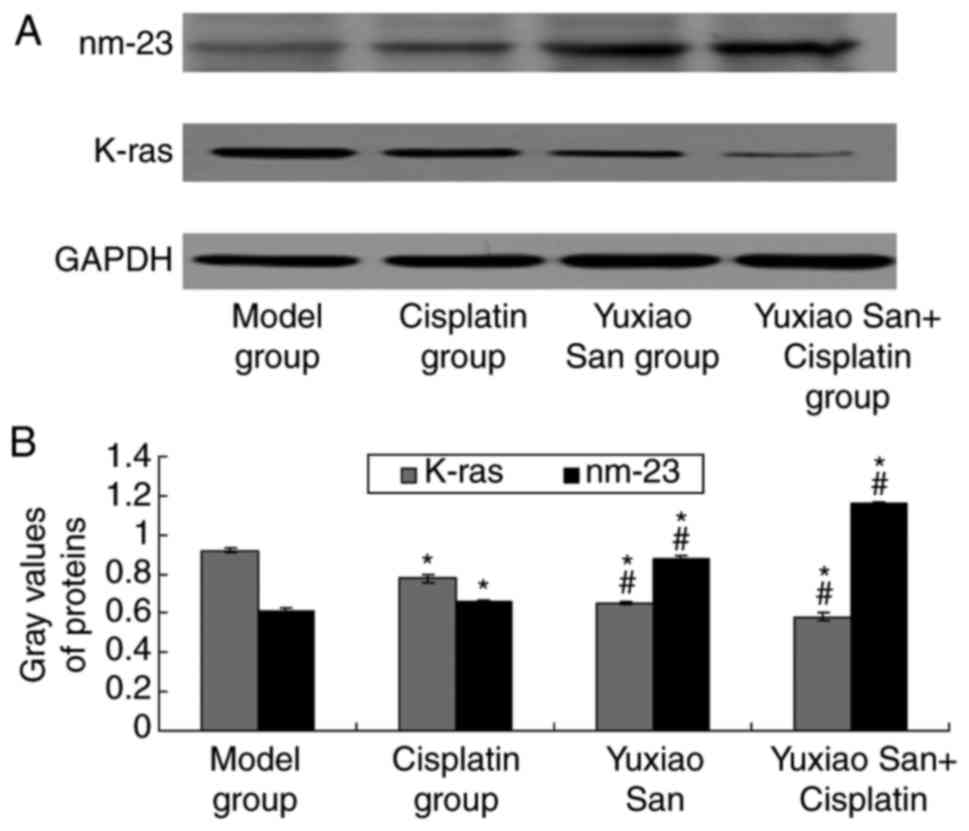
Detection of K-ras and nm-23 in tumor
tissue by western blotting
K-ras expression levels were examined by using
western blotting (Fig. 2A). The
results suggested that K-ras protein expression levels in the
cisplatin group, Yuxiao San group and cisplatin and Yuxiao San
combination group were significantly decreased compared with the
model group (P<0.01; Fig. 2B). In
addition, K-ras expression levels in the Yuxiao San group and the
cisplatin and Yuxiao San combination group were also decreased
compared with the cisplatin group (P<0.01; Fig. 2B).
Protein expression levels of nm-23 in the cisplatin
group, Yuxiao San group, and cisplatin and Yuxiao San combination
group were significantly increased compared with the model group
(P<0.01; Fig. 2B). Protein
expression levels of nm-23 in the Yuxiao San group and cisplatin
and Yuxiao San combination group were also increased compared to
the cisplatin group (P<0.01; Fig.
2B).
