Introduction
Total laryngectomy (TL) ± radiotherapy (RT) had been
the classical treatment for resectable locally-advanced laryngeal
squamous cell carcinoma (LALSCC), which is classified as clinical
T3-T4a disease (1). Various
organ-preserving treatment modalities, including partial
laryngectomy (PL), concurrent chemoradiotherapy (CRT),
chemoselection from induction chemotherapy have been performed for
resectable LALSCC (1–5). We have also showed the efficacy of
alternating chemoradiotherapy (ACRT) with early assessment
following induction of CRT and salvage surgery for residual tumors
in resectable LALSCC (6). Although
the tumor-node metastasis (TNM) classification of resectable LALSCC
is globally used as the prognostic factor of survival outcomes,
such as cancer-specific survival (CSS), after various treatments,
the same TNM classification is unable to predict survival outcomes
(2–4).
Recently, the prognostic factors for resectable LALSCC have been
investigated by various approaches (2–4,7–10).
Treatment package time (TPT), which is similar to
overall treatment time, is calculated from the start of any
treatment to the end of all treatments and had been shown as a
prognostic factor in several cancers (3,4,7–10).
Prolonged TPT of several modalities, including RT alone and surgery
with postoperative RT, has been reported to be a predictor of worse
CSS for head and neck cancer (3).
To the best of our knowledge, the association
between survival outcomes and TPT in patients with resectable
LALSCC, who consecutively underwent various treatments, including
surgery alone and salvage surgery for residual tumor, has not been
previously investigated. In the present study, we investigated
whether TPT was significantly associated with survival outcomes of
resectable LALSCC in patients who consecutively underwent various
treatments, including surgery alone and salvage surgery for
residual tumor.
Materials and methods
Patients
Between 2004 June and 2016 October, 101 patients who
were newly diagnosed as clinical T3-T4a laryngeal squamous cell
carcinoma consecutively underwent definitive treatment at the Aichi
Cancer Center Hospital. After excluding one patient who diagnosed
as cN3 with unresectable disease, a total of 100 patients with
resectable LALSCC were enrolled in this study. This study was
approved by the review board of Aichi Cancer Center Hospital, and
informed consent for the examinations and treatments was obtained
from all of the patients. The subsites of the primary tumor were
the supraglottis (n=64), glottis (n=32), and subglottis (n=4).
Clinical staging based on the TNM classification of the seventh
edition of the International Union Against was determined from
routine physical examination, flexible laryngeal endoscope,
enhanced cervical computed tomography, and 18F-FDG-PET/CT, if
possible. The Charlson comorbidity index, which was a weighted
index, was calculated from 19 comorbid conditions.
Initial treatment
The 100 patients were classified based on initial
treatment of the primary tumor: TL ± RT with or without
chemotherapy (TL, n=27); PL (PL, n=5); concurrent CRT ± salvage
surgery for residual tumor (CRT, n=11); induction CRT followed by
CRT ± salvage surgery for residual tumor to responders or TL ± RT
with or without chemotherapy to non-responders (ACRT, n=27);
induction chemotherapy following concurrent RT with or without
chemotherapy ± salvage surgery for residual tumor to responders or
TL ± RT with or without chemotherapy to non-responders
(chemoselection, n=27); and RT alone ± salvage surgery for residual
tumor (RT alone, n=3). We recommended postoperative RT with or
without chemotherapy to patients with multiple lymph node
metastasis, extranodal extension, and positive resection margin.
Salvage surgery was performed for residual tumor found on
pathological and imaging examination at 1–2 months after the
completion of RT with or without chemotherapy, as described
previously (11). After completion of
the initial treatments, including salvage surgery for residual
tumor, we made an effort to perform salvage surgery based on the
presence of tumor recurrence.
Selection of initial treatment
The recommended treatment of choice was TL, but for
patients who wished not to undergo surgery, we initially selected
concurrent CRT or RT alone in those with advanced age or who
refused chemotherapy. For patients who required maximal organ
preservation without a reduction in treatment efficacy, ACRT was
selected from January 2004 to January 2011 if consistent with the
protocol; after February 2011, most patients underwent
chemoselection. Patients who wished organ preservation and refused
RT underwent PL. The details of the en bloc dissection, RT
procedures, chemotherapy regimens, and the selection of primary
treatment have been reported elsewhere (6,11–14). In brief, definitive RT was given at a
total dose of 66–70 Gy with 2 Gy per fraction; induction
chemotherapy was given mostly by intravenous infusion of
5-fluorouracil and cisplatin, the regimens of concurrent CRT were
triweekly or weekly cisplatin, and weekly cetuximab.
Time to treatment initiation and
TPT
According to previous reports (9,14), the
time to treatment initiation was calculated in days from the time
of pathological diagnosis of LALSCC to the start of any treatment;
TPT was likewise calculated in days from the start of any treatment
to the end of all treatments, including postoperative RT and
salvage surgery for residual tumor.
Statistical analysis
Statistical analyses were carried out using the JMP
software package (version 9; SAS Institute, Inc., Cary, NC, USA).
