Introduction
Non-small cell lung cancer (NSCLC) is the most
common type of cancer and the main cause of cancer-associated
mortalities in China (1). In the
previous decade, the tyrosine kinase inhibitors (TKI) gefitinib and
erlotinib, which specifically target the TK domain of the epidermal
growth factor receptor (EGFR), have demonstrated longer
progression-free survival in patients with NSCLC with EGFR
mutations than in patients with wild-type EGFR (2,3). Thus, it
is important to establish the EGFR mutation status for the
optimization of treatment in patients with NSCLC. However,
sufficient tumor tissues cannot always be obtained for genetic
testing in clinical settings. Therefore, developing noninvasive
techniques to predict the EGFR mutation status may be of clinical
use.
The level of fibrinogen, a major acute phase protein
synthesized by the liver epithelium, increases in the blood in
response to blood clotting, infection, wound healing, inflammation
and neoplasia (4). It is increasingly
recognized that levels of plasma fibrinogen are associated with
human malignancies, such as lung, gastric, esophageal, ovarian and
renal cancer (5–8). Fibrinogen, and other coagulation factors
serve an important function in tumor cell growth, invasion and
metastasis by supporting the sustained adhesion of tumor cells and
by promoting tumor neoangiogenesis. Additionally, fibrinogen itself
is able to directly bind to inflammatory cells, inducing synthesis
of proinflammatory cytokines, and the inflammatory microenvironment
of tumors effectively influences proliferation and migration of
tumor cells. Previous studies have reported that
hyperfibrinogenemia is correlated with lymphatic metastasis in
gastric cancer and gallbladder carcinoma, but not in NSCLC
(9–11). Additionally, no studies have
demonstrated that plasma fibrinogen concentration in patients with
NSCLC may be a predictor for EGFR gene mutation status. The aim of
the present study was to investigate the correlation between plasma
fibrinogen levels and EGFR gene mutation status in patients with
NSCLC. A predictive model was also established for EGFR mutation
status.
Patients and methods
Patient selection
The medical records of patients treated between
January 2010 and December 2013 were retrospectively investigated at
Nanfang Hospital (Guangzhou, China). All patients were confirmed to
exhibit NSCLC and had not yet received any treatment. All patients
met the following entry criteria: The patient underwent EGFR gene
testing subsequent to histologic or cytological diagnosis; plasma
fibrinogen was acquired prior to initiating treatment; positron
emission tomography (PET)/computed tomography (CT) were performed
prior to all treatment; histopathology was reviewed at Nanfang
Hospital, Guangzhou, China. Patient characteristics including age,
sex and smoking history were recorded prior to therapy. Tumor
characteristics such as histology, clinical differentiated degree
and clinical stage were acquired from the clinical pathology
reports, and the clinical stage was specified based on the seventh
edition of the American Joint Committee on Cancer Staging Manual
(AJCC) (12). T1 stage, tumor ≤3 cm
in diameter, surrounded by lung or visceral pleura, without
bronchoscopic evidence of invasion more proximal than the lobar
bronchus. T2 stage, tumor >3 cm but ≤7 cm in diameter, or having
any of the following features: Main bronchus >2 cm from carina,
invades visceral pleura and partial atelectasis. T3 stage, tumor
>7 cm in diameter or directly invading any of the following:
Chest wall, diaphragm, pericardium or mediastinal pleura, or the
main bronchus <2 cm from carina, total atelectasis or separate
nodule(s) in same lobe. T4 stage, tumor of any size that invades
one or more of the following: Mediastinum, heart, large vessels,
carina, trachea, oesophagus, vertebra; separate tumor nodule(s) in
a different ipsilateral lobe.
Fibrinogen measurement
Blood samples from patients with NSCLC were obtained
within the week prior to all treatment. Fibrinogen was measured by
the Clauss method (Sysmex CA-1500; Sysmex Corporation, Kobe,
Japan). Plasma fibrinogen concentrations of 1.8–3.5 g/l were
considered normal, and concentrations of >3.5 g/l were defined
as hyperfibrinogenemic based on the protocol of the
manufacturer.
EGFR mutation analysis
EGFR mutations in exons 18–21 were detected by
direct sequencing in the pathology department of Nanfang Hospital
(Guangzhou, China) between 2010 and 2012. Genomic DNA was extracted
from tumor specimens using the TaKaRa DEXPATTM kit (TaKaRa Bio,
Inc., Ostu, Japan). Genomic DNA sequences were acquired by
polymerase chain reaction (PCR)-based direct sequencing. The
primers used were: Exon 18 sense, CAAATGAGCTGGCAAGTGCCGTGTC and
antisense, GAGTTTCCCAAACACTCAGTGAAAC; Exon 19 sense,
GCAATATCAGCCTTAGGTGCGGCTC and antisense,
CATAGAAAGTGAACATTTAGGATGTG; Exon 20 sense, CCATGAGTACGTATTTTGAAACTC
and antisense, CATATCCCCATGGCAAACTCTTGC; Exon 21 sense,
CTAACGTTCGCCAGCCATAAGTCC and antisense, GCTGCGAGCTCACCCAGAATGTCTGG.