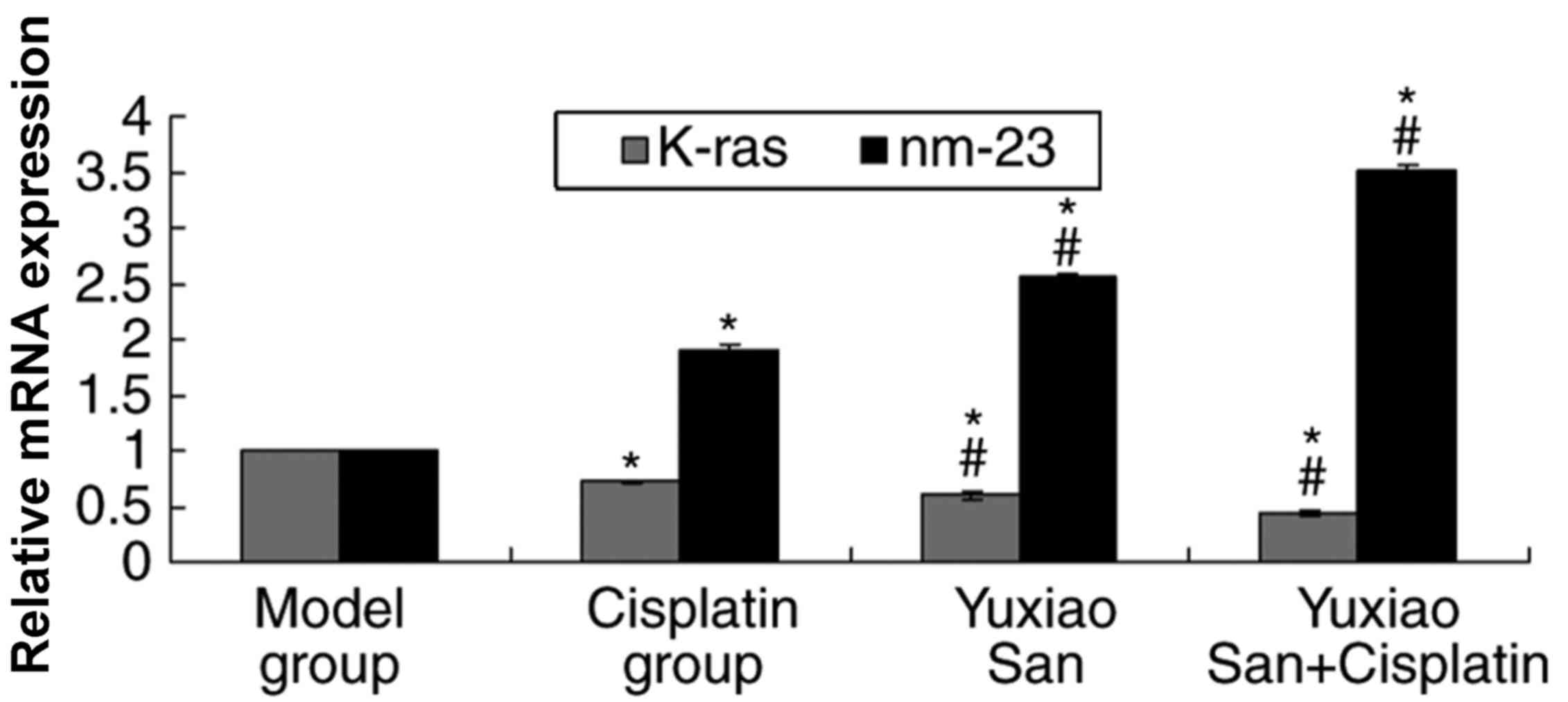
Detection of K-ras and nm-23 in tumor
tissue by RT-qPCR
K-ras mRNA expression levels were examined by using
RT-qPCR (Fig. 3). The results
suggested that K-ras mRNA expression levels in the cisplatin group,
Yuxiao San group and cisplatin and Yuxiao San combination group
were significantly decreased compared with the model group
(P<0.01). K-ras mRNA expression levels in the Yuxiao San group
and cisplatin and Yuxiao San combination group were also decreased
compared with the cisplatin group (P<0.01).