The best cut-off values for time to treatment initiation or TPT for
death due to LALSCC were assessed by receiver-operating
characteristic curve (ROC) analysis, as described previously
(12). ROC analysis in the present
study was performed by using TPT or the time to treatment
initiation, as continuous variables. Survival time, which was
calculated as the number of days from the start of any treatment to
a target event or last contact, was analyzed by the Kaplan-Meier
method. The target events were death for overall survival (OS),
death due to LALSCC for CSS, local or regional recurrence for
locoregional recurrence-free survival (LRRFS), and distant
metastasis for distant metastasis-free survival (DMFS). The
patients were categorized into two groups based on their TPT
(<68 days vs. ≥68 days); differences between the two groups were
compared by univariate survival analysis using a log-rank test.
Another grouping, using, using two models, was based on the initial
treatment of the primary tumor; model 1 was TL (n=27) and the
non-TL (n=73) groups, whereas model 2 compared the induction group,
which included ACRT and chemoselection (n=54), and the
non-induction group (n=46). The associations between the two groups
(TPT <68 days or TPT ≥68 days) with regard to the clinical
characteristics (cT and cN classification, cStage, subsite, vocal
cord fixtation, charlson comorbidity index, gender, age, adjuvant
treatment, initial treatment, treatment group) were compared by
using chi-squared test or Fisher's exact test. Multivariate
analyses for the factors associated with CSS and DMFS used two
Cox's proportional hazards models. Model-1 was adjusted with the cT
(cT3/cT4), cN (cN0-1/cN2-3), TPT (<68 days/≥68 days), and
treatment group (TL/non-TL). Model-2 was adjusted with the cT
(cT3/cT4), cN (cN0-1/cN2-3), TPT (<68 days/≥68 days), and
treatment group (induction/non-induction). P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical characteristics
The clinical characteristics of the population are
shown in Table I. At the end of the
study, the mean ± SD follow-up period was 1317±930 days among all
patients, 1,478±961 days for the 68 patients who remained alive,
976±768 days for the 32 patients who died, and 1,000±716 days for
the 20 patients who died due to LALSCC. The mean ± SD of the time
to treatment initiation and TPT were 26.0±13.1 days and 58.3±51.4
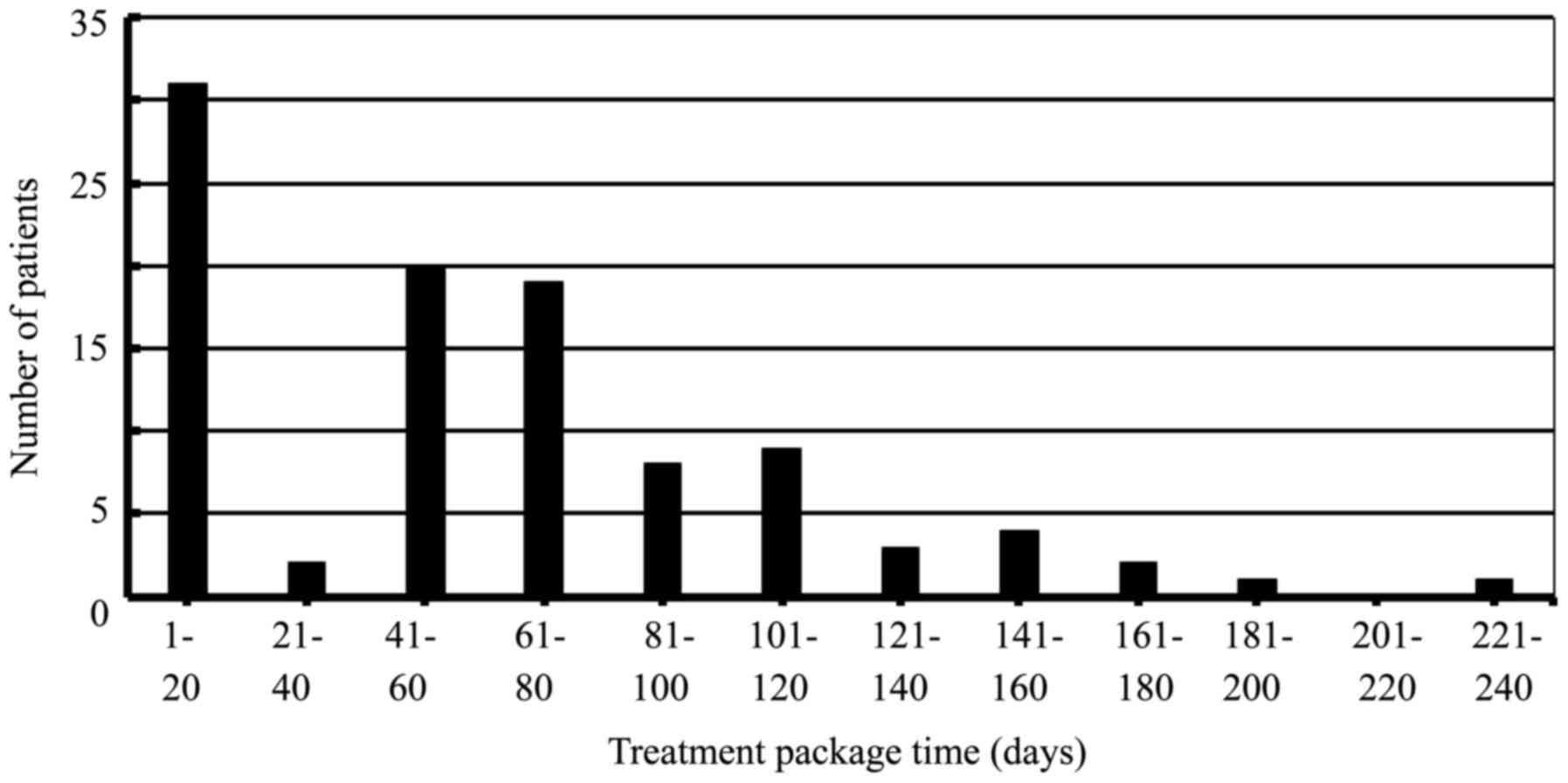
days, respectively. The association between TPT and number of
patients were shown in Fig. 1.