The appropriate PCR products were then sequenced in forward and
reverse directions using the Version 3 ABI PRISM BigDye Terminator
Cycle Sequencing Ready Reaction kit and an ABI PRISM 3730XL Genetic
Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The chromatograms were analyzed by a
professional reviewer. The Amplification Refractory Mutation System
(ARMS) was used to detect exons 18–21 in all samples sourced from
2013. All ARMS primer pairs were utilized for PCR with the
following criteria: Concentration of 1 µM, control reaction primers
at concentration 0.1 µM and TaqMan probes at concentration 0.5 µM.
DNA sequences were amplified by PCR.
The PCR cycling conditions were as follows: An
initial denaturation at 95°C for 5 min followed by six cycles of
95°C for 30 sec, an annealing phase at 60°C for 90 sec, decreasing
by 0.5°C every cycle until reaching 57°C, and an elongation step of
72°C for 90 sec. This was followed by an additional 45 cycles as
described herein, with an annealing temperature of 57°C, and
finally an elongation step at 72°C for 10 min. According to
standard protocols, PCR products were sequenced in both sense and
anti-sense directions using an ABI PRISM® 9700 Genetic
Analyzer (Applied Biosystems, Foster City, CA, USA). The
quantification cycle (Cq) values of the target gene and reference
genes were obtained from the PCR data by a DxS EGFR mutation test
kit (Manchester, UK), according to the manufacturer's
protocols.
Statistical analysis
Plasma fibrinogen was analyzed as a categorical
variable and a continuous variable subsequent to grouping by normal
levels and hyperfibrinogenemia. Continuous covariates were analyzed
using a Wilcoxon rank-sum test or a Student's t-test. Categorical
covariates were analyzed with a Fisher's exact test or Pearson
chi-squared test as appropriate. Logistic regression analysis was
performed to test the variables which were associated with EGFR
mutation status in univariate analysis. Subsequently, the area
under the curve (AUC) of the receiver operator characteristic (ROC)
curve was used for measuring the predictive value. P<0.05, which
derived from two-sided tests, and was considered to indicate a
statistically significant difference. The values are given as mean
± standard deviation if appropriate. All experiments were repeated
three times. Analyses were performed with SPSS 21.0 (IBM SPSS,
Armonk, NY, USA) for all statistical calculations.
Results
Plasma fibrinogen concentration and
characteristics of patients
The association between plasma fibrinogen and the
general characteristics of patients is summarized in Table I. A total of 178 patients (58.7%)
exhibited low plasma fibrinogen concentrations (≤3.5 g/l), whereas
125 (41.3%) exhibited high serum fibrinogen concentrations (>3.5
g/l). Low plasma fibrinogen concentration was significantly