In addition, nm-23 mRNA expression levels in the
cisplatin group, Yuxiao San group and cisplatin and Yuxiao San
combination group were significantly increased compared with the
model group (P<0.01; Fig. 3).
nm-23 mRNA expression levels in the Yuxiao San group and cisplatin
and Yuxiao San combination group were increased compared with the
cisplatin group (P<0.01).
Discussion
The traditional Chinese Medicine holds the view that
tumors are associated with the depression and stress indicated in
patients. Lung cancer also belongs to a kind of pulmonary disease,
according to a Chinese medical theory, indicating an abscess in the
lung (8). Yuxiao San powder is a
common application for tumors and is composed of Curcuma
aromatica, mint, rhubarb, pubescent holly root, dandelion and
Glauber's salt (9,10). Modern medical research has established
that the effective constituent of Curcuma aromatica
(8), mint (9) and dandelion (10) work against tumor growth and induce
cell apoptosis.
The K-ras gene causes cancer when mutated. K-ras
genes make proteins, which are involved in cell signaling, growth
and death (apoptosis). The Ras oncogene is well known to be
involved in cancer development, including H-RAS, K-RAS and N-RAS,
which encode a family of 21 kDa guanosine triphosphate-binding
proteins called p21.
In physiologic conditions, these proteins may be
activated by binding with guanosine triphosphate (GTP) and initiate
cell proliferation via the Ras-dependent kinase cascade.
Subsequently, GTP is hydrolyzed to guanosine diphosphate by its
intrinsic GTPase activity and Ras proteins return to an inactive
state. However, in tumors, when a point mutation occurs in these
genes, which often results in loss of its intrinsic GTPase
activity, Ras proteins may acquire transforming potential, leading
to continuous activation of Ras signaling (11).
K-ras gene mutations occur frequently in non-small
cell lung cancer (NSCLC), mainly in adenocarcinoma, and rarely in
squamous cell carcinoma (12).
Approximately 80% of K-ras mutations in NSCLC involve codon 12,
others are located in codons 13 or 61 (13). Evidence from animal model studies of
NSCLC demonstrated that K-ras mutations enhance cellular
proliferation and induce malignant transformation, and their
continuous activation serves a key role in tumor development and
maintenance. The carcinogenesis of K-ras mutations was also
demonstrated in human NSCLC (14).
Targeted knockdown of K-ras in NSCLC cell lines has
provided critical preclinical confirmation of the role of this
driver in tumorigenesis, resulting in the suppression of tumor
growth and sensitization to inhibitors of other signaling pathways
(15). However, in practical terms,
K-ras itself is an intractable target for the development of
therapeutics (16), and considerable
effort has thus focused on inhibition of its downstream effectors
to perturb persistent activation of oncogenic signaling pathways.
The canonical RAF/MEK/ERK kinase cascade is the primary mitogenic
pathway stimulated by K-ras under both physiologic and pathologic
conditions (17,18).
The nm-23 gene was first discovered as a cancer
metastasis suppressor in murine melanoma cell lines. As many as
five nm-23 genes in human have been identified, including nm-23-h1
to nm-23-h5. Protein nm-23 gene products are known as abnormal wing
disc, which is found in Drosophila (19). Factor 1 (inhibitor of differentiation)
and c-myc purine-binding transcription factor (PUF) are the
transcription factors that regulate c-myc. Factor 1 and PUF include
a large family of proteins that have activity for nucleoside
diphosphate kinase (NDPK). NDPK is found in almost all cells and
serves to catalyze the phosphorylation of 5′triphosphate nucleoside
to 5′diphosphate nucleoside, through an intermediary mechanism
involving high-energy oxidative phosphorylation enzymes (19,20).
