Introduction
Osteosarcoma (OS) is an aggressive primary bone
sarcoma, which occurs mainly in adolescents and young adults. OS
has been reported to comprise 2.4% of all types of malignancy in
paediatric patients and ~20% of all primary types of bone cancer
(1). As the most common primary
malignant bone tumour, OS has been reported to have a high
metastatic rate and patients with metastasis have poorer prognosis
with a lower 5-year survival rate (2). The prognosis and treatment of OS remains
a challenge due to local relapse or metastases following surgical
resection and intensive-chemotherapy (3). Therefore, it is essential to illustrate
the underlying mechanism of OS progression regulation, to improve
the prognosis, and to identify novel therapeutic targets and
approaches for OS treatment.
microRNAs (miRNAs) are endogenous single-stranded,
19–22 nucleotide-long, noncoding RNAs that regulate
post-transcriptional silencing by binding with partial
complementarity to the 3′-untranslated region (3′-UTR) of the
target mRNA (4). It has been reported
that miRNAs are highly conserved and are able regulate ~30% of
human genes (5). miRNAs have been
demonstrated to be involved in multiple fundamental cell processes,
including stem cell development, autophagy, transformation,
proliferation control, cell differentiation, regulation of the cell
cycle and cell apoptosis, as well as tumorigenesis (6,7). It has
been validated that dysregulation of miRNAs can induce a number of
human diseases and the abnormal expression of miRNAs has been
closely associated with tumour progression, making them potential
markers for cancer prognosis and diagnosis (8). Particularly, downregulation of
miR-142-5p expression has been observed in OS human samples and
compared with controls (9).
Group XVI phospholipase A2 (PLA2G16) expression,
also known as H-REV-107, Ha-RAS like suppressor 3 (HRASLS3) and
adipose specific PLA2 (AdPLA2), has been reported to be observed in
the majority of healthy tissues (10)
and was first identified as a class II tumour suppressor by its
inhibition of Ras-mediated transformation (11). Furthermore, PLA2G16 overexpression
suppresses proliferation and promotes apoptosis (12). PLA2G16 expression has been reported to
be absent in various types of human cancer, including breast,
ovary, kidney and testicular germ cells (13). Nevertheless, increased levels of
PLA2G16 expression have been observed in lung, colon, stomach and
rectal cancer, suggesting that PLA2G16 may serve an oncogenic role
in the aforementioned tumours (14).
It has also been reported that PLA2G16 is an important prognostic
factor in patients with primary OS, predicting the development of
metastases and poor survival and is associated with pulmonary
metastasis and poor prognosis in human OS (15). Therefore, in the present study, the
regulatory role of miR-142-5p was examined in proliferation and
apoptosis, as well as its association with PLA2G16 expression, in
the human OS cell line, HOS.
Materials and methods
Tissue samples and cell lines
OS tissue samples (n=21) and adjacent noncancerous
tissues (n=21) were collected from patients at Honghui Hospital of
Xi'an Jiaotong University (Xi'an, China) with written informed
consent, in accordance with the Declaration of Helsinki of the
World Medical Association. Diagnosis was confirmed by
immunohistochemical analysis by pathologists from Honghui Hospital
of Xi'an Jiaotong University (Xi'an, China) prior to the
experimentation of the present study. The present study was
approved by the Institutional Review Board and Human Ethics
Committee of Xi'an Jiaotong University (Xi'an, China).
The human OS cell line, HOS, the human fetal
osteoblastic cell line, hFOB1.19 and the 293T cell line were
obtained from the American Type Culture Collection (Manassas, VA,
USA). The hFOB1.19 cells were cultured in Dulbecco's modified
Eagle's medium/F12 (DMEM/F12; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and HOS cells were cultured in DMEM medium
(Gibco; Thermo Fisher Scientific, Inc.) all of which were
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2. The 293T cells
were cultured in DMEM medium supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), high glucose (Gibco; Thermo Fisher
Scientific, Inc.), L-glutamine and sodium pyruvate (all from Thermo
Fisher Scientific, Inc.) at 37°C with 5% CO2.
Cell transfection
The miR-NC mimics (5′-GUGUAACACGUCUAUACGCCCA-3′) and
miR-142-5p mimics (5′-CAUAAAGUAGAAAGCACUACU-3′) were designed and
chemically synthesized (Shanghai GenePharma Co., Ltd., Shanghai,
China). Cultured cells (4×105 cells/well) were seeded
into 6-well plates (Corning Incorporated, Corning, NY, USA) for 24
h and transfected with 50 nM miR-NC or miR-142-5p mimics using
Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The transfection
efficiency was measured using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assays. Cells were then
subjected to further analysis 72 h following transfection as
indicated in the results section. In addition, PLA2G16 coding
sequence without the 3′-UTR was selected and cloned into pcDNA3.1
(Invitrogen; Thermo Fisher Scientific, Inc.) to overexpress
PLA2G16. The plasmids were transfected into HOS cells using
Lipofectamine® 2000, according to the manufacturer's
protocol.
MTT assay
Proliferation was determined using the MTT assay. A
total of 5×103 cells/well were seeded into 96-well
plates (Corning Incorporated) following transfection. The cells
were grown for 24, 48 and 72 h, and subsequently 20 µl of modified
tetrazolium salt (5 mg/ml) was added to each well and incubated for
4 h at 37°C. The supernatant was subsequently removed and 100 µl
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was added to dissolve the formazan crystals. The absorbance was
evaluated at a wavelength of 490 nm with a Bio-Rad Benchmark
micro-plate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
5-Bromo-2′-deoxyuridine (BrdU)
incorporation assay
BrdU incorporation during DNA synthesis was
determined by ELISA using the BrdU kit (cat. no., C0088L; Beyotime
Institute of Biotechnology, Shanghai, China) 72 h following
transfection, according to the manufacturer's protocol. The assay
was conducted in triplicate and the absorbance was detected at a
wavelength of 450 nm with a microplate reader.
