Introduction
Breast cancer is a gynecological malignant tumor
caused by hormone secretion imbalance, nutritional imbalance, viral
stimulation, radiation factors and genetic background (1). As social life rhythm accelerates and
working methods change, the incidence of breast cancer has
increased year by year, which has already ranked first in the
incidence of female malignant tumors, seriously threatening women's
physical and mental health. The clinical manifestations of breast
cancer are mainly orange-like changes in the skin and contour of
the breast, with lumps in the breast, nipple discharge and swollen
lymph nodes (2). Its early cure rate
is extremely high, so early detection and early diagnosis are very
important for its prognosis. Moreover, different stages of breast
cancer are treated differently. Therefore, the accurate diagnosis
of different stages is also very important for breast cancer
treatment. With the development and improvement of imaging
technologies such as ultrasound (US) and magnetic resonance imaging
(MRI), imaging methods have played an increasingly important role
in the diagnosis of breast cancer (1,3). At
present, TNM staging is the main staging method of breast cancer
(4). Currently, physical means,
physical examination and molybdenum palladium are mainly used for
staging clinically, with not enough sensitivity (5). Both US and MRI are commonly used imaging
methods for the early diagnosis and staging of breast cancer. There
are studies on the clinical value of MRI combined with US in the
diagnosis of breast cancer staging, but most studies are on T
staging, with few studies on N and M staging (6,7).
Therefore, in this study, the diagnostic value of US combined with
MRI in the T, N and M staging of breast cancer was analyzed, in
order to provide a more effective, sensitive and accurate detection
program for the early diagnosis and accurate staging, improving the
efficacy of patients' subsequent treatment and the prognoses.
Patients and methods
Research subjects
A total of 140 breast cancer patients diagnosed in
Heping Hospital Affiliated to Changzhi Medical College (Changzhi,
China) from June 2016 to June 2018 were collected, studied as the
breast cancer group, 80 patients with benign breast tumor treated
in Heping Hospital Affiliated to Changzhi Medical College in the
same period were the benign group. Tumor size, breast cancer
classification and other tumor lesions and the diagnosis of breast
cancer are subject to pathological results. Inclusion criteria of
the breast cancer group were: those confirmed as breast cancer by
pathology; females >18 years old; those who signed the informed
consent form. Exclusion criteria of the breast cancer group were:
those with severe fungal bacterial virus infections; those with
other severe basic diseases such as heart, liver and kidney; those
with mental illness and mental disorders; MRI and US
contraindications; pregnant or lactating women; those with
incomplete clinical data; those unsatisfied with US and MRI
examination images acquired; those with incomplete pathological
histological data; those who had received any breast cancer-related
treatment within 3 months. Inclusion criteria of the benign group
were: those with clinical symptoms such as painless lump, nipple
discharge and nipple change; those diagnosed as benign breast tumor
by pathology and clinical palpation; females >18 years old;
those who signed the informed consent form. Exclusion criteria of
the benign group were: those with severe fungal bacterial virus
infections; those with other severe basic diseases such as heart,
liver and kidney; those with mental illness and mental disorders;
MRI and US contraindications; pregnant or lactating women; those
with incomplete clinical data; those unsatisfied with US and MRI
examination images acquired; those with incomplete pathological
histological data. This study has been approved by the Ethics
Committee of Heping Hospital Affiliated to Changzhi Medical
College.
Examination time
The breast lump tissue was surgically resected for
pathological examination, and US and MRI were performed at 1 week
before operation. US, MRI and the combination of the two were used
to confirm the diagnosis of breast cancer, and then the TNM staging
was perform of the confirmed pathology.
US examination
The Antares US diagnostic apparatus (Beijing
Oriental Mairun Medical Devices Co., Ltd., Beijing, China) has a
probe frequency of 8–15 MHz. The two-dimensional US was used to
first investigate the shape, size, margin, internal posterior echo
and internal calcification of the lesion for judging benign and
malignant according to the BI-RADS standard. The Color Doppler Flow
Imaging (CDFI) was used to observe the blood flow distribution
inside and around the lesion, with a blood flow resistance index
(RI) of malignant lesions of ≥0.70. Either the two-dimensional US
or CDFI detected the lesion as malignant and that was diagnosed as
breast cancer.