NDPK serves a function in signal transduction from
the cell membrane into the cell nucleus via a G protein activation
pathway and p21, and serves a role in cell division and maintenance
of cell shape (21). Protein nm-23
gene products have implications in the proliferation and
differentiation of cells, and also serves a role in cancer
(22). The nm-23 protein is
distributed to the cytosol, mitochondria, plasma membrane and
nucleus (23). The nm-23 gene family
is highly conserved among a wide variety of eukaryotic species
(24,25). The identification of nm23 as a tumor
suppressor gene indicated the existence of genes that specifically
regulate metastasis (26,27).
The function of nm23 in human cancer is currently
unconfirmed (22,28–31), and
certain reports have indicated there is a link between high
expression of the nm-23 gene and its protein product with low
metastatic potential of cancer (28).
Nm-23 is known to be associated with early onset of familial breast
and ovarian cancer (28).
Furthermore, an inverse association between nm-23 expression and
metastasis was also observed in various types of cancer (22). Although the mechanism by which nm-23
regulates metastasis is not fully understood, experimental data
have shown that nm23 serves an important role in the regulation of
metastasis in a number of human cancer types (29). It was also reported that nm-23
expression was a significant factor for predicting a favorable
prognosis, suggesting the anti-metastatic potential of nm-23 in
NSCLC (30,31). The expression of nm23 in normal lung
tissue was much higher than in cancer tissues, and was associated
with staging and lymphatic metastasis, and this may suggest that
the absence of nm23 expression may serve an important role in the
incidence of NSCLC.
The present study determined the effects of Yuxiao
San on Lewis lung cancer in mice. This study was conducted in
vivo and in vitro, and there were four groups, including
the model group, cisplatin group, Yuxiao San group and the
combination group (Yuxiao San and cisplatin). The Lewis lung cancer
model in C57BL/6 mice was established and mice were treated with
Yuxiao San and cisplatin, before determining any side effects of
treatment. Comparisons between tumor weight, tumor volume and tumor
inhibition rate were made, and the methods of immunohistochemistry,
western blotting and RT-qPCR were used to detect K-ras and nm23
gene expression levels in tumor tissue.
Results of the present study revealed that Yuxiao
San may reduce tumor weight and tumor volume. Yuxiao San may also
decrease K-ras gene expression levels and increase nm-23 gene
expression levels. It was found that Yuxiao San downregulated the
expression of K-ras, and upregulating expression of nm-23 in Lewis
lung cancer mice tissues. The expression levels of nm-23 in normal
lung tissue was much higher compared with that in cancer tissues.
Combining cisplatin and Yuxiao San was better than the use of
single drug treatment, as compared with the model group, the tumor
volume, weight and lung metastases of tissues treated with Yuxiao
San and cisplatin group in combination were decreased. In addition,
compared with the model group, the expression levels of K-ras via
immunohistochemical staining and RT-qPCR were decreased. Expression
levels of nm-23 in tumors were increased via immunohistochemistry
staining, western blotting and RT-qPCR.
Although this study revealed some notable results,
there were a number of limitations. Firstly, the sample sizes were
relatively small. In a subsequent study, a large sample size should
be utilized. Furthermore, the down-stream factors of nm-23 and
K-ras have not been clarified. The mechanism for the effect of
Yuxiao San following the changes of nm-23 and K-ras should be
investigated. Additionally, the effects of Yuxiao San have not been
examined in combination with the other chemotherapy drugs, except
for cisplatin. In a follow-up study, the combined effects of Yuxiao
San with other chemotherapy drugs should be assessed.