Locoregional recurrence and distant metastasis were found in 25 and
17 patients, respectively. TL on initial treatment, including
salvage surgery, was performed on 46 patients with residual tumor
and on nine patients with recurrence tumor. The overall five-year
rates for OS, CSS, LRRFS, and DMFS were 68.9, 78.0, 70.9, and
76.3%, respectively.
 | Table I.Characteristics of initial treatment
in 100 patients with laryngeal squamous cell carcinoma. |
Table I.
Characteristics of initial treatment
in 100 patients with laryngeal squamous cell carcinoma.
|
|
| Initial
treatment |
|---|
|
|
|
|
|---|
| Characteristics | Total | TL | PL | Concurrent RT | ACRT | Chemo-selection | RT alone |
|---|
|
| 100 | 27 | 5 | 11 | 27 | 27 | 3 |
| cT, cT3/cT4 | 72/28 | 19/8 | 4/1 | 11/0 | 20/7 | 16/11 | 2/1 |
| cN |
|
|
|
|
|
|
|
|
cN0/cN1 | 59/13 | 17/4 | 5/0 | 8/0 | 14/5 | 12/4 | 3/0 |
|
cN2/cN3 | 26/2 | 5/1 | 0/0 | 3/0 | 8/0 | 10/1 | 0/0 |
| cStage,
cStageIII/cStageIV | 57/43 | 17/10 | 4/1 | 8/3 | 15/12 | 11/16 | 2/1 |
| Charlson comorbidity
index, 0/≥1 | 37/63 | 10/17 | 1/4 | 1/10 | 15/12 | 8/19 | 1/2 |
| Sex, male/female | 94/6 | 24/3 | 5/0 | 11/0 | 25/2 | 26/1 | 3/0 |
| Subsite,
supraglottis/glottis/subglottis | 64/32/4 | 18/8/1 | 1/4/0 | 8/3/0 | 16/10/1 | 19/6/2 | 2/1/0 |
| Vocal cord fixation,
presence/absence | 44/56 | 18/9 | 0/5 | 3/8 | 16/11 | 19/8 | 1/2 |