MRI examination
A 5T magnetic resonance scanner (Shenzhen Siemens
Magnetic Resonance Co., Ltd., Shenzhen, China) and a bilateral
breast surface coil were used in MRI, with a contrast agent as
gadodiamide injection (Shanghai General Electric Pharmaceutical
Shanghai Co., Ltd., Shanghai, China; SFDA approval number:
J20140164). MRI scan was used to observe the shape, margin and
internal structure of the tumor, and breast cancer scan showed more
lobulated or burr signs. The low signal of T1WI and high signal of
T2WI, with uneven internal signal, were the enhanced performance of
the ‘mesh’ or ‘island’ MRI enhanced scanning for observing the
tumor. The breast cancer lump showed a ring-enhanced change.
TNM staging
The breast lump tissue was surgically resected for
pathological examination, and breast cancer was staged according to
the TNM staging (8). The T staging is
mainly based on tumor size, N staging on the swollen regional lymph
node and metastasis, and M staging on the distant metastasis of the
tumor. The criteria of T, N and M staging are shown in Table I.
 | Table I.Criteria of T, N and M staging. |
Table I.
Criteria of T, N and M staging.
| Staging | Tumor conditions |
|---|
| T staging |
|
| Tis in
situ carcinoma | No palpable lump in
the breast |
| T1 | Maximum diameter of
tumor: <2 cm |
| T2 | Maximum diameter of
tumor: 2–5 cm |
| T3 | Maximum diameter of
tumor: >5 cm |
| T4 | Regardless of tumor
size, it has already invaded the chest wall or skin |
| N staging |
|
| N0 | No palpable regional
lymph node |
| N1 | Ipsilateral axillary
lymph nodes swollen with activity |
| N2 | Ipsilateral axillary
lymph nodes swollen, fused to each other and even adhered to other
tissues |
|
| N3 | Ipsilateral internal
mammary lymph nodes with metastasis |
| M staging |
|
| M0 | No distant
metastasis |
| M1 | Distant metastasis
including lymph node metastasis on ipsilateral clavicle |
Comparison indicators
Pathological results were used as the gold standard
to compare the diagnostic coincidence of US and MRI in breast
cancer patients in different stages of T, N and M. The sensitivity
(SEN), specificity (SPE), negative predictive value (NPV), positive
predictive value (PPV) and Youden index (YI) of each group were
compared. YI = SEN + SPE - 1.
Statistical analysis
SPSS19.0 software system (IBM, SPSS, Chicago, IL,
USA) was used for data analysis. Measurement data were expressed as
mean ± SD and tested by t-test. Count data were expressed as % and
tested by Chi-square test. The level of significance is α=0.05.
Results
General clinical data of patients
There was no difference between breast cancer group
and benign group in general clinical data such as age, menopausal
status, lesion size and number and tumor site (P>0.05), but
significant differences in the expression of estrogen receptor
(ER), progesterone receptor (PR) and human epidermal growth factor
receptor 2 (Her-2). The positive expression case rates of ER and PR
were significantly higher in breast cancer group than those in
benign group, but that of Her-2 was significantly lower in breast
cancer group than that in benign group (all P<0.05) (Table II).
 | Table II.General clinical data of patients. |
Table II.
General clinical data of patients.