RT-qPCR
Total RNA, including miRNAs, from tissue samples or
cells, was isolated by the miRNA Isolation kit (Ambion; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The miR-142-5p expression level was measured using the TaqMan miRNA
assay (Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The miRNA expression
levels were quantified to RNU48 expression. The relative miR-142-5p
expression level was calculated by the 2−ΔΔCq method
(16). The RT-qPCR reaction condition
was as follows: Pre-degeneration at 95°C for 3 min, denaturation at
95°C for 30 sec, annealing at 60°C for 30 sec and extension at 72°C
for 30 sec with a total of 40 cycles. The sequence of miR-142-5p
primer was designed as follows: Forward, 5′-GGCCCATAAAGTAGAAAGC-3′
and reverse, 5′-TTTGGCACTAGCACATT-3′. Endogenous control RNU48 was
amplified using forward, 5′-TGATGATGACCCCAGGTAACTC-3′ and reverse,
5′-GAGCGCTGCGGTGATG-3′. The above experiment was performed in
triplicate. The sample that lacked cDNA was used as a negative
control.
Caspase-3 (CASP3) activity assay
CASP3 activity was measured by the CASP3 kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocols. Aliquots of protein were incubated with
corresponding CASP3 substrate Ac-DEVD-pNA for 2 h at 37°C. The
colorimetric release of phosphorylated (p)-nitroaniline from the
Ac-DEVD-pNA substrate was recorded at wavelength of 405 nm.
Luciferase reporter assay
The potential binding site between miR-142-5p and
PLA2G16 was initially obtained and analyzed online (http://www.targetscan.org/). The 3′-UTR of human
PLA2G16, containing the miR-142-5p targeting sequence, was inserted
into the pMIR-REPORT™ miRNA Expression Reporter Vector
system (Ambion; Thermo Fisher Scientific, Inc.). Subsequently, the
reporter plasmids, PLA2G16-wt 3′-UTR and PLA2G16-mut 3′-UTR, were
co-transfected with miR-NC or miR-142-5p mimics into 293T cells
using Lipofectamine® 2000. Cultured cells were collected
following 48 h to measure reporter activity in comparison with
Renilla luciferase activity using a Renilla Promega
luciferase assay kit (Promega Corperation, Madison, WI, USA),
according to the manufacturer's protocols.
Flow cytometry
The apoptotic rate was detected by flow cytometry
analysis using the Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection kit (R&D
Systems Europe, Ltd., Abingdon, UK), according to the
manufacturer's protocols. Briefly, cultured HOS cells were
collected and suspended in annexin-binding buffer and then exposed
to AnnexinV-FITC and PI for 12 min in the dark. The apoptotic rate
was analyzed and quantified using a FACSCalibur flow cytometer
equipped with CellQuest 6.0 software (BD Biosciences, Franklin
Lakes, NJ, USA).
Western blot analysis
Cells were lysed using radioimmunoprecipitation
assay buffer [50 mM Tris hydrochloride, pH 7.4, 1% Nonidet-P40,
0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM
NaVO4, 2 mM NaF, Complete protease inhibitor cocktail
(Roche Diagnostics, Indianapolis, IN, USA)], and the protein
concentrations were examined by the Pierce BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols. Equal amounts of protein (50 µg) were subsequently
separated by 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). Subsequent
to blocking using 5% BSA (Sangon Biotech Co. Ltd., Shanghai,
China). for 1 h at room temperature, the membranes were incubated
with primary antibodies against PLA2G16 (cat. no. 10337, Cayman
Chemical Company, Ann Arbor, MI, USA) at a 5 ug/ml dilution; BCL2
associated X, apoptosis regulator (Bax; cat. no. sc20067), BCL2,
apoptosis regulator (BCL2; cat. no. sc23960), proto-oncogene,
serine/threonine kinase 1 (Raf-1; cat. no. sc373722) and p-Raf-1
(cat. no. sc271928) (dilution, 1:500; all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); CASP3 (cat. no. 9662),
p-extracellular signal-regulated kinase (ERK) 1/2 (cat. no. 4370),
ERK1/2 (cat. no. 4695), p-mitogen-activated protein kinase kinase
(MEK; cat. no. 9154), MEK (cat. no. 4694) and β-actin (cat. no.
4970) (dilution, 1:1,000; all from Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C overnight. Following incubation with the
secondary antibodies, including anti-rabbit (cat. no. A0208) or
anti-mouse (cat. no. A0216) immunoglobulin G conjugated to
horseradish peroxidase (dilution, 1:5,000; Beyotime Institute of
Biotechnology) at 37°C for 1 h, the protein bands were visualized
by Enhanced Chemiluminescent (ECL) kit (Beyotime Institute of
Biotechnology), according to manufacturer's protocols. Band
intensity was quantified and analyzed with Image J software 1.6
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The results of the present study were obtained from
three independent experiments and analyzed using GraphPad Prism 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA). The results
are presented as the mean ± standard deviation. Statistical
analysis was conducted by the conventional Student's t-test or
paired Student's t-test. Multiple comparisons were performed with
one-way analysis of variance followed by Least Significant
Difference test. P<0.05 was considered to indicate a
statistically significant difference compared with the respective
control.