associated with several variables, including female sex, never
smokers, adenocarcinoma histopathology, well-differentiated tumor
grading and AJCC stage I tumors (Table
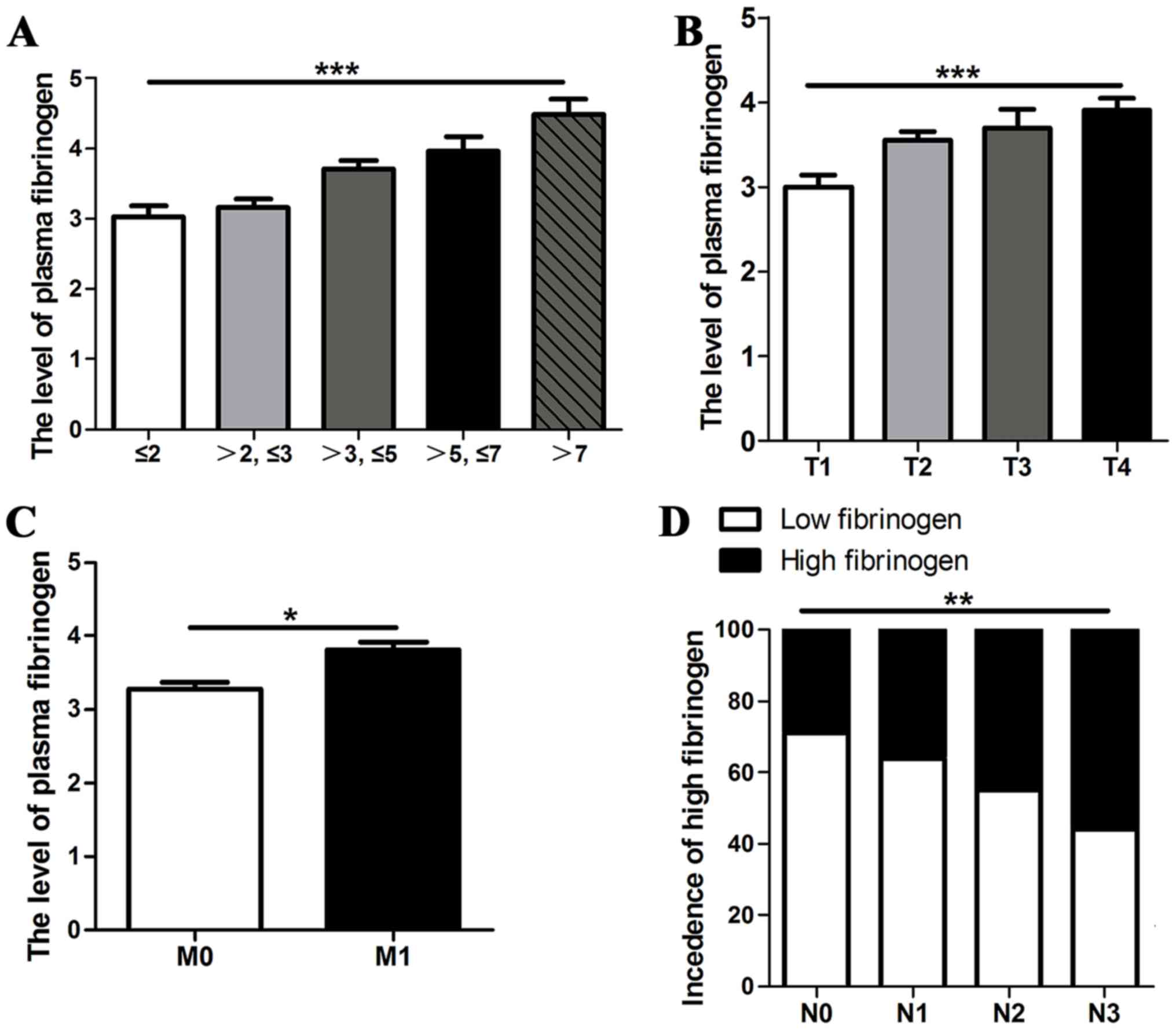
I). When the primary tumor sizes were classified into five
groups, ≤2, 2.01–3, 3.01–5, 5.01–7, and >7 cm, there was a
positive correlation between plasma fibrinogen concentration and
tumor size, 3.03±1.18, 3.16±1.09, 3.71±1.12, 3.96±1.24 and
4.48±1.19, respectively (P<0.001), as demonstrated in Fig. 1A. The level of plasma fibrinogen was
significantly higher in T2, T3 or T4 stage tumors compared with T1
stage tumors, 3.55±1.09, 3.70±1.29 and 3.90±1.29 vs. 3.01±1.19,
respectively (P<0.001), as illustrated in Fig. 1B, and was significantly higher in
M1 stage tumors compared with M0 stage
tumors, 3.81±1.33 vs. 3.28±1.07, respectively (P=0.025), as
demonstrated in Fig. 1C. The
proportion of patients with high fibrinogen concentration was
significantly higher in N2 and N3 stage tumors compared with N0 and
N1 stage tumors, 45.2 and 56.5 vs. 29.2 and 36.0%, respectively
(P=0.001), as illustrated in Fig.
1D.
 | Table I.Associations between serum fibrinogen
level with clinical factors in patients with NSCLC. |
Table I.
Associations between serum fibrinogen
level with clinical factors in patients with NSCLC.
| Characteristic | Low fibrinogen
(n=178) (≤3.5 g/l) n (%) | High fibrinogen
(n=125) (>3.5 g/l) n (%) | P-value |
|---|
| Age, mean (SD) | 59 (11.73) | 61 (11.23) | 0.174 |
| Sex |
|
| 0.045 |
| Male | 113 (54.9) | 93 (45.1) |
|
|
Female | 65 (67.0) | 32 (33.0) |
|
| Smoking history |
|
| <0.001 |
| Ever | 74 (47.7) | 81 (52.3) |
|
|
Never | 104 (70.3) | 44 (29.7) |
|
| Histopathology |
|
| <0.001 |
|
Adenocarcinoma | 152 (65.5) | 80 (34.5) |
|
| Squamous
cell carcinoma | 16 (32.7) | 33 (67.3) |
|
|
Others | 10 (45.5) | 12 (54.5) |
|
| Tumor
gradea |
|
| 0.001 |
|
Well-differentiated | 39 (83.0) | 8 (17.0) |
|
|
Moderately differentiated | 43 (58.1) | 31 (41.9) |
|
| Poorly
differentiated | 70 (52.6) | 63 (47.4) |
|
| AJCC stage |
|
| 0.002 |
| I | 54 (78.3) | 15 (21.7) |
|
| II | 12 (46.2) | 14 (53.8) |
|
| III | 37 (57.8) | 27 (42.2) |
|
| IV | 75 (52.1) | 69 (47.9) |
|
Patient characteristics and EGFR
mutation status
The baseline characteristics of the patients are
listed in Table II. In total, 303
histologically verified patients with NSCLC, with a mean age of
60±11.6 years, 97 females and 206 males, were included. A total of
121 patients, 39.9%, were positive for an EGFR mutation. The EGFR
mutations were more frequent in female patients compared with
males, 62.9 vs. 29.1%, respectively (P<0.001). A total of 148
patients, 48.8%, were never smokers, followed by 127 patients,
41.9%, who were smokers and 28 patients, 9.2%, who used to smoke.