| Clinical data | Breast cancer group
(n=140) | Benign group
(n=80) | χ2
value | P-value |
|---|
| Age | 35.34±16.54 | 32.13±13.24 |
1.577 | 0.117 |
| Menstrual status [n
(%)] |
|
|
2.020 | 0.155 |
| Before
menopause | 110 (78.57) | 56 (70.00) |
|
|
| After
menopause | 30
(21.43) | 24 (30.00) |
|
|
| Lesion size (cm) | 0.42–5.44 | 0.59–5.22 |
0.229 | 0.819 |
| Lesion number [n
(%)] |
|
|
7.356 | 0.007 |
| Single
lesion | 74 (52.86) | 56 (70.00) |
|
|
| Multiple
lesions | 66 (47.14) | 24 (30.00) |
|
|
| Tumor site |
|
|
0.094 | 0.759 |
| Left
breast | 74 (52.86) | 44 (55.00) |
|
|
| Right
breast | 66 (47.14) | 36 (45.00) |
|
|
| ER |
|
| 36.780 | <0.001 |
|
Negative | 49 (35.00) | 62 (77.50) |
|
|
|
Positive | 91(65.00) | 18 (22.50) |
|
|
| PR |
|
|
6.890 | 0.009 |
|
Negative | 60 (42.86) | 49 (61.25) |
|
|
|
Positive | 80 (57.14) | 31 (38.75) |
|
|
| Her-2 |
|
| 55.210 | <0.001 |
|
Negative | 92 (65.71) | 11 (13.75) |
|
|
|
Positive | 48 (34.29) | 69 (86.25) |
|
|
| Pathological
classification [n (%)] |
|
| – | – |
| (WHO classification
criteria for breast cancer) |
| Invasive
ductal carcinoma | 32 (22.86) | – |
|
|
|
Intraductal carcinoma | 20 (14.29) | – |
|
|
| Medullary
carcinoma | 5 (3.57) | – |
|
|
| Papillary
carcinoma | 36 (25.71) | – |
|
|
| Simple
carcinoma | 33 (23.57) | – |
|
|
| Apocrine
carcinoma | 14 (10.00) | – |
|
|
Comparison of breast cancer diagnosis
between US and MRI
Among 140 patients diagnosed as breast cancer by
pathology, 107 patients were diagnosed by US, 125 patients by MRI
and 129 patients by US combined with MRI (+). Specific results are
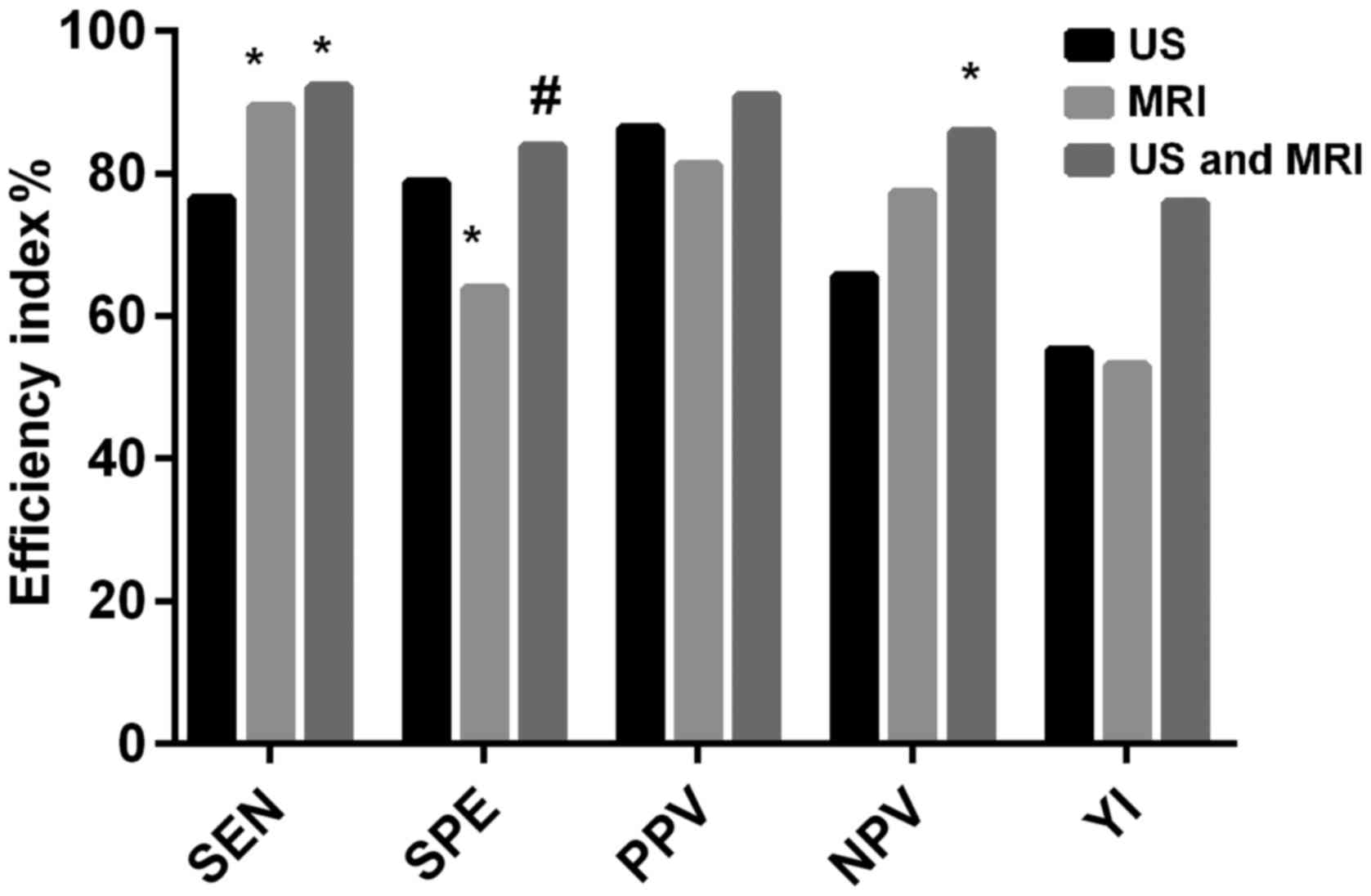
shown in Table III. The SEN of MRI
alone was not different from that of US combined with MRI
(P>0.05), but that of MRI alone and US combined with MRI was
higher than that of US alone (P<0.05). The SPE of US alone was
not different from that of US combined with MRI (P>0.05), but
that of MRI alone was lower than that of US alone and US combined
with MRI (P<0.05). The difference in the PPV among the three
methods was not statistically significant (P>0.05). The NPV of