| Age | 67.4±8.1 | 72.9±8.6 | 69.2±6.7 | 69.7±6.9 | 63.1±7.3 | 65.9±5.4 | 61.7±9.0 |
| Treatment package
time, days | 58.3±51.4 | 8.8±24.0 | 1±0 | 65.3±32.6 | 77.9±41.2 | 92.6±46.4 | 87.3±61.2 |
| Time to
initiation | 26.0±13.1 | 31.4±18.7 | 33.2±3.0 | 29.3±7.3 | 20.3±8.9 | 24.0±8.4 | 21.7±14.2 |
| Adjuvant treatment,
PORT(POCRT)/salvage surgery/no | 6/10/84 | 3/0/24 | 0/0/5 | 0/3/8 | 0/3/24 | 3/3/23 | 0/1/2 |
| Outcomes |
|
|
|
|
|
|
|
|
Follow-up, days | 1317±930 | 1012±727 | 524±262 | 846±680 | 2123±1031 | 1189±663 | 1016±882 |
|
Laryngectomy, initial/salvage
for recurrence/no | 46/9/45 | 27/0/0 | 0/0/5 | 0/2/9 | 5/5/17 | 12/2/13 | 2/0/1 |
| Recurrence or
metastasis, locoregional/distant/none | 25/17/58 | 5/4/18 | 1/0/4 | 4/1/6 | 5/3/19 | 9/8/10 | 1/1/1 |
| Survival,
alive/cancer death/others death | 68/20/12 | 16/5/6 | 5/0/0 | 6/2/3 | 20/4/3 | 20/7/0 | 1/2/0 |
ROC analysis
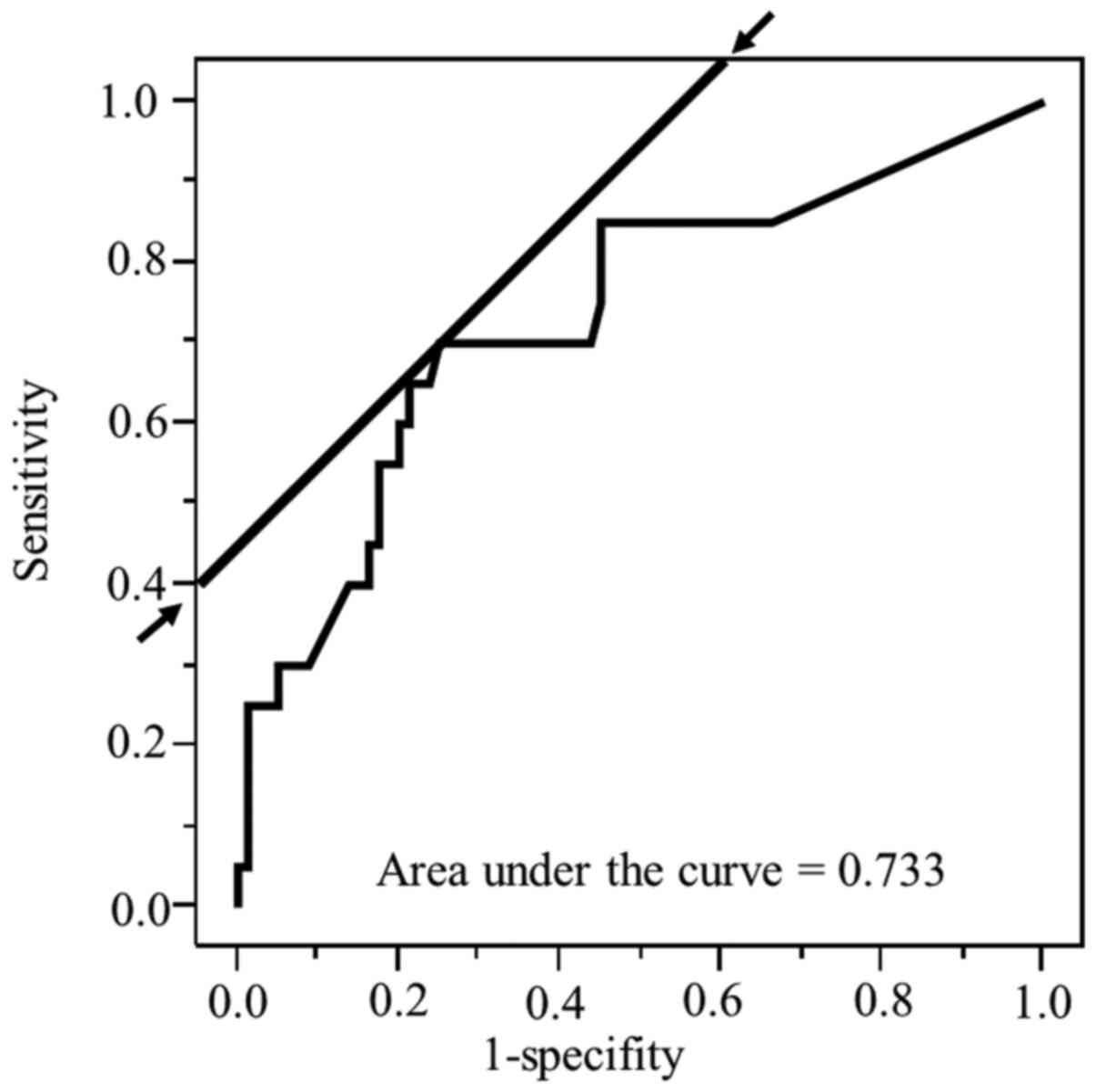
From the ROC analyses, the best cut-off values for
time to treatment initiation and TPT to detection of death from
LALSCC were 15 days (AUC=0.50, P=0.81) and 68 days (AUC=0.73,
P<0.01), respectively. The ROC, AUC of the ROC, sensitivity, and
1-specificity of TPT for death from LALSCC are shown in Fig. 2; the time to treatment initiation for
death from LALSCC was additionally determined (data not shown).