For statistical analyses, the smokers and who used to smoke were
combined into an smoking group (n=155; 51.2%). The EGFR mutations
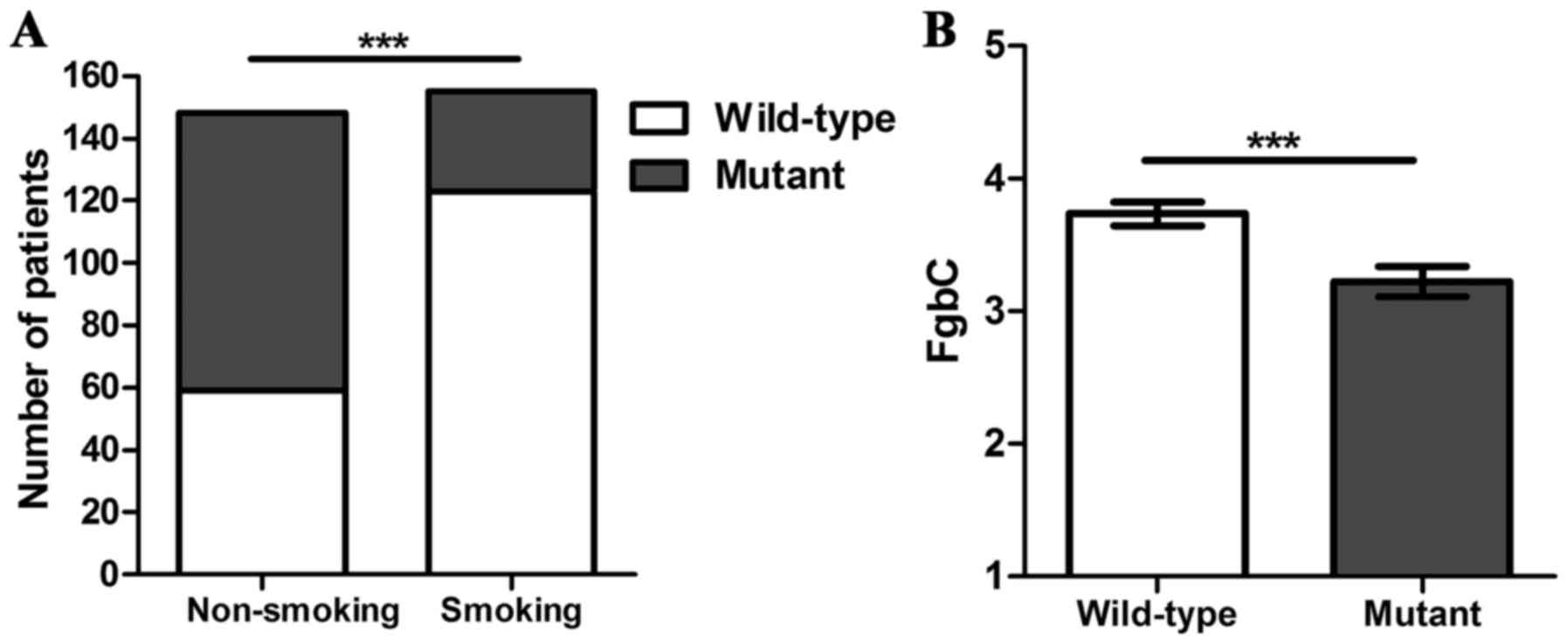
were more frequent in non-smokers compared with smokers, 60.1 vs.
20.6%, respectively (P<0.001), as demonstrated in Fig. 2A. There were 232 patients, 76.6%, with
adenocarcinoma. EGFR mutations were more frequent in patients with
adenocarcinoma (P<0.001). A total of 133 patients, 43.9%, were
diagnosed as poorly differentiated, whereas 47 patients, 15.5%, and
74 patients, 24.4%, were diagnosed as well-differentiated and
moderately differentiated, respectively. In 49 patients, 16.2%,
information about the tumor grade was missing. Almost half of the
patients were diagnosed at stage IV (n=144; 47.5%), whereas 69
patients (22.8%) 26 patients (8.6%) and 64 patients (21.1%) were
detected at stages I, II and III, respectively.
 | Table II.Associations between clinical
features and EGFR mutation. |
Table II.
Associations between clinical
features and EGFR mutation.
| Characteristic | Wild-type EGFR
(n=182) n (%) | Mutant EGFR (n=121)
n (%) | P-value |
|---|
| Age, median
(range) | 60 (24–87) | 60 (30–82) | 0.89 |
| Sex |
|
| <0.001 |
|
Male | 146 (70.9) | 60 (29.1) |
|
|
Female | 36 (37.1) | 61 (62.9) |
|
| Smoking
history |
|
| <0.001 |
|
Ever | 123 (79.4) | 32 (20.6) |
|
|
Never | 59 (39.9) | 89 (60.1) |
|
| Histopathology |
|
| <0.001 |
|
Adenocarcinoma | 124 (53.4) | 108 (46.6) |
|
|
Squamous cell carcinoma | 41 (83.7) | 8 (16.3) |
|
|
Others | 17 (77.3) | 5 (22.7) |
|
| Tumor
gradea |
|
| 0.02 |
|
Well-differentiated | 19 (40.4) | 28 (59.6) |
|
|
Moderately differentiated | 44 (59.5) | 30 (40.5) |
|
| Poorly
differentiated | 88 (66.2) | 45 (33.8) |
|
| AJCC stage |
|
| 0.01 |
| I | 39 (56.5) | 30 (43.5) |
|
| II | 19 (73.1) | 7 (26.9) |
|
|
III | 48 (75.0) | 16 (25.0) |
|
| IV | 76 (52.8) | 68 (47.2) |
|
Association between plasma fibrinogen
and EGFR mutation status
Plasma fibrinogen levels were significantly lower in
patients with EGFR mutations compared with the wild-type EGFR gene,
2.95 g/l (range, 0.84–8.61 g/l) vs. 3.57 g/l (range, 1.38–7.44
g/l), respectively (P<0.001), as demonstrated in Fig. 2B. Patients were allocated into two
groups with a cut-off level of normal fibrinogen level of 3.5 g/l.