US alone and US combined with MRI was not different from that of
MRI alone (P>0.05). The NPV of US combined with MRI was
significantly higher than that of US alone (P<0.05). The YI of
US combined with MRI was significantly higher than that of US alone
and MRI alone, and that of US alone was not significantly different
from that of MRI alone (Fig. 1 and
Table IV).
 | Table III.Results of US, MRI and pathology for
breast cancer diagnosis. |
Table III.
Results of US, MRI and pathology for
breast cancer diagnosis.
| US/MRI detection
results | Pathology results
(+) | Pathology results
(−) | Total |
|---|
| US (+) | 107 | 17 | 124 |
| US (−) | 33 | 63 | 96 |
| Total | 140 | 80 | 220 |
| MRI (+) | 125 | 29 | 154 |
| MRI (−) | 15 | 51 | 66 |
| Total | 140 | 80 | 220 |
| US combined with
MRI (+) | 129 | 13 | 142 |
| US combined with
MRI (−) | 11 | 67 | 78 |
| Total | 140 | 80 | 220 |
 | Table IV.Comparison of SEN, SPE, PPV and NPV
among groups. |
Table IV.
Comparison of SEN, SPE, PPV and NPV
among groups.
| Detection
indicators | US (%) | MRI (%) | US combined with
MRI (%) | χ2
value | P-value |
|---|
| SEN | 76.43 | 89.29a | 92.14a | 16.25 | <0.001 |
| SPE | 78.75 | 63.75a | 83.75b | 9.349 | <0.001 |
| PPV | 86.29 | 81.17 | 90.85 | 5.746 | 0.057 |
| NPV | 65.63 | 77.27 | 85.90a | 9.709 | 0.008 |
| YI | 55.18 | 53.04 | 75.89 | – | – |
Comparison of T staging diagnosis of
breast cancer between US and MRI
The evaluation of T staging and pathology of 140
patients before operation was compared among US, MRI and US
combined with MRI. The results showed that the difference in the
diagnosis of T1 and T3 among three methods was not statistically
significant (P>0.05), with coincidence rates of 100% in the
evaluation of T4. In the diagnosis of T2, the coincidence rates of
MRI alone and US combined with MRI were significantly higher than
that of US alone (P<0.05), and that of MRI alone was not
significantly different than that of US combined with MRI
(P>0.05) (Table V).
 | Table V.Results of breast cancer T staging in
US and MRI. |
Table V.