Univariate survival analysis
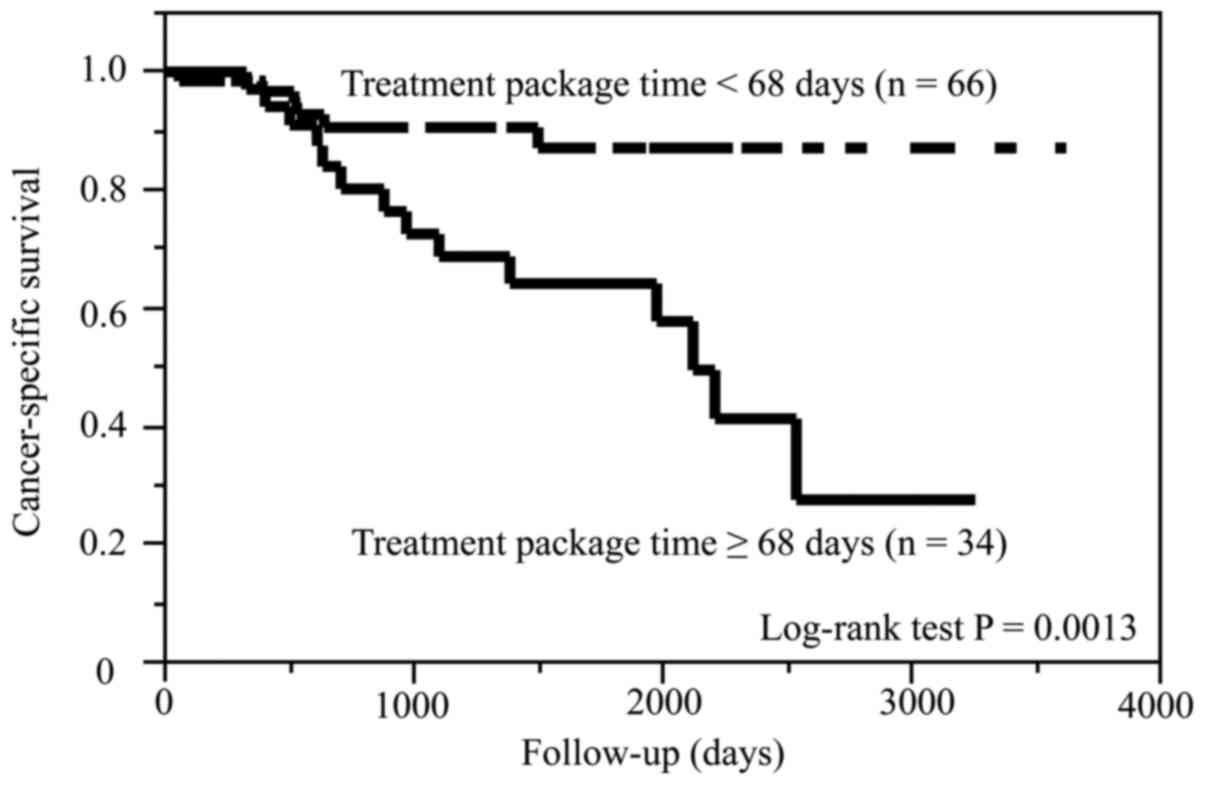
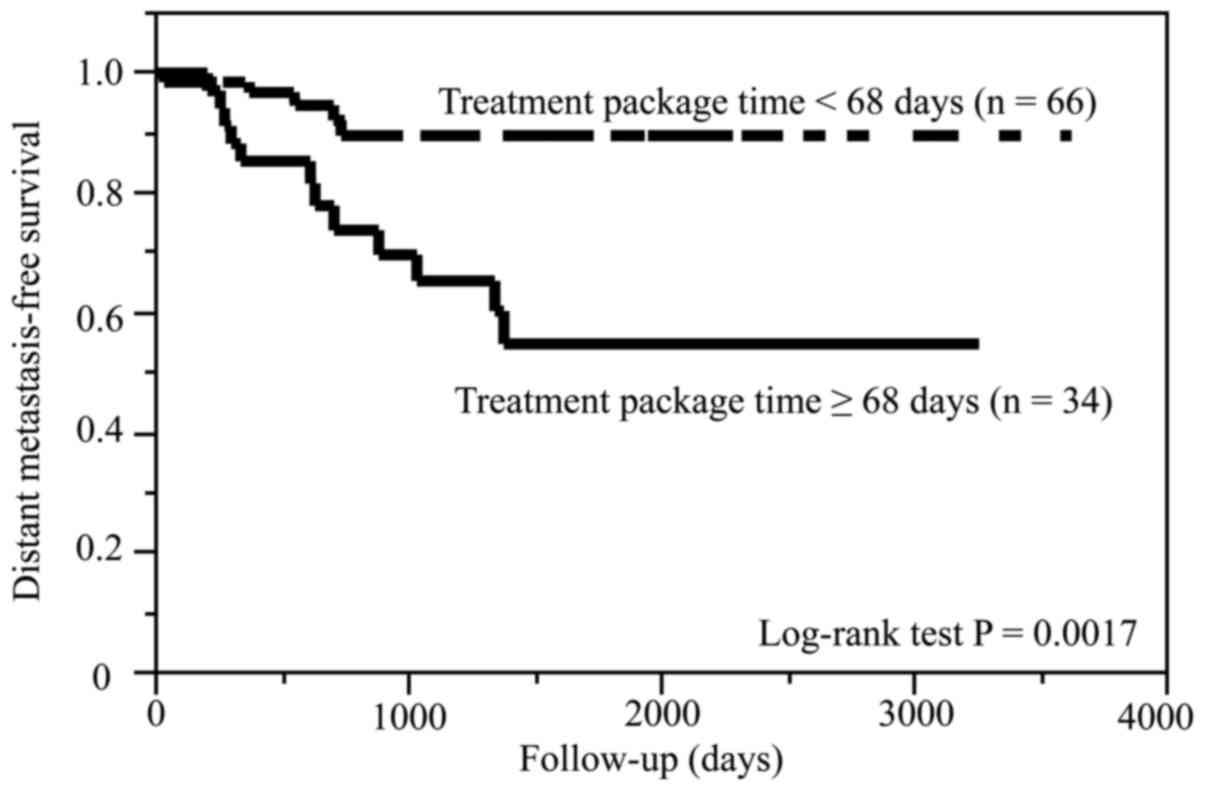
Compared with patients with TPT <68 days, those
with TPT ≥68 days had significantly shorter CSS (P=0.0013) and DMFS
(P=0.0017). On the other hand, the two groups of TPT had no
difference in the OS (P=0.08) and LRRFS (P=0.16). The Kaplan-Meier
curves for CSS and DMFS are shown in Figs. 3 and 4,
respectively.