Results
miR-142-5p downregulation and PLA2G16
upregulation in human OS tissues and HOS cells
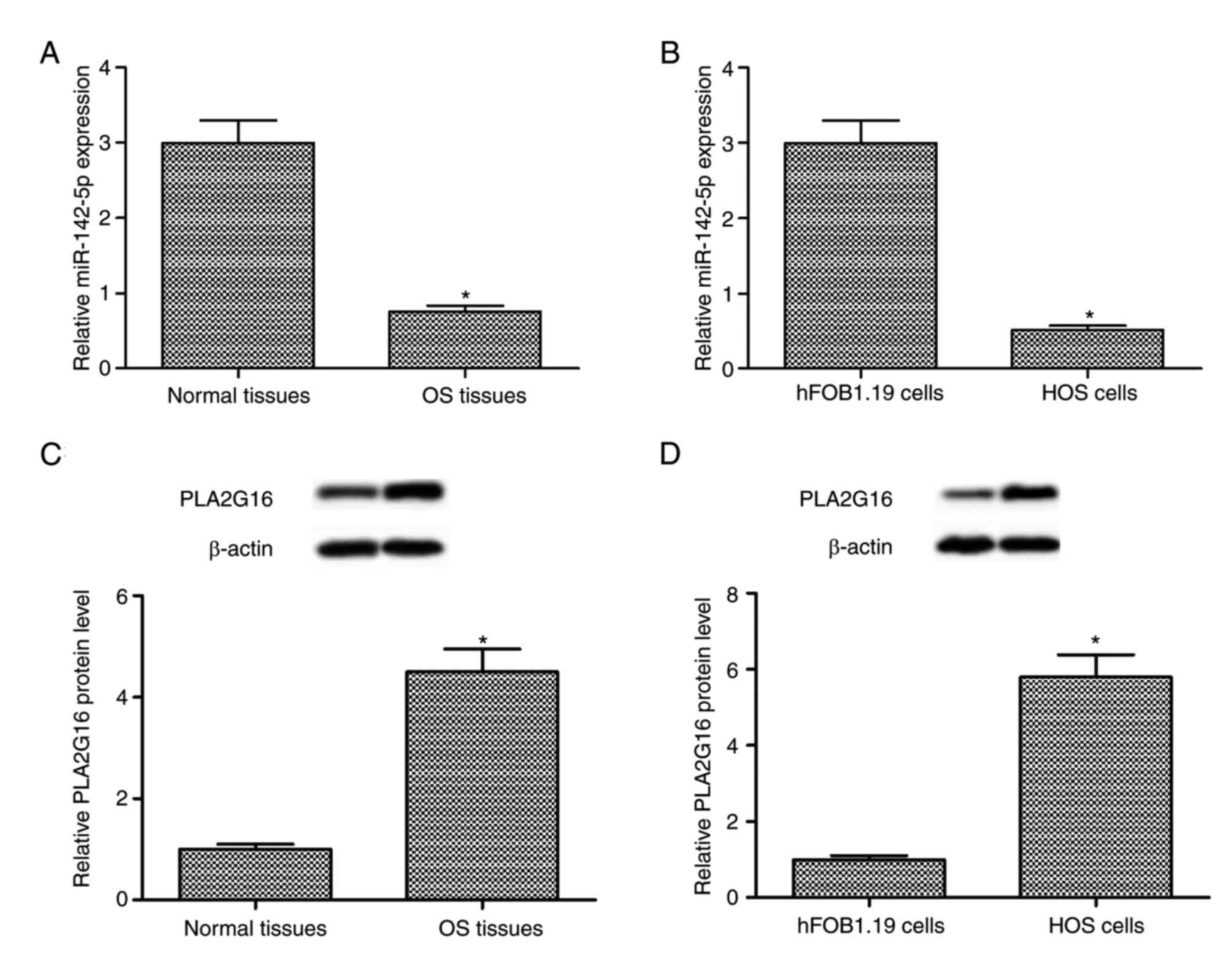
The results of RT-qPCR and western blot analysis
indicated that the expression level of miR-142-5p was downregulated
and the expression level of PLA2G16 protein was upregulated in
human OS tissues when compared with the adjacent healthy tissues
(P<0.05; Fig. 1A and C). Compared
with the human fetal osteoblastic hFOB1.19 cells, a similar
expression pattern was observed in the human OS HOS cells
(P<0.05; Fig. 1B and D). The
aforementioned findings suggested that miR-142-5p downregulation
and PLA2G16 upregulation was associated with the progression of
human OS.
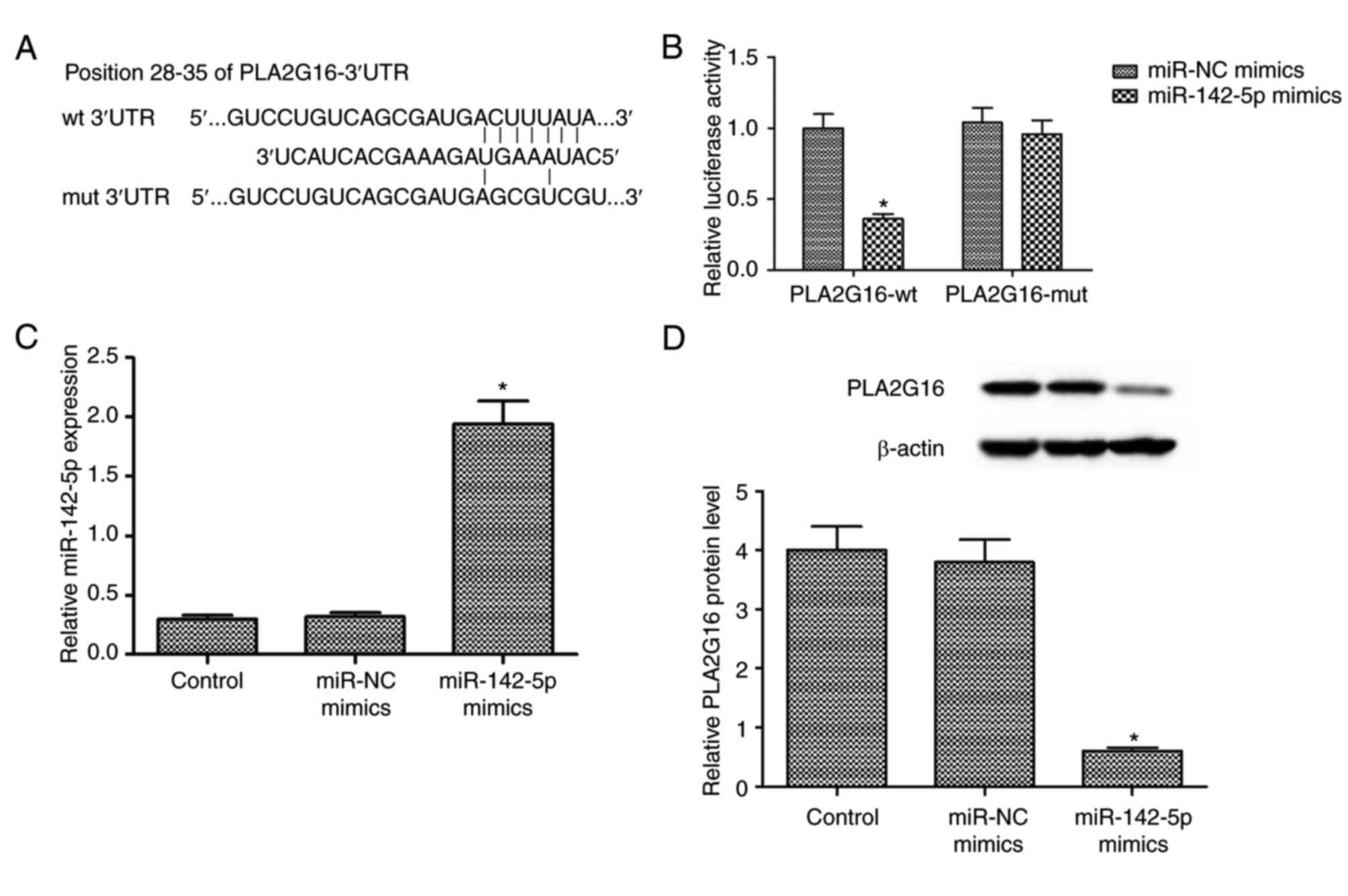
PLA2G16 is a direct target of
miR-142-5p
The potential underlying mechanism between
miR-142-5p and PLA2G16 was investigated by bioinformatics
prediction (http://www.targetscan.org). The
results indicated one putative binding site between miR-142-5p and
3′-UTR of PLA2G16 (Fig. 2A).