Results of breast cancer T staging in
US and MRI.
| Pathological T
staging | US consistent with
pathology, n (%) | MRI consistent with
pathology, n (%) | US combined with
MRI consistent with pathology, n (%) | χ2
value | P-value |
|---|
| T1 (n=13) | 7 (53.85) | 10 (76.92) | 11 (84.62) |
3.292 | 0.193 |
| T2 (n=72) | 50 (69.44) | 62
(86.11)a | 64
(88.89)a,b | 10.550 | 0.005 |
| T3 (n=44) | 39 (88.64) | 42 (95.45) | 43 (97.73) |
4.827 | 0.090 |
| T4 (n=11) | 11 (100.00) | 11 (100.00) | 11 (100.00) | – | – |
| Total (n=140) | 107 (76.43) | 125
(89.29)a | 129
(92.14)a,b | 16.250 | <0.001 |
Comparison of N staging diagnosis of
breast cancer between US and MRI
The evaluation of N staging and pathology of 140
patients before operation was compared among US, MRI and US
combined with MRI. The results showed that the difference in N1 and
N3 among three methods was not statistically significant
(P>0.05). In the evaluation of N0, the coincidence rates of MRI
alone and US combined with MRI were higher than that of US alone
(P<0.05), and that of MRI alone was not different than that of
US combined with MRI (P>0.05). In the diagnosis of N2, that of
US combined with MRI was significantly higher than those of US
alone and MRI alone (P<0.05), and that of MRI alone was not
significantly different than that of US alone (P>0.05) (Table VI).
 | Table VI.Results of breast cancer N staging in
US and MRI. |
Table VI.
Results of breast cancer N staging in
US and MRI.
| Pathological T
staging | US consistent with
pathology, n (%) | MRI consistent with
pathology, n (%) | US combined with
MRI consistent with pathology, n (%) | χ2
value | P-value |
|---|
| N0 (n=75) | 60 (80.00) | 69
(92.00)a | 69
(92.00)a |
4.485 | 0.034 |
| N1 (n=37) | 30 (81.08) | 32 (86.49) | 34 (91.89) |
1.850 | 0.397 |
| N2 (n=20) | 13 (65.00) | 17 (85.00) | 19
(95.00)a |
6.234 | 0.044 |
| N3 (n=8) | 4 (50.00) | 7 (87.50) | 7 (87.50) |
4.200 | 0.135 |
| Total (n=140) | 107 (76.43) | 125
(89.29)a | 129
(92.14)a | 16.250 | <0.001 |
Comparison of M staging diagnosis of
breast cancer between US and MRI
The evaluation of M staging and pathology of 140
patients before operation was compared among US, MRI and US
combined with MRI. The results showed that in stages of M0 and M1
among the three methods, the coincidence rates of MRI alone and US
combined with MRI were higher than that of US alone (P<0.05),
and that of MRI alone was not significantly different from that of
US combined with MRI (P>0.05) (Table
VII).
 | Table VII.Results of breast cancer M staging in
US and MRI. |
Table VII.
Results of breast cancer M staging in
US and MRI.
| Pathological T
staging | US consistent with
pathology, n (%) | MRI consistent with
pathology, n (%) | US combined with
MRI consistent with pathology, n (%) | χ2
value | P-value |
|---|
| M0 (n=124) | 98
(79.03) | 111
(89.52)a | 114
(91.94)a | 10.200 | 0.006 |
| M1 (n=16) | 9
(56.25) | 14
(87.50)a | 15
(93.75)a |
7.832 | 0.020 |
| Total (n=140) | 107 (76.43) | 125
(89.29)a | 129
(92.14)a | 16.250 | <0.001 |
Discussion
In recent years, as its incidence occurs at younger
age and mortality has gradually increased, breast cancer has become
the number one killer threatening female health (9,10).
Clinical features of early breast cancer are atypical, with high
misdiagnosis and missed diagnosis rates, so most of breast cancer
patients are in the advanced stage when diagnosed. At present,
there is no clear and effective primary prevention method.
Therefore, the early detection and accurate preoperative staging
and treatment of breast cancer are crucial to improve the prognosis
(11,12). Many studies have been reported on the
value of US combined with MRI detection in breast cancer staging,
but most of them only focus on T staging and diagnosis efficiency,
few only on N and M staging (13).
Therefore, in this study, the diagnostic value of US combined with
MRI in the T, N and M staging of breast cancer was analyzed, in
order to provide a more effective, sensitive and accurate detection
program for the early diagnosis and accurate staging, improving the
efficacy of patients' subsequent treatment and the prognoses.