A total of 125 patients were allocated to the elevated fibrinogen
group (>3.5 g/l) and 178 patients were allocated to the
non-elevated fibrinogen group (≤3.5 g/l). The incidence of EGFR
mutations in the non-elevated fibrinogen group was higher compared
with the elevated fibrinogen group, 51.1 vs. 24.0%, respectively
(P<0.001). In the univariate logistic regression analysis, the
ORs of EGFR mutations (OR, 0.30; 95% CI, 0.18–0.50; P<0.001)
were significantly decreased in patients in the elevated fibrinogen
group. ROC curve analysis was performed to demonstrate that the
fibrinogen cut-off point was 3.5, as illustrated in Table II, and the AUC of the ROC curve was
0.64 (95% CI, 0.57–0.70; Fig. 3). For
additional analyses, fibrinogen levels were dichotomized into
groups according to the 75th percentile of the total study
population. An elevated level of fibrinogen, with a cut-off of 4.2
g/l, was present in 228 patients, 25%. The ORs of EGFR mutations
(OR, 0.41; 95% CI, 0.23–0.75; P=0.004) were significantly decreased
in patients with elevated fibrinogen group. ROC curve analysis was
performed to show that the fibrinogen cutoff point was 3.5, as
illustrated in Table III, and AUC
of the ROC curve was 0.57 (95% CI, 0.51–0.64), as demonstrated in
Fig. 3.
 | Table III.Multivariate analysis for various
predictive factors of epithelial growth factor receptor
mutations. |
Table III.
Multivariate analysis for various
predictive factors of epithelial growth factor receptor
mutations.
| Variable | Odds ratio | 95% confidence
interval | P-value |
|---|
| Never-smokers | 5.07 | 3.01–8.53 | <0.001 |
| Normal
fibrinogen | 2.63 | 1.53–4.51 | <0.001 |
An elevated level of fibrinogen was associated with
patients who smoked (P<0.001). The majority of smoking patients
(n=74, 91.4%) in the elevated fibrinogen group (>3.5 g/ml) was
associated with wild-type EGFR (P<0.001). However, in nonsmoking
patients, no statically significant association was observed
between fibrinogen and EGFR mutation status. The incidence of EGFR
mutations of non-elevated fibrinogen group (≤3.5 g/ml) was high
compared with the elevated fibrinogen group in adenocarcinoma (55.3
vs. 30.0%; P<0.001), whereas no statistically significant
association was observed between fibrinogen and EGFR mutation
status in squamous cell carcinoma and others, determined via
histopathology. The incidence of EGFR mutations of non-elevated
fibrinogen group (≤3.5 g/ml) was higher compared with the elevated
fibrinogen group in poorly differentiated (47.1 vs. 19.0%;
P=0.001), whereas no statistically significant association was
observed between fibrinogen and EGFR mutation status in the other
tumor grade. At stage I, III and IV, the incidence of EGFR
mutations of non-elevated fibrinogen group was significantly higher
compared with the elevated fibrinogen group in adenocarcinoma
(P<0.05). There was a trend of lower incidence of EGFR mutations
in the elevated fibrinogen group compared with the non-elevated
fibrinogen group, though it was not statistically significant at
stage II.
Multivariate analysis of various
predictive factors of EGFR mutation status
In the univariate analysis, EGFR mutation status was
significantly associated with sex, smoking status, tumor histology,
tumor differentiated degree, tumor stage and fibrinogen, as
summarized in Table II. In order to
clarify which was the independent predictive factor of EGFR
mutations, a multivariate analysis was performed. Logistic
regression was utilized and the model was adjusted for sex, smoking
history, histopathology, tumor grade, stage and fibrinogen.
Fibrinogen (OR 2.5, CI 1.53–4.51, P<0.001) and smoking status
(OR 5.07, CI 3.01–8.53, P<0.001) were revealed to be independent
predictive factors subsequent to multivariate analyses, whereas
sex, tumor histology, tumor differentiated degree and tumor stage
exhibited no independent predictive impact on EGFR mutation status
in the study population.