Luciferase reporter assays revealed that miR-142-5p overexpression
significantly reduced the luciferase activity of the PLA2G16-wt
3′-UTR-transfected cells, whereas PLA2G16-mut 3′-UTR blocked this
effect (P<0.05; Fig. 2B).
Following transfection with miR-142-5p mimics and miR-NC, RT-qPCR
analysis revealed that miR-142-5p transfection upregulated the
expression of mature miR-142-5p in HOS cells (P<0.05; Fig. 2C). Furthermore, miR-142-5p
overexpression significantly reduced the expression level of
PLA2G16 protein in HOS cells (Fig.
2D). The aforementioned data illustrated that PLA2G16 was a
direct target of miR-142-5p.
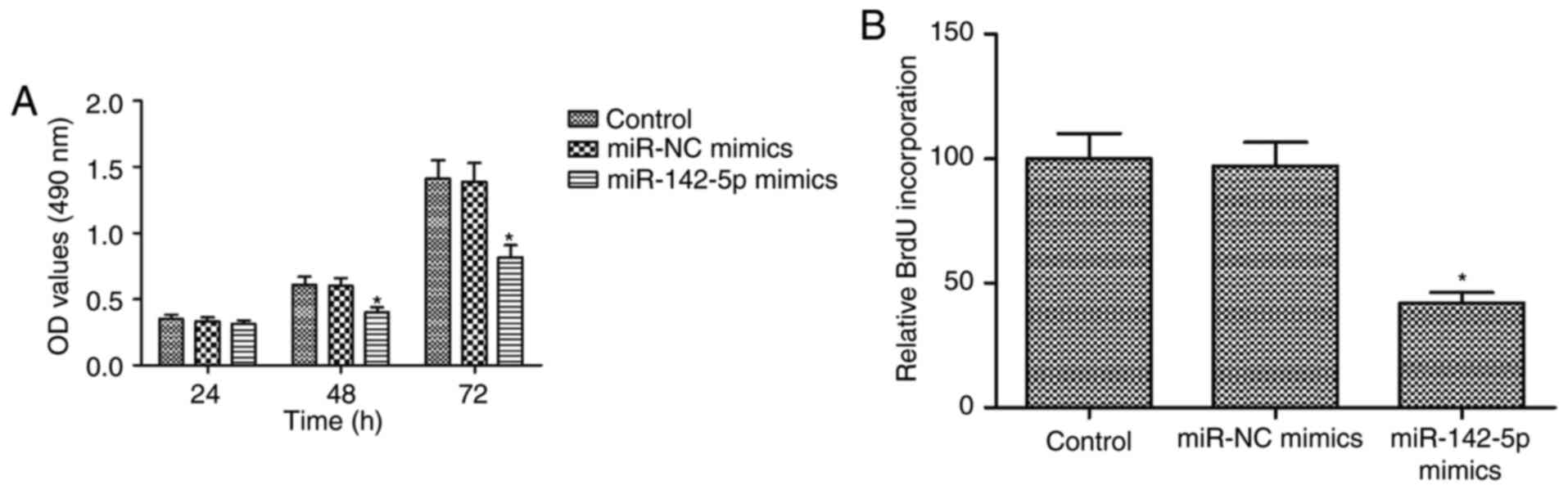
miR-142-5p overexpression suppresses
proliferation and promotes apoptosis in HOS cells
The effects of miR-142-5p on proliferation and
apoptosis were examined in HOS cells transfected with miR-NC or
miR-142-5p mimics. MTT assays demonstrated that miR-142-5p
overexpression significantly inhibited in a time-dependent manner
the proliferation of HOS cells (P<0.05; Fig. 3A). The results of BrdU incorporation
assays confirmed that miR-142-5p overexpression inhibited DNA
synthesis in HOS cells (P<0.05; Fig.
3B). A significant increase of CASP3 activity was observed
following miR-142-5p overexpression (P<0.05; Fig. 4A). Additionally, miR-142-5p
overexpression significantly elevated the percentage of apoptotic
cells (P<0.05; Fig. 4B). Western
blot analysis indicated that miR-142-5p overexpression
significantly promoted apoptosis, along with BCL2 reduction and
elevation of CASP3 and Bax expression level in HOS cells
(P<0.05; Fig. 4C). Therefore, the
results revealed that miR-142-5p overexpression suppressed
proliferation and promoted apoptosis in HOS cells.