The positive expression case rates of ER and PR were
significantly higher in breast cancer group than those in benign
group, but that of Her-2 was significantly lower in breast cancer
group than that in benign group. This is consistent with the
findings of Li et al (14), in
the study of ER, PR and HER-2 expression in breast cancer and their
relationship with tumor staging and lymph node metastasis. ER, PR
and HER-2 are important indicators for judging the prognosis of the
patient.
In the evaluation of breast cancer T staging, US,
MRI and US combined with MRI had coincidence rates of 100% in the
evaluation of T4. The coincidence rates of MRI alone and US
combined MRI in the diagnosis of T2 were significantly higher than
that of US alone, with no statistically significant difference in
other stages. The accuracy of MRI and US for tumor lesion and tumor
size in newly diagnosed non-high-risk breast cancer patients was
compared by Segara et al (15). The results have shown that the
difference in tumor size is not significant between MRI and
pathological findings. Among molybdenum palladium, US and MRI,
breast cancer size is the most accurate when measured by MRI
(15). In the evaluation of breast
cancer N staging, the coincidence rates of MRI alone and US
combined with MRI were higher than that of US alone in the
evaluation of N0. In the diagnosis of N2, that of US combined with
MRI was significantly higher than those of US alone and MRI alone.
In stage N2, the ipsilateral axillary lymph nodes of breast cancer
patients were swollen, fused to each other and even adhered to
other tissues. Therefore, the key point in the examination is to
judge the breast lymph node metastasis and the organ invasion
around the breast. MRI soft tissue has high resolution and high
field NMR. It can be multi-sequence and multi-angle imaging, more
accurate for observing breast lymph nodes and breast circumference.
Nevertheless, there are still some difficulties in identifying
smaller lymph nodes. After the use of developer, identifying the
site and shape of the primary lesion, US can also evaluate the
obvious contrast effect between the strong echogenic surface
produced by ultrasound shadow agent and the breast structure and
the tissue around the breast, so as to significantly improve the
image quality and better observe breast conditions. However, the
axillary lymph in the stage of N2 fuse with each other and even
adhere to other tissues, causing a certain degree of interference
to acoustic shadow, so US examination has certain difficulties
(16,17). In stages of M0 and M1 among the three
methods, the coincidence rates of MRI alone and US combined with
MRI were higher than that of US alone, and that of MRI alone was
not significantly different from that of US combined with MRI.
Clearly displaying the local irregular thickening of the breast,
MRI can determine whether there is tumor invasion and liver
metastasis outside the breast, and tumor recurrence. US for
observing the hierarchical structure of the breast can determine
the depth of lesion invasion and lymph node metastasis around the
breast, but it may be difficult to distinguish when ulcer or tumor
lesions occur (18,19).
In summary, US combined with MRI for the diagnosis
of breast cancer has higher SEN and SPE, with better accuracy rate
for the identification of each stage. Reducing the incidence of
missed diagnosis and misdiagnosis that may be caused by single
diagnosis and treatment, it is conducive to clinical screening and
guiding clinical symptomatic treatment and worthy of clinical
promotion.
Acknowledgements
Not applicable.
Funding
This study was supported by the project of Changzhi
Medical College Doctoral Scientific Research Start-up Fund (no.
BS15002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QP was responsible for US examination. JJ analyzed
the data of US and MRI examination. Both authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Heping Hospital Affiliated to Changzhi Medical College (Changzhi,
China). Patients who participated in this study, signed the
informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Merckel LG, Knuttel FM, Deckers R, van
Dalen T, Schubert G, Peters NH, Weits T, van Diest PJ, Mali WP,
Vaessen PH, et al: First clinical experience with a dedicated
MRI-guided high-intensity focused ultrasound system for breast
cancer ablation. Eur Radiol. 26:4037–4046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Onitilo AA, Engel JM, Greenlee RT and
Mukesh BN: Breast cancer subtypes based on ER/PR and Her2
expression: Comparison of clinicopathologic features and survival.