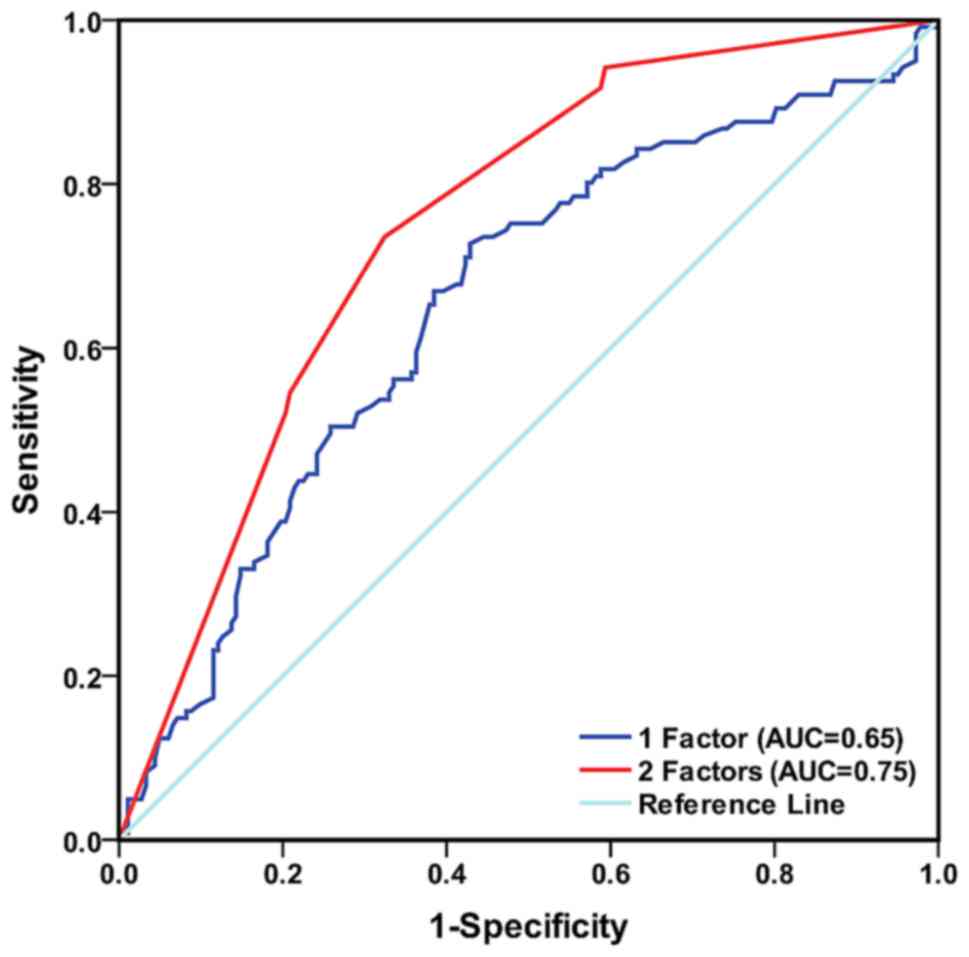
Finally, the sensitivity and specificity of plasma
fibrinogen levels and smoking status in predicting EGFR mutation
status were investigated and ROC analysis was performed. When the
predictive model included either plasma fibrinogen levels or
smoking status, the AUC for prognosticating EGFR mutation status
was 0.65 (95% CI: 0.59–0.72) and 0.71 (95% CI: 0.65–0.77),
respectively. When the predictive model included plasma fibrinogen
levels and smoking status together, the AUC for prognosticating
EGFR mutation status was 0.75 (95% CI: 0.70–0.81), indicating that
the model with two factors possessed higher accuracy for predicting
EGFR mutation status compared with the model with only one factor
in NSCLC, as demonstrated in Fig.
3.
Discussion
The association between the progression of
malignancies and coagulation has been investigated (13). The activation of coagulation and
fibrinolysis in patients with NSCLC is closely associated with
tumor invasion, metastasis and poorer prognosis (14). Hypercoagulability is an indication of
more aggressive disease states. Amongst patients with NSCLC, high
plasma concentrations of fibrinogen, platelet counts and D-dimers
are associated with poor prognosis (15). Additionally, anticoagulant drugs such
as heparin with low molecular weights exhibited a significant
antitumor effect without increasing the incidence of venous
thromboembolism (16).
In the present study, plasma fibrinogen
concentration was significantly associated with clinical
characteristics. Hyperfibrinogenemia was associated with male sex
and squamous cell carcinoma histopathologies. In addition, plasma
fibrinogen concentration was higher in smokers compared with in
nonsmokers, whilst a previous study reported that high fibrinogen
levels were associated with smoking-associated types of cancer
(17). High fibrinogen concentration
was associated with poorly differentiated and advanced stage
tumors. Notably, an association was identified between high
fibrinogen levels and advanced T stage, which was also consistent
with a previous study (11).
Elevated coagulation may be a key factor of the
possibility of metastasis, such as circulating tumor cells.
Fibrinogen promotes platelets to adhere to tumor cells, and the
aggregates of platelet-fibrin-tumor cell complexes may lead to
potential metastasis by preventing the natural killer cells from
eliminating tumor cells (18). In
addition, animal models have confirmed that fi In addition, anim
may strongly diminish tumor size, but did not prevent lung
metastasis (19). This phenomenon is
due to the fact that fibrinogen maintains adhesion to the
circulating tumor cells (20). These
data are important as they suggest an association between
fibrinogen and M stage. This association was also demonstrated in
the present study. Notably, it was revealed that
hyperfibrinogenemia was associated with lymphatic metastasis,
similar with the results of previous studies in gastric and
gallbladder cancer (9,10). Additionally, Palumbo et al
(21) demonstrated that the incidence
of metastases in regional lymph nodes was markedly reduced in
fibrinogen-deficient mice with subcutaneously inoculated Lewis lung
cell carcinoma. However, Jones et al (11) revealed that hyperfibrinogenemia was
not associated with lymphatic metastasis in NSCLC. The reasons for
these conflicting results may be associated with the fact that the
previous study was based on a relatively small sample and the
majority of the patients (92.5%) were of N0 stage:
Additionally, all of the patients underwent CT scanning instead of
PET/CT scanning. Therefore, hyperfibrinogenemia may be associated
with distant organ metastasis and lymphatic metastasis.