TPT and clinical characteristics
The associations between the TPT and clinical
characteristics are shown in Table
II. Compared with patients with TPT <68 days, those with TPT
≥68 days had significantly higher number of patients of cN2-3
disease (P=0.02), who received adjuvant treatment (P<0.01),
initial treatment (P<0.01), in the TL group (P<0.01) and the
induction therapy group (P<0.01).
 | Table II.Association between treatment package
time (<68 days/≥68 days) and clinical characteristics in
laryngeal squamous cell carcinoma. |
Table II.
Association between treatment package
time (<68 days/≥68 days) and clinical characteristics in
laryngeal squamous cell carcinoma.
|
|
| Treatment package
time |
|
|---|
|
|
|
|
|
|---|
| Clinical
characteristics | Total, n=100 | <68 days,
n=66 | ≥68 days, n=34 | P-value |
|---|
| cT, cT3/cT4 | 72/28 | 47/19 | 25/9 | 1.00 |
| cN,
cN0-1/cN2-3 | 72/28 | 53/13 | 19/15 | 0.02 |
| cStage,
cStageIII/cStageIV | 57/43 | 41/25 | 16/18 | 0.20 |
| Subsite,
glottis/others | 32/68 | 25/41 | 7/27 | 0.11 |
| Vocal cord
fixtation, presence/absence | 44/56 | 30/36 | 14/20 | 0.83 |
| Charlson
comorbidity index, 0/≥1 | 37/63 | 25/41 | 12/22 | 0.83 |
| Gender,
male/female | 94/6 | 62/4 | 32/2 | 1.00 |
| Age,
<68/≥68 | 49/51 | 28/38 | 21/13 | 0.09 |
| Adjuvant treatment,
presence/absence | 16/84 | 3/63 | 13/21 |
<0.01a |
| Treatment
group |
|
|
|
|
| Total
laryngectomy/non-total laryngectomy | 27/73 | 26/40 | 1/33 |
<0.01a |
|
Induction
therapy/non-induction therapy | 54/46 | 24/42 | 30/4 |
<0.01a |
Multivariate survival analysis
The results of the multivariate analyses for CSS and
DMFS are shown in Table III. In
model 1, cN0-1 (P=0.02), TPT <68 days (P<0.01), and the
non-TL group (P=0.02) were significantly associated with a longer
CSS, and cN0-1 (P<0.01), TPT <68 days (P<0.01), and the
non-TL group (P=0.03) were significantly associated with a longer
DMFS. In mode 2, cN0-1 (P=0.01), TPT <68 days (P<0.01), and
the induction therapy group (P<0.01) were significantly
associated with longer CSS, and cN0-1 (P<0.01), TPT <68 days
(P<0.01), and the induction therapy group (P=0.02) were
significantly associated with longer DMFS.
 | Table III.Multivariate analysis for CSS and
DMFS in laryngeal squamous cell carcinoma. |
Table III.
Multivariate analysis for CSS and
DMFS in laryngeal squamous cell carcinoma.
| A, Model-1 |
|---|
|
|---|
|
| CSS | DMFS |
|---|
|
|
|
|
|---|
| Clinical
characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| cT, cT3/cT4 | 0.89 | 0.34–2.47 | 0.81 | 0.96 | 0.35–2.74 | 0.93 |
| cN,
cN0-1/cN2-3 | 0.33 | 0.12–0.86 | 0.02a | 0.11 | 0.03–0.36 |
<0.01a |
| Treatment package
time, <68 days/≥68 days | 0.14 | 0.03–0.45 |
<0.01a | 0.15 | 0.03–0.59 |
<0.01a |
| Treatment group,
TL/non-TL | 5.50 | 1.31–23.0 | 0.02a | 5.94 | 1.16–32.2 | 0.03a |
|
| B,
Model-2 |
|
|
| CSS | DMFS |
|
|
|
|
| Clinical
characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| cT, cT3/cT4 | 0.64 | 0.23–1.86 | 0.40 | 0.77 | 0.27–2.28 | 0.63 |
| cN,
cN0-1/cN2-3 | 0.30 | 0.11–0.79 | 0.01a | 0.11 | 0.03–0.36 |
<0.01a |
| Treatment package
time, <68 days/≥68 days | 0.08 | 0.02–0.28 |
<0.01a | 0.16 | 0.04–0.56 |
<0.01a |
| Treatment group,
induction/non-induction | 0.09 | 0.02–0.30 |
<0.01a | 0.19 | 0.05–0.72 | 0.02a |
Discussion
In the present study, we showed for the first time
that a TPT of ≥68 days was significantly associated with shorter
CSS and DMFS in patients with resectable LALSCC who consecutively
underwent various treatments, including surgery alone and salvage
surgery for residual tumor.