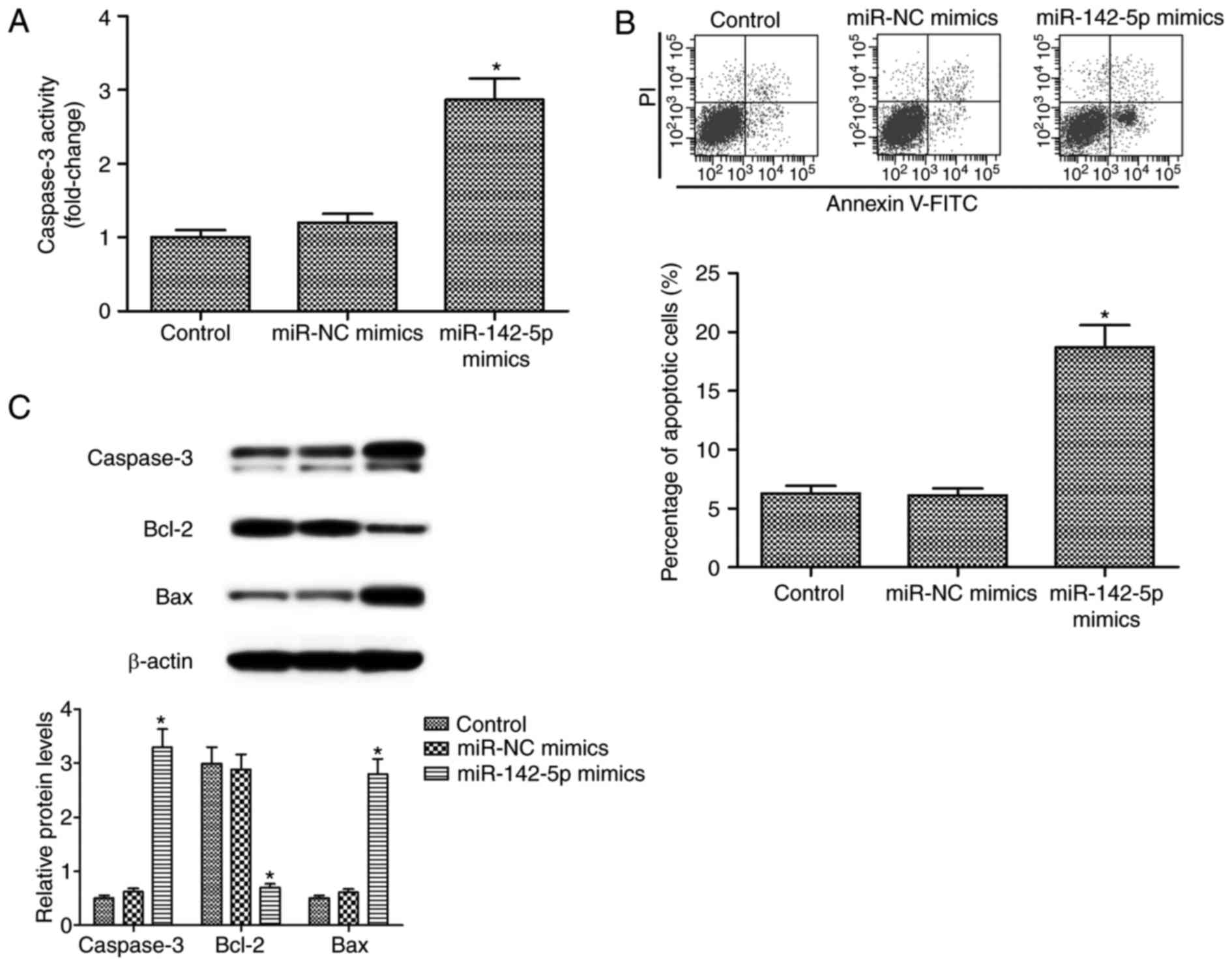 | Figure 4.Effect of miR-142-5p on apoptosis of
HOS cells. (A) Increased CASP3 activity was observed following
miR-142-5p overexpression in HOS cells transfected with miR-142-5p
and miR-NC mimics. (B) Apoptotic percentage measured by flow
cytometry in HOS cells transfected with miR-142-5p and miR-NC
mimics. (C) The expression level of CASP3, BCL2, Bax and β-actin
proteins were detected and measured. miR-142-5p overexpression
promoted apoptosis, reduced BCL2 expression and elevated CASP3 and
Bax expression levels in HOS cells. *P<0.05 compared with
control. Bax, BCL2-associated X, apoptosis regulator; BCL2, BCL2,
apoptosis regulator; CASP3, Caspase-3; miR, microRNA; Annexin
V-FITC, Annexin V-fluorescein isothiocyanate; PI, propidium iodide;
NC, negative control. |
Restoration of PLA2G16 reverses the
tumour-suppressive roles of miR-142-5p overexpression in HOS cells
through ERK1/2 signaling pathway
The results of the western blot analysis
demonstrated that miR-142-5p transfection significantly decreased
the expression levels of p-Raf-1, p-MEK, and p-ERK1/2 proteins in
HOS cells (P<0.05; Fig. 5A). To
further examine whether the role of miR-142-5p in OS progression
was regulated through PLA2G16, the pCDNA3.1-PLA2G16 vector was
transfected into HOS cells. The results demonstrated that PLA2G16
expression was significantly enhanced in HOS cells following
transfection with pCDNA3.1-PLA2G16 and PLA2G16 overexpression
rescued the reduced expression level of p-ERK1/2 protein associated
with miR-142-5p transfection in HOS cells (P<0.05; Fig. 5B). MTT and CASP3 activity assays were
conducted in HOS cells following transfection with miR-142-5p
mimics either with or without pCDNA3.1-PLA2G16. The results
revealed that restoration of PLA2G16 reversed the
tumour-suppressive roles of miR-142-5p overexpression in HOS cells
(P<0.05; Fig. 5C and D). The
aforementioned findings suggest that miR-142-5p overexpression
suppressed proliferation and promoted apoptosis in HOS cells by the
ERK1/2 signaling pathway.