In the present study, EGFR mutation status was
significantly associated with sex, smoking history, histopathology,
tumor grade and clinical stage, which was also verified by previous
studies (22,23). EGFR mutations were associated with the
nonsmoking study population. These data were consistent with those
of previous studies. EGFR gene mutations located between the
CAAGGAA repeats were preponderantly in-frame deletions in NSCLC of
non-smoking patients with NSCLC (24). In the present study, the proportion of
EGFR gene mutations were low in smoking patients, which was
consistent with previous studies.
To our knowledge, this is the first study
investigating the association between plasma fibrinogen
concentration and EGFR gene mutation status. Hyperfibrinogenemia
was revealed to be associated with the wild-type EGFR.
Additionally, logistic regression analysis revealed that plasma
fibrinogen and smoking history were independent predictive factors
for EGFR gene mutation status in patients with NSCLC. The genotype
of a non-smoking patient who exhibited low plasma fibrinogen
concentration (≤3.5) always indicated EGFR gene mutations,
particularly in females and patients with adenocarcinoma.
Conversely, the genotype of a smoking patient who exhibited
hyperfibrinogenemia was generally wild-type EGFR, particularly in
males and patients with squamous carcinoma. However, the reason for
the association between elevated plasma fibrinogen and EGFR gene
mutation status of NSCLC remains unknown.
Interleukin-6 (IL-6) is a cytokine which serves an
important role in numerous inflammatory diseases, and IL-6R is one
subtype of interleukin-6. Previous studies have confirmed that the
activation of IL-6R/Janus kinase 1/signal transducer and activator
of transcription 3 signaling via autocrine IL-6 production induced
resistance to EGFR-TKIs in NSCLC (24,25).
Yamaguchi et al (26) revealed
a positive correlation between plasma fibrinogen concentration and
serum IL-6. Therefore, hyperfibrinogenemia may result in resistance
to EGFR-TKIs in patients with EGFR mutations. This requires
additional studies with larger sample sizes.
In summary, the present study demonstrates for the
first time that plasma fibrinogen is an independent predictive
factor for EGFR gene mutation status. Fibrinogen levels are
moderately accurate in predicting EGFR gene mutation status when
plasma fibrinogen measurements are combined with smoking history in
patients with NSCLC. In addition, hyperfibrinogenemia is associated
with distant organ metastasis and lymphatic metastasis, suggesting
that patients with NSCLC with notably elevated fibrinogen level may
benefit from aggressive multimodality therapy. However, additional
independent studies are required to fully characterize the
molecular mechanisms between fibrinogen, EGFR gene mutation status
and metastasis.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81300029); Science and
technology projects in Guangdong Province (grant no.
2012B031800262); Natural Science Foundation of Guangdong Province
(grant no. S2013040013505); President Foundation of Nanfang
Hospital, Southern Medical University (grant no. 2013Z010);
President Foundation of Nanfang Hospital, Southern Medical
University (grant no. 2012Z002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LYL and LC designed the study. CQ, QL, MC, YaZ, YD,
LL and YuZ collected the data. MY collected and corrected the data.
NX performed statistical data processing. and wrote the manuscript.
JG participated in the design of the study, interpreted the data,
drafted and revised the manuscript, approved the version of the
manuscript for publication publication version and is responsible
for all aspects of the work and to ensure proper investigation and
resolution of issues relating to the accuracy or completeness of
any part of the work.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of NanFang Hospital (approval no. NFEC-2015-009;
Guangzhou, China).
Patient consent for publication
Written informed consent for data to be published is
was obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mosesson MW: Fibrinogen and fibrin
structure and functions. J Thromb Haemost. 3:1894–1904. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma XK, Wen JY, Chen ZH, Lin Q, Li X, Dong
M, Wei L, Chen J, Wang TT, Ruan DY, et al: Clinical significance of
plasma fibrinogen level in patients with prostate cancer. J Clin
Oncol. 31:2013.