Clin Med Res. 7:4–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Y, Chen K, Xiao X, Nie Y, Qu S, Gong
C, Su F and Song E: Pretreatment neutrophil-to-lymphocyte ratio is
correlated with response to neoadjuvant chemotherapy as an
independent prognostic indicator in breast cancer patients: A
retrospective study. BMC Cancer. 16:3202016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee
SH, Chie EK, Han W, Kim DW, Moon WK, et al: Prognostic impact of
clinicopathologic parameters in stage II/III breast cancer treated
with neoadjuvant docetaxel and doxorubicin chemotherapy:
Paradoxical features of the triple negative breast cancer. BMC
Cancer. 7:2032007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siroy A, Abdul-Karim FW, Miedler J, Fong
N, Fu P, Gilmore H and Baar J: MUC1 is expressed at high frequency
in early-stage basal-like triple-negative breast cancer. Hum
Pathol. 44:2159–2166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abe H, Schacht D, Kulkarni K, Shimauchi A,
Yamaguchi K, Sennett CA and Jiang Y: Accuracy of axillary lymph
node staging in breast cancer patients: An observer-performance
study comparison of MRI and ultrasound. Acad Radiol. 20:1399–1404.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuhl C, Kuhn W, Braun M and Schild H:
Pre-operative staging of breast cancer with breast MRI: One step
forward, two steps back? Breast. 16 (Suppl 2):S34–S44. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fouad TM, Barrera AMG, Reuben JM, Lucci A,
Woodward WA, Stauder MC, Lim B, DeSnyder SM, Arun B, Gildy B, et
al: Inflammatory breast cancer: A proposed conceptual shift in the
UICC-AJCC TNM staging system. Lancet Oncol. 18:e228–e232. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gahlaut R, Bennett A, Fatayer H, Dall BJ,
Sharma N, Velikova G, Perren T, Dodwell D, Lansdown M and Shaaban
AM: Effect of neoadjuvant chemotherapy on breast cancer phenotype,
ER/PR and HER2 expression - Implications for the practising
oncologist. Eur J Cancer. 60:40–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao ZX, Lu LJ, Wang RJ, Jin LB, Liu SC, Li
HY, Ren GS, Wu KN, Wang DL and Kong LQ: Discordance and clinical
significance of ER, PR, and HER2 status between primary breast
cancer and synchronous axillary lymph node metastasis. Med Oncol.
31:7982014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nik-Zainal S, Davies H, Staaf J,
Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB,
Martin S, Wedge DC, et al: Landscape of somatic mutations in 560
breast cancer whole-genome sequences. Nature. 534:47–54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tseng J, Kyrillos A, Liederbach E, Spear
GG, Ecanow J, Wang CH, Czechura T, Kantor O, Miller M, Winchester
DJ, et al: Clinical accuracy of preoperative breast MRI for breast
cancer. J Surg Oncol. 115:924–931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Jia M, Qin X, Hu J, Zhang X and Zhou
G: Harmful effect of ERβ on BCRP-mediated drug resistance and cell
proliferation in ERα/PR-negative breast cancer. FEBS J.
280:6128–6140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Segara D, Krop IE, Garber JE, Winer E,
Harris L, Bellon JR, Birdwell R, Lester S, Lipsitz S, Iglehart JD,
et al: Does MRI predict pathologic tumor response in women with
breast cancer undergoing preoperative chemotherapy? J Surg Oncol.
96:474–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heijnsdijk EA, Warner E, Gilbert FJ,
Tilanus-Linthorst MM, Evans G, Causer PA, Eeles RA, Kaas R, Draisma
G, Ramsay EA, et al: Differences in natural history between breast
cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI
screening-MRISC, MARIBS, and Canadian studies combined. Cancer
Epidemiol Biomarkers Prev. 21:1458–1468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DeLeo MJ III, Domchek SM, Kontos D, Conant
E, Chen J and Weinstein S: Breast MRI fibroglandular volume and
parenchymal enhancement in BRCA1 and BRCA2 mutation carriers before
and immediately after risk-reducing salpingo-oophorectomy. AJR Am J
Roentgenol. 204:669–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rudat V, Nour A, Almuraikhi N, Ghoniemy I,
Brune-Erber I, Almasri N and El-Maghraby T: MRI and ultrasonography
for assessing multifocal disease and tumor size in breast cancer:
Comparison with histopathological results. Gulf J Oncol. 1:65–72.
2015.
|
|
19
|
Partridge SC, Nissan N, Rahbar H, Kitsch
AE and Sigmund EE: Diffusion-weighted breast MRI: Clinical
applications and emerging techniques. J Magn Reson Imaging.
45:337–355. 2017. View Article : Google Scholar : PubMed/NCBI
|