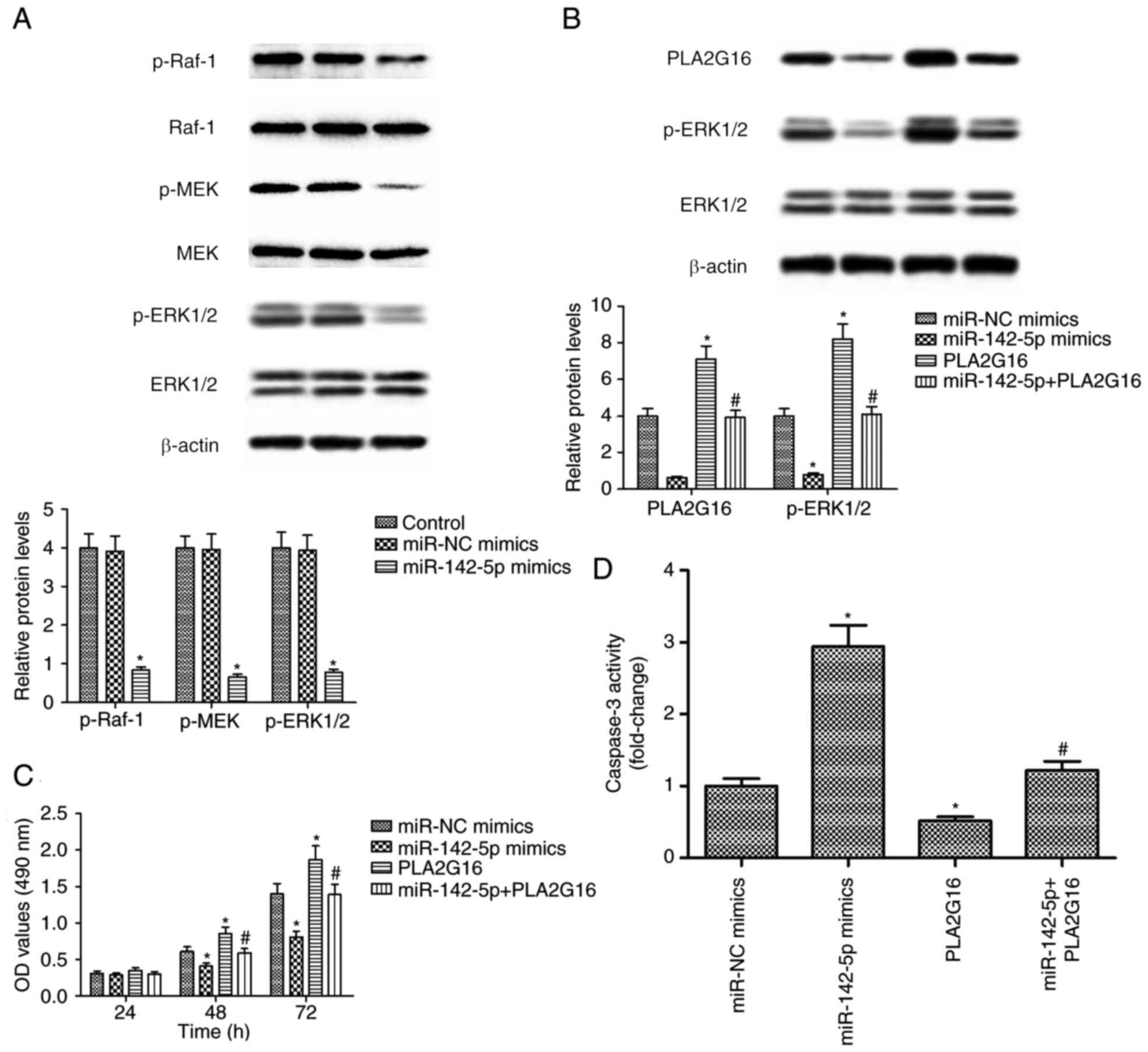 | Figure 5.Effects of miR-142-5p and PLA2G16 on
p-ERK1/2 protein expression, proliferation and apoptosis of HOS
cells. HOS cells were transfected with miR-142-5p, miR-NC mimics
and pcDNA3.1-PLA2G16 plasmids. (A) The expression level of p-Raf-1,
Raf-1, p-MEK, MEK, p-ERK1/2, ERK1/2 and β-actin proteins.
miR-142-5p transfection decreased the expression levels of p-Raf-1,
p-MEK, and p-ERK1/2 proteins in HOS cells. (B) The expression
levels of PLA2G16, p-ERK1/2, ERK1/2 and β-actin proteins were
detected and measured. The PLA2G16 expression level was enhanced in
HOS cells following transfection with pCDNA3.1-PLA2G16 and PLA2G16
overexpression rescued the reduced expression level of p-ERK1/2
protein associated with miR-142-5p transfection in HOS cells. (C)
Apoptotic rate of HOS cells following transfection with miR-142-5p
mimics either with or without pCDNA3.1-PLA2G16 at 24, 48 and 72 h.
(D) CASP3 activity assays were conducted in HOS cells following
transfection with miR-142-5p mimics either with or without
pCDNA3.1-PLA2G16. *P<0.05 compared with control.
#P<0.05 compared with miR-142-5p mimics. OD, optical
density; p, phosphorylated; Raf-1, proto-oncogene, serine/threonine
kinase 1, CASP3, Caspase-3; ERK1/2, extracellular signal-regulated
kinase 1/2; MEK, mitogen-activated protein kinase kinase; NC,
negative control; PLA2G16, group XVI phospholipase A2. |
Discussion
Despite OS being an uncommon malignant mesenchymal
neoplasm, it is the third most common cancer-associated disease
among children and adolescents (17).
An increasing number of novel miRNAs have been identified, which
mediate complex processes, including osteogenic differentiation and
osteoblastic bone formation (18).
However, the dysregulation and abnormal expression of miRNAs have
been reported to accelerate the development of OS (19,20).
Therefore, it is essential to investigate the abnormal expression
of miRNAs as the first step to characterize the miRNA-mediated
pathways involved in human OS progression.
Accumulating evidence has indicated that miR-142-5p
expression levels are upregulated in a number of pathological
contexts, including small bowel inflammation (21), Hashimoto's thyroiditis (22), simian immunodeficiency virus
encephalitis (23) and
atherosclerosis (24). It has been
demonstrated that miR-142-5p is a potential tumour suppressor gene
in numerous types of cancer, including colon cancer (25). Reduced miR-142-5p expression levels
have been reported to be associated with a high frequency of
recurrence and a poor survival rate in patients with gastric cancer
(26). It has been demonstrated that
miR-142-5p expression is downregulated in transgenic lung cancer
and that miR-142-5p overexpression inhibits proliferation in lung
cancer (27). It has also been
reported that miR-142-5p overexpression suppresses hepatocellular
carcinoma proliferation and promotes apoptosis by regulating
forkhead box O (28). Furthermore,
reduced miR-142-5p expression has been observed in
osteosarcomagenesis, as it is used as an miRNA signature to
indicate the expression identity of OS (9). In addition, miR-142-5p has been reported
to contribute to bone repair by sustaining osteoblast activity
(29). In the present study, the
observed levels of miR-142-5p expression were downregulated in
human OS tissues and HOS cells when compared with adjacent healthy
tissues and human fetal osteoblastic hFOB1.19 cells. In addition,
it was indicated that miR-142-5p overexpression suppressed
proliferation and promoted apoptosis in human osteosarcoma HOS
cells.