To conclude, Yuxiao San and cisplatin injection by
external application may effectively inhibit growth of transplanted
tumors in mice. The underlying mechanism may be associated with
nm-23 upregulation and K-ras downregulation.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Clinical
Research Base of traditional Chinese medicine Construction in 2015
(second special research projects; grant no. JDZX2015073) and
Project of Chongqing Education Bureau (grant no. yjg143075).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MT, SW, YW performed the tests and experiments. YW
analyzed the data. MT and JF wrote the manuscript and were the
major contributors to the design of the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed following the
guidelines of the Care and Use of Laboratory Animals of National
Institute of Health (NIH) and was approved by the Ethics Committee
of Chongqing Medical University (Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ye L, Jia Y, Ji KE, Sanders AJ, Xue K, Ji
J, Mason MD and Jiang WG: Traditional Chinese medicine in the
prevention and treatment of cancer and cancer metastasis. Oncol
Lett. 10:1240–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li F and Zhang W: Role of traditional
Chinese medicine and its chemical components in anti-tumor
metastasis. J Cancer Res Ther. 10 Suppl 1:S20–S26. 2014. View Article : Google Scholar
|
|
3
|
Yang H, Liu J and Dou QP: Targeting tumor
proteasome with traditional Chinese medicine. Curr Drug Discov
Technol. 7:46–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi Y, Izumi Y, Matsutani N, Dejima
H, Nakayama T, Okamura R, Uehara H and Kawamura M: Optimized
magnitude of cryosurgery facilitating anti-tumor immunoreaction in
a mouse model of Lewis lung cancer. Cancer Immunol Immunother.
65:973–982. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu C, Wang Y, Xia Y, He S, Wang Z, Chen Y,
Shu Y and Jiang J: Wilms' tumor 1 enhances Cisplatin-resistance of
advanced NSCLC. FEBS Lett. 588:4566–4572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Gu JF, Zou X, Wu J, Zhang MH, Jiang
J, Qin D, Zhou JY, Liu BX, Zhu YT, et al: The anti-lung cancer
activities of steroidal saponins of P. polyphylla Smith var.
chinensis (Franch.) Hara through enhanced immunostimulation in
experimental Lewis tumor-bearing C57BL/6 mice and induction of
apoptosis in the A549 cell line. Molecules. 18:12916–12936. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang F, Xu L, Qu X, Zhao M, Jin B, Kang
J, Liu Y and Hu X: Synergistic antitumor effect of β-elemene and
etoposide is mediated via induction of cell apoptosis and cell
cycle arrest in non-small cell lung carcinoma cells. Mol Med Rep.
4:1189–1193. 2011.PubMed/NCBI
|
|
9
|
Journigan VB and Zaveri NT: TRPM8 ion
channel ligands for new therapeutic applications and as probes to
study menthol pharmacology. Life Sci. 92:425–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loh CH, Inbaraj BS, Liu MH and Chen BH:
Determination of chlorophylls in Taraxacum formosanum by
high-performance liquid chromatography-diode array detection-mass
spectrometry and preparation by column chromatography. J Agric Food
Chem. 60:6108–6115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma Y, Guo FC, Wang W, Shi HS, Li D and
Wang YS: K-ras gene mutation as a predictor of cancer cell
responsiveness to metformin. Mol Med Rep. 8:763–768. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodenhuis S, van de Wetering ML, Mooi WJ,
Evers SG, van Zandwijk N and Bos JL: Mutational activation of the
K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of
the lung. N Engl J Med. 317:929–935. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weiss GJ, Ganeshan B, Miles KA, Campbell
DH, Cheung PY, Frank S and Korn RL: Noninvasive image texture
analysis differentiates K-ras mutation from pan-wildtype NSCLC and
is prognostic. PLoS One. 9:e1002442014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piva S, Ganzinelli M, Garassino MC, Caiola
E, Farina G, Broggini M and Marabese M: Across the universe of
K-RAS mutations in non-small-cell-lung cancer. Curr Pharm Des.
20:3933–3943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sunaga N, Shames DS, Girard L, Peyton M,
Larsen JE, Imai H, Soh J, Sato M, Yanagitani N, Kaira K, et al:
Knockdown of oncogenic KRAS in non-small cell lung cancers
suppresses tumor growth and sensitizes tumor cells to targeted
therapy. Mol Cancer Ther. 10:336–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Young A, Lyons J, Miller AL, Phan VT,
Alarcón IR and McCormick F: Ras signaling and therapies. Adv Cancer
Res. 102:1–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dalpa E, Gourvas V, Soulitzis N and
Spandidos DA: K-Ras, H-Ras, N-Ras and B-Raf mutation and expression
analysis in Wilms tumors: Association with tumor growth. Med Oncol.