Various organ-preserving treatments modalities are
being offered in clinical practice, and several trials have
reported on concurrent CRT vs. induction following CRT (1–5). We have
also shown the efficacy of ACRT with early assessment for laryngeal
squamous cell carcinoma and that of chemoselection for several
cancer sites, including the hypopharynx and cervical esophagus
(6,11–13).
The TPT as time factor of several treatment
modalities, including RT alone, concurrent CRT, and surgery with
postoperative CRT, has been shown to be a prognostic factor in
several types of cancer, including laryngeal cancer (3,7–10). We also investigated the association
between survival outcomes and the time factors of several
treatments, including ACRT, in hypopharyngeal cancer (11). To the best of our knowledge, the
association between survival outcomes and TPT of various
treatments, including surgery alone, chemoselection, and ACRT with
salvage surgery for residual tumor, has not been previously
investigated in resectable LALSCC. Thus, we considered that there
was a need for such an analysis.
Overall treatment time, which is calculated the same
as the TPT, had been shown by several authors to be a significant
prognostic factor in various types of cancer (3,4,7,8,10). For example, an overall treatment time
of <80 days for chemoselection was significantly correlated with
longer CSS in patients with squamous cell carcinomas of pharynx and
larynx (3). The findings of the
present study revealed significant associations between prolonged
TPT and shorter CSS and are in good agreement with the results of
the previous study (3).
Although the present study for patients with
consecutively various treatments investigated in comparison with
previous study for head and neck cancer with adjuvant
chemoradiotherapy following surgery, both present and previous
studies showed similar findings of the significant association
between better survival outcomes and shorter TPT (15).
Soyfer et al (8), reported that the duration of
postoperative RT in days was significantly correlated with distant
recurrence in gastric cancer. The findings of the present study
revealed significant associations between prolonged TPT and shorter
DMFS and are in good agreement with the results of the previous
study (8).
Because distant metastasis is directly correlated
with CSS in LALSCC (2), we
hypothesized that TPT is correlated with DMFS. Indeed, in the
present study, we showed that prolonged TPT was significantly
associated with shorter DMFS. The findings from the present study
suggested that the TPT in LALSCC is a prognostic factor for
identifying groups that are at a high-risk of developing distant
metastasis. Moreover, we thought that the significant association
between a TPT of ≥68 days and a shorter CSS was caused by a shorter
rate of DMFS.
In the present study, the TPT <68 days with good
prognosis shows that definitive treatment with shorter TPT is
important. We considered that the cut off at 68 days is an
important day, because this contained not only surgery but also
surgery and RT or CRT. We considered that TPT 68≥ days results in a
shorter prognosis in the present study, because the patients with
TPT 68≥ days was significantly associated with more frequent cN2-3
and adjuvant treatment. From the significant association between
TPT and survival outcomes in the present study, we suggest that it
is essential to perform definitive treatment with shorter TPT as
the true meaning in the head and neck treatment. In the present
study, the cut-off date (68 days) was experimentally determined by
ROC analysis using TPT as continuous variables.
The present study is examined whether the TPT
predicts CSS and distant metastasis in laryngeal cancer. A
limitation of this study is that the backgrounds of patients in the
compared groups are very different. Patients with advanced cancer
may have cancer specific-deaths and metastases at a higher rate
than those with less advanced cancer. The limitations of this study
were the retrospective design and the relatively small number of
subjects. A future prospective study on a large cohort in a
multi-institutional setting would yield more accurate results. In a
future study regarding the effect of TPT, the background factors of
patients in the study should be matched.
In conclusion, the present study demonstrated that a
prolonged TPT was a prognostic factor of shorter CSS and DMFS in
patients with resectable LALSCC who consecutively underwent various
treatments modalities, including surgery alone and salvage surgery
for residual tumor.
Acknowledgements
Not applicable.
Funding
The present study was by the JSPS KAKENHI (grant no.
16K11253).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available on reasonable request from the corresponding
author.