Evidence suggests that PLA2G16 is associated with
tumour progression (14), and that it
may contribute to tumour progression through altered metabolic
pathways (30). As a phospholipase,
PLA2G16 produces lysophosphatidic acid (LPA) and free fatty acid
(FFA) from phosphatidic acid, which has been reported to promote
proliferation, migration and metastasis in cancer progression
(31). It has been demonstrated that
LPA promotes tumour progression by modulating cytoskeletal changes,
cell-cell contacts, cell survival, proliferation, invasion and
metastasis through activating multiple signaling pathways,
including HRAS, MAPK, Rac family small GTPase, rhodopsin,
phospholipase C, AKT, serine/threonine kinase and Hippo-YAP
pathways (32,33). It has been demonstrated that FFAs
contribute to the production of prostaglandin Es, which serves an
important role in cancer pathogenesis (10). Furthermore, PLA2G16 has been reported
to promote OS progression and metastasis in mouse and human OS
cells (15). Similarly, the findings
of the present study indicated that compared to adjacent healthy
tissues and human fetal osteoblastic hFOB1.19 cells, the expression
levels of the PLA2G16 protein were upregulated in human OS tissues
and HOS cells. Furthermore, it was demonstrated that PLA2G16 was a
direct target of miR-142-5p and that miR-142-5p overexpression
suppressed proliferation and promoted apoptosis by targeting
PLA2G16 in human osteosarcoma HOS cells.
It has been demonstrated that the MAPK/ERK pathway
is involved in the processes of proliferation, differentiation,
migration, senescence and apoptosis (34). As a key signal transduction pathway,
the ERK signaling pathway has been reported to be frequently
aberrantly activated in cancer cells and considered central to a
number of signaling pathways (35).
miR-142-5p has been reported to mediate certain target genes in
numerous oncogenic signaling pathways, including MAPK (26). The underlying mechanism of miR-142
function in mediating ERK phosphorylation has been illustrated and
decreased miR-142 expression levels lead to enhanced levels of ERK
phosphorylation, contributing to cell differentiation in mouse
embryonic stem cells (36,37). It has also been demonstrated that
enhanced PLA2G16 expression promotes OS metastasis through
activating the MAPK pathway and may be a novel therapeutic target
for various types of cancer (38).
The findings of the present study indicated that miR-142-5p
transfection decreased the phosphorylation of Raf-1/MEK/ERK1/2
proteins in HOS cells, suggesting that miR-142-5p may act by the
Raf/MEK/ERK1/2 signaling pathway. Additionally, PLA2G16
overexpression restored the expression levels of p-ERK1/2 protein
reduced by miR-142-5p overexpression. PLA2G16 restoration reversed
the tumour-suppressive roles of miR-142-5p overexpression in HOS
cells.
In conclusion, the results of the present study
confirmed the tumor-suppressive role of miR-142-5p in human OS
progression. The aforementioned findings suggested that miR-142-5p
suppressed proliferation and promoted apoptosis in human
osteosarcoma HOS cells by targeting PLA2G16 by the ERK1/2 signaling
pathway. Therefore, the present study provided novel insight into
the underlying mechanisms of OS progression, suggesting that
miR-142-5p may be used as a novel candidate therapeutic target in
OS. Considering that only a single OS cell line was used to
elucidate the effects of miR-142-5p, further studies are required,
in order to fully elucidate the roles of miR-142-5p and PLA2G16 in
proliferation and apoptosis of OS cells. Additional research is
also required to determine the potential of miR-142-5p in clinical
applications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
The Institutional Review Board and Human Ethics
Committee of Xi'an Jiaotong University (Xi'an, China) approved the
study. All patients signed written informed consent for
participation in the present study, which was conducted according
to the Declaration of Helsinki.
Authors' contributions
DC and LH designed the study, collected the
literature, analyzed and interpreted the data, and prepared the
manuscript. JL collected, analyzed and interpreted the data, and
prepared the manuscript. LZ and LH collected the literature, and
analyzed and interpreted the data. All authors approved the final
manuscript.
Patient consent for publication
Patient consent was obtained for the publication of
the present study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OS
|
Osteosarcoma
|
|
miRNAs
|
microRNAs
|
|
3′UTR
|
3′-untranslated region
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
PLA2G16
|
Group XVI phospholipase A2
|
|
HRASLS3
|
Ha-RAS like suppressor 3
|
|
AdPLA2
|
adipose specific PLA2
|
|
ATCC
|
American Type Culture Collection
|
|
MTT
|
modified tetrazolium salt
3-(4,5-dimethyl-2-thiazolyl)-2,5-dipheny-2
H-tetrazolium-bromide
|
|
DMSO
|
dimethyl sulfoxide
|
|
BrdU
|
5-Bromo-2′-deoxyuridine
|
|
PVDF
|
polyvinylidene fluoride membranes
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
SD
|
standard deviation
|
|
LPA
|
lysophosphatidic acid
|
|
FBS
|
fetal bovine serum
|
|
FFA
|
free fatty acid
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
MAPK/ERK
|
mitogen-activated protein
kinases/extracellular signal-regulated kinase
|
References
|
1
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aljubran AH, Griffin A, Pintilie M and
Blackstein M: Osteosarcoma in adolescents and adults: Survival
analysis with and without lung metastases. Ann Oncol. 20:1136–1141.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferreira CG, de Melo AC and
Nogueira-Rodrigues A: The adolescent and young adult with cancer:
State of the art-epithelial cancer. Curr Oncol Rep. 15:287–295.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li S, Wang L, Fu B, Berman MA, Diallo A
and Dorf ME: TRIM65 regulates microRNA activity by ubiquitination
of TNRC6. Proc Natl Acad Sci USA. 111:6970–6975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu S, Yin F, Zhang J, Wicha MS, Chang AE,
Fan W, Chen L, Fan M and Li Q: Regulatory roles of miRNA in the
human neural stem cell transformation to glioma stem cells. J Cell
Biochem. 115:1368–1380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng G: Circulating miRNAs: Roles in
cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev.