|
|
6
|
Son HJ, Park JW, Chang HJ, Kim DY, Kim BC,
Kim SY, Park SC, Choi HS and Oh JH: Preoperative plasma
hyperfibrinogenemia is predictive of poor prognosis in patients
with nonmetastatic colon cancer. Ann Surg Oncol. 20:2908–2913.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuda S, Takeuchi H, Kawakubo H, Fukuda
K, Nakamura R, Takahashi T, Wada N, Saikawa Y, Omori T and Kitagawa
Y: Cumulative prognostic scores based on plasma fibrinogen and
serum albumin levels in esophageal cancer patients treated with
transthoracic esophagectomy: Comparison with the Glasgow prognostic
score. Ann Surg Oncol. 22:302–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verheul HM, Hammers H, van Erp K, Wei Y,
Sanni T, Salumbides B, Qian DZ, Yancopoulos GD and Pili R: Vascular
endothelial growth factor trap blocks tumor growth, metastasis
formation, and vascular leakage in an orthotopic murine renal cell
cancer model. Clin Cancer Res. 13:4201–4208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamashita H, Kitayama J and Nagawa H:
Hyperfibrinogenemia is a useful predictor for lymphatic metastasis
in human gastric cancer. Jpn J Clin Oncol. 35:595–600. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao
Y, Wang XA, Zhang F, Xiang SS, Li HF, et al: Clinical and
prognostic significance of preoperative plasma hyperfibrinogenemia
in gallbladder cancer patients following surgical resection: A
retrospective and in vitro study. BMC cancer. 14:5662014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jones JM, McGonigle NC, McAnespie M, Cran
GW and Graham AN: Plasma fibrinogen and serum C-reactive protein
are associated with non-small cell lung cancer. Lung Cancer.
53:97–101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene F and Trotti A: AJCC cancer staging manual. 7th. New York,
NY: Springer; 2010
|
|
13
|
Gatt A, Bonello F, Buttigieg R, Debono S,
Brincat P, Grima C, Gatt P, Lofaro T and Laspina S: Flow cytometry
and thromboelastography to assess platelet counts and coagulation
in patients with haematological malignancies. Blood Transfus.
12:479–484. 2014.PubMed/NCBI
|
|
14
|
Kurup A, Lin C, Murry DJ, Dobrolecki L,
Estes D, Yiannoutsos CT, Mariano L, Sidor C, Hickey R and Hanna N:
Recombinant human angiostatin (rhAngiostatin) in combination with
paclitaxel and carboplatin in patients with advanced non-small-cell
lung cancer: A phase II study from Indiana University. Ann Oncol.
17:97–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Altiay G, Ciftci A, Demir M, Kocak Z, Sut
N, Tabakoglu E, Hatipoglu ON and Caglar T: High plasma D-dimer
level is associated with decreased survival in patients with lung
cancer. Clin Oncol (R Coll Radiol). 19:494–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuderer NM, Khorana AA, Lyman GH and
Francis CW: A meta-analysis and systematic review of the efficacy
and safety of anticoagulants as cancer treatment: Impact on
survival and bleeding complications. Cancer. 110:1149–1161. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
dos Santos Silva I, De Stavola BL, Pizzi C
and Meade TW: Circulating levels of coagulation and inflammation
markers and cancer risks: Individual participant analysis of data
from three long-term cohorts. Int J Epidemiol. 39:699–709. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M and Degen JL:
Platelets and fibrin(ogen) increase metastatic potential by
impeding natural killer cell-mediated elimination of tumor cells.
Blood. 105:178–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Palumbo JS, Potter JM, Kaplan LS, Talmage
K, Jackson DG and Degen JL: Spontaneous hematogenous and lymphatic
metastasis, but not primary tumor growth or angiogenesis, is
diminished in fibrinogen-deficient mice. Cancer Res. 62:6966–6972.
2002.PubMed/NCBI
|
|
20
|
Fahham D, Merquiol E, Gilon T, Marx G and
Blum G: Insoluble fibrinogen particles for harvesting and expanding
attachment-dependent cells and for trapping suspended cancer cells
in the presence of blood. Biomed Mater. 10:0250102015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palumbo JS, Kombrinck KW, Drew AF, Grimes
TS, Kiser JH, Degen JL and Bugge TH: Fibrinogen is an important
determinant of the metastatic potential of circulating tumor cells.
Blood. 96:3302–3309. 2000.PubMed/NCBI
|
|
22
|
Wu KL, Tsai MJ, Yang CJ, Chang WA, Hung
JY, Yen CJ, Shen CH, Kuo TY, Lee JY, Chou SH, et al: Liver
metastasis predicts poorer prognosis in stage IV lung
adenocarcinoma patients receiving first-line gefitinib. Lung
Cancer. 88:187–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soria JC, Wu YL, Nakagawa K, Kim SW, Yang
JJ, Ahn MJ, Wang J, Yang JC, Lu Y, Atagi S, et al: Gefitinib plus
chemotherapy versus placebo plus chemotherapy in
EGFR-mutation-positive non-small-cell lung cancer after progression
on first-line gefitinib (IMPRESS): A phase 3 randomised trial.
Lancet Oncol. 16:990–998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamichi S, Kubota K, Horinouchi H, Kanda
S, Fujiwara Y, Nokihara H, Yamamoto N and Tamura T: Successful
EGFR-TKI rechallenge of leptomeningeal carcinomatosis after
gefitinib-induced interstitial lung disease. Jpn J Clin Oncol.
43:422–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishiguro Y, Ishiguro H and Miyamoto H:
Epidermal growth factor receptor tyrosine kinase inhibition
up-regulates interleukin-6 in cancer cells and induces subsequent
development of interstitial pneumonia. Oncotarget. 4:550–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaguchi T, Yamamoto Y, Yokota S,
Nakagawa M, Ito M and Ogura T: Involvement of interleukin-6 in the
elevation of plasma fibrinogen levels in lung cancer patients. Jpn
J Clin Oncol. 28:740–744. 1998. View Article : Google Scholar : PubMed/NCBI
|