34:62017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JT Jr, Steelman LS and McCubrey JA:
Modulation of Raf/MEK/ERK kinase activity does not affect the
chemoresistance profile of advanced prostate cancer cells. Int J
Oncol. 26:1637–1644. 2005.PubMed/NCBI
|
|
19
|
Conery AR, Sever S and Harlow E:
Nucleoside diphosphate kinase Nm23-H1 regulates chromosomal
stability by activating the GTPase dynamin during cytokinesis. Proc
Natl Acad Sci USA. 107:15461–15466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong L, Wu Z, Guo L, Li L, Zhao R, Zhu D
and Zhou Q: Metastasis suppressor Nm23-H1 inhibits STAT3 signaling
via a negative feedback mechanism. Biochem Biophys Res Commun.
434:541–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujita Y, Fujiwara K, Zenitani S and
Yamashita T: Acetylation of NDPK-D regulates its subcellular
localization and cell survival. PLoS One. 10:e01396162015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu L, Liang L, Su J and Yang Z: Inhibitory
effect of upregulated DR-nm23 expression on invasion and metastasis
in colorectal cancer. Eur J Cancer Prev. 22:512–522. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Radović S, Dorić M, Hukić A, Babić M,
Kuskunović S and Spahović N: Immunohistochemical expression and
significance of NM23 suppressor protein in primary gastric
adenocarcinoma. Bosn J Basic Med Sci. 13:72–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schlattner U, Tokarska-Schlattner M, Epand
RM, Boissan M, Lacombe ML, Klein-Seetharaman J and Kagan VE:
Mitochondrial NM23-H4/NDPK-D: A bifunctional nanoswitch for
bioenergetics and lipid signaling. Naunyn Schmiedebergs Arch
Pharmacol. 388:271–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Durán E, Cárdenas JM, Reina MA and Arriazu
R: Loss of Nm23 is associated with a more favorable tumor
microenvironment in patients with breast cancer. Histol
Histopathol. 30:345–352. 2015.PubMed/NCBI
|
|
26
|
Yokdang N, Nordmeier S, Speirs K, Burkin
HR and Buxton IL: Blockade of extracellular NM23 or its endothelial
target slows breast cancer growth and metastasis. Integr Cancer Sci
Ther. 2:192–200. 2015.PubMed/NCBI
|
|
27
|
Niitsu N: The association of nm23-H1
expression with a poor prognosis in patients with peripheral T-cell
lymphoma, not otherwise specified. J Clin Exp Hematop. 54:171–177.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Fu LJ, Liu XQ, Hu ZY, Jiang Y,
Gao RF, Feng Q, Lan X, Geng YQ, Chen XM, et al: nm23 regulates
decidualization through the PI3K-Akt-mTOR signaling pathways in
mice and humans. Hum Reprod. 31:2339–2351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fiore LS, Ganguly SS, Sledziona J, Cibull
ML, Wang C, Richards DL, Neltner JM, Beach C, McCorkle JR, Kaetzel
DM and Plattner R: c-Abl and Arg induce cathepsin-mediated
lysosomal degradation of the NM23-H1 metastasis suppressor in
invasive cancer. Oncogene. 33:4508–4520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
You J, Chang R, Liu B, Zu L and Zhou Q:
Nm23-H1 was involved in regulation of KAI1 expression in
high-metastatic lung cancer cells L9981. J Thorac Dis. 8:1217–1226.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu Y, Li Y, Zhao X, Dong D, Tang C, Li E
and Geng Q: Combined detection of the expression of Nm23-H1 and p53
is correlated with survival rates of patients with stage II and III
colorectal cancer. Oncol Lett. 13:129–136. 2017. View Article : Google Scholar : PubMed/NCBI
|