Authors' contributions
HS designed the study, acquired and analyzed the
data, drafted the manuscript, and is accountable for all aspects of
the study. HTe, NH, DN, YusK, SB, TK, HTac, YutK and HTan acquired
the data and critically revised the manuscript. YH designed the
study and acquired the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the review board
of Aichi Cancer Center Hospital (Nagoya, Japan), and informed
consent for the examinations and treatments was obtained from all
of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACRT
|
alternating chemoradiotherapy
|
|
CRT
|
concurrent chemoradiotherapy
|
|
CSS
|
cancer-specific survival
|
|
DMFS
|
distant metastasis-free survival
|
|
LALSCC
|
locally-advanced laryngeal squamous
cell carcinoma
|
|
LRRFS
|
locoregional recurrence-free
survival
|
|
OS
|
overall survival
|
|
PL
|
partial laryngectomy
|
|
RT
|
radiotherapy
|
|
TL
|
total laryngectomy
|
|
TNM
|
tumor-node metastasis
|
|
TPT
|
treatment package time
|
References
|
1
|
Sanabria A, Chaves ALF, Kowalski LP, Wolf
GT, Saba NF, Forastiere AA, Beitler JJ, Nibu KI, Bradford CR,
Suárez C, et al: Organ preservation with chemoradiation in advanced
laryngeal cancer: The problem of generalizing results from
randomized controlled trials. Auris Nasus Larynx. 44:18–25. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eskiizmir G, Toker Tanyeri G, Celik O,
Gunhan K, Tan A and Ellidoluz H: Predictive and prognostic factors
for patients with locoregionally advanced laryngeal carcinoma
treated with surgical multimodality protocol. Eur Arch
Otorhinolaryngol. 274:1701–1711. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Semrau S, Schmidt D, Lell M, Waldfahrer F,
Lettmaier S, Kuwert T, Iro H and Fietkau R: Results of
chemoselection with short induction chemotherapy followed by
chemoradiation or surgery in the treatment of functionally
inoperable carcinomas of the pharynx and larynx. Oral Oncol.
49:454–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sher DJ, Posner MR, Tishler RB, Sarlis NJ,
Haddad RI, Holupka EJ and Devlin PM: Relationship between radiation
treatment time and overall survival after induction chemotherapy
for locally advanced head-and-neck carcinoma: A subset analysis of
TAX 324. Int J Radiat Oncol Biol Phys. 81:e813–e818. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Budach W, Bölke E, Kammers K, Gerber PA,
Orth K, Gripp S and Matuschek C: Induction chemotherapy followed by
concurrent radio-chemotherapy versus concurrent radio-chemotherapy
alone as treatment of locally advanced squamous cell carcinoma of
the head and neck (HNSCC): A meta-analysis of randomized trials.
Radiother Oncol. 118:238–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakata Y, Ijichi K, Hanai N, Nishikawa D,
Suzuki H, Hirakawa H, Kodaira T, Fujimoto Y, Fujii T, Miyazaki T,
et al: Treatment results of alternating chemoradiotherapy with
early assessment for advanced laryngeal cancer: A
multi-institutional phase II study. Auris Nasus Larynx. 44:104–110.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaikh T, Handorf EA, Murphy CT, Murphy
CT, Mehra R, Ridge JA and Galloway TJ: The impact of radiation time
on survival in patients with head and neck cancer. Int J Radiat
Oncol Biol Phys. 96:967–975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soyfer V, Geva R, Michelson M, Inbar M,
Shacham-Shmueli E and Corm BW: The impact of overall radiotherapy
treatment time and delay in initiation of radiotherapy on local
control and distant metastases in gastric cancer. Radiat Oncol.
9:812014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peters LJ and Withers HR: Applying
radiobiological principles to combined modality treatment of head
and neck cancer-the time factor. Int J Radiat Oncol Biol Phys.
39:831–836. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guttmann DM, Kobie J, Grover S, Lin A,
Lukens JN, Mitra N, Rhodes KV, Feng W and Swisher-McClure S:
National disparities in treatment package time for resected locally
advanced head and neck cancer and impact on overall survival. Head
Neck. 40:1147–1155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takehana K, Kodaira T, Tachibana H, Kimura
K, Shimizu A, Makita C, Tomita N, Nishikawa D, Suzuki H, Hirakawa
H, et al: Retrospective analysis of the clinical efficacy of
definitive chemoradiotherapy for patients with hypopharyngeal
cancer. Jpn J Clin Oncol. 46:344–349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki H, Hanai N, Nishikawa D, Fukuda Y
and Hasegawa Y: Complication and surgical site infection for
salvage surgery in head and neck cancer after chemoradiotherapy and
bioradiotherapy. Auris Nasus Larynx. 44:596–601. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakata Y, Hanai N, Nishikawa D, Suzuki H,
Koide Y, Fukuda Y, Nomura M, Kodaira T, Shimizu T and Hasegawa Y:
Comparison between chemoselection and definitive radiotherapy in
patients with cervical esophageal squamous cell carcinoma. Int J
Clin Oncol. 22:1034–1041. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Polesel J, Furlan C, Birri S, Giacomarra
V, Vaccher E, Grando G, Gobitti C, Navarria F, Schioppa O, Minatel
E, et al: The impact of time to treatment initiation on survival
from head and neck cancer in north-eastern Italy. Oral Oncol.
67:175–182. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghanem AI, Mannari A, Schymick MA,
Burmeister C, Ghanem T, Chang S and Siddiqui F: The effect of
treatment package time in head and neck cancer patients treated
with adjuvant radiation therapy and concurrent systemic therapy.
Int J Radiat Oncol Biol Phys. 99:E3382017. View Article : Google Scholar
|