81:75–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duncan RE, Sarkadi-Nagy E, Jaworski K,
Ahmadian M and Sul HS: Identification and functional
characterization of adipose-specific phospholipase A2 (AdPLA). J
Biol Chem. 283:25428–25436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hajnal A, Klemenz R and Schafer R:
Subtraction cloning of H-rev107, a gene specifically expressed in
H-ras resistant fibroblasts. Oncogene. 9:479–490. 1994.PubMed/NCBI
|
|
12
|
Nazarenko I, Schafer R and Sers C:
Mechanisms of the HRSL3 tumor suppressor function in ovarian
carcinoma cells. J Cell Sci. 120:1393–1404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siegrist S, Feral C, Chami M, Solhonne B,
Mattei MG, Rajpert-De Meyts E, Guellaen G and Bulle F: hH-Rev107, a
class II tumor suppressor gene, is expressed by post-meiotic
testicular germ cells and CIS cells but not by human testicular
germ cell tumors. Oncogene. 20:5155–5163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nazarenko I, Kristiansen G, Fonfara S,
Guenther R, Gieseler C, Kemmner W, Schafer R, Petersen I and Sers
C: H-REV107-1 stimulates growth in non-small cell lung carcinomas
via the activation of mitogenic signaling. Am J Pathol.
169:1427–1439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang S, Ren Z, Han X, Yang J, Shan L, Li
L, Wang B, Zhang Q, Mu T, Chen K, et al: PLA2G16 expression in
human osteosarcoma is associated with pulmonary metastasis and poor
prognosis. PLoS One. 10:e01272362015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolf NK, Largaespada DA and Moriarity BS:
Coping with cancer genes altered by copy number. Oncotarget.
6:35155–35156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei J, Shi Y, Zheng L, Zhou B, Inose H,
Wang J, Guo XE, Grosschedl R and Karsenty G: miR-34s inhibit
osteoblast proliferation and differentiation in the mouse by
targeting SATB2. J Cell Biol. 197:509–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Yan YG, Wang C, Zhang SJ, Yu XH
and Wang WJ: MicroRNAs in osteosarcoma. Clin Chim Acta. 444:9–17.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sampson VB, Yoo S, Kumar A, Vetter NS and
Kolb EA: MicroRNAs and potential targets in osteosarcoma: Review.
Front Pediatr. 3:692015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schaefer JS, Montufar-Solis D, Vigneswaran
N and Klein JR: Selective upregulation of microRNA expression in
peripheral blood leukocytes in IL-10-/- mice precedes expression in
the colon. J Immunol. 187:5834–5841. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu J, Zhang Y, Zhang W, Fan L, Wang L,
Liu Y, Liu S, Guo Y, Wang Y, Yi J, et al: MicroRNA-142-5p
contributes to hashimoto's thyroiditis by targeting CLDN1. J Transl
Med. 14:1662016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chaudhuri AD, Yelamanchili SV, Marcondes
MC and Fox HS: Up-regulation of microRNA-142 in simian
immunodeficiency virus encephalitis leads to repression of
sirtuin1. FASEB J. 27:3720–3729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu R, Bi C, Song J, Wang L, Ge C, Liu X
and Zhang M: Upregulation of miR-142-5p in atherosclerotic plaques
and regulation of oxidized low-density lipoprotein-induced
apoptosis in macrophages. Mol Med Rep. 11:3229–3234. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi D, Zhai B, Zheng Y, Ren R, Han M and
Wang X: Transcatheter arterial infusion chemotherapy increases
expression level of miR-142-5p in stage III colorectal cancer.
Indian J Cancer. 52 Suppl 2:e47–e55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Yan Z, Zhang J, Gong L, Li W, Cui
J, Liu Y, Gao Z, Li J, Shen L and Lu Y: Combination of hsa-miR-375
and hsa-miR-142-5p as a predictor for recurrence risk in gastric
cancer patients following surgical resection. Ann Oncol.
22:2257–2266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Sempere LF, Galimberti F,
Freemantle SJ, Black C, Dragnev KH, Ma Y, Fiering S, Memoli V, Li
H, et al: Uncovering growth-suppressive MicroRNAs in lung cancer.
Clin Cancer Res. 15:1177–1183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lou K, Chen N, Li Z, Zhang B, Wang X, Chen
Y, Xu H, Wang D and Wang H: MicroRNA-142-5p overexpression inhibits
cell growth and induces apoptosis by regulating FOXO in
hepatocellular carcinoma cells. Oncol Res. 25:65–73. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tu M, Tang J, He H, Cheng P and Chen C:
MiR-142-5p promotes bone repair by maintaining osteoblast activity.
J Bone Miner Metab. 35:255–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jaworski K, Ahmadian M, Duncan RE,
Sarkadi-Nagy E, Varady KA, Hellerstein MK, Lee HY, Samuel VT,
Shulman GI, Kim KH, et al: AdPLA ablation increases lipolysis and
prevents obesity induced by high-fat feeding or leptin deficiency.
Nat Med. 15:159–168. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nomura DK, Long JZ, Niessen S, Hoover HS,
Ng SW and Cravatt BF: Monoacylglycerol lipase regulates a fatty
acid network that promotes cancer pathogenesis. Cell. 140:49–61.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SC, Fujiwara Y and Tigyi GJ:
Uncovering unique roles of LPA receptors in the tumor
microenvironment. Receptors Clin Investig. 2:e4402015.PubMed/NCBI
|
|
33
|
Wang H, Liu W, Wei D, Hu K, Wu X and Yao
Y: Effect of the LPA-mediated CXCL12-CXCR4 axis in the tumor
proliferation, migration and invasion of ovarian cancer cell lines.
Oncol Lett. 7:1581–1585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu Z, Ye S, Hu G, Lv M, Tu Z, Zhou K and
Li Q: The RAF-MEK-ERK pathway: Targeting ERK to overcome obstacles
to effective cancer therapy. Future Med Chem. 7:269–289. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sladitschek HL and Neveu PA: The bimodally
expressed microRNA miR-142 gates exit from pluripotency. Mol Syst
Biol. 11:8502015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shrestha A, Mukhametshina RT, Taghizadeh
S, Vasquez-Pacheco E, Cabrera-Fuentes H, Rizvanov A, Mari B,
Carraro G and Bellusci S: MicroRNA-142 is a multifaceted regulator
in organogenesis, homeostasis, and disease. Dev Dyn. 246:285–290.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li L, Liang S, Wasylishen AR, Zhang Y,
Yang X, Zhou B, Shan L, Han X, Mu T, Wang G and Xiong S: PLA2G16
promotes osteosarcoma metastasis and drug resistance via the MAPK
pathway. Oncotarget. 7:18021–18035. 2016.PubMed/NCBI
|